Abstract
Context:
In autosomal dominant hypophosphatemic rickets (ADHR), fibroblast growth factor 23 (FGF23) resists cleavage, causing increased plasma FGF23 levels. The clinical phenotype includes variable onset during childhood or adulthood and waxing/waning of hypophosphatemia. Delayed onset after puberty in females suggests iron status may be important.
Objective:
Studies were performed to test the hypothesis that plasma C-terminal and intact FGF23 concentrations are related to serum iron concentrations in ADHR.
Design and Setting:
Cross-sectional and longitudinal studies of ADHR and a cross-sectional study in healthy subjects were conducted at an academic medical center.
Participants:
Participants included 37 subjects with ADHR mutations from four kindreds and 158 healthy adult controls.
Main Outcome Measure:
The relationships of serum iron concentrations with plasma C-terminal and intact FGF23 concentrations were evaluated.
Results:
Serum phosphate and 1,25-dihydroxyvitamin D correlated negatively with C-terminal FGF23 and intact FGF23 in ADHR but not in controls. Serum iron was negatively correlated to both C-terminal FGF23 (r = −0.386; P < 0.05) and intact FGF23 (r = −0.602; P < 0.0001) in ADHR. However, control subjects also demonstrated a negative relationship of serum iron with C-terminal FGF23 (r = −0.276; P < 0.001) but no relationship with intact FGF23. Longitudinally in ADHR subjects, C-terminal FGF23 and intact FGF23 concentrations changed negatively with iron concentrations (P < 0.001 and P = 0.055, respectively), serum phosphate changed negatively with C-terminal FGF23 and intact FGF23 (P < 0.001), and there was a positive relationship between serum iron and phosphate (P < 0.001).
Conclusions:
Low serum iron is associated with elevated FGF23 in ADHR. However, in controls, low serum iron was also associated with elevated C-terminal FGF23, but not intact FGF23, suggesting cleavage maintains homeostasis despite increased FGF23 expression.
Mutations in the fibroblast growth factor 23 (FGF23) gene cause autosomal dominant hypophosphatemic rickets (ADHR), which is characterized by impaired cleavage of the intact hormone (1). Osteoblasts/osteocytes produce FGF23, a hormone that reduces renal NPT2a (sodium phosphate co-transporter 2a), NPT2c (sodium phosphate co-transporter 2c), and vitamin D 1α-hydroxylase expression and stimulates 24-hydroxylase expression (2, 3), resulting in decreased renal phosphate reabsorption and decreased serum phosphate and 1,25-dihydroxyvitamin D (1,25D) concentrations. A proportion of FGF23 is normally cleaved before secretion, resulting in both intact (active) hormone and biologically inactive C-terminal and N-terminal fragments in the circulation (4, 5). Four different FGF23 mutations have been reported causing ADHR, each resulting in an amino acid change at an 176RXXR179/S180 consensus cleavage site (R176Q, R176W, R179Q, and R179W) (1, 6). These amino acid changes cause partial resistance to proteolytic cleavage of intact FGF23 (7) and increased plasma FGF23 concentrations (8), resulting in hypophosphatemia and low or inappropriately normal serum 1,25D.
In contrast to other inherited disorders of FGF23 excess (e.g. X-linked hypophosphatemia), ADHR shows incomplete penetrance and variable time of onset of the clinical phenotype. Some affected patients present in childhood with rickets and hypophosphatemia. Of these patients, some normalize serum phosphate spontaneously as they age. On the other hand, some patients grow normally and do not have hypophosphatemia but present in adolescence or adulthood with hypophosphatemic osteomalacia. Furthermore, adult ADHR patients with delayed onset can spontaneously normalize serum phosphate with resolution of clinical symptoms. Thus, there is evidence of the disease waxing and waning in ADHR, which coincides with, and is likely due to, variations in plasma FGF23 concentrations (8).
Under normal conditions in mice and humans, hypophosphatemia suppresses FGF23 production (9–11). For unclear reasons, subjects with an ADHR mutation cannot down-regulate FGF23 production during active disease, resulting in FGF23 concentrations that are inappropriately elevated with concomitant hypophosphatemia. However, some subjects regain normal regulation of plasma FGF23 concentrations, resulting in correction of hypophosphatemia (8). The cause for waxing and waning of plasma FGF23 concentrations in ADHR is not known.
Delayed onset of ADHR phenotype occurs primarily in postpubertal females (6, 8, 12). Iron deficiency is more common in women (38–40%) than men (6%) (13, 14). Thus, we hypothesized that plasma FGF23 concentrations are related to serum iron concentrations in subjects with ADHR but not in healthy controls. To test this hypothesis, a cross-sectional and longitudinal study of plasma FGF23 and iron status in patients with ADHR and a cross-sectional study in healthy men and premenopausal women were performed.
Materials and Methods
Study design
Samples were obtained during an ongoing observational study of families with ADHR. ADHR subjects were recruited from known kindreds. This study included a cross-sectional and longitudinal sample of subjects with ADHR and a cross-sectional sample of healthy subjects and evaluated the relationship between iron status and plasma FGF23. It was conducted in accordance with the Declaration of Helsinki and was approved under Indiana University-Purdue University at Indianapolis/Clarian Institutional Review Board. Written informed consent was obtained from all subjects.
Subjects
Only ADHR subjects with established FGF23 mutations were included in this analysis. ADHR subjects were from kindreds previously described (8, 12), with the addition of one newly identified kindred. Subjects from the observational study were selected for the cross-sectional study if sufficient serum and plasma samples were available. Fifteen subjects with ADHR mutations provided longitudinal samples during the observational protocol. The study sample was composed of members of one kindred with R176Q mutations (31 subjects), two kindreds with R179Q mutations (three subjects), and one kindred with R179W mutation (three subjects). Disease activity was assessed by serum phosphate concentrations; phosphate concentrations below 2.5 mg/dl were considered hypophosphatemic. Samples from healthy Caucasian controls (79 men and 79 premenopausal women) were selected randomly, obtained from a study on genetics of peak bone mass (15, 16).
Mutation detection
Mutations were determined by direct sequencing or with restriction fragment length polymorphism for FGF23 exon 3 for some patients with R176Q, as previously described (1).
Measurements
Fasting blood samples were stored at −80 C until analysis. Serum biochemistries were measured with the Roche Cobas Mira S (Roche, Indianapolis, IN) for phosphate, creatinine, alkaline phosphatase, iron, and total iron-binding capacity (TIBC). Reagents for phosphate, creatinine, and alkaline phosphatase were from Thermo Scientific (Waltham, MA) and for iron and TIBC from Pointe Scientific, Inc. (Canton, MI). Iron deficiency was defined as iron concentration below 50 μg/dl. PTH and 1,25D were measured using RIA from DiaSorin (Stillwater, MN).
FGF23 was measured in EDTA-plasma using two different ELISA. Plasma intact FGF23 was measured with an ELISA from Kainos Laboratories (Tokyo, Japan), using monoclonal antibodies that bind to either side of the 176RXXR179/S180 cleavage site. This assay measures intact FGF23 in picograms per milliliter. FGF23 was also measured using an ELISA with polyclonal antibodies directed against two different epitopes on the C-terminal portion of the molecule. The C-terminal assay thus detects the combination of both C-terminal fragments and full-length FGF23 (Immutopics, San Clemente, CA). The C-terminal assay measures FGF23 in relative units (RU) per milliliter. The coefficient of variation was 4.4% for intact FGF23 and 4.0% for C-terminal FGF23.
Statistics
The distribution of variables was examined, and appropriate normalizing transformations were performed where needed (for PTH, 1,25D, C-terminal FGF23, and intact FGF23). Differences between ADHR and control groups were tested with t tests for means and χ2 tests for proportions. Regression was used to test differences in means while controlling for age and sex. To investigate pair-wise relationships between variables, multiple regression with an appropriate interaction term was used to test whether the relationship differed significantly between ADHR and controls. When significant interactions were detected, pair-wise relationships between variables were examined within each group using scatter plots and Pearson correlation. To investigate the relationship between two variables changing longitudinally within individuals, a mixed-effects model for one variable was developed with the individual subjects as random effects and the other variable as a fixed effect. The P values for longitudinal relationships were reported without R2, which is not well defined in mixed models. Statistical analysis was made using SAS version 9.2.
Results
Cross-sectional data
All subjects in the ADHR group had mutations in FGF23, and subjects with and without active hypophosphatemic disease were included. The 37 subjects with ADHR were older (mean age 45 yr) with a wider age range (14–85 yr) than the 158 healthy controls (mean age 28 yr, age range 20–51 yr) (Table 1). Two adolescent subjects were in the ADHR group, aged 14 and 17 yr.
Table 1.
Baseline biochemical characteristics
| Controlsa | ADHR | |
|---|---|---|
| n | 158 | 37 |
| Male/female | 79/79 | 16/21 |
| Age (yr) | 27.7 ± 7.3 | 44.7 ± 16.4b |
| Creatinine (mg/dl) | 0.9 ± 0.2 | 1.0 ± 0.2 |
| Phosphate (mg/dl) | 3.4 ± 0.5 | 2.7 ± 0.6b |
| Phosphate <2.5 mg/dl [n (%)] | 4 (3%) | 11 (30%)b |
| Alkaline phosphatase (U/liter) | 58 ± 16 | 88 ± 30b |
| 1,25D (pg/ml)a | 44.6 ± 17.0 | 47.3 ± 15.1 |
| Log 1,25Da | 1.6 ± 0.2 | 1.7 ± 0.1 |
| PTH (pg/ml)a | 28.8 ± 10.6 | 35.5 ± 16.0c |
| Log PTHa | 1.4 ± 0.1 | 1.5 ± 0.2c |
| Iron (μg/dl) | 93.6 ± 40.5 | 92.7 ± 31.6 |
| Iron <50 μg/dl [n (%)] | 18 (11%) | 3 (8%) |
| Iron >160 μg/dl [n (%)] | 9 (6%) | 2 (5%) |
| TIBC (μg/dl) | 329 ± 63 | 340 ± 45 |
| Iron % saturation | 29.1 ± 12.7 | 27.9 ± 10.8 |
| Iron saturation <20% [n (%)] | 37 (23%) | 9 (24%) |
| Intact FGF23 (pg/ml) | 40 ± 13 | 43 ± 25 |
| Log intact FGF23 (log of pg/ml) | 1.58 ± 0.14 | 1.59 ± 0.20 |
| C-terminal FGF23 (RU/ml) | 83 ± 52 | 105 ± 215 |
| Log C-terminal FGF23 (log of RU/ml) | 1.86 ± 0.20 | 1.78 ± 0.33 |
Unless indicated otherwise, results are given as mean ± sd. To convert the values for phosphate to millimoles per liter, multiply by 0.32. To convert calcium to millimoles per liter, multiply by 0.25. To convert creatinine to micromoles per liter, multiply by 76.26. To convert 1,25D to picomoles per liter, multiply by 2.6. To convert PTH to picomoles per liter, multiply by 0.1053. To convert iron to micromoles per liter, multiply by 0.179. To convert TIBC to micromoles per liter, multiply by 0.179.
For controls, n = 129 for PTH, and n = 152 for 1,25D measurements.
P <0.0001.
P <0.05.
Mean serum phosphate concentrations were lower in ADHR subjects compared with controls (P < 0.0001), and mean serum alkaline phosphatase concentrations were higher in ADHR subjects (P < 0.0001) (Table 1). However, not all the ADHR subjects had active disease as determined by serum phosphate concentration. Although 11 of 37 subjects with ADHR had hypophosphatemia (serum phosphate <2.5 mg/dl), over half of the ADHR subjects had serum phosphate concentrations higher than 2.5 mg/dl. There was no difference between groups for serum 1,25D concentration, but the PTH concentration was slightly higher in ADHR subjects than controls (P < 0.05). There were no differences between ADHR patients and controls in serum creatinine, iron, TIBC, and percent iron saturation.
Because the FGF23 mutation affects cleavage, FGF23 was measured using two assays: one that detects only biologically active (intact FGF23) and another that detects both intact FGF23 and inactive C-terminal fragments (C-terminal FGF23). Plasma intact FGF23 and C-terminal FGF23 concentrations were normally distributed in controls but not in ADHR subjects; thus, log transformation was performed. The mean plasma FGF23 concentration and mean log FGF23 concentration for both C-terminal FGF23 and intact FGF23 were not different between ADHR patients and control subjects (Table 1), consistent with previous findings in ADHR patients (8). Mean log C-terminal FGF23 was slightly lower in ADHR patients (P = 0.025) when controlling for age and sex. Visual inspection of ADHR data indicated two subjects with very high FGF23 concentrations, who were the most severely affected based on lower serum phosphate and higher serum alkaline phosphatase concentrations. These subjects had log intact FGF23 more than 2.5 sd above the mean and log C-terminal FGF23 more than 3 sd above the mean. Both had serum phosphate below 2 mg/dl. No control subject had log intact FGF23 higher than 2.5 sd above the control mean, but two had log C-terminal FGF23 more than 3 sd above the mean. The log C-terminal FGF23 and log intact FGF23 were more strongly correlated in the ADHR group (r = 0.616; P < 0.01) than in the control group (r = 0.279; P < 0.01), although the difference in regression slopes did not reach statistical significance (P = 0.07).
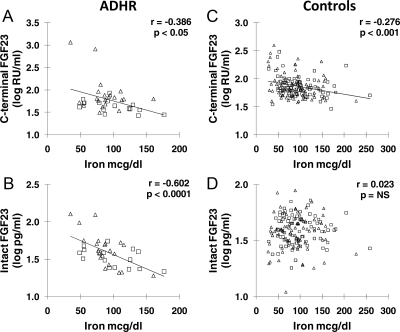
Log intact FGF23 and log C-terminal FGF23 were correlated with iron concentration in ADHR and in control subjects separately. Serum iron correlated negatively with both log C-terminal FGF23 (r = −0.386; P < 0.05) and log intact FGF23 (r = −0.602; P < 0.0001) in ADHR (Fig. 1, A and B). Percent iron saturation also correlated negatively with log C-terminal FGF23 (r = −0.453; P < 0.01) and log intact FGF23 (r = −0.502; P < 0.01). When excluding the two ADHR subjects with the highest serum intact FGF23 and lowest serum phosphate, the relationships of serum iron concentration with plasma log intact FGF23 remained significant (P < 0.001), but the relationship with plasma log C-terminal FGF23 was no longer significant (P = 0.13).
Fig. 1.
Iron and FGF23. Cross-sectional plots are shown of serum iron with plasma log intact FGF23 and log C-terminal FGF23 for ADHR and control subjects. A and B, ADHR subjects; C and D, healthy controls (▵, females; □, males). To easily view the distribution of variables, different scales are used for some axes between ADHR and controls.
Similarly, control subjects also demonstrated a significant negative relationship between serum iron and log C-terminal FGF23 (r = −0.276; P < 0.001), but in contrast to ADHR patients, there was no relationship with log intact FGF23 (Fig. 1, C and D). Although the relationship between serum iron and log C-terminal FGF23 was negative for both ADHR and controls, the slopes differed significantly between groups (P = 0.03). The relationship between serum iron and log intact FGF23 was different between groups (P < 0.0001) with a negative relationship in ADHR (r = −0.602) but no relationship in controls. The percent iron saturation also correlated negatively to log C-terminal FGF23 (r = −0.296; P < 0.001) but not to log intact FGF23 in controls. Males and females had similar negative relationships between serum iron and log C-terminal FGF23 concentrations in both the ADHR and control groups. Thus, log transformed intact and C-terminal FGF23 concentrations correlated negatively with iron concentration in ADHR patients, whereas in control subjects, C-terminal FGF23 was negatively related to iron concentrations, but there was no relationship between intact FGF23 and iron.
For ADHR patients (n = 22) with serum iron below 94 μg/dl (the control mean), the relationship between iron and log C-terminal FGF23 was no longer significant, but iron was still significantly correlated to log intact FGF23 (r = −0.508; P = 0.02). In control subjects (n = 89) with serum iron below 94 μg/dl, the relationship between serum iron and log C-terminal FGF23 was slightly stronger (r = −0.335; P = 0.001), compared with the whole control group (r = −0.276), but iron was still not related to log intact FGF23.
Because intact FGF23 is necessary for the pathogenesis of ADHR, the relationships between plasma FGF23, serum phosphate, 1,25D, PTH, and alkaline phosphatase were examined. In ADHR, serum phosphate correlated negatively with log C-terminal FGF23 (r = −0.607; P < 0.0001), but this correlation did not reach statistical significance with log intact FGF23 (r = −0.278; P = 0.096) (Fig. 2, A and B), although the correlations were driven by the two outliers with the highest log C-terminal FGF23. Among hypophosphatemic ADHR subjects (serum phosphate below 2.5 mg/dl, n = 11), the negative correlation between log intact FGF23 and serum phosphate was significant(r = −0.727; P = 0.006). In this group, the relationships of iron with log C-terminal FGF23 (r = −0.702) and log intact FGF23 (r = −0.677) also remained significant (both P = 0.02). In control subjects, serum phosphate did not correlate with either log C-terminal FGF23 or log intact FGF23 (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). Regression analyses demonstrated that the relationships of intact FGF23 and C-terminal FGF23 with phosphate differed significantly between ADHR and controls (P < 0.001).
Fig. 2.
FGF23, phosphate, 1,25D, and alkaline phosphatase in ADHR. Cross-sectional plots are shown for ADHR relationships of plasma log C-terminal FGF23 and log intact FGF23 with serum phosphate (A snd B), alkaline phosphatase (C and D), and 1,25D (E and F) as dependent variables (▵, females; □, males).
In ADHR subjects, log 1,25D negatively correlated with log C-terminal FGF23 (r = −0.554; P < 0.001), but the log 1,25D correlation with log intact FGF23 did not reach statistical significance (r = −0.300; P = 0.072) (Fig. 2, E and F). In control subjects, log 1,25D was not significantly related to either log C-terminal FGF23 or to log intact FGF23 (Supplemental Fig. 1).
Alkaline phosphatase is frequently elevated in ADHR patients with active hypophosphatemic osteomalacia (Table 1). Serum alkaline phosphatase was positively related in ADHR to both log C-terminal FGF23 (r = 0.678; P < 0.0001) and log intact FGF23 (r = 0.337; P = 0.041) (Fig. 2, C and D). There was also a slight positive relationship in controls between alkaline phosphatase and log C-terminal FGF23 (r = 0.159; P = 0.046) but not with log intact FGF23 (Supplemental Fig. 1). Although in ADHR, the relationship between FGF23 and alkaline phosphatase is likely mediated through effects of hypophosphatemia, the reason for a correlation between C-terminal FGF23 and alkaline phosphatase in controls is not clear, but both may reflect osteoblastic activity.
Log PTH was negatively related to serum phosphate concentrations in both ADHR (r = −0.475) and in controls (r = −0.247; both P < 0.01). However, in ADHR subjects, log PTH was not related to log C-terminal or intact FGF23 or to serum iron concentrations. In controls, log PTH was positively related to log C-terminal FGF23 (r = 0.205; P < 0.05) and negatively related to serum iron concentrations (r = −0.329; P < 0.001).
In multiple regression, log PTH did not predict log intact FGF23 or log C-terminal FGF23 when adjusting for serum iron concentrations in either ADHR or controls. However, log 1,25D did remain a significant predictor of log C-terminal FGF23 in ADHR (P < 0.001) when adjusting for serum iron concentrations and an interaction factor for iron with 1,25D. However, this may be caused by action of FGF23 on 1,25D metabolism directly.
Serum phosphate and 1,25D concentrations are biochemical measures of ADHR disease activity. Serum phosphate was not directly related to iron or iron saturation in ADHR patients in cross-sectional analysis. However, log 1,25D was positively related to serum iron concentrations (r = 0.334; P = 0.04) in ADHR subjects. Neither serum phosphate nor log 1,25D was related to serum iron concentration in control subjects.
Longitudinal data
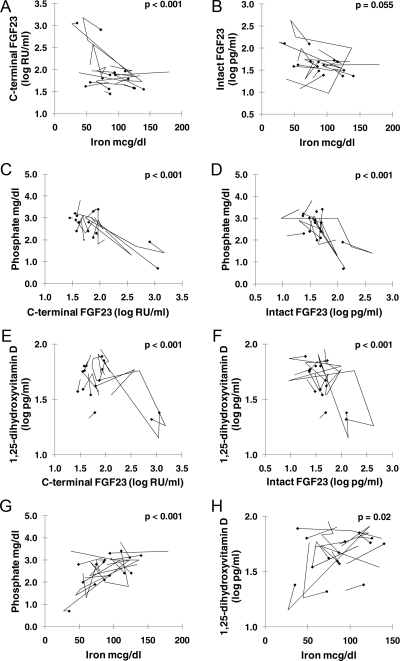
To examine whether the cross-sectional relationships between iron and FGF23 in ADHR were corroborated in longitudinal studies, a subset of 15 subjects within the ADHR group were studied. Each subject had two to six blood samples taken over 1–8 yr. Figure 3 shows how serum iron, plasma log C-terminal FGF23, log intact FGF23, and serum phosphate and 1,25D concentrations traverse within individuals over sequential measurements. Analyses indicated that plasma log C-terminal FGF23 and log intact FGF23 changed negatively with serum iron within individuals (log C-terminal FGF23 P < 0.001, and log intact FGF23 P = 0.055) (Fig. 3, A and B). The two subjects with the highest initial log C-terminal FGF23 had large FGF23 changes associated with changes in serum iron concentration that dominated the overall negative relationship. Serum phosphate concentrations moved negatively with log intact FGF23 and log C-terminal FGF23 within individuals (P < 0.001) (Fig. 3, C and D). There was a positive relationship between serum phosphate and iron concentrations (P < 0.001) within individuals in the longitudinal sample (Fig. 3G) that was not detected in the cross-sectional sample. Log PTH (n = 14) was not significantly related to serum iron concentrations, log intact FGF23, or log 1,25D. However, log PTH was positively related to log C-terminal FGF23 (P = 0.04) and negatively related to serum phosphate concentrations (P = 0.03). Log 1,25D (n = 14) was positively related to serum iron concentrations (Fig. 3H) (P = 0.02) and negatively related to log intact FGF23 and log C-terminal FGF23 (Fig. 3, E and F) (P < 0.001). Thus, longitudinally, the changes in plasma FGF23 in relation to serum iron concentrations occurred with concomitant changes in serum phosphate and 1,25D concentrations.
Fig. 3.
ADHR longitudinal data: iron, FGF23, 1,25D, and phosphate. A and B, Longitudinal plots are shown for relationships of serum iron to plasma log C-terminal FGF23 (A) and plasma log intact FGF23 (B). C and D illustrate the relationships of plasma log C-terminal FGF23 and log intact FGF23 to serum phosphate, longitudinally. Note similarities in distribution with the cross-sectional graphs in Figs. 1 and 2. E and F illustrate the relationships of plasma log C-terminal FGF23 and log intact FGF23 to serum 1,25D, longitudinally. G and H, Longitudinal plot of the relationships of serum iron and serum phosphate (G) and longitudinal plots of the relationship of serum iron and 1,25D (H). For all measurements, n = 15, except 1,25D (n = 14). For each graph, the diamonds indicate the initial sample, and the lines indicate changes in parameters over sequential measurements.
Discussion
This study demonstrates a negative relationship between serum iron and plasma FGF23 concentrations in healthy controls and in ADHR. However, despite similar occurrences of iron deficiency, there were differences in the patterns of relationships of serum iron concentrations with C-terminal and intact FGF23 concentrations between controls and ADHR patients. These data suggest that changes in serum iron concentrations are involved in regulating FGF23 concentrations and in the pathogenesis of delayed onset and waxing/waning of the ADHR phenotype.
In healthy controls, serum iron was negatively related to plasma C-terminal FGF23 (which includes inactive fragments) but not to intact FGF23. If low iron concentrations increase FGF23 cleavage, phosphate and 1,25D feedback loops should stimulate higher expression and secretion of FGF23 to maintain normal intact FGF23 concentrations and normal phosphate and 1,25D homeostasis. Thus, increased expression and cleavage could explain the accumulation of C-terminal FGF23 in controls. However, it appears that low iron concentration alone is not sufficient to generate inappropriately elevated intact FGF23 concentrations and hypophosphatemia.
In contrast, ADHR patients demonstrated negative relationships of serum iron concentrations to both C-terminal and intact FGF23 concentrations. Furthermore, longitudinal changes in serum iron concentrations corresponded inversely to intact FGF23 concentrations with accompanying significant changes in both serum phosphate and 1,25D. During normophosphatemia, ADHR patients appeared to regulate FGF23 appropriately, and as a group, mean intact FGF23 concentrations were not higher than in controls. Only a subset of ADHR patients had elevated FGF23 concentrations.
Although iron levels altering FGF23 cleavage could explain the control results, this does not explain the elevated intact FGF23 in ADHR patients. If low iron increased cleavage of FGF23 at the 176RXXR179/S180 motif, the ADHR mutation would likely protect against that cleavage, and there would not be a compensatory increased production of FGF23. Consequently, neither C-terminal nor intact FGF23 concentrations would become elevated. However, if low iron caused increased cleavage at alternate sites in the protein, ADHR patients would develop a similar relationship of serum iron and FGF23 to control subjects, and like control subjects, they would not develop excess intact FGF23 or hypophosphatemia.
Thus, increased FGF23 expression and secretion during low iron states is the most likely explanation for both the ADHR and control data. PTH did not appear to mediate this process in regression analysis. FGF23 likely does not regulate iron metabolism directly because iron deficiency is not part of the phenotype of other disorders associated with excess plasma FGF23 (e.g. X-linked hypophosphatemia and tumor-induced osteomalacia).
Both iron deficiency and excess are associated with altered skeletal metabolism. Iron deficiency is associated with stress fractures (14). Conversely, in vitro, excess iron inhibits markers of osteoblast differentiation (17). Iron overload in hereditary hemochromatosis, even without hypogonadism, is associated with osteoporosis (18). On bone biopsies from eugonadal men with hemochromatosis, lower osteoblastic surface area and bone formation rates were found in those who were not yet treated with phlebotomy (19). Because FGF23 is expressed in osteoblast/osteocyte lineage cells, iron may have a direct or indirect impact on FGF23 metabolism.
One previous study reported an inverse relationship between serum ferritin and C-terminal FGF23 but not with the intact FGF23 assay used in the current study (20). Although the authors did not measure iron, the very low ferritin concentrations were consistent with iron deficiency. Similarly, in our healthy controls, we found a relationship between iron and C-terminal FGF23 but not with intact FGF23. One difference between these studies is that we measured FGF23 in EDTA-plasma as opposed to serum. EDTA binds iron as well as calcium, eliminating potential confounding effects of iron concentration on the assay itself.
Iron polymaltose infusions were recently demonstrated to raise intact FGF23 concentrations causing hypophosphatemia and osteomalacia (21–23). In one case, cessation of serial iron polymaltose infusions led to normalization of FGF23 and phosphate, improving the osteomalacia (21). These authors also prospectively demonstrated that single iron polymaltose infusions increased intact FGF23 and decreased plasma phosphate in eight iron-deficient subjects (22). However, not all iron preparations are associated with renal phosphate wasting and osteomalacia in mice or humans (23, 24).
The above findings appear to be counter to our findings, where low iron concentration is related to elevated C-terminal FGF23 concentrations. However, it should be noted that iron infusions are only administered to iron-deficient patients. A direct effect of iron on FGF23 production is possible. However, additional factors related to both iron deficiency and the response to iron infusion may be interacting to affect FGF23. Possibly, iron infusion might also impact the degradation pathway transiently, increasing intact FGF23 in subjects who may have already been primed for increased FGF23 expression due to iron deficiency.
Limitations of this study include inability to determine the prevalence of anemia or to directly assess other factors such as estrogen. Correction in analysis for sex only partially adjusts for effects of estrogen. Furthermore, comprehensive assessment of TmP/GFR (tubular maximum reabsorption of phosphate per unit glomerular filtration rate) was not possible. In addition, the relationship between the C-terminal FGF23 assay and the intact FGF23 assay is imperfect, and the relative molar contribution of intact or fragments of FGF23 cannot be precisely determined with these methods. In fact, in some control and ADHR subjects, the C-terminal FGF23 concentration was numerically less than the intact FGF23 concentration.
A strength of this study is the inclusion of subjects with ADHR mutations both with and without active hypophosphatemia, allowing analysis of iron status across a range of disease activity. Although the study population of ADHR subjects is small, it is the largest known group of ADHR subjects under study in the world (1, 8, 12). In addition, longitudinal follow-up on a number of ADHR patients confirmed a similar pattern over time for changes in serum iron, FGF23, 1,25D, and phosphate.
These data have both mechanistic and clinical importance. The results suggest a novel hormone regulatory mechanism for FGF23. In this regard, an aberrant stimulus (low iron levels) results in increased FGF23 mRNA and protein production. However, plasma intact FGF23 elevations that are inappropriate to the patient's phosphate status are prevented by cleavage to inactive fragments. This also suggests that FGF23 cleavage is a regulated step. Normal homeostasis of the hormone's primary targets (phosphate and 1,25D) is thus maintained, unless a mutation causes resistance to cleavage. This offers a potential explanation for the observed clinical fluctuation of FGF23 concentrations, hypophosphatemia, and osteomalacia in ADHR. Low-dose iron supplementation might benefit ADHR patients, but testing should be done in ADHR mice before attempting human trials, because of the observed increase in FGF23 associated with iron polymaltose infusions.
Furthermore, these results have relevance to the clinical utility of FGF23 assays. Patients may develop elevations of plasma C-terminal FGF23 due to iron deficiency but have normal intact FGF23. In fact, of 10 control subjects with the highest C-terminal FGF23 concentrations (ranging from 177–392 RU/ml), half had serum iron below 50 μg/dl, all but one had normal intact FGF23 concentrations (25, 26), and all had normal serum phosphate and creatinine concentrations.
In conclusion, we demonstrate a novel relationship between iron and FGF23 in the pathophysiology of ADHR. Likewise, low iron levels are associated with elevated C-terminal FGF23 but not intact FGF23 in controls, suggesting increased expression, but cleavage to maintain homeostasis. These data suggest that iron deficiency may be the trigger for dysregulation of FGF23 in ADHR. Additional studies are necessary to determine the underlying mechanism of this relationship.
Acknowledgments
This study was funded by National Institutes of Health Grants K23AR057096, R01AR42228, P01 AG18397, and UL1RR025761.
Data from this manuscript were presented in part at the 2009 Annual Meeting of the American Society for Bone and Mineral Research in Denver, CO.
Disclosure Summary: M.J.E. receives royalties from Kyowa Hakko Kirin Pharma, Inc., and E.A.I. and M.P. participate in a clinical trial with Kyowa Hakko Kirin Pharma, Inc. All other authors have no disclosures regarding this manuscript.
Footnotes
- ADHR
- Autosomal dominant hypophosphatemic rickets
- 1,25D
- 1,25-dihydroxyvitamin D
- FGF23
- fibroblast growth factor 23
- RU
- relative units
- TIBC
- total iron-binding capacity.
References
- 1. ADHR Consortium 2000. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 26:345–348 [DOI] [PubMed] [Google Scholar]
- 2. Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. 2004. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19:429–435 [DOI] [PubMed] [Google Scholar]
- 3. Larsson T, Marsell R, Schipani E, Ohlsson C, Ljunggren O, Tenenhouse HS, Jüppner H, Jonsson KB. 2004. Transgenic mice expressing fibroblast growth factor 23 under the control of the α1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology 145:3087–3094 [DOI] [PubMed] [Google Scholar]
- 4. Benet-Pagès A, Lorenz-Depiereux B, Zischka H, White KE, Econs MJ, Strom TM. 2004. FGF23 is processed by proprotein convertases but not by PHEX. Bone 35:455–462 [DOI] [PubMed] [Google Scholar]
- 5. Shimada T, Muto T, Urakawa I, Yoneya T, Yamazaki Y, Okawa K, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. 2002. Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology 143:3179–3182 [DOI] [PubMed] [Google Scholar]
- 6. Gribaa M, Younes M, Bouyacoub Y, Korbaa W, Ben Charfeddine I, Touzi M, Adala L, Mamay O, Bergaoui N, Saad A. 2010. An autosomal dominant hypophosphatemic rickets phenotype in a Tunisian family caused by a new FGF23 missense mutation. J Bone Miner Metab 28:111–115 [DOI] [PubMed] [Google Scholar]
- 7. White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ. 2001. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int 60:2079–2086 [DOI] [PubMed] [Google Scholar]
- 8. Imel EA, Hui SL, Econs MJ. 2007. FGF23 concentrations vary with disease status in autosomal dominant hypophosphatemic rickets. J Bone Miner Res 22:520–526 [DOI] [PubMed] [Google Scholar]
- 9. Antoniucci DM, Yamashita T, Portale AA. 2006. Dietary phosphorus regulates serum FGF-23 concentrations in healthy men. J Clin Endocrinol Metab 91:3144–3149 [DOI] [PubMed] [Google Scholar]
- 10. Burnett SM, Gunawardene SC, Bringhurst FR, Jüppner H, Lee H, Finkelstein JS. 2006. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res 21:1187–1196 [DOI] [PubMed] [Google Scholar]
- 11. Perwad F, Azam N, Zhang MY, Yamashita T, Tenenhouse HS, Portale AA. 2005. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology 146:5358–5364 [DOI] [PubMed] [Google Scholar]
- 12. Econs MJ, McEnery PT. 1997. Autosomal dominant hypophosphatemic rickets/osteomalacia: clinical characterization of a novel renal phosphate-wasting disorder. J Clin Endocrinol Metab 82:674–681 [DOI] [PubMed] [Google Scholar]
- 13. Heath AL, Skeaff CM, Williams S, Gibson RS. 2001. The role of blood loss and diet in the aetiology of mild iron deficiency in premenopausal adult New Zealand women. Public Health Nutr 4:197–206 [DOI] [PubMed] [Google Scholar]
- 14. Merkel D, Moran DS, Yanovich R, Evans RK, Finestone AS, Constantini N, Israeli E. 2008. The association between hematological and inflammatory factors and stress fractures among female military recruits. Med Sci Sports Exerc 40:S691–S697 [DOI] [PubMed] [Google Scholar]
- 15. Ichikawa S, Koller DL, Johnson ML, Lai D, Xuei X, Edenberg HJ, Klein RF, Orwoll ES, Hui SL, Foroud TM, Peacock M, Econs MJ. 2006. Human ALOX12, but not ALOX15, is associated with BMD in white men and women. J Bone Miner Res 21:556–564 [DOI] [PubMed] [Google Scholar]
- 16. Koller DL, Ichikawa S, Lai D, Padgett LR, Doheny KF, Pugh E, Paschall J, Hui SL, Edenberg HJ, Xuei X, Peacock M, Econs MJ, Foroud T. 2010. Genome-wide association study of bone mineral density in premenopausal European-American women and replication in African-American women. J Clin Endocrinol Metab 95:1802–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamasaki K, Hagiwara H. 2009. Excess iron inhibits osteoblast metabolism. Toxicol Lett 191:211–215 [DOI] [PubMed] [Google Scholar]
- 18. Valenti L, Varenna M, Fracanzani AL, Rossi V, Fargion S, Sinigaglia L. 2009. Association between iron overload and osteoporosis in patients with hereditary hemochromatosis. Osteoporos Int 20:549–555 [DOI] [PubMed] [Google Scholar]
- 19. Diamond T, Stiel D, Posen S. 1989. Osteoporosis in hemochromatosis: iron excess, gonadal deficiency, or other factors? Ann Intern Med 110:430–436 [DOI] [PubMed] [Google Scholar]
- 20. Durham BH, Joseph F, Bailey LM, Fraser WD. 2007. The association of circulating ferritin with serum concentrations of fibroblast growth factor-23 measured by three commercial assays. Ann Clin Biochem 44:463–466 [DOI] [PubMed] [Google Scholar]
- 21. Schouten BJ, Doogue MP, Soule SG, Hunt PJ. 2009. Iron polymaltose-induced FGF23 elevation complicated by hypophosphataemic osteomalacia. Ann Clin Biochem 46:167–169 [DOI] [PubMed] [Google Scholar]
- 22. Schouten BJ, Hunt PJ, Livesey JH, Frampton CM, Soule SG. 2009. FGF23 elevation and hypophosphatemia after intravenous iron polymaltose: a prospective study. J Clin Endocrinol Metab 94:2332–2337 [DOI] [PubMed] [Google Scholar]
- 23. Shimizu Y, Tada Y, Yamauchi M, Okamoto T, Suzuki H, Ito N, Fukumoto S, Sugimoto T, Fujita T. 2009. Hypophosphatemia induced by intravenous administration of saccharated ferric oxide: another form of FGF23-related hypophosphatemia. Bone 45:814–816 [DOI] [PubMed] [Google Scholar]
- 24. Sanai T, Oochi N, Okada M, Imamura K, Okuda S, Iida M. 2005. Effect of saccharated ferric oxide and iron dextran on the metabolism of phosphorus in rats. J Lab Clin Med 146:25–29 [DOI] [PubMed] [Google Scholar]
- 25. Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, Takeuchi Y, Fujita T, Nakahara K, Yamashita T, Fukumoto S. 2002. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab 87:4957–4960 [DOI] [PubMed] [Google Scholar]
- 26. Imel EA, Peacock M, Pitukcheewanont P, Heller HJ, Ward LM, Shulman D, Kassem M, Rackoff P, Zimering M, Dalkin A, Drobny E, Colussi G, Shaker JL, Hoogendoorn EH, Hui SL, Econs MJ. 2006. Sensitivity of fibroblast growth factor 23 measurements in tumor-induced osteomalacia. J Clin Endocrinol Metab 91:2055–2061 [DOI] [PubMed] [Google Scholar]