Abstract
Context:
The type, dose, and route of 17β-estradiol (E2) used to feminize girls with Turner syndrome (TS) is not well established.
Objective:
The objective of the study was to characterize pharmacokinetics and pharmacodynamics of oral vs. transdermal E2.
Setting:
The study was conducted at a clinical research center.
Subjects:
Ten girls with TS, mean age 17.7 ± 0.4 (se) yr and 20 normally menstruating controls (aged 16.8 ± 0.4 yr) participated in the study.
Interventions:
TS subjects were randomized 2 wk each to: low-dose daily oral (0.5 mg) and biweekly transdermal E2 (0.0375 mg) with 2 wk washout in between or high-dose oral (2.0 mg) and transdermal (0.075 mg), studied for 24 h each. Tandem mass spectrometry E2 and estrone (E1) assays and a recombinant cell bioassay were used.
Results:
Controls consisted of the following: E2, 96 ± 11 pg/ml (se), E1, 70 ± 7 (mean follicular/luteal). TS consisted of the following: E2, average concentration on low-dose oral, 18 ± 2.1 pg/ml, low-dose transdermal, 38 ± 13, high-dose oral, 46 ± 15, high-dose transdermal, 114 ± 31 pg/ml. E1 concentrations were much higher on oral E2 (low or high dose) than transdermal in TS and higher than controls. Bioestrogen was closest to normal in the high-dose transdermal group. LH and FSH decreased more in transdermal than oral low-dose routes and similarly in the high-dose oral and transdermal groups. IGF-I concentrations were variable (P = NS) among groups, and low-density lipoprotein/high-density lipoprotein cholesterol responses were variable.
Conclusions:
Transdermal E2 results in E2, E1, and bioestrogen concentrations closer to normal and achieves greater suppression of LH/FSH in lower doses compared with normal. Whether the long-term metabolic effects of estrogen differ using the same form of E2, depending on route, awaits further study in TS.
Physiologically, estrogen is necessary for feminization during puberty and to allow achievement of peak bone mass and normal bone health in the female (1). Estrogen primarily lowers bone resorption in adults, yet the effects in children primarily result in bone maturation, skeletal consolidation, and skeletal growth, although they eventually also cause epiphyseal fusion and completion of linear growth. Hence, proper replacement is important in hypogonadal girls.
There is wide variation in the types of estrogens used for replacement as well as in doses and routes of administration. Although ethinyl estradiol has been commonly used as replacement therapy in research studies in Turner syndrome (2–4), since 2004 this is no longer commercially available as a single agent in the United States. The predominant type of estrogen used clinically to replace hypogonadal girls until recently in the United States has been conjugated equine estrogen (CEE) (5), which has more than 100 individual estrogenic compounds of different biologic potency and cannot be measured in conventional assays. In the postmenopausal literature, a number of studies have suggested that estrogen given orally (PO) has deleterious effects on body composition and lipid oxidation compared with transdermal (TD) (6, 7) and that it could serve as a GH antagonist (8), although these findings have not been consistently confirmed (9, 10). However, the studies used not only different routes but also different forms of estrogen altogether [CEE oral vs. 17β-estradiol (E2) TD], and bioequivalencies are not well established and have been based mostly on degree of suppression of gonadotropins and vaginal cytology (11–13).
Girls with Turner syndrome represent an important case study for these issues because they have early primary gonadal insufficiency or failure many years before the achievement of peak bone mass. Micronized E2, identical with the product of the ovary, is available both orally and TD and should be considered when replacing young hypogonadal women because it is perhaps the most physiological form of estrogen available. In a previous study, we used the same type of E2 orally and TD in girls with Turner syndrome in 16-wk experiments and found no differences in rates of protein synthesis, degradation, or lipid oxidation or whole-body lypolysis between the estrogens, depending on route (14). This new study seeks to characterize the pharmacokinetics (PK) and pharmacodynamics (PD) of the exact same form of E2 using a very sensitive liquid chromatography mass spectrometry/mass spectrometry (LCMSMS) assay and a recombinant cell bioassay using a transformed yeast expressing the estrogen receptor in girls with Turner syndrome.
Subjects and Methods
These studies were approved by the Wolfson Children's Hospital Institutional Review Committee. Informed written consent/assent was obtained before study entry from the parents/guardians and subjects (if older than 18 yr) and children's assent. The PK/PD study was the first arm of a larger ongoing trial registered [in Clinicaltrial.gov (NCT00837616)].
Study subjects
Ten girls with Turner syndrome (45X and related karyotypes), between the ages of 13 and 20 yr, were recruited. Study subjects were referred to the Nemours Children's Clinic (Jacksonville, FL) from several pediatric endocrine clinics and through web-based advertising. By design, subjects had completed or nearly completed their linear growth (defined as a bone age equal or more than 14 yr and a growth velocity of <2 cm/yr) and any previous GH therapy discontinued at least 6 months before study participation. All were hypogonadal as indicated by elevated FSH concentrations. Any subject on estrogen replacement therapy had medications discontinued for at least 6 wk before baseline studies. Subjects with significant obesity (body mass index >36 kg/m2) or history of systemic illness were excluded from participation.
Twenty healthy, normally menstruating (every 28–30 d) girls on no medications and not on oral contraceptives were recruited for comparison.
Study design
A physical examination including pubertal staging and laboratory studies were performed at baseline in all the girls. Blood samples for measurement of plasma lipids, estradiol and estrone concentrations, LH, FSH, IGF-I, and highly sensitive C-reactive protein (hsCRP) were obtained. Subjects were randomized into two study groups, assigned to take estrogenic compounds as follows. The lower-dose group took either E2 0.5 mg orally (Estrace; Bristol-Myers Squibb, New York, NY) daily for 2 wk or E2 0.0375 mg twice weekly TD for 2 wk (Vivelle TD system; Novartis Pharmaceuticals, East Hanover, NJ). The higher-dose group took either E2 2.0 mg orally daily or twice-weekly E2 0.075 mg TD for 2 wk. After the baseline samples were obtained, subjects were started on daily doses of the assigned oral estrogen preparations or twice a week changed estradiol patches for 2 wk (transdermal patches delivered stated concentrations of estradiol daily). On the morning of d 15, each patient was admitted to the clinical research center while on the estrogen formulation and blood samples collected at 0, 4, 8, 12, 16, and 24 h after dosing. Estradiol was then stopped for 2 wk to achieve washout of estrogen and blood was withdrawn again; then the second assigned dose/form of estrogen was started for 2 more weeks, at which time another PK/PD study was again repeated at the same time intervals. Each study lasted 6 wk. Doses chosen are within the therapeutic spectrum of prescribed Food and Drug Administration-approved dosing and presumably raise E2 concentrations within physiological range of younger females in postmenopausal women.
Healthy girls serving as controls were examined and sampled twice, once in the early follicular (within 1 wk following menses) and again in the luteal phase (∼3 wk after menses) of their menstrual cycle for determination of estradiol and estrone concentrations using the same assays.
Assays
E2 and estrone (E1) concentrations were measured in serum at the mass spectrometry laboratory at Mayo Clinic Rochester (Rochester, MN) using LCMSMS methodology as previously described (15) with a quantitation limit of 2.5 pg/ml and an intrassay coefficient variation of 20%. This assay has proven superior in accuracy and specificity over commercial RIA and other assays (16–18). In addition, total bioactive estrogens were measured in plasma by a recombinant cell bioassay with a sensitivity of 0.2 pg/ml (0.73 pmol/liter) as previously described (19).
LH, FSH, IGF-I, and lipids were measured by standard assays (Luminex, ELISA, and Beckman DXC 800; Beckman Instruments, Fullerton, CA) at the study's core laboratory at Nemours Children's Clinic-Jacksonville and at Baptist Hospital-Jacksonville (Jacksonville, FL). The intraassay coefficient variations for IGF-I was 4.3% and for LH and FSH 6.3 and 7.2%, respectively. HsCRP was measured by immunonephelometry, glucose by a glucose oxidase method using a YSI (Yellow Springs Instruments, Yellow Springs, OH), and insulins by RIA in our laboratory.
Calculations
The PK analysis of E2 after oral and TD administration were derived using the program WinNonLin (version 2.0; Pharsight Corp., Palo Alto, CA). Outcome variables derived for each subject at steady state included maximum and minimum E2 plasma concentration, time of maximal concentration (Tmax), and area under the curve (AUC) calculated by the linear trapezoidal rule.
Statistical analysis
Descriptive statistics were used to summarize each PK/PD parameter for each dose and route of delivering estrogen; these included the number of subjects, mean, median, se, and per-treatment group. For AUC0-t24, in addition to the above-mentioned summary statistics, the geometric mean was calculated because AUC data are non-Gaussian. Estradiol concentrations were tabulated by treatment and time for all subjects who completed the study. For each 2 × 2 crossover design, natural log-transformed AUC0-t24 and maximum concentration (Cmax) were analyzed using a mixed-effects model containing fixed effects for sequence, period, and treatment and random effects for subjects (within sequence). For pharmacodynamic analyses of estradiol administration, a mixed-effect model with random intercept and an appropriate covariance structure were used to compare the mean changes (within and between doses) over time in LH, FSH, IGF-I, and lipids from baseline/washout baseline. The change from baseline/washout baseline concentration was used as the response variable, and the dose-group, time points, period (before and after washout), and interactions of dose-group with period and time points were used as independent factors. Models were adjusted for baseline/washout baseline concentrations. The least squared means, with se, are presented. Multiple comparisons, with Bonferroni adjustment, were performed to detect the pairs of doses that were significantly different. Power to detect differences in estrogen concentrations between oral and TD, the main study outcomes, with an n = 5 in each group was estimated at 80% for E2 and 90% for E1 at a P < 0.05. Similarly power estimates for changes in LH and FSH in response to estradiol administration were about 80% with an n = 5 per group. The power was less (73%) for IGF-I. All tests were two tailed with a significance level of 0.05. The statistical packages SAS version 9.1.2 (SAS Institute Inc., Cary, NC) and SPSS version 17.0 (SPSS Inc., Chicago, IL) were used for data analyses.
Results
Ten subjects with Turner syndrome (45 X or related karyotypes), who met the inclusion criteria participated in the studies, five in each groups A and B, and 20 healthy controls. Their clinical characteristics are summarized in Table 1. Subjects were well matched for age, and as expected, the girls with Turner syndrome were much shorter than controls and had castrate levels of FSH. Body mass index (BMI) was higher in Turner syndrome (P = 0.03). There was no order effect in the girls with Turner syndrome; hence, data were grouped by route/dose for analysis. Compliance was assessed by frequent phone calling and pill and patch counting. Because of the short-term nature of these studies, we believe all girls were taking the stated doses of estradiol.
Table 1.
Clinical characteristics of study subjects
| Turner syndrome | Healthy controls | |
|---|---|---|
| n | 10 | 20 |
| Age (yr) | 17.7 ± 0.4 | 16.8 ± 0.4 |
| BMI (kg/m2) | 24.9 ± 1.6 | 21.7 ± 0.5 |
| Height SDS | −1.8 ± 0.3 | 0.0 ± 0.2 |
| Years off GH | 2.0 ± 0.4 | N/A |
| FSH (mIU/ml) | 125 ± 25 | N/A |
| Karyotypes | 45XO (n = 4) | N/A |
| Mosaic (n = 6)a |
NA, Not available or not applicable.
One each of: 46X,i(Xq)(del Xp,dup Xq); 46,X, i(Xq); 45X/46,Xr(X); 45X/46X,iXq; 46,Xi(Xq) [26]/45,X[4]; 46,Xi(Xq)/45X/45,i(Xq).
Estrogen concentrations in healthy controls
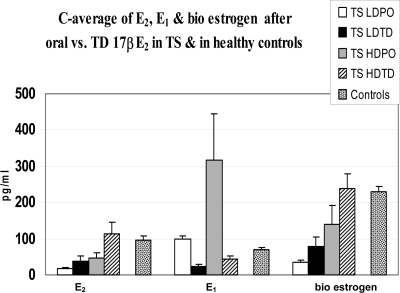
Mean estradiol concentrations during the follicular and luteal phases were 51 ± 6 pg/ml (range 23–140) and 142 ± 17 (33–306), respectively; mean E1 concentrations in the follicular and luteal phases were 44 ± 4 (range 25–87) and 86 ± 10 (41–192) (to convert estradiol to SI units, multiply by 3.671 to convert to picomoles per liter, estrone by 3.699 to picomoles per liter). Because adolescent Turner syndrome patients do not ovulate, we elected to average the follicular and luteal phase concentrations in the healthy controls to obtain integrated mean levels over the cycle to use as physiological targets to achieve in the Turner syndrome patients. Integrated mean levels were found to be 96 ± 11 pg/ml for E2 and 70 ± 7 pg/ml for E1. The total estrogen bioassay concentrations were 219 ± 20 and 266 ± 12 pg/ml in the follicular and luteal phase, respectively; hence, the monthly average bioestrogen concentration used was 231 ± 12 pg/ml. This compares with total estrogen concentrations (E2 + E1) by mass spectrometry assay of 166 pg/ml. The difference likely represents additional estrogenic metabolites or serum factors in the bioassay.
Pharmacokinetics
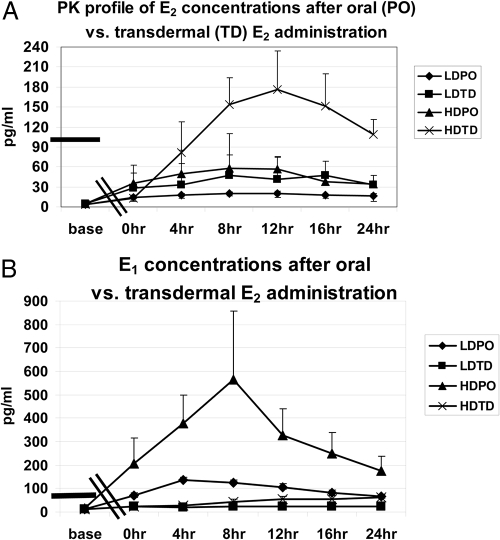
PK analysis of E2 concentrations after E2 administration showed a dose-dependent Cmax achieved after high-dose TD estradiol (Table 2 and Fig. 1). Average concentration (C-average) levels achieved were as follows: low-dose PO, 18 ± 2.1 pg/ml; low-dose TD, 38 ± 13 pg/ml; high-dose PO, 46 ± 15 pg/ml; and high-dose TD: 114 ± 31 pg/ml. Both low- and high-dose oral E2 resulted in significantly higher E1 concentrations compared with those achieved after TD estradiol in girls with Turner syndrome and compared with controls (C-average for E1: low-dose PO, 98 ± 10 pg/ml; low-dose TD, 23 ± 6 pg/ml; high-dose PO, 317 ± 126 pg/ml; high-dose TD, 43 ± 9 pg/ml). Bioestrogen concentrations measured by recombinant cell bioassay were as follows: low-dose PO, 36 ± 5 pg/ml; low-dose TD, 79 ± 25 pg/ml; high-dose PO, 140 ± 53 pg/ml; high-dose TD, 238 ± 40 pg/ml. Average concentrations vs. controls are shown for E2, E1, and bioestrogen in Fig. 2. Spearman rank correlation coefficient of bioestrogen concentrations and E2 by LCMSMS showed a strong correlation between the two assays (R = 0.9, P < 0.001).
Table 2.
Pharmacokinetic parameters (mean ± se)
| E2 |
E1 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Oral | TD | Oral | TD | Oral | TD | Oral | TD | |
| Dose (mg) | 0.5 | 0.0375 | 2.0 | 0.075 | 0.5 | 0.0375 | 2 | 0.075 |
| Cmax (pg/ml) | 23 ± 2.3 | 71 ± 25 | 64 ± 19 | 183 ± 57 | 139 ± 8 | 30 ± 8 | 592 ± 283 | 63 ± 33 |
| Tmax (h) | 8.8 ± 1.5 | 12.8 ± 4.1 | 7.2 ± 1.5 | 14.4 ± 2.7 | 4.8 ± 0.8 | 8.0 ± 5.1 | 7.2 ± 1.5 | 22.4 ± 3.6 |
| AUC (pg/ml · h) | 434 ± 51 | 960 ± 331 | 1096 ± 355 | 3014 ± 854 | 2362 ± 240 | 553 ± 155 | 7294 ± 2680 | 1109 ± 563 |
To convert E2 to SI units, multiply by 3.671 to convert to picomoles per liter, E1 by 3.699 to picomoles per liter. n = 5 in each group.
Fig. 1.
E2 plasma concentration over time in the top panel (A) and E1 concentrations in the lower panel (B) after PO vs. TD estradiol in low (LD) and high-doses (HD). The highest E2 concentration was observed after high-dose (0.075 mg) TD E2 approximately 12 h after administration. E1 concentrations were much higher after oral E2 than TD. Bars on the left represent estradiol or E1 concentrations in healthy age-matched controls averaged from follicular and luteal phase values. Double bar on the x-axis indicates the 2 wk gap between baseline and the clinical research center admission 2 wk after dosing.
Fig. 2.
Average E2, E1, and bioestrogen concentrations (C-average) in girls with Turner syndrome treated with low-dose (LD) oral (0.5 mg) and transdermal (0.0375 mg) E2 or high-dose (HD) oral (2.0 mg) or transdermal (0.075 mg) E2. Concentrations of the same types of estrogen from a group of age-matched normally menstruating controls were shown for comparison (data represent mean of follicular and luteal phase concentrations).
Pharmacodynamics
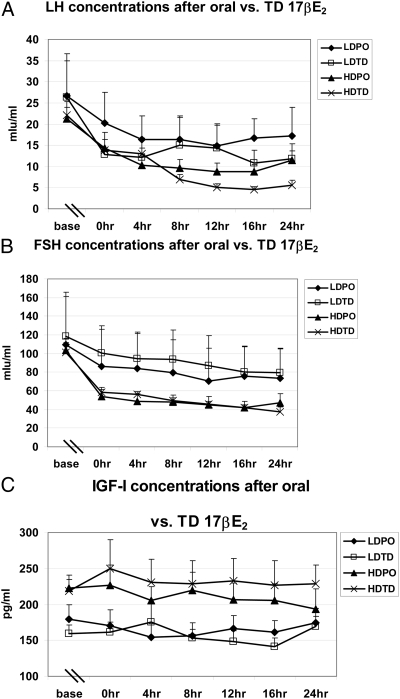
PD analysis for LH, FSH, and IGF-I concentrations after administration of E2 orally or TD was performed. The mean baseline levels of LH and FSH for the entire Turner syndrome group was 24.1 ± 3.2 mIU/ml and 122.3 ± 17.2 mIU/ml, respectively, with very low E2 concentrations of 3.8 ± 0.5 pg/ml; there were no differences in these concentrations at baseline among the groups. The overall mean changes over time were significant (P < 0.001) for each dose with maximum overall reduction in LH and FSH in high-dose TD and the minimum overall reduction in low-dose PO (Fig. 3). Pairwise comparisons of the changes in LH between the different groups showed significantly greater reductions in LH in the TD vs. oral route in the low-dose (P = 0.028) but not the high-dose (P = 0.28) estradiol administration. Similar comparisons of changes in FSH between the different groups showed significantly greater reductions in FSH in the TD vs. oral route only in the low-dose group (P = 0.009), with similar reductions in the high-dose oral and TD groups (P > 0.99). Spearman correlation analysis of the E2 concentrations vs. LH showed a negative correlation (R = 0.42, P = 0.067) vs. FSH (R = −0.478, P = 0.033). Similar analysis showed a significant negative correlation between bio estrogen and FSH concentrations (R = −0.5, P = 0.026).
Fig. 3.
Pharmacodynamics of E2 administration on LH (A), FSH (B), and IGF-I concentrations (C) measured over 24 h, 2 wk after initiation of treatment. Pairwise comparisons of the changes in LH between the different groups showed significantly greater reductions in LH in the TD vs. oral route in both low (P = 0.028) but not the high-dose (P = 0.28) estradiol administration. Similar comparisons of changes in FSH between the different groups showed significantly greater reductions in FSH in the TD vs. oral route only in the low-dose group (P = 0.009), with similar reductions in the high-dose oral and TD groups (P > 0.99). IGF-1 concentrations did not change significantly in any of the groups.
IGF-I concentrations did not change significantly in any of the groups and remained within normal physiological range in all groups.
There were significant (P < 0.05) within-group mean decreases over time in total cholesterol and low-density lipoprotein (LDL) cholesterol concentrations in the transdermal groups (low dose TD, high dose TD) and in the high-dose PO group (not low dose PO). These were accompanied by similar decreases in high-density lipoprotein (HDL) cholesterol and a significant increase in mean triglyceride concentration in low-dose groups (oral and TD). The difference in mean concentrations over time among doses were not significant however, although there was considerable variability. There were no between-dose significant differences in mean natural log-transformed AUC of these four markers. There was a decreasing trend in mean AUC of HDL cholesterol over the higher level of doses (Table 3).
Table 3.
Changes in plasma lipid concentrations during E2 administration
| Assay | Dose | Baseline | LS-mean changes at 2 wk | AUC (mg/dl · min) at 2 wk |
|---|---|---|---|---|
| Cholesterol (mg/dl) | Low-dose PO | 157 ± 7 | 0.5 ± 5.5 | 3728 ± 393 |
| Low-dose TD | 168 ± 12 | −21.0 ± 5.6a | 3289 ± 184 | |
| High-dose PO | 156 ± 11 | −12.4 ± 5.5a | 3457 ± 179 | |
| High-dose TD | 159 ± 9 | −14.7 ± 5.5a | 3462 ± 160 | |
| P | 0.80 | 0.08 | 0.07 | |
| LDL cholesterol (mg/dl) | Low-dose PO | 98 ± 4 | −4.8 ± 4.7 | 2117 ± 277 |
| Low-dose TD | 102 ± 10 | −19.5 ± 4.7a | 1835 ± 229 | |
| High-dose PO | 98 ± 9 | −5.8 ± 4.7 | 2212 ± 135 | |
| High-dose TD | 100 ± 8 | −7.8 ± 4.7 | 2203 ± 114 | |
| P | 0.93 | 0.14 | 0.11 | |
| HDL cholesterol (mg/dl) | Low-dose PO | 47 ± 5 | 1.2 ± 2.4 | 1124 ± 150 |
| Low-dose TD | 54 ± 8 | −6.6 ± 2.7a | 1015 ± 157 | |
| High-dose PO | 46 ± 3 | −6.4 ± 2.4a | 929 ± 299 | |
| High-dose TD | 43 ± 4 | −5.5 ± 2.4a | 930 ± 83 | |
| P | 0.53 | 0.11 | 0.42 | |
| TG (mg/dl) | Low-dose PO | 78 ± 14 | 20.7 ± 9.3a | 2445 ± 368 |
| Low-dose TD | 66 ± 10 | 21.1 ± 9.3a | 2182 ± 412 | |
| High-dose PO | 59 ± 10 | 2.5 ± 9.6 | 1587 ± 224 | |
| High-dose TD | 78 ± 17 | −6.5 ± 9.2 | 1640 ± 399 | |
| P | 0.70 | 0.11 | 0.26 |
LS-mean change, Least squared mean changes over time; P value, differences between doses. To convert to SI units for LH/FSH, multiply cholesterol, LDL-C, and HDL-C by 0.0259 for millimoles per liter and triglycerides by 0.0113 for millimoles per liter. Mixed model was used to compare natural log-transformed AUC between doses.
Significant within-group mean change over time vs. baseline.
Fasting plasma glucose concentrations were similar before and after 2 wk of estradiol in all groups (low dose PO: 74 ± 3, 75 ± 4 mg/dl; low dose TD: 83 ± 3, 79 ± 3 mg/dl; high dose PO: 83 ± 4, 80 ± 2 mg/dl; high dose TD: 82 ± 4, 80 ± 2 mg/dl, P = NS before and after estradiol, respectively). Fasting insulin concentrations were variable with no consistent pattern during estrogen treatment (low dose PO: 11 ± 2, 15 ± 3 μU/ml; low dose TD: 12 ± 1, 19 ± 1 μU/ml; high dose PO: 16 ± 2, 12 ± 2 μU/ml; high dose TD: 23 ± 6, 14 ± 3 μU/ml, before and after estradiol, respectively).
HsCRP was measured only twice, once before initiation of estradiol and again once after 2 wk of either oral or TD administration. Median change in concentrations were: low-dose PO: −0.58 (−0.66, 1.16) mg/liter, low-dose TD: −0.86 (−3.52, 2.00) mg/liter, high-dose PO: −1.48 (−28, −0.52) mg/liter, high-dose TD: −0.61 (−1.59, 4.20) mg/liter. These differences within and between groups were not significant. (To convert to SI units for LH/FSH, multiply by 1 for units per liter, for cholesterol, LDL cholesterol, and HDL cholesterol by 0.0259 for millimoles per liter, for triglycerides by 0.0113 for millimoles per liter, for IGF-I by 1 for micrograms per liter, and for hsCRP by 10 for milligrams per liter.)
Discussion
This is the first study to our knowledge to assess pharmacokinetics and pharmacodynamics of the same form of E2 given orally and transdermally in girls with Turner syndrome. We first sampled a group of normally menstruating healthy adolescent girls the same age as those with Turner syndrome to establish normative data for the E2. BMI in the Turner syndrome group was higher than controls; however, BMI is a poor measure of adiposity in Turner syndrome due to their very short size (20, 21). We measured estradiol by LCMSMS and by a recombinant cell bioassay. The LCMSMS assay has proven particularly sensitive to small concentrations of estradiol and is highly specific (15–18). Girls with Turner syndrome had baseline E2, FSH, and LH concentrations comparable with menopausal women and offer a unique model to study PK of estradiol, eliminating confounding effects of other products of the intact gonad. A previous study in a group of girls with Turner syndrome aimed to compare routes of administration (oral and TD) but again used two different types of estrogen, CEE orally and E2 TD, making any conclusions regarding route uncertain (22). We chose to use E2 instead of CEE because the latter has a large number of estrogenic compounds of different potency, which cannot be readily measured and which could have highly variable effects, depending on the target tissue.
PK analysis of E2 concentrations after E2 administration showed a dose-dependent Cmax and C-average within each route (oral and TD) with the highest concentrations achieved after high-dose TD and the lowest after low-dose oral administration. The high-dose TD E2 group concentrations were the closest to those in the normally menstruating girls, the latter chosen as the average of the midfollicular and luteal phase estradiol concentrations. This average is similar to the integrated mean of the concentrations throughout the entire cycle in normal women and is a reasonable estimate of integrated estradiol concentrations based on analysis of available data (Ref. 23 and data provided by Santen, R., and J. Symons, personal communications in healthy normally menstruating women, data not shown). Tmax was much shorter (∼7–8 h) with the oral E2 doses than the TD (∼13–14 h), most likely reflecting differences in absorption, much slower in the TD route. However, the AUC at steady state, a measure of total drug exposure, was much higher in the high-dose TD group, with very comparable levels in the high-dose PO and low-dose TD groups and lowest in the low-dose PO. These results are similar to concentrations achieved in postmenopausal women (11–13) and reflect the lack of first-pass hepatic metabolism when estradiol is administered by the TD route. Of note in this PK profile is the time-dependent estradiol concentrations achieved after dosing, with the high-dose TD in particular again reflecting differences in absorption in the transdermal route (Fig. 1A). On the other hand, the levels are more stable with oral (low and high dose) and low-dose TD, potentially facilitating monitoring with measurement of sensitive E2 levels. Regardless of route, the ability to monitor plasma estradiol levels after several weeks of stable dosing is an advantage of using E2 preparations compared with ethinyl estradiol and CEE; this has important clinical implications in the follow-up of patients with hypogonadism.
E1 concentrations in the oral route groups, particularly the high-dose PO, were significantly higher than those in healthy controls, with much lower concentrations in the TD groups (low and high dose). This is expected after oral estrogen due to gut absorption and first passage through the liver with consequent E2 to E1 conversion. Estrone, however, has much less biological potency than estradiol; hence, the overall impact of these concentrations on the metabolic effects of E2 is likely small.
The use of a recombinant cell bioassay offers the theoretical advantage of measuring estrogen action and relative potency by turning on the estrogen receptor in the transformed yeast (19, 24). Using this assay bioestrogen concentrations were clearly dose dependent, lowest in low-dose PO and low-dose TD, and highest in the high-dose PO and high-dose TD groups with the closest concentrations compared with healthy controls in the high-dose TD group. The high oral dose was 4 times higher than the low dose, yet the AUC and C-average was only 2.5 fold higher, which suggests bioavailability may be dose dependent. The fact that bioestrogen concentrations in the high-dose PO group were not higher than high-dose TD or controls supports the notion that higher E1 concentrations generated via the oral route are of negligible estrogenic potency. Absolute concentrations were significantly higher using the bioestrogen assay compared with LCMSMS, likely reflecting the bioassay picking up other estrogenic molecules or serum factors; however, there was a substantially strong correlation between the bioestrogen concentration and E2 measured by LCMSMS (R = 0.9, P < 0.001) .
The degree of suppression of circulating gonadotropins has been used previously as a surrogate measure of the relative biological potency of different estrogens (1, 11–13). In our studies gonadotropin concentrations decreased as expected after the administration of all doses of E2. Suppression of LH and FSH was greater in the TD vs. oral route in the low-dose but similar in the high-dose group in both routes. Although gonadotropins decreased markedly, the levels did not fully normalize, particularly FSH concentrations, likely due to the lack of inhibin in these patients with primary gonadal dysfunction. Among the four groups the high-dose PO (2 mg) dose was comparable with the low-dose TD (0.0375 mg) dose, raising E2 concentrations; however, they had a different degree of suppression of gonadotropins (greater in the TD group). Spearman correlation analysis of the E2 concentrations vs. LH (R = 0.42, P = 0.067) and vs. FSH (R = 0.478, P = 0.033) suggests that degree of suppression of gonadotropins cannot be accurately used to judge bioequivalency.
The impact of the route of administration of estrogen on circulating IGF-I concentrations has previously shown mixed results. IGF-I levels decreased after oral CEE but remained constant during E2 TD in postmenopausal women (6, 7), and postmenopausal hypopituitary women needed more GH to normalize IGF-I when taking CEE orally than E2 TD (8). However, in girls with Turner syndrome whom we studied using the same form of E2 orally and TD in short-term experiments, there was no clinically significant difference in IGF-I concentrations between the two routes (14). In the present studies, IGF-I concentrations did not change significantly in any of the study groups and were maintained within physiological range. These data suggest that unconjugated, purer types of estrogen may be also preferable in girls to avoid impact to IGF-I; however, further long-term studies are needed in these and younger girls, particularly those with height potential, before firm conclusions can be derived. The power to detect subtle differences in IGF-I in our relatively small sample size may also be a limitation that awaits larger studies.
The issue of the status of plasma lipids in Turner syndrome per se has shown somewhat conflicting results. Cooley et al. (25) have implicated that haploinsufficiency of some yet-identified gene in the X chromosome confers an atherogenic lipid profile; however, this concept has been challenged by others who postulate it is the estrogen deficiency the principal culprit (26). Investigators observed higher total cholesterol and LDL in those with Turner syndrome vs. healthy eugonadal controls (27) and vs. women with premature ovarian failure (28). Others have reported higher total cholesterol but normal LDL and HDL concentrations compared with controls (29) or the cholesterol elevation mostly in older subjects (30). However, other series have shown either no difference in lipid levels with controls (31) or differences accounted for when normalized by BMI (32). In our own experience in a previous study of the metabolic effects of oral and TD estradiol in GH-treated girls with Turner syndrome, we observed normal plasma lipid concentrations and no effect of estradiol administration either oral or TD on those values (14). The fact that in the present studies the same girls with Turner syndrome took the estradiol orally and TD makes this a robust and useful model to address the effect of the estrogen on lipid metabolism without the confounding effects of other products of the intact gonad. The effects on lipids by different forms of estrogen therapy, on the other hand, have been variable, but in general oral estrogens have been associated with a greater beneficial effect on lipids (decreased LDL cholesterol and increased HDL cholesterol) than TD preparations (1, 11–13). In the present study, there were mild decreases in LDL cholesterol in the low-dose TD group only, accompanied by decreases in HDL in both oral and TD (high dose) E2 treatments. Triglycerides increased mildly but were still within normal range in both oral and TD groups in low doses of E2. Overall there were no significant differential effects among the dosing groups in their effects on plasma lipids.
In general, estrogen replacement via different routes has been found to be beneficial to carbohydrate metabolism with fasting insulin and glucose concentrations often lower during treatment (32–37), raising the possibility that estrogens may be cardioprotective. However, in our previous study in GH-treated girls with Turner syndrome given oral vs. TD E2, there were no differences in glucose concentrations after 6 wk each of treatment (14). In the present studies, glucose concentrations did not change (with variable insulin concentrations), regardless of route and dose, suggestive that estrogens are not clinically important modulators of carbohydrate metabolism in this age group. Longer treatment would be needed to better assess the impact of pure estradiol in insulin concentrations however.
HsCRP is a proinflammatory marker produced by the liver, and elevated levels may be indicative of incipient cardiovascular disease. The effects of estrogen on hsCRP have been variable. Yilmazer et al. (38) showed that in postmenopausal women, C-reactive protein levels increased over time after oral E2 and CEE but not with transdermal E2. Bukoswka et al. (39), also observed higher C-reactive protein values when using oral hormone replacement (estriol/estradiol valerate plus levonogestrel), compared with transdermal E2 after 3 months. In our present studies, there were no significant changes in hsCRP with either dose or route.
One of the limitations of this study is using a model of estrogen deficiency in girls with Turner syndrome, which may not be extrapolated to normal physiology. It is possible that the chromosomal anomaly per se could affect the responsivity to the different forms of estradiol. However, although we are not testing the effects of estrogen in normal females, the qualitative responses reported here are comparable with those of postmenopausal women. The fact that each girl received E2 orally and transdermally, we believe, makes the comparisons of the effect of estrogen valid in this model of hypogonadism. In addition, Turner syndrome is one of the most common conditions that requires estrogen replacement in childhood.
In summary, the availability of sensitive and specific E2 assays (LCMSMS) and purer forms of estrogen (E2) available for administration now permit direct comparison of the biological effects of estrogen, depending on route and dose, as well as monitoring of plasma concentrations, a significant advantage over conjugated oral estrogens. High-dose TD (0.075 mg) E2 resulted in a PK profile of E2 and E1 concentrations closer to that of normally menstruating adolescents compared with low-dose TD, high-dose PO, and low-dose PO. These doses also suppressed LH and FSH more in the low-dose groups, yet in the high-dose groups, LH and FSH suppressed similarly in the oral and TD routes. High-dose TD dosing achieved bioestrogen concentrations closest to normal. None of the doses used via either route affected IGF-I concentrations, and all affected cholesterol minimally and hsCRP concentrations similarly, although power to detect differences in the latter may be reduced and requires more data. In conclusion, among identical forms of estradiol, high-dose transdermal (0.075 mg) was the most physiological. Whether the long-term metabolic effects of estrogen differ using the same form of estradiol (E2), depending on route, awaits further study in Turner syndrome.
Acknowledgments
The authors are grateful to Shiela Smith and the expert nursing staff of the Wolfson Children's Hospital Clinical Research Center for their dedicated care of our subjects; Shawn Sweeten and Hilary E. Blair for laboratory assistance; and Katie Black for research support. We thank Drs. H. Hsiang, M. Kummer, and J. Daaboul for referring patients to these studies. We also thank our patients and their families for their participation in these studies.
This work was supported by the Genentech Center for Clinical Research in Endocrinology (to N.M.), the Nemours Research Programs, and Mayo Clinic CTSA Grant UL1 RR024150-05-NIH/NCRR.
Disclosure Summary: Authors M.T., J.L., J.H., and K.O.K. have nothing to disclose. R.S. serves on the advisory boards of Pfizer, Novo-Nordisk, and MedAccess and is a consultant related to estrogen for Pfizer, Novo-Nordisk, and TEVA Women's Health. N.M. has a grant from Genentech for the conducting of these studies.
Footnotes
- AUC
- Area under the curve
- BMI
- body mass index
- C-average
- average concentration
- CEE
- conjugated equine estrogen
- Cmax
- maximum concentration
- E1
- estrone
- E2
- 17β-estradiol
- HDL
- high-density lipoprotein
- hsCRP
- highly sensitive C-reactive protein
- LCMSMS
- liquid chromatography mass spectrometry/mass spectrometry
- LDL
- low-density lipoprotein
- PD
- pharmacodynamics
- PK
- pharmacokinetics
- PO
- orally
- TD
- transdermal
- Tmax
- time of maximal concentration.
References
- 1. Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. 2002. Production and actions of estrogens. N Engl J Med 346:340–352 [DOI] [PubMed] [Google Scholar]
- 2. Ross JL, Quigley CA, Cao D, Feuillan P, Kowal K, Chipman JJ, Cutler GB., Jr 2011. Growth hormone plus childhood low-dose estrogen in Turner Syndrome. N Engl J Med 364:1230–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ross JL, Cassorla FG, Skerda MC, Valk IM, Loriaux DL, Cutler GB., Jr 1983. A preliminary study of the effect of estrogen dose on growth in Turner syndrome. N Engl J Med 309:1104–1106 [DOI] [PubMed] [Google Scholar]
- 4. Mauras N, Rogol AD, Veldhuis JD. 1990. Increased hGH production rate after low-dose estrogen therapy in prepubertal girls with Turner syndrome. Pediatr Res 28:626–630 [DOI] [PubMed] [Google Scholar]
- 5. Drobac S, Rubin K, Rogol AD, Rosenfield RL. 2006. A workshop on pubertal hormone replacement options in the United States. J Pediatr Endocrinol Metab 19:55–64 [DOI] [PubMed] [Google Scholar]
- 6. O'Sullivan AJ, Crampton LJ, Freund J, Ho KK. 1998. The route of estrogen replacement therapy confers divergent effects on substrate oxidation and body composition in postmenopausal women. J Clin Invest 102:1035–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leung KC, Johannsson G, Leong GM, Ho KK. 2004. Estrogen regulation of growth hormone action. Endocr Rev 25:693–721 [DOI] [PubMed] [Google Scholar]
- 8. Cook DM, Ludlam WH, Cook MB. 1999. Route of estrogen administration helps to determine growth hormone (GH) replacement dose in GH-deficient adults. J Clin Endocrinol Metab 84:3956–3960 [DOI] [PubMed] [Google Scholar]
- 9. Hanggi W, Lippuner K, Jaeger P, Birkhauser MH, Horber FF. 1998. Differential impact of conventional oral or transdermal hormone replacement therapy or tibolone on body composition in postmenopausal women. Clin Endocrinol (Oxf) 48:691–699 [DOI] [PubMed] [Google Scholar]
- 10. Gravholt CH, Hjerrild BE, Naeraa RW, Engbaek F, Mosekilde L, Christiansen JS. 2005. Effect of growth hormone and 17β-oestradiol treatment on metabolism and body composition in girls with Turner syndrome. Clin Endocrinol (Oxf) 62:616–622 [DOI] [PubMed] [Google Scholar]
- 11. Chetkowski RJ, Meldrum DR, Steingold KA, Randle D, Lu JK, Eggena P, Hershman JM, Alkjaersig NK, Fletcher AP, Judd HL. 1986. Biologic effects of transdermal estradiol. N Engl J Med 314:1615–1620 [DOI] [PubMed] [Google Scholar]
- 12. Maschack CA, Lobo RA, Dozono-Takano R, Eggena P, Nakamura RM, Brenner PF, Mishell DR., Jr 1982. Comparison of pharmacodynamic properties of various estrogen formulations. Am J Obstet Gynecol 144:511–518 [DOI] [PubMed] [Google Scholar]
- 13. Powers MS, Schenkel L, Darley PE, Good WR, Balestra JC, Place VA. 1985. Pharmacokinetics and pharmacodynamics of transdermal dosage forms of 17β-estradiol: comparison with conventional oral estrogens used for hormone replacement. Am J Obstet Gynecol 152:1099–1106 [DOI] [PubMed] [Google Scholar]
- 14. Mauras N, Shulman D, Hsiang HY, Balagopal P, Welch S. 2007. Metabolic effects of oral versus transdermal estrogen in growth hormone-treated girls with Turner syndrome. J Clin Endocrinol Metab 92:4154–4160 [DOI] [PubMed] [Google Scholar]
- 15. Nelson RE, Grebe SK, OKane DJ, Singh RJ. 2004. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem 50:373–384 [DOI] [PubMed] [Google Scholar]
- 16. Stanczyk FZ, Lee JS, Santen RJ. 2007. Standardization of steroid hormone assays: why, how, and when? Cancer Epidemiol Biomarkers Prev 16:1713–1719 [DOI] [PubMed] [Google Scholar]
- 17. Santen RJ, Demers L, Ohorodnik S, Settlage J, Langecker P, Blanchett D, Goss PE, Wang S. 2007. Superiority of gas chromatography/tandem mass spectrometry assay (GC/MS/MS) for estradiol for monitoring of aromatase inhibitor therapy. Steroids 72:666–671 [DOI] [PubMed] [Google Scholar]
- 18. Santen RJ, Lee JS, Wang S, Demers LM, Mauras N, Wang H, Singh R. 2008. Potential role of ultra-sensitive estradiol assays in estimating the risk of breast cancer and fractures. Steroids 73:1318–1321 [DOI] [PubMed] [Google Scholar]
- 19. Klein KO, Baron J, Colli MJ, McDonnell DP, Cutler GB., Jr 1994. Estrogen levels in childhood determined by an ultrasensitive recombinant cell bioassay. J Clin Invest 94:2475–2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blackett PR, Rundle AC, Frane J, Blethen SL. 2000. Body mass index (BMI) in Turner syndrome before and during growth hormone (GH) therapy. Int J Obes Relat Metab Disord 24:232–235 [DOI] [PubMed] [Google Scholar]
- 21. Isojima T, Yokoya S, Ito J, Horikawa R, Tanaka T. 2009. Inconsistent determination of overweight by two anthropometric indices in girls with Turner syndrome. Acta Paediatr 98:513–518 [DOI] [PubMed] [Google Scholar]
- 22. Nabhan ZM, Dimeglio LA, Qi R, Perkins SM, Eugster EA. 2009. Conjugated oral versus transdermal estrogen replacement in girls with Turner syndrome: a pilot comparative study. J Clin Endocrinol Metab 94:2009–2014 [DOI] [PubMed] [Google Scholar]
- 23. Groome NP, Illingworth PJ, O'Brien M, Pai R, Rodger FE, Mather JP, McNeilly AS. 1996. Measurement of dimeric inhibin B throughout the human menstrual cycle. J Clin Endocrinol Metab 81:1401–1405 [DOI] [PubMed] [Google Scholar]
- 24. Wang S, Paris F, Sultan CS, Song RX, Demers LM, Sundaram B, Settlage J, Ohorodnik S, Santen RJ. 2005. Recombinant cell ultrasensitive bioassay for measurement of estrogens in postmenopausal women. J Clin Endocrinol Metab 90:1407–1413 [DOI] [PubMed] [Google Scholar]
- 25. Cooley M, Bakalov V, Bondy CA. 2003. Lipid profiles in women with 45,X vs 46,XX primary ovarian failure. JAMA 290:2127–2128 [DOI] [PubMed] [Google Scholar]
- 26. Taylor HS. 2004. Genetic vs hormonal factors in lipid metabolism in women. JAMA 291:424–425 [DOI] [PubMed] [Google Scholar]
- 27. Kozlowska-Wojciechowska M, Jez W, Zdrojewski T, Chwojnicki K. 2006. Are young women with Turner syndrome at greater risk of coronary artery disease? Eur J Cardiovasc Prevent Rehab 13:467–469 [DOI] [PubMed] [Google Scholar]
- 28. Van PL, Bakalov VK, Bondy CA. 2006. Monosomy for the X-chromosome is associated with an atherogenic lipid profile. J Clin Endocrinol Metab 91:2867–2870 [DOI] [PubMed] [Google Scholar]
- 29. Giordano R, Forno D, Lanfranco F, Manieri C, Ghizzoni L, Ghigo E. 2011. Metabolic and cardiovascular outcomes in a group of adult patients with Turner syndrome under hormonal replacement therapy. Eur J Endocrinol 164:819–826 [DOI] [PubMed] [Google Scholar]
- 30. Ross JL, Feuillan P, Long LM, Kowal K, Kushner H, Cutler GB., Jr 1995. Lipid abnormalities in Turner syndrome. J Pediatr 126:242–245 [DOI] [PubMed] [Google Scholar]
- 31. Lanes R, Gunczler P, Palacios A, Villaroel O. 1997. Serum lipids, lipoprotein lp (a), and plasminogen activator inhibitor-1 in patients with Turner syndrome before and during growth hormone and estrogen therapy. Fertil Steril 68:473–477 [DOI] [PubMed] [Google Scholar]
- 32. Elsheikh M, Bird R, Casadei B, Conway GS, Wass JA. 2000. The effect of hormone replacement therapy on cardiovascular hemodynamics in women with Turner syndrome. J Clin Endocrinol Metab 85:614–618 [DOI] [PubMed] [Google Scholar]
- 33. Crespo CJ, Smit E, Snelling A, Sempos CT, Andersen RE; NHANES III 2002. Hornome replacement therapy and its relationship to lipid and glucose metabolism in diabetic and nondiabetic postmenopausal women: results from the Third National Health and Nutrition Examination Survey (NHANES III). Diabetes Care 25:1675–1680 [DOI] [PubMed] [Google Scholar]
- 34. Kanaya AM, Herrington D, Vittinghoff E, Lin F, Grady D, Bittner V, Cauley JA, Barrett-Connor E; Heart and Estrogen/progestin Replacement Study 2003. Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 138:1–9 [DOI] [PubMed] [Google Scholar]
- 35. Li C, Samsioe G, Borgfeldt C, Bendahl PO, Wilawan K, Aberg A. 2003. Low-dose hormone therapy and carbohydrate metabolism. Fertil Steril 79:550–555 [DOI] [PubMed] [Google Scholar]
- 36. Alves ST, Gallichio CT, Guimarães MM. 2006. Insulin resistance and body composition in Turner syndrome: effect of sequential change in the route of estrogen administration. Gynecol Endocrinol 22:590–594 [DOI] [PubMed] [Google Scholar]
- 37. Ostberg JE, Storry C, Donald AE, Attar MJ, Halcox JP, Conway GS. 2007. A dose-response study of hormone replacement in young hypogonadal women: effects on intima media thickness and metabolism. Clin Endocrinol (Oxf) 66:557–564 [DOI] [PubMed] [Google Scholar]
- 38. Yilmazer M, Fenkci V, Fenkci S, Sonmezer M, Aktepe O, Altindis M, Kurtay G. 2003. Hormone replacement therapy, C-reactive protein, and fibrinogen in healthy postmenopausal women. Maturitas 46:245–253 [DOI] [PubMed] [Google Scholar]
- 39. Bukowska H, Stanosz S, Zochowska E, Millo B, Sieja K, Chelstowski K, Naruszewicz M. 2005. Does the type of hormone replacement therapy affect lipoprotein (a), homocysteine, and C-reactive protein levels in postmenopausal women? Metabolism 54:72–78 [DOI] [PubMed] [Google Scholar]