Abstract
Context:
Cycling androgens has been reported by athletes to improve physical performance by enhancing muscle mass and strength, a paradigm that has not been studied, and may have clinical value in older men being treated with testosterone.
Objective:
We investigated the efficacy of a monthly cycled testosterone regimen that uses half the testosterone dose as the current standard of care continuous therapy on body composition and muscle strength in older men.
Design, Setting, and Patients:
Twenty-four community-dwelling older men 70 ± 2 yr of age with total testosterone levels below 500 ng/dl were randomized at the Institute for Translational Sciences-Clinical Research Center into a 5-month double-blind placebo-controlled trial.
Intervention:
Subjects were dosed weekly for 5 months, receiving continuous testosterone (TE, n = 8; 100 mg testosterone enanthate, im injection), monthly cycled testosterone (MO, n = 8; alternating months of testosterone and placebo), or placebo (PL, n = 8).
Main Outcome Measures:
Main outcomes included body composition by dual-energy x-ray absorptiometry and upper and lower body muscle strength. Secondary outcomes included body weight, serum hormones, and mixed-muscle protein fractional synthesis rate (FSR).
Results:
Total lean body mass was increased and percent fat was reduced after 5 months in TE and MO (P < 0.05). Upper body muscle strength increased in TE, and lower body muscle strength increased in TE and MO (P < 0.05). FSR increased in TE and MO (P < 0.05) but not in PL.
Conclusions:
Cycled testosterone improved body composition and increased muscle strength compared with placebo and increased FSR similarly to continuous testosterone.
The use of testosterone is increasing in middle-aged and older men in the United States (1). Testosterone is used to enhance athletic performance, with cycled dosing being a common practice among athletes (2, 3). However, the mechanisms through which cycled testosterone exerts its action on skeletal muscle are poorly understood. In previous studies, the short-term (≤1 month) anabolic effect of testosterone is consistently associated with an increase in basal (fasting) rates of muscle protein synthesis (4–7). However, as the duration of testosterone administration increases, the potential contributions of fasting rates of muscle protein synthesis and breakdown to the overall anabolic response is less clear (5, 8) and may involve a shift from an initial promotion of muscle protein synthesis to a later slowing of protein degradation (5). Because cycling androgens is highly successful among athletes at enhancing muscle mass and strength, we sought to determine whether a cycled testosterone administration (i.e. using half the dose) would be similarly efficacious in healthy older men, because they represent the most clinically treated age group and suffer the greatest risk of testosterone side effects. Therefore, we conducted a randomized, double-blinded trial in older men to test the efficacy of testosterone, administered for 5 months in a monthly cycled on-off fashion. We hypothesized that monthly cycled testosterone administration would enhance body composition and muscle strength via a preferential stimulation of muscle protein synthesis.
Subjects and Methods
Ethics statement
The study was approved by The University of Texas Medical Branch (UTMB) Institutional Review Board and complied with the Declaration of Helsinki. Written informed consent was obtained from all subjects.
Subjects
Twenty-four healthy, community-dwelling older men (60–85 yr) with endogenous levels of serum total testosterone in the lower half of the normal range (between 280 and 500 ng/dl) (Table 1) were recruited through the Sealy Center on Aging Volunteer Registry. Subjects underwent a battery of tests including a history and physical examination, complete blood count, metabolic panel including fasting serum glucose and insulin, an electrocardiogram, plasma electrolytes, prostate-specific antigen, and liver and renal function and lipid panel. Subjects able to provide regular transportation to the Institute for Translational Sciences-Clinical Research Center (ITS-CRC) at UTMB were included. Subject exclusion criteria followed those recommended by the Clinical Guidelines Subcommittee Task Force of The Endocrine Society (9) and those used in our previously published trials with testosterone and older men (5, 7).
Table 1.
Subject characteristics and outcome measures
| PL |
MO |
TE |
||||
|---|---|---|---|---|---|---|
| Mean ± sd | n | Mean ± sd | n | Mean ± sd | n | |
| Baseline characteristics of the subjects | ||||||
| Demographic factors | ||||||
| Age (yr) | 65 ± 3a | 8 | 72 ± 8b | 8 | 73 ± 8b | 8 |
| Race or ethnic group (%)c | ||||||
| Caucasian | 87.5% | 7 | 100% | 8 | 87.5% | 7 |
| Minority | 12.5% | 1 | 0% | 0 | 12.5% | 1 |
| Body composition | ||||||
| Weight (kg) | 94 ± 19 | 8 | 81 ± 11 | 8 | 84 ± 9 | 8 |
| Body mass index (kg/m2) | 31 ± 6 | 8 | 28 ± 3 | 8 | 27 ± 2 | 8 |
| LBM (kg)e | 62 ± 10 | 8 | 57 ± 7 | 8 | 58 ± 6 | 8 |
| Fat mass (kg)e | 30 ± 11 | 8 | 22 ± 5 | 8 | 23 ± 4 | 8 |
| Body fat (%)e | 31 ± 5 | 8 | 27 ± 5 | 8 | 28 ± 3 | 8 |
| Muscle strength | ||||||
| Arm curl (kg) | 39 ± 12 | 8 | 39 ± 10 | 8 | 32 ± 9 | 8 |
| Leg curl (kg) | 46 ± 6 | 8 | 44 ± 7 | 8 | 40 ± 9 | 8 |
| Arm extension (kg) | 40 ± 9 | 8 | 41 ± 14 | 8 | 38 ± 9 | 8 |
| Leg extension (kg) | 70 ± 21 | 8 | 73 ± 15 | 8 | 66 ± 16 | 8 |
| Serum biochemical levels | ||||||
| Hematocrit (%) | 43 ± 3 | 8 | 40 ± 1 | 8 | 41 ± 3 | 8 |
| Fasting glucose (mg/dl) | 92 ± 7 | 7 | 92 ± 7 | 8 | 86 ± 7 | 8 |
| Alkaline phosphatase (U/liter) | 73 ± 12 | 8 | 69 ± 10 | 8 | 66 ± 14 | 8 |
| Alanine transferase (U/liter) | 44 ± 20 | 8 | 38 ± 10 | 8 | 32 ± 15 | 8 |
| Aspartate aminotransferase (U/liter) | 34 ± 16 | 8 | 29 ± 5 | 8 | 26 ± 11 | 8 |
| Albumin (g/dl) | 3.8 ± 0.2 | 8 | 3.9 ± 0.2 | 8 | 3.7 ± 0.3 | 8 |
| Hormones, lipids, and PSA | ||||||
| Total testosterone (ng/dl) | 344 ± 85 | 8 | 357 ± 103 | 8 | 341 ± 85 | 8 |
| Estradiol (pg/ml) | 38 ± 12 | 8 | 30 ± 6 | 8 | 30 ± 6 | 8 |
| IGF-I (ng/ml) | 101 ± 33 | 8 | 107 ± 38 | 8 | 104 ± 62 | 8 |
| SHBG (ng/dl) | 35 ± 10 | 8 | 36 ± 18 | 8 | 32 ± 13 | 8 |
| Total cholesterol (mg/dl) | 172 ± 30 | 8 | 181 ± 32 | 8 | 166 ± 49 | 8 |
| HDL cholesterol (mg/dl) | 40 ± 7 | 8 | 44 ± 9 | 8 | 43 ± 10 | 8 |
| LDL cholesterol (mg/dl) | 104 ± 26 | 8 | 106 ± 32 | 8 | 100 ± 43 | 8 |
| Triglycerides (mg/dl) | 142 ± 55 | 8 | 154 ± 52 | 8 | 116 ± 24 | 8 |
| PSA (mg/dl) | 1.24 ± 1.20 | 7 | 1.58 ± 1.04 | 8 | 2.09 ± 1.09 | 8 |
| Mean changes between baseline and month 5 | ||||||
| Body composition | ||||||
| Weight (kg) | 0.54 ± 1.55 | 8 | 1.70 ± 1.22f | 8 | 1.66 ± 1.58f | 8 |
| Body mass index (kg/m2) | 0.16 ± 0.50 | 8 | 0.57 ± 0.41g | 8 | 0.56 ± 0.52 | 8 |
| LBM (kg)d,e | −1.09 ± 1.64 | 8 | 2.67 ± 1.37f,g | 8h | 3.12 ± 2.06f,g | 8 |
| Fat mass (kg)e | 1.20 ± 1.87 | 8 | −0.80 ± 0.87 | 8h | −1.24 ± 0.95g | 8 |
| Body fat (%)e | 1.53 ± 1.77 | 8 | −1.69 ± 1.10g | 8h | −2.09 ± 1.11g | 8 |
| Muscle strength | ||||||
| Arm curl (kg)d | 0.56 ± 4.58 | 8 | 5.95 ± 7.36 | 8 | 4.99 ± 3.63g | 7j |
| Leg curl (kg)d | 2.83 ± 5.16 | 8 | 6.15 ± 4.08g | 7i | 9.72 ± 6.34g | 7j |
| Arm extension (kg)d | 1.98 ± 2.58 | 8 | 4.53 ± 5.44 | 8 | 6.74 ± 1.84g | 7j |
| Leg extension (kg)d | 2.26 ± 9.54 | 8 | 11.98 ± 10.96 | 7i | 13.48 ± 7.25g | 7j |
| Serum biochemical levels | ||||||
| Hematocrit (%) | −1.09 ± 1.00g | 8 | 1.89 ± 0.47f,g | 8 | 2.80 ± 1.93f,g | 8 |
| Fasting glucose (mg/dl) | −2.88 ± 5.24 | 8 | 3.38 ± 4.32 | 8 | −1.75 ± 4.45 | 8 |
| Alkaline phosphatase (U/liter) | −2.38 ± 6.81 | 8 | −6.62 ± 5.01g | 8 | −6.50 ± 5.62 | 8k |
| Alanine transferase (U/liter) | −3.63 ± 7.63 | 8 | −4.63 ± 4.67 | 8 | −3.15 ± 5.15 | 8k |
| Aspartate aminotransferase (U/liter) | −0.38 ± 5.76 | 8 | −1.00 ± 2.79 | 8 | −2.63 ± 5.00 | 8k |
| Albumin (g/dl) | −0.09 ± 0.11 | 8 | −0.34 ± 0.16g | 8 | −0.33 ± 0.18g | 8 |
| Hormones, lipids, and PSA | ||||||
| Total testosterone (ng/dl) | −45.01 ± 50.46 | 8 | 307.69 ± 193.74f,g | 8 | 317.39 ± 172.07f,g | 8 |
| Estradiol (pg/ml) | −7.00 ± 3.87g | 8 | 24.50 ± 11.95f,g | 8 | 33.25 ± 15.94f,g | 8 |
| IGF-I (ng/ml) | −6.21 ± 20.68 | 8 | 14.25 ± 13.31 | 8 | 19.69 ± 28.72 | 8 |
| SHBG (ng/dl) | −1.16 ± 6.14 | 8 | −1.19 ± 6.69 | 8 | −2.60 ± 5.45 | 8 |
| Total cholesterol (mg/dl) | −1.50 ± 6.01 | 8 | −6.00 ± 28.24 | 8 | −28.0 ± 24.71 | 8 |
| HDL cholesterol (mg/dl) | −1.63 ± 3.14 | 8 | −7.36 ± 5.18g | 8 | −4.75 ± 3.41g | 8 |
| LDL cholesterol (mg/dl) | 3.75 ± 7.15 | 8 | 1.25 ± 22.99 | 8 | −19.25 ± 22.15 | 8k |
| Triglycerides (mg/dl) | −18.25 ± 23.28 | 8 | 6.13 ± 41.11 | 8 | −25.13 ± 11.70g | 8 |
| PSA (mg/dl) | 0.06 ± 0.13 | 8 | 0.28 ± 0.33 | 8k | 0.54 ± 0.29f,g | 8 |
Results are shown as mean ± sd unless indicated otherwise. LDL, Low-density lipoprotein; PSA, prostate-specific antigen.
Means with different letters are significantly different at baseline.
Self-reported.
The category is a primary outcome variable.
Body composition variables were measured by DXA.
Change is significantly different from PL (ANOVA, P ≤ 0.05).
Significant change between months 0 and 5 (t test, P ≤ 0.05).
DXA data were not available at month 5 for one subject, and data from month 4 were used instead.
Leg strength measures were uncomfortable for one subject and were discontinued.
Strength measures were not performed by one subject at months 4 and 5.
Serum biochemical levels were not available at month 5 for one subject, and month 4 data were used instead.
Study design
All men were randomized into the following groups: 1) continuous testosterone (TE, n = 8; 100 mg testosterone-enanthate im), 2) monthly cycled testosterone (MO, n = 8; alternating months of 100 mg testosterone-enanthate and placebo), or 3) placebo (PL, n = 8) by the UTMB Investigational Drug Service pharmacist. Subjects were dosed weekly for 5 months by a research nurse at the ITS-CRC, and all injections were administered only after obtaining a safety blood specimen and completion of scheduled outcome measures. Dual-energy x-ray absorptiometry (DXA) (Hologic, Bedford, MA) and one-repetition maximal voluntary strength tests (Cybex, Medway, MA) were performed monthly on the day before the metabolic study. Fasting blood draws were performed in the morning, and a metabolic stable isotope study was then conducted. Injections were given after each metabolic study.
Metabolic study
Postabsorptive mixed-muscle fractional synthesis rate (FSR) was measured monthly using a pulse bolus stable isotope technique (10). Subjects were admitted to the ITS-CRC at 1200 h the day before the metabolic study. Nutritional intake and timing of the evening meal was controlled, and each subject fasted from 2200 h through the end of the study the following day. The morning of the study, a polyethylene catheter was inserted into the antecubital vein for infusion of stable isotopes and collection of background blood samples. Bolus injections of l-[ring-13C6] Phe and l-[15N]Phe, dissolved in 0.9% saline and filtered through 2-μm filters, were administered iv at 0 and 30 min, respectively. At 5 and 60 min, muscle biopsies (∼100–200 mg) were taken from the vastus lateralis, 15–20 cm above the knee (11, 12). Muscle samples were processed and analyzed (13, 14), and mixed-muscle FSR was calculated using the precursor-product model as previously described (10, 13).
Statistical analysis
All outcomes were continuous measures or change scores from a baseline of continuous measures. Upon inspection of the data distributions, the assumption of approximate normality was made for all outcomes. Group differences at baseline were evaluated using ANOVA (Table 1). Estimated group mean differences from baseline at month 5 and 95% confidence limits are reported. Also reported in Table 1 are the results of ANOVA on the group mean change scores (month 5 minus baseline), with statistically different pairwise comparisons vs. PL indicated. Results of paired t tests of 5-month compared with baseline values, by group, are shown in the figures.
Results
Subject characteristics and outcome measures
Subject characteristics at baseline and changes after 5 months are shown in Table 1. Randomization resulted in an unanticipated, but significantly younger PL group than MO or TE. No other differences were seen in any between-group measures at baseline. Adverse events during the testosterone replacement phase of this study were reported to the UTMB Institutional Review Board and were not considered attributable to study participation. These adverse events were minimal and not limited to a specific treatment group.
Serum total testosterone
All subjects entered the study with similar concentrations of circulating total testosterone in the lower half of the normal range (348 ± 88 ng/dl). Total testosterone remained at baseline concentrations after each month of placebo treatment and increased after each month of testosterone treatment regardless of group (Fig. 1A). Among all groups, testosterone concentrations never went out of the normal upper physiological range in response to testosterone treatment (range between months 0 and 5, 157–1303 ng/dl).
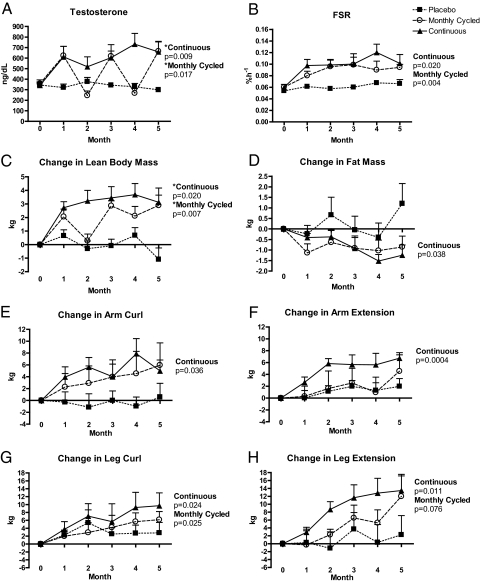
Fig. 1.
A, Total testosterone; B, mixed-muscle FSR; C, change in LBM; D, change in fat mass; E, change in arm curl; F, change in arm extension; G, change in leg curl; H, change in leg extension. *, Significantly different from PL (ANOVA, P < 0.05). P values indicate changes between months 0 and 5 (t test). One MO subject missed his month-2 study visit due to a personal scheduling conflict, and three subjects missed study visits due to Hurricane Ike (two TE subjects missed months 2 and 3, respectively, and one MO subject missed month 4). DXA data from one subject were unavailable at months 3 and 5 due to a technical issue with the DXA machine. Month-4 DXA data from this subject were used for before vs. after comparisons and did not impact our findings. DXA data include four individuals (one PL and three TE) that were analyzed on a different machine at month 5 vs. month 0. Exclusion of these data strengthened the statistical significance of our results, and these data were included in our final statistical analysis offering the most conservative results.
Fractional synthetic rate
Postabsorptive mixed-muscle FSR at baseline were similar between groups and remained unchanged in PL (Fig. 1B). FSR increased in both TE and MO at month 1 and remained elevated above baseline for the remainder of the study.
Lean body mass (LBM) and fat mass
LBM increased in both TE and MO after the first month of testosterone, which was sustained in TE across the 5 months (Fig. 1C). Although LBM returned back to near baseline levels in MO around month 2 (i.e. between the first cycle of placebo and second cycle of testosterone), the subsequent months showed a sustained increase in LBM. The decline in fat mass was significant only in TE (Fig. 1D).
Muscle strength
Muscle strength for arm curl (Fig. 1E), arm extension (Fig. 1F), leg curl (Fig. 1G), and leg extension (Fig. 1H) increased in TE. Leg curl strength increased in MO, and there was a trend for increased leg extension (P = 0.076). There were no changes in one-repetition maximal voluntary strength tests in PL.
Discussion
Cycling of androgens has long been employed by athletes to build muscle strength and mass and improve performance without careful scientific study (2, 3). This study determined 1) the efficacy of cycled testosterone to increase muscle mass and strength in older men and 2) whether cycled testosterone promotes skeletal muscle anabolism by a preferential stimulation of muscle protein synthesis. We found that monthly cycles of testosterone preferentially stimulated muscle protein synthesis, likely due to the repeated removal and reintroduction of the anabolic stimulus testosterone. The rationale for conducting this study came from our previous work in older men where we showed that skeletal muscle protein synthesis accounted for the anabolic effects of testosterone at 1 month, but at 6 months, net anabolism resulted from inhibition of skeletal muscle breakdown (5, 15).
Our healthy older men provided a unique model to study cycling testosterone because their total testosterone concentrations were in the lower half of the normal range and increased to the upper half of the normal range with cycled testosterone administration, therefore enabling us to assess the mechanism by which testosterone promotes muscle anabolism by increasing testosterone while still maintaining serum values within the normal range for men. Interestingly, Fig. 1A shows that older men in the MO group returned to baseline testosterone serum concentrations during the off months without testosterone. Although LBM declined somewhat during the initial month without testosterone, it recovered with the subsequent month of replacement and did not decline during the subsequent month without testosterone, staying nearly identical to the TE group at month 5 (Fig. 1C). More importantly, skeletal muscle protein synthesis remained elevated during the cycled withdrawing of testosterone (Fig. 1B). The change in total fat mass followed a similar inverse pattern to LBM (Fig. 1D) and contributed to the significant decline in percent fat mass in both groups receiving testosterone. Although changes in muscle strength in MO reached significance only in a single muscle group, strong positive trends were seen in all other muscle groups (Fig. 1, E–H).
Our findings of decreased high-density lipoprotein (HDL) cholesterol in TE and MO are consistent with the observed decreases in HDL cholesterol after testosterone treatment in healthy older men by others (16). It is not clear why triglycerides decreased only in the TE group, but it is possible that, although not significant, the lower triglyceride concentrations in this group at baseline indicate some predisposition in this group for a further decline in circulating lipids. Because previous studies have shown that testosterone side effects increase with increasing doses, it is possible to infer that a therapeutic approach using half the testosterone dose would result in reduced side effects (17, 18).
With respect to efficacy and mechanism, the major difference in the two treatment paradigms appears to be a slowing of the gains in lean mass and strength in MO relative to TE during the initial months of treatment. Importantly, however, by 5 months, gains in lean mass were similar in MO and TE, and gains in strength in MO approached or equaled those of TE. Notably, the month-to-month variation in LBM in the MO group was much greater than that of FSR, which remained relatively constant once treatment was initiated during month 1. This suggests that testosterone's anabolic effects are not entirely explained by changes in fasting muscle protein synthesis but may also involve changes in fasting rates of muscle protein breakdown or fed-state muscle protein metabolism and, furthermore, that changes in these variables are responsible for the relatively slower rate of adaptation to the MO dosing regimen.
Finally, our data show that by raising testosterone concentrations from the lower half to the upper half of the normal range in a monthly cycled paradigm, skeletal muscle FSR remains consistently elevated in healthy older men. Thus, if monthly on/off cycles of testosterone can consistently increase muscle protein synthesis and LBM without an increase in side effects, then this paradigm offers a significant treatment for preventing sarcopenia in older men. However, larger and longer-term studies are needed to assess the cumulative effects of this dosing paradigm on efficacy, safety, and functions of daily living such as sexual function, vitality, and overall quality of life.
Acknowledgments
We thank the men who volunteered their time for the successful completion of this study; the ITS-CRC staff and faculty; James Angel, R.N.; Neel L. Shah, M.D.; and the staff and faculty of UT-Houston's Clinical Research Center for helping us complete this important study after Hurricane Ike. After Hurricane Ike, and a mandatory evacuation of all of Galveston Island and the subsequent closing of all UTMB hospitals, all subjects that were enrolled at that time resumed study activities at the UT-Houston Clinical Research Center and Memorial Hermann Hospital in Houston, TX, until clinical services reopened at UTMB. When possible, arrangements were made to continue DXA and one-repetition measures at available surrounding facilities between Galveston and Houston, TX. Previous arrangements were made to ensure that the injections continued as scheduled, and no doses were missed.
This work was supported by National Institutes of Health (NIH) National Institute on Aging Grant R01 AG022023 (R.J.U.) and The Moody Endowment. Studies were conducted at the ITS-CRC at UTMB, funded by Grant M01 RR 00073 from the National Center for Research Resources, NIH, U.S. Public Health Service, and by The Claude D. Pepper Older Americans Independence Center, funded by Grant P30 AG024832.
R.J.U. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. R.J.U. and M.S.-M. were responsible for the design and conduct of the study; E.L.D., S.L.C., C.R.G., D.P.-J., W.J.D., and J.J.G. for the collection, management, analysis, and interpretation of the data; and E.L.D., M.S.-M., W.J.D., and R.J.U. for preparation, review, and approval of the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- DXA
- Dual-energy x-ray absorptiometry
- FSR
- fractional synthesis rate
- HDL
- high-density lipoprotein
- LBM
- lean body mass
- MO
- monthly cycled testosterone
- PL
- placebo
- TE
- continuous testosterone.
References
- 1. Bassil N, Morley JE. 2010. Late-life onset hypogonadism: a review. Clin Geriatr Med 26:197–222 [DOI] [PubMed] [Google Scholar]
- 2. Basaria S. 2010. Androgen abuse in athletes: detection and consequences. J Clin Endocrinol Metab 95:1533–1543 [DOI] [PubMed] [Google Scholar]
- 3. Graham MR, Davies B, Grace FM, Kicman A, Baker JS. 2008. Anabolic steroid use: patterns of use and detection of doping. Sports Med 38:505–525 [DOI] [PubMed] [Google Scholar]
- 4. Chen F, Lam R, Shaywitz D, Hendrickson RC, Opiteck GJ, Wishengrad D, Liaw A, Song Q, Stewart AJ, Cummings CE, Beals C, Yarasheski KE, Reicin A, Ruddy M, Hu X, Yates NA, Menetski J, Herman GA. 2011. Evaluation of early biomarkers of muscle anabolic response to testosterone. J Cachex Sarcopenia Muscle 2:45–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, Lieberman SA, Tipton K, Wolfe RR, Urban RJ. 2002. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab 282:E601–E607 [DOI] [PubMed] [Google Scholar]
- 6. Ferrando AA, Tipton KD, Doyle D, Phillips SM, Cortiella J, Wolfe RR. 1998. Testosterone injection stimulates net protein synthesis but not tissue amino acid transport. Am J Physiol 275:E864–E871 [DOI] [PubMed] [Google Scholar]
- 7. Urban RJ, Bodenburg YH, Gilkison C, Foxworth J, Coggan AR, Wolfe RR, Ferrando A. 1995. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol 269:E820–E826 [DOI] [PubMed] [Google Scholar]
- 8. Brodsky IG, Balagopal P, Nair KS. 1996. Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men: a clinical research center study. J Clin Endocrinol Metab 81:3469–3475 [DOI] [PubMed] [Google Scholar]
- 9. Committee on Assessing the Need for Clinical Trials of Testosterone Replacement Therapy 2004. Executive summary. In: Liverman C, Blazer D. eds. Testosterone and aging: clinical research directions. Washington, DC: National Academies Press; 1–10 [PubMed] [Google Scholar]
- 10. Zhang XJ, Chinkes DL, Wolfe RR. 2002. Measurement of muscle protein fractional synthesis and breakdown rates from a pulse tracer injection. Am J Physiol Endocrinol Metab 283:E753–E764 [DOI] [PubMed] [Google Scholar]
- 11. Bergström J, Fürst P, Norée LO, Vinnars E. 1974. Intracellular free amino acid concentration in human muscle tissue. J Appl Physiol 36:693–697 [DOI] [PubMed] [Google Scholar]
- 12. Sheffield-Moore M, Urban RJ, Wolf SE, Jiang J, Catlin DH, Herndon DN, Wolfe RR, Ferrando AA. 1999. Short-term oxandrolone administration stimulates net muscle protein synthesis in young men. J Clin Endocrinol Metab 84:2705–2711 [DOI] [PubMed] [Google Scholar]
- 13. Wolfe RR, Chinkes DL. 2005. Isotope tracers in metabolic research: principles and practice of kinetic analysis. 2nd ed New York: Wiley-Liss [Google Scholar]
- 14. Calder AG, Anderson SE, Grant I, McNurlan MA, Garlick PJ. 1992. The determination of low d5-phenylalanine enrichment (0.002–0.09 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass Spectrom 6:421–424 [DOI] [PubMed] [Google Scholar]
- 15. Ferrando AA, Sheffield-Moore M, Paddon-Jones D, Wolfe RR, Urban RJ. 2003. Differential anabolic effects of testosterone and amino acid feeding in older men. J Clin Endocrinol Metab 88:358–362 [DOI] [PubMed] [Google Scholar]
- 16. Meriggiola MC, Marcovina S, Paulsen CA, Bremner WJ. 1995. Testosterone enanthate at a dose of 200 mg/week decreases HDL-cholesterol levels in healthy men. Int J Androl 18:237–242 [PubMed] [Google Scholar]
- 17. Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, Tricker R, Shirazi A, Casaburi R. 1996. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med 335:1–7 [DOI] [PubMed] [Google Scholar]
- 18. Sattler F, Bhasin S, He J, Chou CP, Castaneda-Sceppa C, Yarasheski K, Binder E, Schroeder ET, Kawakubo M, Zhang A, Roubenoff R, Azen S. 2011. Testosterone threshold levels and lean tissue mass targets needed to enhance skeletal muscle strength and function: the HORMA trial. J Gerontol A Biol Sci Med Sci 66:122–129 [DOI] [PMC free article] [PubMed] [Google Scholar]