Abstract
Context:
The metabolic syndrome (MetS) is associated with increased risk of diabetes and cardiovascular disease (CVD). Numerous groups have shown increased circulating biomarkers of inflammation in MetS. However, there are scanty data on the cellular sources contributing to this low-grade inflammation.
Objective:
The aim of this study was to determine the role of sc adipose tissue (SAT) biology in nascent MetS without concomitant diabetes or CVD.
Patients and Methods:
Subjects with MetS and controls were recruited after informed consent. Fasting blood was collected, and SAT was obtained by biopsy.
Results:
Circulating biomarkers of inflammation and insulin resistance, high-sensitivity C-reactive protein (hsCRP), IL-6, IL-1β, leptin, serum amyloid A, and retinol-binding protein-4 (RBP-4) concentrations were significantly higher in the MetS subjects than controls, whereas adiponectin concentrations were lower. In SAT, leptin, RBP-4, CRP, serum amyloid A, plasminogen activator inhibitor-1, IL-1, IL-6, IL-8, and monocyte chemotactic protein-1 (MCP-1) levels were significantly higher in MetS than controls. These differences except for RBP-4 persisted after adjusting for waist circumference. In addition, there were significantly increased numbers of macrophages infiltrating the SAT of MetS and increased numbers of crown-like structures compared with controls. hsCRP correlated positively with homeostasis model assessment and SAT MCP-1 and negatively with adiponectin. Homeostasis model assessment correlated positively with plasminogen activator inhibitor-1, RBP-4, and SAT MCP-1.
Conclusions:
We make the novel observation that SAT of MetS has increased macrophage recruitment with cardinal crown-like structure features and contributes to the increased cellular inflammation that produces increased levels of biomarkers that are correlated with both insulin resistance and low-grade inflammation. These aberrations could contribute to the progression of MetS and the increased risk for diabetes and CVD.
The metabolic syndrome (MetS) comprises a cluster of cardiometabolic risk markers with insulin resistance and adiposity as central features (1–4). Five diagnostic criteria for MetS have been identified [central obesity, dyslipidemia, high triglycerides (TG) and/or low high-density lipoprotein cholesterol (HDL-C), hypertension, and impaired fasting glucose] by the Adult Treatment Panel III criteria of the National Cholesterol and Education Program, and the presence of three features is considered sufficient to diagnose the syndrome (2, 4, 5). Using this definition, the National Health and Nutrition Examination Survey data show that currently approximately 35% of all U.S. adults have MetS (6). Furthermore, MetS confers an increased risk for diabetes and cardiovascular disease (CVD) (7–10). Although it appears that low-grade chronic inflammation could be a central feature to help explain the increased risks of CVD and diabetes in MetS, the precise mechanisms remain to be elucidated. The role of sc adipose tissue (SAT) dysregulation could be a potential mechanism.
Numerous investigators have shown increased circulating biomarkers of inflammation in MetS, thus providing support for the proinflammatory state of MetS (2, 4, 11). However, there are scant data on SAT biology in nascent MetS subjects without the confounding presence of diabetes and/or CVD. The relationship between inflammation and MetS is supported by several studies (2, 4, 12, 13), as is the relationship between increased visceral fat mass and MetS (14–16). However, the role of SAT in the pathogenesis of MetS has been debated given inconsistencies in the literature (17). The sc fat, which comprises approximately 80% of adipose tissue and the major source of fatty acids for the liver, is readily accessible to study and has been shown to be metabolically correlated to indices of insulin resistance as well as to visceral adipose tissue (VAT) (18–21). In addition to intraabdominal fat, Salmenniemi et al. (22) have shown that the amount of SAT in subjects with MetS positively correlates with increasing MetS factor scores and negatively correlates with circulating adiponectin levels. Carr et al. (23) have also reported that SAT is significantly associated with MetS and increases with increasing number of MetS features, independent of age and sex.
Furthermore, macrophage infiltration appears to be important in adipose tissue inflammation. Apovian et al. (24) examined abdominal SAT from obese subjects and reported that an inflamed adipose phenotype characterized by tissue macrophage accumulation in crown-like structures (CLS) was associated with systemic hyperinsulinemia and insulin resistance and impaired endothelium-dependent flow-mediated vasodilation. Macrophage retention in fat was also linked to up-regulated tissue CD68 and TNF-α mRNA expression in addition to increased plasma high-sensitivity C-reactive protein (hsCRP).
However, there is a paucity of data examining SAT biology in subjects with MetS, specifically without diabetes and/or CVD compared with age- and gender-matched controls. Thus, the main objective of this study was to determine whether SAT biology in subjects with MetS is dysregulated and contributes to the syndrome's systemic low-grade inflammatory process.
Subjects and Methods
Subjects
Subjects were recruited from Sacramento County using procedures as described previously (25). Subjects were then classified as having MetS or not using the National Cholesterol and Education Program Adult Treatment Panel III criteria (5). Briefly, subjects classified as MetS had at least three risk factors to sustain the diagnosis, including central obesity, hypertension, dyslipidemia (low HDL-C, high TG), and/or hypertension or on antihypertensive medications. The control subject needed to have no more than two features of MetS and not be on blood pressure (BP) medications. Other exclusion criteria for controls were a fasting plasma glucose concentration higher than 100 mg/dl and a fasting TG concentration higher than 200 mg/dl. For both groups, other exclusion criteria were a previous diagnosis of diabetes, clinical atherosclerosis [coronary artery disease (CAD), peripheral vascular disease, CVD, etc.], a TG concentration higher than 400 mg/dl, a hsCRP concentration higher than 10 mg/liter, pregnancy, an abnormal complete blood count, alcohol consumption higher than 1 oz/d, consumption of N-3 polyunsaturated fatty acids, smoking, hypo- or hyperthyroidism, malabsorption, active wounds, recent surgery, inflammatory or malignant disease, anticoagulant therapy, steroid therapy, the current use of antiinflammatory drugs, statins and/or other hypolipidemic agents, hypoglycemic agents, angiotensin receptor blockers, oral contraceptives, and antioxidant supplements (in the previous six months), postmenopausal women on estrogen replacement therapy, and chronic high-intensity exercisers (exercise >100 min/wk). Informed consent was obtained from all participants in the study, and the study was approved by the Institutional Review Board at the University of California, Davis (UC Davis). After a history and physical examination, a fasting blood sample was obtained. A complete blood count, plasma lipid and lipoprotein profile, urea nitrogen, creatinine, aspartate aminotransferase, alanine aminotransferase, glucose and TSH were assayed by standard laboratory techniques in the Clinical Pathology Laboratory (UC Davis). Insulin levels were assayed by ELISA (Linco Biosystems, Lincoln, NE), and the homeostasis model assessment (HOMA) of insulin resistance was calculated from the fasting glucose and insulin levels (26).
Biomarkers of inflammation
Blood levels of hsCRP, adiponectin, and plasminogen activator inhibitor-1 (PAI-1) were assayed by ELISA. Also, IL-1β, TNF-α, IL-6, IL-8, IL-18, monocyte chemotactic protein-1 (MCP-1) were assayed by multiplex cytokine array (Bioplex, San Jose, CA).
SAT biopsy and fat analysis
SAT biopsies were performed on all subjects at the UC Davis Clinical and Translational Science Center's Clinical Research Center. Briefly, the gluteal region was sterilized, and 4 ml 2% lidocaine was injected to desensitize the area. A 14-gauge needle connected to a 20-ml syringe was then inserted at the site of lidocaine injection. Fat was aspirated as suction was applied while the syringe and needle were repeatedly rotated. Approximately 4–6 ml fat and fluid were aspirated, washed, and then placed in the transport medium. The area was cleaned and dressed. The aspirated SAT was weighed in a balance, then minced into fine (<10 mg) pieces and resuspended in 5 mm glucose culture medium. The SAT was then incubated in multiple wells for 24 h. The cell supernatants were collected and cells lysed in 0.1 n NaOH for protein measurement. All ELISA results are reported normalized to the amount of fat in grams and/or milligrams cell protein. Biomarkers that were examined included adiponectin, CRP, serum amyloid A (SAA), leptin, PAI-1, retinol-binding protein-4 (RBP-4), and chemokines (MCP-1 and IL-8) as well as cytokines (IL-1, TNF, IL-18, and IL-6). Adiponectin, SAA, RBP-4, and leptin levels were measured using reagents from Linco. CRP and PAI-1 were measured as previously described (27). IL-1β, TNF-α, IL-6, and IL-8 were measured using a multiplex cytokine/chemokine array. In addition, three to four fragments of SAT were fixed in 10% buffered formalin after cleaning and processed for immunohistochemistry in the Cytopathology Section of our department. The SAT sections were stained for CD68 (a macrophage marker) and T cells (CD3 and CD5) to assess macrophage/T cell infiltration into adipose tissue. The number of macrophages and CLS per high-power field (hpf) were counted in a blinded fashion. At least 10 fields were selected randomly and counted for analysis.
Statistical analysis
Parametric data are expressed as mean ± sd, and nonparametric data are expressed as median ± interquartile ranges. Parametric data were compared using the Student's t test, whereas the nonparametric data were compared with the Wilcoxon signed rank tests. The Pearson and Spearman correlations were computed between variables of interest.
Results
Study cohort
The characteristics of the study population (including anthropometric measurements, BP, fasting glucose levels, fasting lipid levels, and circulating levels of adipocytokines and other biomarkers of inflammation) are shown in Table 1. There was no significant difference in the ages or male to female ratio between the controls and subjects with MetS. Waist circumference, body mass index, BP, fasting glucose concentrations, total cholesterol concentrations, TG concentrations, and HOMA were higher in the MetS subjects than in controls, whereas HDL-C concentrations were lower. In addition, hsCRP, IL-6, IL-1β, leptin, SAA, and RBP-4 concentrations were significantly higher in the MetS subjects than in controls, whereas the adiponectin concentrations were lower.
Table 1.
Subject characteristics
| Control (n = 26) | MetS (n = 39) | P value | |
|---|---|---|---|
| Age (yr) | 44 ± 10 | 49 ± 11 | 0.12 |
| Waist (cm) | 94 ± 17 | 108 ± 17 | <0.001 |
| Male to female ratio | 10:16 | 14:25 | |
| BMI (kg/m2) | 29 ± 7 | 34 ± 6 | <0.01 |
| Systolic BP (mm Hg) | 118 ± 18 | 135 ± 18 | <0.001 |
| Diastolic BP (mm Hg) | 77 ± 12 | 86 ± 17 | <0.001 |
| Fasting glucose (mg/dl) | 88 ± 10 | 102 ± 12 | <0.001 |
| Total cholesterol (mg/dl) | 187 ± 34 | 194 ± 38 | <0.05 |
| HDL-C (mg/dl) | 68 ± 12 | 44 ± 19 | <0.001 |
| Non-HDL-C (mg/dl) | 126 ± 22 | 148 ± 27 | <0.01 |
| TG (mg/dl) | 78 (66–94) | 129 (106–149) | <0.001 |
| HOMA | 1.1 (0.9–2.6) | 2.1 (1.7–4.9) | <0.0001 |
| hsCRP (mg/liter) | 1.2 (0.5–2.8) | 3.4 (1.6–5.2) | <0.01 |
| Leptin (ng/ml) | 34 (24–55) | 79 (51–104) | <0.001 |
| RBP-4 (μg/ml) | 41 ± 13 | 51 ± 19 | <0.02 |
| Adiponectin (μg/ml) | 7.8 (5.4–12.9) | 5.4 (3.8–8.2) | <0.02 |
| SAA (μg/ml) | 6.5 ± 2.7 | 9.5 ± 3.2 | <0.001 |
| IL-1β (pg/ml) | 9.7 (3.1–11.8) | 20.6 (5.8–34.2) | <0.05 |
| IL-6 (pg/ml) | 1.2 (0.4–2.7) | 2.9 (1.1–4.5) | <0.01 |
| TNF (pg/ml) | 1.7 (0.9–2.9) | 2.3 (1.1–3.5) | >0.05 |
Data are expressed as mean ± sd or median (25–75th percentile) for skewed data. BMI, Body mass index.
SAT biomarkers
The concentrations of several biomarkers in SAT from controls and subjects with MetS are shown in Table 2. Expressed per gram of SAT, the amount of leptin, RBP-4, CRP, SAA, PAI-1, and MCP-1 were significantly higher in subjects with MetS than controls. In addition, release of IL-1β, IL-6, IL-8, and MCP-1, as expressed per milligram protein, was higher in SAT from subjects with MetS than controls.
Table 2.
SAT biomarker levels
| Controls | MetS | Adjusted P value | |
|---|---|---|---|
| Adiponectin (ng/g) | 4.2 (1.3–5.6) | 3.7 (1.2–4.6) | 0.077 |
| Leptin (ng/g) | 3.0 (2.1–6.2) | 7.3 (3.8–18.6)a | <0.05 |
| RBP-4 (ng/g) | 11.1 (6.4–18.4) | 29.1 (16.2–33.7)b | 0.069 |
| CRP (ng/g) | 2.5 (2.3–7.9) | 5.4 (3.4–19.1)a | <0.05 |
| SAA (ng/g) | 14.8 (5.1–34.2) | 25.3 (14.5–55.7)a | <0.05 |
| PAI-1 (ng/g) | 3.2 (2.2–6.5) | 5.6 (3.1–9.9)b | <0.001 |
| MCP-1 (ng/g) | 6.7 (4.3–9.1) | 22.1 (11.8–33.5)b | <0.01 |
| IL-1β (ng/mg protein) | 31.1 (21.2–45.1) | 39.7 (24.8–61.5)a | <0.05 |
| TNF (ng/mg protein) | 3.7 (1.9–4.6) | 3.8 (2.9–5.3) | >0.05 |
| IL-6 (ng/mg protein) | 16.5 (10.6–24.5) | 18.7 (12.7–33.2)a | <0.05 |
| IL-8 (ng/mg protein) | 10.9 (5.1–14.2) | 17.4 (14.5–27.3)b | <0.02 |
Data are expressed as median (25–75th percentile). P values are compared with controls and adjusted for WC.
P < 0.05.
P < 0.001.
SAT composition and architecture
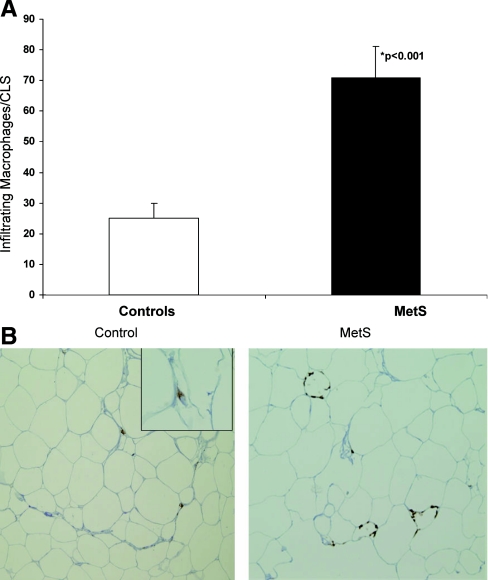
Using CD3 and CD5 staining, no lymphocyte populations were observed in any of the SAT specimens. However, there were significantly increased numbers of macrophages infiltrating the SAT of MetS subjects compared with controls as demonstrated by positive CD68 staining (Fig. 1). Furthermore, there were significantly increased numbers of CLS in the SAT of MetS subjects than those from controls (3-fold more in MetS subjects compared with controls) (controls, five CLS per 10 hpf; MetS, 14 CLS/10 hpf; P < 0.001).
Fig. 1.
A, Number of infiltrating macrophages in adipose tissue of controls and subjects with MetS. The number of macrophages including CLS per 10 hpf were counted after immunohistochemistry and staining with CD68 as detailed in Subjects and Methods. *, P < 0.001 compared with control. B, Representative SAT biopsy specimens from controls and subjects with MetS (hematoxylin and eosin stain). Note the macrophages arranged adjacent to the adipocyte cell membrane forming a CLS in the SAT from the MetS subject. The images shown are at ×20 magnification; inset of the control is at ×40 magnification.
Adjusted analyses
Because the patients with MetS in our cohort had significantly greater waist circumferences (WC) than the controls, all the analytes reported above were repeated with WC as a covariate. Importantly, in these adjusted analyses, all reported differences between the two groups persisted except for RBP-4 (Table 2). Undertaking a subgroup analysis with a median WC of 42 in. revealed higher levels of only leptin and RBP-4 with WC over 42 vs. WC under 42 in. (for WC < 42 in., leptin was 71 (49–83) ng/ml vs. 82 (55–89) ng/ml for WC > 42 in.; for WC < 42 in., RBP-4 was 25 (11–31) ng/mg protein vs. 33 (17–37) ng/mg protein for WC > 42 in.; P < 0.05 compared with WC < 42 in.).
Correlations
Because both insulin resistance and low-grade inflammation are typical features of MetS, we used HOMA and hsCRP as the representative biomarkers for these conditions to probe relevant correlations. hsCRP correlated positively with HOMA (r = 0.39; P = 0.03) and adipose tissue MCP-1 (r = 0.46; P = 0.03) and negatively with adipose tissue adiponectin (r = −0.44; P = 0.01). Furthermore, there was a significant correlation between circulating and adipose tissue levels of CRP (r = 0.49; P = 0.01), IL-1β (r = 0.56; P = 0.002), and IL-6 (r = 0.76; P = 0.001). HOMA correlated positively with PAI-1 (r = 0.56; P = 0.020), RBP-4 (r = 0.49; P = 0.03), and SAT MCP-1 (r = 0.039; P = 0.040). Although there were significantly more CLS in patients with MetS, there were no significant correlations with biomarkers of inflammation.
Discussion
We demonstrate in this study, SAT biomarkers and architecture differ markedly in subjects with MetS compared with matched controls. We show that patients with MetS without the confounding conditions of diabetes and/or CVD have increased levels of adipocytokines and decreased adiponectin that are related to insulin resistance and inflammation. Furthermore, we show that this dysregulation of adipokines could not be accounted for simply by increased adiposity, suggesting that other aspects of MetS contribute to both the pro-inflammatory state and insulin resistance. More importantly, we document a significant increase in macrophages in SAT and abundant CLS that appear to surround a hypoxic environment triggered by adipocyte death (28–30). For reasons unclear to us, there were no significant correlations between CLS and biomarkers of inflammation. One can speculate that in humans, these CLS could be predominantly of the M2 macrophage phenotype participating in tissue remodeling. This will be the subject of a future study. Also, the chemokine MCP-1 was increased in SAT from MetS subjects and clearly facilitates homing of macrophages to such tissue depots. As such, the SAT may indeed be a key player in MetS and its associated comorbidities. Moreover, because SAT is easy to access and SAT biopsies can be performed in large-scale clinical studies, and the expression of inflammatory genes in SAT compares well with VAT (19, 20), the evaluation of SAT in subjects with and without MetS may provide novel insights into the syndrome's pathogenesis and serious sequelae.
Our data are consistent with some but not all studies that have reported mRNA/gene expression profiles in SAT in subjects with MetS (31, 32). Specifically, Gormez et al. (31) reported increased mRNA levels of TNF-α and leptin but not adiponectin in SAT from subjects with MetS vs. controls, whereas our data showed only increased amounts of SAT secreted and circulating leptin. However, it needs to be emphasized that Gormez et al. (31) studied MetS patients with CAD with 88% of the subjects having concomitant diabetes and dyslipidemia. Thus, one cannot ascribe their findings to MetS alone, and the additional confounder of medications cannot be excluded. Furthermore, Sacks et al. (32) reported no changes with respect to IL-1β gene expression in SAT from 15 subjects with MetS with CAD vs. controls, whereas our data (using a much larger sample size) showed an increased amount of IL-1β released from SAT and in plasma. Thus, unlike the present report, these groups focused on a few selective biomediators/biomarkers. Also, these differences could easily be the result of posttranscriptional processes and highlight the problem with inferring that gene expression directly correlates with protein expression. Thus, the novelty of our study is that we report on patients with MetS but without diabetes and/or CVD and assayed both plasma and secreted levels of adipokines. We did not examine expression of these adipokines in stromal vascular vs. adipocyte fractions due to the confounding effects of collagenase treatment on inflammatory gene expression, and this could be perceived as a limitation of the study. However, we expressed levels of these biomediators per gram of fat mass as well as per milligram protein to examine whether any of these changes were per se due to changes in fat mass. As shown in Table 2, differences in adipokines were observed when expressed either per gram of fat or per milligram protein. It is the consensus that these circulating adipokines function as biomediators that contribute to the increased risk for both diabetes and CVD in MetS subjects (33–35). Also, it is important to note that CRP levels are significantly increased from adipose tissue of MetS subjects compared with controls. Although a large part of CRP appears to be produced in the liver, previous investigators have shown that there is increased CRP gene expression from adipose tissue (36) and vascular endothelium (37), a component of the stromal vascular fraction. These data support the idea that MetS is a proinflammatory state and that the adipose tissue of MetS contributes to the increased inflammation in these subjects.
The essential role of SAT in metabolic homeostasis has best been described in lipodystrophic syndromes, where its absence leads to ectopic fat accumulation in the liver and skeletal muscle with concomitant insulin resistance (38). And although a deficiency in SAT has been associated with MetS (39), our data demonstrate that the SAT may also be a key player in the pathogenesis of MetS, consistent with findings from the Framingham Heart Study showing that larger volumes of SAT were associated with more cardiometabolic risk factors (16).
Adipose tissue dysfunction has been shown to be a mediator in the development of obesity-associated complications (40), which include MetS. Thus, given that recent studies have shown that molecular adaptations in SAT are as discriminating as those in VAT in conditions of worsening metabolic status (41), we specifically chose to study SAT in subjects with and without MetS given its easy accessibility. Importantly, our study was designed not to address the levels of biomarker gene expression in subjects with MetS, as has been done before but suffers from the confounding of the comorbidities of diabetes and CVD (31, 32, 41), but instead to measure the amounts of biomarker proteins present in the SAT and secreted because it is the secreted proteins that mediate biological effects. This type of analysis thus bypasses the potential problems with assuming that gene expression correlates with protein expression and reveals the amount of protein that is actually present. Another important observation from our study is that MCP-1 appears to correlate well with insulin resistance and inflammation. Previous studies have shown, in mice, that knockout of the receptor for MCP-1, i.e. CCR2, results in decreased hepatic inflammation and steatosis (42) and also results in decreased adipose tissue macrophages (43), underscoring the importance of SAT MCP-1 in MetS subjects. The exact mechanisms by which MCP-1 contributes to both insulin resistance and increased inflammation in MetS need to be elucidated in future studies.
Our study also has some limitations. First, VAT samples were not obtained in our study population, so the correlation between SAT and VAT findings could not be corroborated. However, in future studies with our bariatric surgery colleagues, we intend to investigate this important tissue. Second, although we report on the comprehensive repertoire of biomarkers in MetS to date, the list is by no means exhaustive; however, most if not all of the biomarkers evaluated have biological plausibility and credence in the pathogenesis of MetS and or its sequelae. Also, we examined biomarkers in adipose tissue after 24 h incubation, whereas some investigators have examined these levels for up to 5 d after incubation. Nonetheless, we can be confident that our novel data support the notion that the dysregulation of SAT physiology/biology in MetS is important and could have implications for its pathogenesis and greater risk for diabetes and CVD. In future studies, we will focus on the role of the pivotal cell of innate immunity, the monocyte.
Conclusion
In this study, we make the novel observation that the SAT in subjects with MetS uncomplicated by the comorbidities of diabetes and/or CVD has increased macrophage recruitment with cardinal CLS in greater abundance. Furthermore, these cells in SAT conspire to produce increased levels of biomarkers that correlate with both insulin resistance and low-grade inflammation, potentially presaging the subsequent increased risk for diabetes and CVD.
Acknowledgments
We are thankful to Long Wang, Ph.D., for help with subject recruitment and Nancy Fitch, R.N., for assistance in procuring adipose tissue biopsies.
This work was supported by the American Diabetes Association and National Institutes of Health Clinical and Translational Science Center.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BP
- Blood pressure
- CAD
- coronary artery disease
- CLS
- crown-like structures
- CVD
- cardiovascular disease
- HDL-C
- high-density lipoprotein cholesterol
- HOMA
- homeostasis model assessment
- hpf
- high-power field
- hsCRP
- high-sensitivity C-reactive protein
- MCP-1
- monocyte chemotactic protein-1
- MetS
- metabolic syndrome
- PAI-1
- plasminogen activator inhibitor-1
- RBP-4
- retinol-binding protein-4
- SAA
- serum amyloid A
- SAT
- sc adipose tissue
- TG
- triglycerides
- VAT
- visceral adipose tissue
- WC
- waist circumference.
References
- 1. Reaven GM. 2005. The insulin resistance syndrome: definition and dietary approaches to treatment. Annu Rev Nutr 25:391–406 [DOI] [PubMed] [Google Scholar]
- 2. Eckel RH, Grundy SM, Zimmet PZ. 2005. The metabolic syndrome. Lancet 365:1415–1428 [DOI] [PubMed] [Google Scholar]
- 3. Haffner S, Cassells HB. 2003. Metabolic syndrome: a new risk factor of coronary heart disease? Diabetes Obes Metab 5:359–370 [DOI] [PubMed] [Google Scholar]
- 4. Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE, Wang H, Eckel RH. 2008. The metabolic syndrome. Endocr Rev 29:777–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) 2001. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 285:2486–2497 [DOI] [PubMed] [Google Scholar]
- 6. Mozumdar A, Liguori G. 2011. Persistent increase of prevalence of metabolic syndrome among U.S. adults: NHANES III to NHANES 1999–2006. Diabetes Care 34:216–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alexander CM, Landsman PB, Teutsch SM, Haffner SM. 2003. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes 52:1210–1214 [DOI] [PubMed] [Google Scholar]
- 8. Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. 2002. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 288:2709–2716 [DOI] [PubMed] [Google Scholar]
- 9. Hanson RL, Imperatore G, Bennett PH, Knowler WC. 2002. Components of the “metabolic syndrome” and incidence of type 2 diabetes. Diabetes 51:3120–3127 [DOI] [PubMed] [Google Scholar]
- 10. Assmann G, Nofer JR, Schulte H. 2004. Cardiovascular risk assessment in metabolic syndrome: view from PROCAM. Endocrinol Metab Clin North Am 33:377–392 [DOI] [PubMed] [Google Scholar]
- 11. Devaraj S, Dasu MR, Jialal I. 2010. Diabetes is a proinflammatory state: a translational perspective. Expert Rev Endocrinol Metab 5:19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hotamisligil GS. 2006. Inflammation and metabolic disorders. Nature 444:860–867 [DOI] [PubMed] [Google Scholar]
- 13. Monteiro R, Azevedo I. 2010. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm pii:289645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Despres JP, Lemieux S, Lamarche B, Prud'homme D, Moorjani S, Brun LD, Gagne C, Lupien PJ. 1995. The insulin resistance-dyslipidemic syndrome: contribution of visceral obesity and therapeutic implications. Int J Obes Relat Metab Disord 19(Suppl 1):S76–S86 [PubMed] [Google Scholar]
- 15. Després JP. 2006. Is visceral obesity the cause of the metabolic syndrome? Ann Med 38:52–63 [DOI] [PubMed] [Google Scholar]
- 16. Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D'Agostino RB, Sr, O'Donnell CJ. 2007. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 116:39–48 [DOI] [PubMed] [Google Scholar]
- 17. Porter SA, Massaro JM, Hoffmann U, Vasan RS, O'Donnel CJ, Fox CS. 2009. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care 32:1068–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. 1997. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes 46:1579–1585 [DOI] [PubMed] [Google Scholar]
- 19. Abate N, Garg A, Peshock RM, Stray-Gundersen J, Grundy SM. 1995. Relationships of generalized and regional adiposity to insulin sensitivity in men. J Clin Invest 96:88–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abate N, Garg A, Peshock RM, Stray-Gundersen J, Adams-Huet B, Grundy SM. 1996. Relationship of generalized and regional adiposity to insulin sensitivity in men with NIDDM. Diabetes 45:1684–1693 [DOI] [PubMed] [Google Scholar]
- 21. Ferreira I, Henry RM, Twisk JW, van Mechelen W, Kemper HC, Stehouwer CD. 2005. The metabolic syndrome, cardiopulmonary fitness, and subcutaneous trunk fat as independent determinants of arterial stiffness: the Amsterdam Growth and Health Longitudinal Study. Arch Intern Med 165:875–882 [DOI] [PubMed] [Google Scholar]
- 22. Salmenniemi U, Ruotsalainen E, Pihlajamäki J, Vauhkonen I, Kainulainen S, Punnonen K, Vanninen E, Laakso M. 2004. Multiple abnormalities in glucose and energy metabolism and coordinated changes in levels of adiponectin, cytokines, and adhesion molecules in subjects with metabolic syndrome. Circulation 110:3842–3848 [DOI] [PubMed] [Google Scholar]
- 23. Carr DB, Utzschneider KM, Hull RL, Kodama K, Retzlaff BM, Brunzell JD, Shofer JB, Fish BE, Knopp RH, Kahn SE. 2004. Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes 53:2087–2094 [DOI] [PubMed] [Google Scholar]
- 24. Apovian CM, Bigornia S, Mott M, Meyers MR, Ulloor J, Gagua M, McDonnell M, Hess D, Joseph L, Gokce N. 2008. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol 28:1654–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jialal I, Devaraj S, Singh U, Huet BA. 2010. Decreased number and impaired functionality of endothelial progenitor cells in subjects with metabolic syndrome: implications for increased cardiovascular risk. Atherosclerosis 211:297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. 1985. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- 27. Devaraj S, Xu DY, Jialal I. 2003. C-reactive protein increases plasminogen activator inhibitor-1 expression and activity in human aortic endothelial cells: implications for the metabolic syndrome and atherothrombosis. Circulation 107:398–404 [DOI] [PubMed] [Google Scholar]
- 28. Anderson EK, Gutierrez DA, Hasty AH. 2010. Adipose tissue recruitment of leukocytes. Curr Opin Lipidol 21:172–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Surmi BK, Hasty AH. 2008. Macrophage infiltration into adipose tissue: initiation, propagation and remodeling. Future Lipidol 3:545–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wood IS, de Heredia FP, Wang B, Trayhurn P. 2009. Cellular hypoxia and adipose tissue dysfunction in obesity. Proc Nutr Soc 68:370–377 [DOI] [PubMed] [Google Scholar]
- 31. Gormez S, Demirkan A, Atalar F, Caynak B, Erdim R, Sozer V, Gunay D, Akpinar B, Ozbek U, Buyukdevrim AS. 2011. Adipose Tissue Gene Expression of Adiponectin, Tumor Necrosis Factor-alpha and Leptin in Metabolic Syndrome Patients with Coronary Artery Disease. Intern Med 50:805–810 [DOI] [PubMed] [Google Scholar]
- 32. Sacks HS, Fain JN, Cheema P, Bahouth SW, Garrett E, Wolf RY, Wolford D, Samaha J. 2011. Inflammatory genes in epicardial fat contiguous with coronary atherosclerosis in the metabolic syndrome and type 2 diabetes: changes associated with pioglitazone. Diabetes Care 34:730–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hajer GR, van Haeften TW, Visseren FL. 2008. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J 29:2959–2971 [DOI] [PubMed] [Google Scholar]
- 34. Fantuzzi G, Mazzone T. 2007. Adipose tissue and atherosclerosis: exploring the connection. Arterioscler Thromb Vasc Biol 27:996–1003 [DOI] [PubMed] [Google Scholar]
- 35. Deng Y, Scherer PE. 2010. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann NY Acad Sci 1212:E1–E19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ouchi N, Kihara S, Funahashi T, Nakamura T, Nishida M, Kumada M, Okamoto Y, Ohashi K, Nagaretani H, Kishida K, Nishizawa H, Maeda N, Kobayashi H, Hiraoka H, Matsuzawa Y. 2003. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation 107:671–674 [DOI] [PubMed] [Google Scholar]
- 37. Venugopal SK, Devaraj S, Jialal I. 2005. Macrophage conditioned medium induces the expression of C-reactive protein in human aortic endothelial cells: potential for paracrine/autocrine effects. Am J Pathol 166:1265–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huang-Doran I, Sleigh A, Rochford JJ, O'Rahilly S, Savage DB. 2010. Lipodystrophy: metabolic insights from a rare disorder. J Endocrinol 207:245–255 [DOI] [PubMed] [Google Scholar]
- 39. Monajemi H, Stroes E, Hegele RA, Fliers E. 2007. Inherited lipodystrophies and the metabolic syndrome. Clin Endocrinol (Oxf) 67:479–484 [DOI] [PubMed] [Google Scholar]
- 40. Blüher M. 2009. Adipose tissue dysfunction in obesity. Exp Clin Endocrinol Diabetes 117:241–250 [DOI] [PubMed] [Google Scholar]
- 41. Klimcáková E, Roussel B, Márquez-Quiñones A, Kovácová Z, Kováciková M, Combes M, Siklová-Vítková M, Hejnová J, Srámková P, Bouloumié A, Viguerie N, Stich V, Langin D. 2011. Worsening of obesity and metabolic status yields similar molecular adaptations in human subcutaneous and visceral adipose tissue: decreased metabolism and increased immune response. J Clin Endocrinol Metab 96:E73–E82 [DOI] [PubMed] [Google Scholar]
- 42. Mitchell C, Couton D, Couty JP, Anson M, Crain AM, Bizet V, Rénia L, Pol S, Mallet V, Gilgenkrantz H. 2009. Dual role of CCR2 in the constitution and the resolution of liver fibrosis in mice. Am J Pathol 174:1766–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ito A, Suganami T, Yamauchi A, Degawa-Yamauchi M, Tanaka M, Kouyama R, Kobayashi Y, Nitta N, Yasuda K, Hirata Y, Kuziel WA, Takeya M, Kanegasaki S, Kamei Y, Ogawa Y. 2008. Role of CC chemokine receptor 2 in bone marrow cells in the recruitment of macrophages into obese adipose tissue. J Biol Chem 283:35715–35723 [DOI] [PubMed] [Google Scholar]