Abstract
Context:
The family risk ratio for papillary thyroid carcinoma (PTC) is among the highest of all cancers. Collectively, familial cases (fPTC) and sporadic cases (sPTC) are not known to show molecular differences. However, one study reported that telomeres were markedly shorter and the telomerase reverse transcriptase (TERT) gene was amplified and up-regulated in germline DNA from patients with fPTC compared with sPTC.
Objective:
The aim of this study was to evaluate telomere length and TERT gene amplification and expression in blood samples of fPTC and sPTC patients in a genetically distinct population from the previous study.
Design:
In 42 fPTC and 65 sPTC patients, quantitative real-time PCR was employed to measure the relative telomere length (RTL) and TERT gene copy number and RNA level. To validate the results using alternative methods, we further studied a subset of the original cohort consisting of randomly chosen fPTC (n = 10) and sPTC (n = 14) patients and controls (n = 21) by assessing both telomere length by flow fluorescent in situ hybridization and TERT gene expression by quantitative real-time PCR.
Results:
RTL and TERT gene copy number did not differ between fPTC and sPTC (P = 0.957 and P = 0.998, respectively). The mean RTL and TERT gene expression were not significantly different among the groups of the validation series (P = 0.169 and P = 0.718, respectively).
Conclusion:
Our data show no difference between familial and sporadic PTC with respect to telomere length, TERT copy number, or expression in our cohort. Further investigations in additional cohorts of patients are desirable.
Thyroid carcinoma is the most common endocrine malignancy. Papillary thyroid carcinoma (PTC) is the most common type accounting for approximately 80% of all thyroid cancers (1). Among all cancers, PTC ranks as one of the highest in terms of heritability; in other words, genetic predisposition is a major risk factor (2, 3). Nevertheless, only one predisposing gene has been firmly established so far (4, 5). In addition, a putative RNA gene in chromosome 8q24 has been implicated as well as two anonymous single nucleotide polymorphisms (6, 7). It appears that the genetic predisposition to thyroid cancer is heterogeneous, and the genes so far implicated display low penetrance. Approximately 5–10% of PTC are familial in that the proband has at least one first- or second-degree relative with PTC (3, 8). According to some studies the familial type (fPTC) has slightly different clinical features from the sporadic form (sPTC), but as far as we know, molecular differences have not been demonstrated (9, 10). However, one study reported fundamental differences between patients with fPTC and sPTC (11). Five groups of individuals were analyzed: patients with fPTC, unaffected siblings of fPTC patients, sPTC cases, nodular goiter cases, and healthy subjects. The results showed no difference in relative telomere length (RTL) among the groups except for the fPTC patients who had distinctly shorter RTL than the other groups. The difference was clear-cut in that there was almost no overlap in RTL between familial and sporadic cases. Gene amplification and expression (both at the mRNA and protein levels) of the telomerase reverse transcriptase (TERT) gene were measured and showed a clear increase in fPTC cases compared with unaffected siblings, sPTC cases, nodular goiter cases, and healthy subjects. In 2010, another publication from the same research group showed significant differences between familial cases (n = 63) and unaffected siblings (n = 41) (12).
If these fundamental differences between fPTC and sPTC could be confirmed, they might provide clues to help unravel the hitherto elusive genetic mechanisms that appear to strongly predispose to PTC. In this study, we used a variety of methods to confirm the previous findings, but our data do not support the results reported by others.
Patients and Methods
Patients and controls
All samples included in this study were collected at The Ohio State University (OSU) as part of ongoing studies approved by the Institutional Review Board at OSU. All subjects gave written informed consent before participation. In the primary test series, familial cases (n = 42) were defined as those in which the proband had at least two first- or second-degree relatives with the disease. Sporadic cases (n = 65) had no first- or second-degree relatives with the disease. Only one fPTC sample per kindred was included in the study. The female to male ratio for fPTC and sPTC was 2:1 and 4:1, respectively. The mean age at diagnosis of fPTC and sPTC was 38.1 ± 14.2 (sd) and 42.4 ± 17.4 yr, respectively. The mean age at the time of blood collection for fPTC was 42.1 ± 13.5 and 44.2 ± 17.1 yr for sPTC (Table 1). All cancer cases were histologically of the papillary type. The age- and gender-matched controls were chosen randomly from a large collection of samples from central Ohio residents not affected with cancer, assembled after internal review board approval at the Division of Human Genetics, OSU. The majority of the tested population were Caucasians [main series familial: Caucasian n = 39 (92.9%), Hispanic n = 3 (7.1%); main series sporadic: Caucasian n = 59 (90.8%), Hispanic n = 2 (3.1%), Asian n = 2 (3.1%), Native American/Alaskan n = 1 (1.5%), and multiracial n = 1 (1.5%); validation series: all Caucasian, only one Asian (4.8%) of 21 controls].
Table 1.
Characteristics of the subjects included in the study
| Main series |
Validation series |
||||||
|---|---|---|---|---|---|---|---|
| fPTC, n = 42 | sPTC, n = 65 | P | fPTC, n = 10 | sPTC, n = 14 | Controls, n = 21 | P | |
| Age at diagnosis (yr) | 38.1 ± 14.2 | 42.4 ± 17.4 | 0.306 | 35.3 ± 11.7 | 42.0 ± 12.6 | 0.204 | |
| Age at blood collection (yr) | 42.1 ± 13.5 | 44.2 ± 17.1 | 0.756 | 48.1 ± 12.9 | 44.4 ± 10.3 | 46.0 ± 11.2 | 0.672 |
| Gender | |||||||
| Females [% (n)] | 64.3 (27) | 78.5 (51) | 0.123 | 60 (6) | 71 (10) | 42 (13) | 0.849 |
| Males [% (n)] | 35.7 (15) | 21.5 (14) | 40 (4) | 29 (4) | 38 (8) | ||
Telomere length measurement by quantitative real-time PCR (q-PCR)
The measurement of telomere length was performed by the same method as in the previous study (11). The method was originally developed by Cawthon (13) and applied here without major modifications. Briefly, copy numbers of the telomere (T) repeat and of a single-copy (S) number control gene (36B4, acidic ribosomal phosphoprotein P0) were determined by q-PCR. The result was expressed as the T/S ratio. The RTL was calculated by dividing the T/S ratio of each experimental sample by the T/S ratio of a reference DNA consisting of a mixture of DNA samples from three healthy individuals matched for age and gender. The TERT gene amplification assay was performed by q-PCR. We used the 2−ΔΔCt method to calculate the TERT/36B4 gene copy ratio [ΔΔcycle threshold (Ct) = (Ct TERTsample − Ct 36B4sample) − (Ct TERTcalibrator − Ct 36B4calibrator)] (14). All samples were run in duplicate.
Flow fluorescent in situ hybridization (FISH) procedure
We wished to validate the results by performing flow cytometry and TERT expression measurement in the available previously studied patients. To accomplish this, we collected fresh blood samples from 14 of the sporadic and 10 of the familial cases chosen randomly from those studied previously. Additionally, we collected 21 samples from subjects with no diagnosed cancer as controls. As shown in Table 1, all three groups were matched for age and gender.
The telomere length was measured by FISH and flow cytometry as previously described (15). Bovine thymocytes (used as internal control for flow cytometric analysis) were obtained from calf thymus and prepared as described by Baerlocher et al. (15).
Flow cytometric analysis
Flow cytometric analysis of all samples and controls was performed in the clinical flow cytometry laboratory at the OSU Medical Center using a FC500 flow cytometer and CXP software version 2.2 as previously described (15).
All samples were run in duplicates and a minimum of 25,000 events acquired per sample. After the acquisition of events X-mean for each sample was derived by subtracting the mean fluorescence intensity (MFI) value for samples with no probe (MFINP) from the corresponding (paired) samples with probe (MFIP). To account for unavoidable experiment to experiment differences in permeabilization, hybridization and washes a duplicate sample of bovine thymocytes (isolated and cryopreserved at −130 C before this study) was included in each experiment. The X-mean for thymocytes was calculated by subtracting the MFI of processed cells without probe (TNP) from the MFI of thymocytes with probe (TP). The derived thymocyte X-mean was then used as a denominator to express final results for patient telomere length for each experiment.
TERT gene expression
A 2.5-ml volume of blood was collected into PAX gene tubes (PreAnalytiX GmbH, Hombrechtikon, Switzerland), and after 2 h incubation at room temperature, the samples were stored at −70 C. RNA extraction was performed according to the manufacturer's protocol, and 500 ng was used to retrotranscribe into cDNA by the High Capacity Reverse Transcriptase kit (Applied Biosystems, Foster City, CA). The TaqMan TERT gene expression assay (Applied Biosystems; catalog item Hs0097265_m1) and TaqMan GAPDH gene expression (Applied Biosystems; catalog item 4352934E) were used in the amplification reaction performed on an ABI Prism 7900HT Sequence Detection System (Applied Biosystems). The conditions for PCR were as follows: 95 C for 10 min to activate the polymerase followed by 40 cycles of denaturation (95 C, 15 sec) and annealing (60 C, 1 min). The formula 2−ΔCt, where ΔCt = Ct(TERT) − Ct(GAPDH) was employed to calculate the relative TERT mRNA level.
For a more detailed description of methodology see Supplemental Methods (published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
Statistical analysis
The statistical analysis was performed using R (version 2.7.1; The R Foundation for Statistical Computing) software. Mann-Whitney U test was used to analyze the difference in RTL and TERT amplification. The telomere length assessed by flow FISH and TERT gene expression obtained by q-PCR were analyzed by Kruskal-Wallis test. ANOVA was used to analyze the difference in age at blood collection, and Fisher's exact test was applied to compare gender and the PTC groups. The P values were two sided; P < 0.05 was considered statistically significant.
Results
Relative telomere length
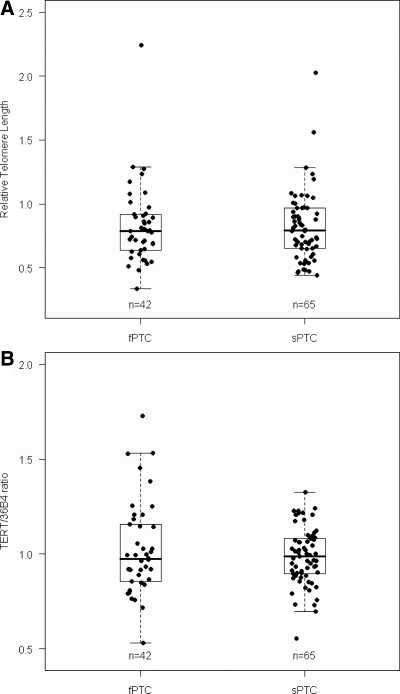
As shown in Fig. 1, the median RTL in fPTC and sPTC was 0.80 (25–75th percentile, 0.65–0.92) and 0.80 (25–75th percentile, 0.66–0.98), respectively, showing no significant difference (P = 0.957). RTL was similar in females and males both within each group of PTC (P = 0.274 and P = 0.956 for fPTC and sPTC, respectively) or as compared between the groups of PTC (RTL, P = 0.637 and P = 0.780 for females and males, respectively).
Fig. 1.
RTL and TERT gene amplification in patients with PTC. A, RTL measured by q-PCR. No difference between fPTC and sPTC cases was found (P = 0.957). B, TERT gene amplification (measured by q-PCR and expressed as TERT/36B4 ratio) in fPTC and sPTC patients. No difference between fPTC and sPTC patients was detected (P = 0.998). Boxes represent the interquartile range of the distribution (25–75th percentile), horizontal line within the box the median, and vertical lines the 5th and 95th percentiles.
Flow FISH
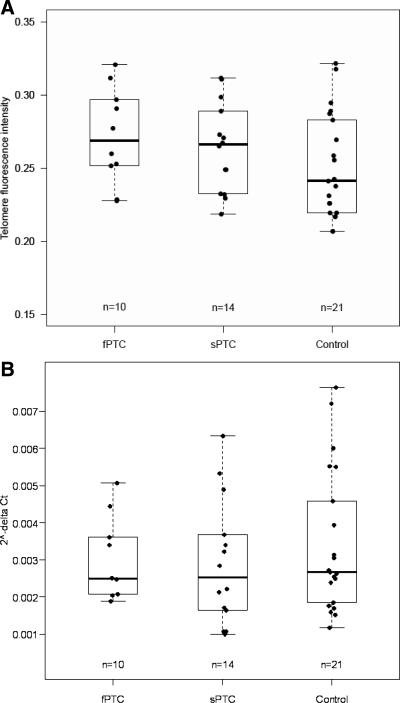
The median telomere length measured by flow FISH in fPTC, sPTC, and controls was 0.27 (25–75th percentile, 0.25–0.30), 0.27 (25–75th percentile, 0.24–0.29), and 0.24 (25–75th percentile, 0.22–0.28), respectively (Fig. 2). No significant difference was detected among the tested groups (P = 0.169).
Fig. 2.
Telomere length measured by flow FISH (A) and TERT gene mRNA level (B) obtained by q-PCR in the validation series of 10 fPTC, 14 sPTC, and 21 controls. No difference was found among the groups (P = 0.169 and P = 0.718 for telomere length and TERT RNA amount, respectively). Boxes represent interquartile range of the distribution (25–75th percentile), horizontal line within the box the median, and vertical lines the 5th and 95th percentiles.
TERT gene amplification and expression
The median TERT/36B4 ratio in fPTC and sPTC was 0.98 (25–75th percentile, 0.86–1.16) and 0.99 (25–75th percentile, 0.90–1.08), respectively. No significant difference was found between the tested groups (P = 0.998). The TERT/36B4 ratio was not significantly different in males and females either within each group of PTC (P = 0.659 and P = 0.075 for fPTC and sPTC, respectively) or as compared between the groups of PTC (P = 0.622 and P = 0.533 for females and males, respectively). The TERT expression level in the groups of validation series was 0.00249 (25–75th percentile, 0.00218–0.00356), 0.00253 (25–75th percentile, 0.00165–0.00361), and 0.00267 (25–75th percentile, 0.00185–0.00458) for fPTC, sPTC, and controls, respectively. No statistically significant difference was detected among the groups for TERT expression (P = 0.718).
Discussion
Contrary to previous studies, the results obtained from testing our cohort did not show any association between short telomere length and fPTC. No differences in TERT gene amplification were found in our population. Data obtained from a validation series using different methodology did not show any difference in the telomere length and the amount of RNA between fPTC and the other groups.
It is known that telomere length depends on many factors such as age, gender, telomerase activity, oxidative stress, and other factors. Hitherto undefined genetic factors also appear to clearly influence the length, particularly at birth (16–18). The pattern of inheritance is controversial; however, it seems to be accepted that transmission of RTL from one generation to the next exists. We cannot exclude the possibility that genetic differences in telomere length and its determination between the Italian population and the U.S. population mainly from Ohio studied by us are somehow responsible for the differences noted. We noticed that fPTC patients from Siena and Rome showed differences in RTL, and to some extent, this was also noticeable for unaffected siblings and sPTC. Whether this is suggestive of genetic differences between inhabitants of Siena and Rome or other hitherto unidentified factors influencing telomere length remains unknown. We note that the ethnic derivation of the Ohio population is predominantly German, British, and French; that is different from the Italians. To avoid biases introduced by the impact of genetic predisposition (e.g. a founder mutation favoring short telomeres) only one fPTC case per kindred was included in our study, whereas in the Italian study, more than one affected family member was tested. Thus, the final results in regard to telomere length could be biased, particularly if an unusual genetic predisposition is present in some probands and may become inadvertently overrepresented through family members.
A factor involved in telomere repeat copy number is age. The telomere length is negatively but nonlinearly correlated with age. However, this correlation is not very strong as witnessed by a distinct variation among similarly aged individuals (19). The mean age at the time of blood collection of fPTC and sPTC cases in our study was 42.1 ± 13.5 (sd) and 44.2 ± 17.1 yr, respectively with no significant difference (P = 0.756). The Italian group stated that all tested groups had been matched for age, but only data regarding age at diagnosis are shown. It was stated that the blood was drawn from 0.5–33 yr after surgery in the Italian study. It remains unknown whether differences in the time of blood collection between groups (if any) could have biased the final results.
Another factor believed to influence telomere length is gender. It has been shown that females have longer telomeres than males of the same age, perhaps because females lose their telomere repeats more slowly than males, but the difference in telomere length between females and males is not very strong (17, 20). The female to male ratio in the patient population studied by us was 2:1 for fPTC and 4:1 for sPTC. Our data showed no significant difference in RTL and TERT gene copy number between females and males either within or between the fPTC and sPTC groups (gender- and age-adjusted ANOVA model analysis also showed no difference, data not shown). We concluded that possible differences in gender distribution between our study and the one by Capezzone et al. (11) are unlikely to have caused the profoundly different results.
In summary, telomere length assessed using different methods was similar in patients with familial and sporadic PTC in our cohort. Although we note some differences between several parameters in the previous study and ours, we cannot clearly state how these differences might have influenced the final results. Further investigations in additional cohorts of patients are desirable to clarify these results.
Acknowledgments
We thank Ilene Comeras, Elizabeth Solinger, and Amy Sturm for collecting samples for the study and Rebecca Pearson for excellent help with flow cytometry. We are grateful to Dr. Krystian Jazdzewski and Dr. Krzysztof Mrózek for advice and Wei Li for technical assistance.
This work was supported by National Cancer Institute Grants P30CA16058 to The Ohio State University Comprehensive Cancer Center and P01CA124570 to M.D.R. (principal investigator) and A.d.l.C. (leader of a project).
Disclosure Summary: The authors have nothing to disclose. Dr. Matthew D. Ringel has previously served on an advisory board for Veracyte and has been an advisor for Astra Zeneca. These were not related to the work presented in the manuscript.
Footnotes
- CV
- coefficient of variation
- Ct
- cycle threshold
- FISH
- fluorescent in situ hybridization
- fPTC
- familial PTC
- MFI
- mean fluorescence intensity
- PTC
- papillary thyroid carcinoma
- q-PCR
- quantitative real-time PCR
- RTL
- relative telomere length
- S
- single-copy gene
- sPTC
- sporadic PTC
- T
- telomere.
References
- 1. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. 2007. Cancer statistics, 2007. CA Cancer J Clin 57:43–66 [DOI] [PubMed] [Google Scholar]
- 2. Dong C, Hemminki K. 2001. Modification of cancer risks in offspring by sibling and parental cancers from 2,112,616 nuclear families. Int J Cancer 92:144–150 [PubMed] [Google Scholar]
- 3. Goldgar DE, Easton DF, Cannon-Albright LA, Skolnick MH. 1994. Systematic population-based assessment of cancer risk in first-degree relatives of cancer probands. J Natl Cancer Inst 86:1600–1608 [DOI] [PubMed] [Google Scholar]
- 4. Jazdzewski K, Liyanarachchi S, Swierniak M, Pachucki J, Ringel MD, Jarzab B, de la Chapelle A. 2009. Polymorphic mature microRNAs from passenger strand of pre-miR-146a contribute to thyroid cancer. Proc Natl Acad Sci USA 106:1502–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jazdzewski K, Murray EL, Franssila K, Jarzab B, Schoenberg DR, de la Chapelle A. 2008. Common SNP in pre-miR-146a decreases mature miR expression and predisposes to papillary thyroid carcinoma. Proc Natl Acad Sci USA 105:7269–7274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. He H, Nagy R, Liyanarachchi S, Jiao H, Li W, Suster S, Kere J, de la Chapelle A. 2009. A susceptibility locus for papillary thyroid carcinoma on chromosome 8q24. Cancer Res 69:625–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gudmundsson J, Sulem P, Gudbjartsson DF, Jonasson JG, Sigurdsson A, Bergthorsson JT, He H, Blondal T, Geller F, Jakobsdottir M, Magnusdottir DN, Matthiasdottir S, Stacey SN, Skarphedinsson OB, Helgadottir H, Li W, Nagy R, Aguillo E, Faure E, Prats E, Saez B, Martinez M, Eyjolfsson GI, Bjornsdottir US, Holm H, Kristjansson K, Frigge ML, Kristvinsson H, Gulcher JR, Jonsson T, Rafnar T, Hjartarsson H, Mayordomo JI, de la Chapelle A, Hrafnkelsson J, Thorsteinsdottir U, Kong A, Stefansson K. 2009. Common variants on 9q22.33 and 14q13.3 predispose to thyroid cancer in European populations. Nat Genet 41:460–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fagin JA. 1997. Familial nonmedullary thyroid carcinoma: the case for genetic susceptibility. J Clin Endocrinol Metab 82:342–344 [DOI] [PubMed] [Google Scholar]
- 9. Ito Y, Kakudo K, Hirokawa M, Fukushima M, Yabuta T, Tomoda C, Inoue H, Kihara M, Higashiyama T, Uruno T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, Miyauchi A. 2009. Biological behavior and prognosis of familial papillary thyroid carcinoma. Surgery 145:100–105 [DOI] [PubMed] [Google Scholar]
- 10. Uchino J, Takayama K, Harada A, Kawakami Y, Inoue H, Curiel DT, Nakanishi Y. 2005. Infectivity enhanced, hTERT promoter-based conditionally replicative adenoviruses are useful for SCLC treatment. Cancer Gene Ther 12:737–748 [DOI] [PubMed] [Google Scholar]
- 11. Capezzone M, Cantara S, Marchisotta S, Filetti S, De Santi MM, Rossi B, Ronga G, Durante C, Pacini F. 2008. Short telomeres, telomerase reverse transcriptase gene amplification, and increased telomerase activity in the blood of familial papillary thyroid cancer patients. J Clin Endocrinol Metab 93:3950–3957 [DOI] [PubMed] [Google Scholar]
- 12. Cantara S, Capuano S, Formichi C, Pisu M, Capezzone M, Pacini F. 2010. Lack of germline A339V mutation in thyroid transcription factor-1 (TITF-1/NKX2.1) gene in familial papillary thyroid cancer. Thyroid Res 3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cawthon RM. 2002. Telomere measurement by quantitative PCR. Nucleic Acids Res 30:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 15. Baerlocher GM, Vulto I, de Jong G, Lansdorp PM. 2006. Flow cytometry and FISH to measure the average length of telomeres (flow FISH). Nat Protoc 1:2365–2376 [DOI] [PubMed] [Google Scholar]
- 16. Nawrot TS, Staessen JA, Gardner JP, Aviv A. 2004. Telomere length and possible link to X chromosome. Lancet 363:507–510 [DOI] [PubMed] [Google Scholar]
- 17. Nordfjäll K, Larefalk A, Lindgren P, Holmberg D, Roos G. 2005. Telomere length and heredity: Indications of paternal inheritance. Proc Natl Acad Sci USA 102:16374–16378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Okuda K, Bardeguez A, Gardner JP, Rodriguez P, Ganesh V, Kimura M, Skurnick J, Awad G, Aviv A. 2002. Telomere length in the newborn. Pediatr Res 52:377–381 [DOI] [PubMed] [Google Scholar]
- 19. Hewakapuge S, van Oorschot RA, Lewandowski P, Baindur-Hudson S. 2008. Investigation of telomere lengths measurement by quantitative real-time PCR to predict age. Leg Med (Tokyo) 10:236–242 [DOI] [PubMed] [Google Scholar]
- 20. Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. 2003. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 361:393–395 [DOI] [PubMed] [Google Scholar]