Abstract
Context:
Recurrent hypoglycemia induces hypoglycemia-associated autonomic failure (HAAF), characterized by deterioration in counterregulatory responses. Endogenous opioids may mediate the development of HAAF, and blockade of opioid receptors with naloxone prevented HAAF in nondiabetic subjects.
Objective:
We hypothesized that opioid receptor blockade with naloxone during antecedent hypoglycemia in patients with type 1 diabetes mellitus (T1DM) would prevent the development of HAAF.
Design, Setting, Participants, and Interventions:
Eight subjects with T1DM (three women, aged 34 ± 7.4 yr, hemoglobin A1c 7.3 ± 1.1%) were studied on 2 consecutive days on three separate occasions. Day 1 consisted of: 1) two 90-min hypoglycemic clamps (60 mg/dl, N−); 2) two 90-min hypoglycemic clamps (60 mg/dl) with concomitant naloxone infusion (N+); or 3) two 90-min euglycemic clamps (90 mg/dl) with concomitant naloxone infusion (control). Day 2 consisted of hyperinsulinemic stepped hypoglycemic clamps (90, 80, 70, and 60 mg/dl plasma glucose steps).
Main Outcome Measures:
Day 2 hypoglycemia counterregulatory hormonal response and glucose turnover [(3-3H)-glucose] as indicators of recovery from hypoglycemia.
Results:
Antecedent hypoglycemia in N− group resulted in a markedly decreased epinephrine response and a lower rate of endogenous glucose production (EGP) during subsequent hypoglycemia compared with control (75 ± 17 vs. 187 ± 21 pg/ml, P < 0.05 and 0.8 ± 0.1 vs. 1.4 ± 0.2 mg/kg · min, P < 0.05, respectively). In contrast, in the N+ studies, plasma epinephrine was 164 ± 18 pg/ml and EGP was 1.3 ± 0.2 mg/kg · min during subsequent hypoglycemia, both levels similar to those seen in control studies (P = NS vs. control). Plasma glucagon did not increase with hypoglycemia.
Conclusions:
Blockade of endogenous opioids with naloxone during antecedent hypoglycemia improves HAAF in patients with T1DM by ameliorating the epinephrine response and restoring EGP.
Maintaining near-normal glycemia represents a main goal in the current diabetes management (1). However, achievement of glycemic control with insulin therapy carries a significant risk of iatrogenic hypoglycemia (2), especially in patients with type 1 diabetes mellitus (T1DM), who lack the physiological feedback necessary to regulate insulin secretion (3). In addition, patients with T1DM suffer from defective hypoglycemia counterregulation, characterized by a compromised glucagon response and a significantly attenuated autonomic/sympathoadrenomedullary response, which results in delayed awareness to, and recovery from, hypoglycemia (3).
Despite significant advances, insulin therapy in diabetes remains imperfect and often results in relative insulin excess and consequently iatrogenic hypoglycemia (2, 4). Recurrent episodes of hypoglycemia have been postulated to lead to further reduction of hypoglycemia counterregulation and decreased awareness to impending hypoglycemia (3, 4), termed hypoglycemia-associated autonomic failure (HAAF). Thus, HAAF leads to further attenuation of the already compromised counterregulatory response in T1DM (5). HAAF can be reproduced experimentally by inducing recurrent hypoglycemia in healthy subjects and T1DM subjects (5–7), providing a robust investigational model for studying its mechanisms.
Although the precise mechanism responsible for HAAF has not yet been elucidated, we and others have proposed that endogenous release of opioids during antecedent hypoglycemia may be implicated in its pathogenesis (7–9). Specifically, blockade of opioid receptors with naloxone during hypoglycemia resulted in augmentation of the sympathoadrenal response in normal subjects and those with T1DM (10). In addition, we have shown that infusion of naloxone during antecedent hypoglycemia resulted in normalization of the glucagon and sympathoadrenal responses to subsequent hypoglycemia in normal subjects (7). Thus, in the present study, we tested the hypothesis that blocking the effects of endogenous opioids with naloxone infusion during antecedent hypoglycemia in subjects with intensively treated T1DM would augment the sympathoadrenomedullary and glucagon responses to subsequent hypoglycemia and hence improve HAAF.
Materials and Methods
We studied eight subjects with intensively treated T1DM (five men, three women, aged 34 ± 7.4 yr, hemoglobin A1c 7.3 ± 1.1%, body mass index 23.4 ± 3.1 kg/m2, average duration of diabetes 11.8 ± 3.3 yr), who were otherwise healthy. Diabetes treatment consisted of pump therapy in all subjects. Inclusion criteria required absence of hypoglycemic episodes for at least 2 wk before the study date. Subjects with a history or symptoms of neuropathy were excluded. Each subject participated in three different studies, in random order, separated from each other by at least 5 wk. All studies were performed after an overnight fast. Each set of studies consisted of 2 consecutive days. Day 1 protocol differed in each of the three study designs. Day 2 was identical in all studies and included a hyperinsulinemic stepped hypoglycemic clamp, with quantification of hormonal responses and glucose kinetics.
The research protocol was approved by the Institutional Review Board of the Albert Einstein College of Medicine, and the informed written consent was obtained in accordance with the institutional review board policy. Subjects were admitted to the Clinical Research Center for each experiment.
Day 1
At 0800 h on the study day, the subjects' insulin pump was disconnected and all subjects had two indwelling cannulae inserted, one placed in an antecubital vein for infusions and the second placed in a retrograde fashion in a distal hand vein of the contralateral forearm for blood sampling. To obtain arterialized venous blood samples, this hand was maintained at 65 C in a thermoregulated sleeve. At t −30 min, a constant insulin infusion (Humulin Regular; Eli Lilly, Indianapolis, IN) was initiated at a rate of 1 mU/kg · min, and a variable infusion of 20% dextrose was administered to maintain the plasma glucose concentration at euglycemia until t = 0 min. Blood samples were collected at 5-min intervals for measurements of plasma glucose. At t = 0, subjects were assigned to 90 min of one of the following: 1) hypoglycemia (plasma glucose 60 mg/dl; to convert from milligrams per deciliter to millimoles per liter of glucose, multiply by 0.05) without naloxone (N−) but with normal saline infused as a naloxone substitute; 2) hypoglycemia (plasma glucose 60 mg/dl) with naloxone (Narcan; DuPont Pharmaceuticals, Wilmington, DE), (N+), administered as a primed continuous infusion at 0.4 μg/kg · min; or 3) euglycemia (plasma glucose 90 mg/dl) with naloxone (control), administered as a primed continuous infusion at 0.4 μg/kg · min, depending on which protocol the subject was randomized to on the study day. Also, at this time, the 20% dextrose infusion was adjusted to maintain the desired plasma glucose concentration, depending on the study performed. At the completion of the 90-min clamp, insulin infusion was decreased to the individual basal rate, naloxone was discontinued, and the plasma glucose was maintained at euglycemia with the infusion of dextrose, as needed, for 90 min. During this time the subjects also received a snack containing 15 g of carbohydrate. At t = 150 min, the experimental conditions were resumed, with subjects assigned to the same conditions as during the first 90 min. At the completion of the second clamp, a meal was provided, and glucose was normalized, the subjects were placed back on their insulin pumps and discharged. In all the groups, blood samples were obtained for the determinations of serum β-endorphin.
Day 2
At 0800 h, the insulin pump was disconnected and subjects had two indwelling cannulae inserted. At t = −120 min, a primed-continuous infusion of HPLC-purified [3-3H] glucose was initiated with a bolus of 21.6 μCi, followed by continuous infusion of 0.15 μCi/min for the entire period of the study. The specific activity of infused dextrose was kept equivalent to plasma glucose specific activity by addition of [3-3H] glucose to the infusate, as previously described by Finegood et al. (11). In addition, during this time period, each subject was maintained on a primed-continuous infusion of insulin, administered at their individual basal rate. At t = 0 min, the insulin infusion rate was increased to 1.0 mU/kg · min for the first 10 min and thereafter was continued at 0.5 mU/kg · min throughout the study. At t = 10 min, a variable infusion of 20% dextrose was also begun to maintain the plasma glucose concentration at 90 mg/dl for 50 min. At t = +50 min and every 50 min thereafter, the plasma glucose concentration was decreased by 10-mg/dl decrements for 50 min each by reducing the dextrose infusion rate accordingly. Plasma glucose was clamped at the desired range according to plasma glucose measured at 5-min intervals with targets of 90, 80, 70, and 60 mg/dl. Blood samples were obtained for the determinations of plasma insulin, C-peptide, glucagon, epinephrine, norepinephrine, and cortisol, as well as glucose turnover.
Plasma glucose was measured with a Beckman glucose analyzer (Beckman Coulter, Fullerton, CA), using the glucose oxidase method. Plasma [3-3H] glucose radioactivity was measured in duplicate on the supernatants of barium hydroxide-zinc sulfate precipitates of plasma samples, after evaporation to dryness to eliminate tritiated water (12). The methods for measurement of plasma insulin, C-peptide, glucagon, cortisol, and their intra- and interassay variations have been previously reported (13). Plasma β-endorphin was measured using ELISA (MD Bioproducts, St. Paul, MN). Plasma epinephrine and norepinephrine levels were determined using RIA (IBL-America, Minneapolis, MN).
Statistical analysis
The data are presented as the mean ± sem. Steele's equation was used for calculation of glucose turnover as described (14). Values for endogenous glucose production (EGP) and glucose uptake, obtained at 10-min intervals, were averaged over the final 30 min of each glucose step. Statistical analyses were performed using repeated-measures ANOVA for multiple comparisons and paired Student's t test for comparing between two means (same subject) before and after an intervention (naloxone infusion). A value of P < 0.05 was considered significant.
Results
Day 1
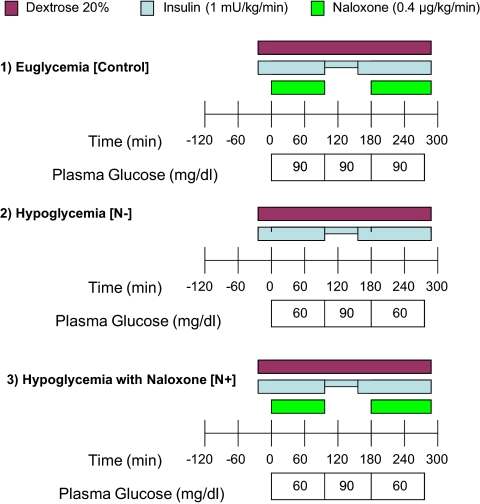
Figure 1 depicts the experimental protocol and the concentrations of plasma glucose on d 1. All studies (N−, N+, and control) achieved the target plasma glucose concentrations.
Fig. 1.
Experimental protocol on d 1.
Plasma β-endorphin concentrations averaged 5.3 ± 2.1 pg/ml in all studies at baseline and increased equally and significantly in the N+ and N− studies at the end of the hypoglycemia clamp (25.0 ± 3.1 pg/ml, P < 0.001 vs. basal). The control (euglycemia) studies showed no significant increase in plasma β-endorphin concentrations (4.7 ± 1.2 pg/ml, P = NS compared with basal). Plasma epinephrine, norepinephrine, and cortisol rose from baseline at the end of the hypoglycemic clamp on d 1. Although the N+ studies demonstrated a higher rise in epinephrine, norepinephrine, and cortisol, the difference was not statistically significant (see Supplemental Table 1 published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). No rise in counterregulatory hormones was noted in the control studies on d 1.
Day 2
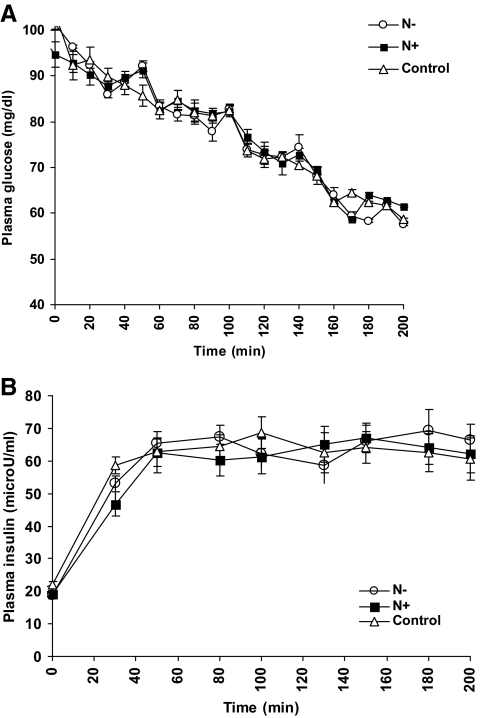
There was no significant difference in plasma glucose concentrations between all the study protocols during the glucose steps on d 2 (Fig. 2A). Plasma insulin concentrations were also similar in all studies at baseline (time 0), averaging 19.9 ± 0.7 μU/ml (to convert from microunits per milliliter to picomoles per liter of insulin, multiply by 6.945), and during the hypoglycemic clamps, averaging 64 ± 1.3 μU/ml (Fig. 2B).
Fig. 2.
A, Plasma glucose concentrations (d 2) at each glucose step. B, Plasma insulin concentrations (d 2) in the N− (white circles), N+ (black squares), and control studies (white triangles).
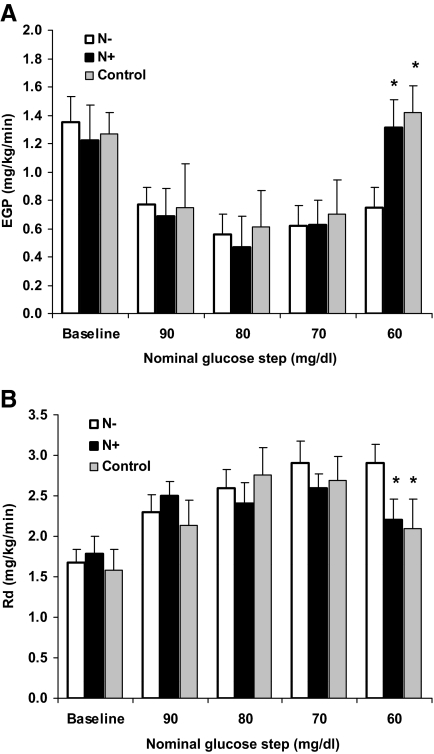
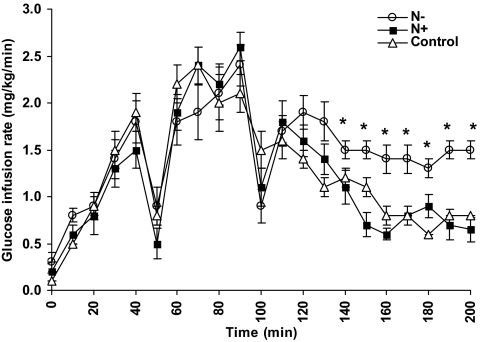
Estimates of EGP and glucose uptake (Rd) derived from isotopic analysis are depicted in Fig. 3, A and B, respectively. [3-3H]-glucose-specific activity was effectively and equally maintained in all sets of studies during the clamps (averaged 892 ± 63 cpm/mg). As expected, a similar decrease in EGP was observed in all studies as the plasma glucose levels were brought down to 70 mg/dl. However, during the 60-mg/dl glucose step, EGP increased significantly in the control and N+ compared with N− studies (1.4 ± 0.2 and 1.3 ± 0.2 mg/kg · min vs. 0.8 ± 0.1 mg/kg · min, respectively, P < 0.05). In concert with these findings, there was a corresponding decrease in Rd during the 60-mg/dl glucose step in the control and N+ studies compared with the N− studies (2.1 ± 0.4 and 2.2 ± 0.3 mg/kg · min vs. 2.9 ± 0.2 mg/kg · min, respectively, P < 0.05). Both the higher EGP and lower Rd in the control and N+ studies contributed to better recovery from hypoglycemia, as reflected by the lower glucose infusion rates necessary to maintain the plasma glucose at 60 mg/dl in these groups (Fig. 4). Thus, glucose infusion rates were similar in all studies during the 90-, 80-, and 70-mg/dl glucose steps. However, during the 60-mg/dl glucose step, average glucose infusion rate was 0.7 ± 0.1 mg/kg · min in the N+ group and 0.8 ± 0 mg/kg · min in the control group, compared with 1.4 ± 0.1 mg/kg · min required in the N− group, P < 0.05 for comparison between control and N+ groups vs. N−.
Fig. 3.
A, EGP (d 2) averaged for the final 30 min of each glucose step. * P < 0.01 vs. N−. B, Rd (d 2) averaged for the final 30 min of each glucose step. *, P < 0.01 vs. N−.
Fig. 4.
Glucose infusion rates (d 2) at each glucose step in the N− (white circles), N+ (black squares), and control studies (white triangles). *, P < 0.01 vs. N+ and control.
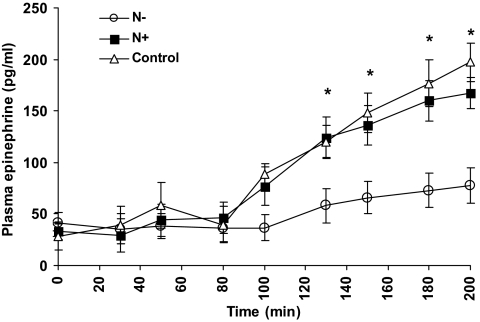
Plasma epinephrine concentrations (Fig. 5) were similar in all studies during the 90-mg/dl glucose step (42 ± 18, 36 ± 14, and 38 ± 12 pg/ml, in the control, N+ and N−, respectively, P = NS; to convert from picograms per milliliter to picomoles per liter of epinephrine, multiply by 5.45). However, during the 60-mg/dl glucose step, plasma epinephrine increased significantly in the control and N+ studies compared with the N− studies (447 and 459% vs. 196% of baseline values, respectively, P < 0.05, Table 1). Plasma norepinephrine and cortisol concentrations were similar in all studies and increased slightly during the 60-mg/dl glucose step (Table 1). Plasma glucagon remained at basal levels during hypoglycemia in all studies (Table 1).
Fig. 5.
Plasma epinephrine concentrations (d 2) at each glucose step in the N− (white circles), N+ (black squares), and control studies (white triangles). *, P < 0.05 vs. N+ and control.
Table 1.
Mean plasma epinephrine, norepinephrine, glucagon, cortisol, and β-endorphin concentrations on d 2 in the N−, N+, and control studies at 90 and 60 mg/dl plasma glucose steps of the hypoglycemic clamp
| N− | N+ | Control | ||||
|---|---|---|---|---|---|---|
| Plasma glucose step (mg/dl)§ | 90 | 60 | 90 | 60 | 90 | 60 |
| Epinephrine (pg/ml) § | 38 ± 12 | 75 ± 17 | 36 ± 14 | 164 ± 18*,# | 42 ± 18 | 187 ± 21*,# |
| Norepinephrine (pg/ml) § | 198 ± 46 | 313 ± 50 | 214 ± 40 | 330 ± 63 | 205 ± 53 | 327 ± 60 |
| Glucagon (pg/ml) § | 45 ± 4 | 38 ± 4 | 38 ± 5 | 38 ± 6 | 34 ± 5 | 35 ± 6 |
| Cortisol (μg/dl) § | 9.1 ± 2.0 | 14.8 ± 2.4 | 9.1 ± 2.1 | 15.0 ± 2.1 | 10.0 ± 2.1 | 15.1 ± 2.1 |
| β-Endorphin (ng/ml) | 4.8 ± 1.2 | 15.4 ± 2.7* | 2.0 ± 0.9 | 13.2 ± 2.1* | 2.9 ± 1.3 | 17.6 ± 3.4* |
To convert glucose from milligrams per deciliter to millimoles per liter, multiply by 0.05; to convert epinephrine from picograms per milliliter to picomoles per liter, multiply by 5.45; to convert norepinephrine from picograms per milliliter to picomoles per liter, multiply by 5.91; to convert glucagon from picograms per milliliter to nanograms per liter, multiply by 1; to convert cortisol from micrograms per deciliter to nanomoles per liter, multiply by 27.58.
P < 0.01 vs. the 90-mg/dl glucose step.
P < 0.01 vs. N−.
Plasma β-endorphin concentrations were similar in all studies at baseline, averaging 3.3 ± 1.1 pg/ml and increased equally and significantly with hypoglycemia to a mean value of 15.4 ± 2.8 pg/ml at the end of the 60-mg/dl glucose step, P < 0.001 vs. baseline (Table 1).
Discussion
Our study demonstrates that blockade of opioid receptors with naloxone at the time of antecedent hypoglycemia in subjects with T1DM prevents the experimentally induced defect in epinephrine release during subsequent hypoglycemia, as evidenced by restoration of the epinephrine response and significant improvement in EGP. Thus, experimental HAAF can be prevented by administration of naloxone during antecedent hypoglycemia, suggesting that endogenous opioids play an important role in the development of HAAF in T1DM subjects and that the immediate effects of the opioid system on hypoglycemia may be acutely reversed. However, the underlying defects in counterregulation (i.e. impaired glucagon, epinephrine, and EGP responses to hypoglycemia) remain attenuated compared with nondiabetic subjects. Although we did not directly compare these subjects with healthy controls, there is a wealth of data from our and others' laboratories to suggest that the T1DM subjects in our study have significant underlying counterregulatory deficits (5, 7, 15). Finally, we did not study the distinct yet important phenomenon of hypoglycemia unawareness because it was beyond the scope of our experimental design.
Despite scrupulous attempts to avoid hypoglycemia in T1DM subjects for at least 2 wk before the study date, the low epinephrine and glucagon levels in the control group at baseline and in response to induced hypoglycemia support that T1DM subjects have underlying counterregulatory dysfunction, independent of acute hypoglycemia (5, 15). The specific findings of improved EGP and epinephrine response during hypoglycemia, as a result of antecedent naloxone infusion, imply that epinephrine has a significant role in stimulating EGP, even in subjects with T1DM who have baseline defective counterregulation. Although others have also noted an increase in EGP and epinephrine response in the setting of naloxone infusion during an induced hypoglycemic episode (10), we demonstrate here, for the first time, that naloxone infusion could attenuate the future development of HAAF.
Furthermore, multiple studies have shown that naloxone is only effective in augmenting the counterregulatory response in the setting of hypoglycemia and has no effect during euglycemia (7, 10, 16). We have previously demonstrated that the counterregulatory responses were identical in healthy euglycemic controls who received either saline only or naloxone on d 1 (7). The fact that the administration of naloxone in the control studies had no specific effects on subsequent hypoglycemia counterregulation excludes the possibility of a carryover effect naloxone could have had in these experiments. However, the increase in hypoglycemia counterregulation on d 1 was more prominent in the N+ studies, supporting previous findings by Caprio et al. (10) of acute naloxone effects.
In the present study, we did not observe either a further impairment in or a rescue by naloxone of glucagon secretion in response to hypoglycemia. However, we have shown that naloxone infusion was effective in rescuing the counterregulatory glucagon response in normal subjects (7), suggesting that the loss of hypoglycemia-induced glucagon secretion in subjects with a prolonged history of T1DM may not be solely dependent on opioid release resulting from hypoglycemia. Thus, in T1DM different mechanisms are likely involved in the regulation of catecholamine and glucagon release in response to hypoglycemia (17).
Although the mechanism of HAAF requires additional study, there is accumulating evidence for the pathophysiological role of endogenous opioids acting centrally and peripherally on the nervous system. Endogenous opioids, including β-endorphin, are secreted by the proopiomelanocortin neurons of the pituitary gland (18), in response to a variety of stressors, one of which is hypoglycemia (8). In addition, animal data indicate that β-endorphin is also released by the adrenal medulla in response to α1-adrenoreceptor stimulation (19, 20) and results in glucose lowering in rats with type 1-like diabetes (21). Furthermore, opioid receptors have been localized to various brain regions involved in glucose sensing (22–25) and adrenal activation (26).
Central nervous system signals that mediate the response to hypoglycemia may be of major importance in glucose counterregulation. In the central nervous system, opioids contribute to the development of HAAF, likely via activation of the δ-, κ-, and μ-opioid receptors, localized to areas in the thalamus and hypothalamus responsible for glucose sensing, including the ventromedial hypothalamus, arcuate nucleus, and dorsal medial thalamus (22–25, 27–29). Administration of exogenous β-endorphin directly into the rat brain was shown to inhibit some of the hypothalamic responses to hypoglycemia (30). Furthermore, recurrent hypoglycemia in rats resulted in the suppression of gene transcription in hypothalamic neurons responsible for the organism's ability to shift from glucose utilization to the use of alternate fuels during hypoglycemia, which resulted in the impaired recovery from hypoglycemia (9). However, this gene suppression was reversed with naloxone (9), thereby implying that endogenous opioids mediate these responses. Because naloxone can cross the blood-brain barrier (31, 32), it could potentially exert its effect on hypoglycemia counterregulation centrally.
Whether endogenous opioids have a direct effect on the hypothalamic response, act indirectly via effects on the peripheral sympathoadrenal system, or contribute to both central and peripheral mechanisms requires further elucidation. However, substantial evidence in animals now suggests that endogenous opioids produced peripherally by the adrenal medulla may lead to glucose lowering in streptozocin-induced diabetic rats by inducing peripheral glucose uptake and reducing hepatic gluconeogenesis (21). Exogenous peripheral administration of β-endorphin has shown similar results (33). Glucose-lowering effects of β-endorphin in a type 1-like diabetic rat model result from enhancement of GLUT4 gene expression leading to increased glucose utilization and attenuation of PEPCK gene expression, causing a decline of hepatic gluconeogenesis (21, 34). Together these effects would be predicted to result in the inability to reverse hypoglycemia and subsequent development of HAAF.
In rats, β-endorphin release from the adrenal is activated via α1-adrenoreceptor stimulation, as indicated by increased β-endorphin levels in response to phenylephrine stimulation and decrease in β-endorphin in response to treatment with α-antagonists (20, 21). Opioid release, in turn, acts to suppress catecholamine secretion from the adrenal (35, 36) via a stabilizing effect on actin filaments in the chromaffin cells (35), suggesting the existence of a negative feedback mechanism between the adrenal catecholamine and opioid secretion. This negative feedback mechanism may serve to protect the organism from deleterious effects of chronic catecholamine exposure, such as psychological stress resulting from recurrent symptoms of adrenal activation and detrimental effects on the cardiovascular system. Although the suppression of the adrenal stress response may serve a beneficial role under chronic stress conditions, it may also be responsible for the disabling symptoms of HAAF.
This opioid effect on the adrenal medulla is reversed in vitro with naloxone administration (36), suggesting that peripherally secreted endogenous opioids may play a contributory role in development of HAAF. These findings are further supported by recent data in humans that show that blunting of the catecholamine response to hypoglycemia could be prevented by pretreatment with adrenergic blockade during antecedent hypoglycemia (37). It is likely that the effect of adrenergic blockade on amelioration of HAAF is due to the blockade of α-adrenergic receptors and subsequent prevention of adrenal β-endorphin release, because the administration of opioid antagonists or induction of μ-receptor knockout mutation abolished the glucose-lowering effect of adrenergic stimulation (21, 34). Furthermore, the fact that we were able to normalize epinephrine with administration of naloxone suggests that β-endorphin exerts a portion of its effect on HAAF at the level of the adrenal gland.
At present, evidence suggests that recurrent hypoglycemia initially induces a rise in both catecholamines and β-endorphin. Stimulation of the endogenous opioid system may inhibit additional release of catecholamines via a negative feedback system or may lead to the development of opioid tolerance, resulting in the attenuation of the catecholamine counterregulatory response, manifesting as HAAF and hypoglycemia unawareness. HAAF could be partially reversed with opioid or adrenergic blockade, with both being effective because the activation of the opioid system appears to be a stepwise process involving initial activation of the adrenergic system. Interestingly, despite naloxone administration on d 1, the β-endorphin levels on d 2 were not higher in the N+ compared with the N− group, as may have been expected if the peripheral opioid-adrenal feedback mechanism was primarily responsible for HAAF. Thus, it is likely that direct neural communications between the central nervous system and adrenal glands control the hypoglycemic response (38) with integration of opioid and adrenergic activation.
Our study has a number of limitations. First is that the investigators were not blinded to the naloxone infusion. The second is that naloxone is a nonspecific opioid receptor antagonist, which may exert its action on hypoglycemia counterregulation via alternate opiate dependent pathways that are hitherto unrecognized.
In summary, here we provide proof of concept that blockade of endogenous opioids by naloxone infusion during antecedent hypoglycemia improves HAAF in subjects with T1DM, primarily by augmenting the epinephrine response and restoring EGP. In light of these results, further investigation into the potential benefits of chronic opioid receptor blockade in the prevention of HAAF in T1DM may be warranted.
Acknowledgments
We are indebted to the staff of the Clinical Research Center for their superb care of the subjects. We thank Ms. Robin Sgueglia and Zhao Hu for laboratory determinations. The results of this study were reported in abstract form at the 70th Annual Scientific Sessions of the American Diabetes Association, Orlando, FL, June 28, 2010.
This work was supported by Grants DK 079974 (to I.G.), RR017313 (to I.G.), DK 20541 (to I.G. and H.S.), and the Clinical and Translational Science Award UL1-RR025750 from the National Institutes of Health.
Disclosure Summary: The authors have no conflict of interest to disclose.
For editorial see page 3357
- EGP
- Endogenous glucose production
- HAAF
- hypoglycemia-associated autonomic failure
- Rd
- glucose uptake
- T1DM
- type 1 diabetes mellitus.
References
- 1. 2011. Standards of medical care in diabetes—2011. Diabetes Care 34(Suppl 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. 1991. Epidemiology of severe hypoglycemia in the diabetes control and complications trial. The DCCT Research Group. Am J Med 90:450–459 [PubMed] [Google Scholar]
- 3. Cryer PE. 2008. The barrier of hypoglycemia in diabetes. Diabetes 57:3169–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cryer PE, Davis SN, Shamoon H. 2003. Hypoglycemia in diabetes. Diabetes Care 26:1902–1912 [DOI] [PubMed] [Google Scholar]
- 5. Dagogo-Jack SE, Craft S, Cryer PE. 1993. Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus. Recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia. J Clin Invest 91:819–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davis MR, Mellman M, Shamoon H. 1992. Further defects in counterregulatory responses induced by recurrent hypoglycemia in IDDM. Diabetes 41:1335–1340 [DOI] [PubMed] [Google Scholar]
- 7. Leu J, Cui MH, Shamoon H, Gabriely I. 2009. Hypoglycemia-associated autonomic failure is prevented by opioid receptor blockade. J Clin Endocrinol Metab 94:3372–3380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nakao K, Nakai Y, Jingami H, Oki S, Fukata J, Imura H. 1979. Substantial rise of plasma β-endorphin levels after insulin-induced hypoglycemia in human subjects. J Clin Endocrinol Metab 49:838–841 [DOI] [PubMed] [Google Scholar]
- 9. Poplawski MM, Mastaitis JW, Mobbs CV. 2011. Naloxone, but not valsartan, preserves responses to hypoglycemia after antecedent hypoglycemia: role of metabolic reprogramming in counterregulatory failure. Diabetes 60:39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Caprio S, Gerety G, Tamborlane WV, Jones T, Diamond M, Jacob R, Sherwin RS. 1991. Opiate blockade enhances hypoglycemic counterregulation in normal and insulin-dependent diabetic subjects. Am J Physiol 260:E852–E858 [DOI] [PubMed] [Google Scholar]
- 11. Finegood DT, Bergman RN, Vranic M. 1987. Estimation of endogenous glucose production during hyperinsulinemic-euglycemic glucose clamps. Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes 36:914–924 [DOI] [PubMed] [Google Scholar]
- 12. Dunn A, Katz J, Golden S, Chenoweth M. 1976. Estimation of glucose turnover and recycling in rabbits using various [3H, 14C]glucose labels. Am J Physiol 230:1159–1162 [DOI] [PubMed] [Google Scholar]
- 13. Mellman MJ, Davis MR, Brisman M, Shamoon H. 1994. Effect of antecedent hypoglycemia on cognitive function and on glycemic thresholds for counterregulatory hormone secretion in healthy humans. Diabetes Care 17:183–188 [DOI] [PubMed] [Google Scholar]
- 14. Steele R. 1959. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann NY Acad Sci 82:420–430 [DOI] [PubMed] [Google Scholar]
- 15. Fanelli C, Pampanelli S, Epifano L, Rambotti AM, Di Vincenzo A, Modarelli F, Ciofetta M, Lepore M, Annibale B, Torlone E, et al. 1994. Long-term recovery from unawareness, deficient counterregulation and lack of cognitive dysfunction during hypoglycaemia, following institution of rational, intensive insulin therapy in IDDM. Diabetologia 37:1265–1276 [DOI] [PubMed] [Google Scholar]
- 16. Bouloux PM, Grossman A, Lytras N, Besser GM. 1985. Evidence for the participation of endogenous opioids in the sympathoadrenal response to hypoglycaemia in man. Clin Endocrinol (Oxf) 22:49–56 [DOI] [PubMed] [Google Scholar]
- 17. Cryer PE. 2005. Mechanisms of hypoglycemia-associated autonomic failure and its component syndromes in diabetes. Diabetes 54:3592–3601 [DOI] [PubMed] [Google Scholar]
- 18. Jordan SD, Könner AC, Brüning JC. 2010. Sensing the fuels: glucose and lipid signaling in the CNS controlling energy homeostasis. Cell Mol Life Sci 67:3255–3273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arefolov VA, Dmitriev AD, Tennov AV, Val'dman AV. 1986. [Detection of the pro-opiomelanocortin peptide fragments—β-endorphin and ACTH—in the adrenals of rats and mice by immunohistochemistry]. Bull Eksp Biol Med 101:445–447 [PubMed] [Google Scholar]
- 20. Cheng JT, Liu IM, Kuo DH, Lin MT. 2001. Stimulatory effect of phenylephrine on the secretion of β-endorphin from rat adrenal medulla in vitro. Auton Neurosci 93:31–35 [DOI] [PubMed] [Google Scholar]
- 21. Liu IM, Chen WC, Cheng JT. 2003. Mediation of beta-endorphin by isoferulic acid to lower plasma glucose in streptozotocin-induced diabetic rats. J Pharmacol Exp Ther 307:1196–1204 [DOI] [PubMed] [Google Scholar]
- 22. Desjardins GC, Brawer JR, Beaudet A. 1990. Distribution of μ, Δ, and κ opioid receptors in the hypothalamus of the rat. Brain Res 536:114–123 [DOI] [PubMed] [Google Scholar]
- 23. Emmerson PJ, Miller RJ. 1999. Pre- and postsynaptic actions of opioid and orphan opioid agonists in the rat arcuate nucleus and ventromedial hypothalamus in vitro. J Physiol 517(Pt 2):431–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang C, Pfaff DW, Kow LM. 1996. Functional analysis of opioid receptor subtypes in the ventromedial hypothalamic nucleus of the rat. Eur J Pharmacol 308:153–159 [DOI] [PubMed] [Google Scholar]
- 25. Zheng SX, Bosch MA, Rønnekleiv OK. 2005. μ-Opioid receptor mRNA expression in identified hypothalamic neurons. J Comp Neurol 487:332–344 [DOI] [PubMed] [Google Scholar]
- 26. Kampa M, Margioris AN, Hatzoglou A, Dermitzaki I, Denizot A, Henry JF, Oliver C, Gravanis A, Castanas E. 1999. κ1-Opioid binding sites are the dominant opioid binding sites in surgical specimens of human pheochromocytomas and in a human pheochromocytoma (KAT45) cell line. Eur J Pharmacol 364:255–262 [DOI] [PubMed] [Google Scholar]
- 27. Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI. 1997. Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J Clin Invest 99:361–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Borg WP, During MJ, Sherwin RS, Borg MA, Brines ML, Shulman GI. 1994. Ventromedial hypothalamic lesions in rats suppress counterregulatory responses to hypoglycemia. J Clin Invest 93:1677–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Borg WP, Sherwin RS, During MJ, Borg MA, Shulman GI. 1995. Local ventromedial hypothalamus glucopenia triggers counterregulatory hormone release. Diabetes 44:180–184 [DOI] [PubMed] [Google Scholar]
- 30. Suda T, Sato Y, Sumitomo T, Nakano Y, Tozawa F, Iwai I, Yamada M, Demura H. 1992. β-Endorphin inhibits hypoglycemia-induced gene expression of corticotropin-releasing factor in the rat hypothalamus. Endocrinology 130:1325–1330 [DOI] [PubMed] [Google Scholar]
- 31. Fishman J, Hahn EF, Norton BI. 1975. Comparative in vivo distribution of opiate agonists and antagonists by means of double isotope techniques. Life Sci 17:1119–1125 [DOI] [PubMed] [Google Scholar]
- 32. Ngai SH, Berkowitz BA, Yang JC, Hempstead J, Spector S. 1976. Pharmacokinetics of naloxone in rats and in man: basis for its potency and short duration of action. Anesthesiology 44:398–401 [DOI] [PubMed] [Google Scholar]
- 33. Cheng JT, Liu IM, Tzeng TF, Tsai CC, Lai TY. 2002. Plasma glucose-lowering effect of β-endorphin in streptozotocin-induced diabetic rats. Horm Metab Res 34:570–576 [DOI] [PubMed] [Google Scholar]
- 34. Hsu JH, Wu YC, Liou SS, Liu IM, Huang LW, Cheng JT. 2004. Mediation of endogenous β-endorphin by tetrandrine to lower plasma glucose in streptozotocin-induced diabetic rats. Evid Based Complement Alternat Med 1:193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dermitzaki E, Gravanis A, Venihaki M, Stournaras C, Margioris AN. 2001. Opioids suppress basal and nicotine-induced catecholamine secretion via a stabilizing effect on actin filaments. Endocrinology 142:2022–2031 [DOI] [PubMed] [Google Scholar]
- 36. Venihaki M, Gravanis A, Margioris AN. 1996. Opioids inhibit dopamine secretion from PC12 rat pheochromocytoma cells in a naloxone-reversible manner. Life Sci 58:75–82 [DOI] [PubMed] [Google Scholar]
- 37. Ramanathan R, Cryer PE. 2011. Adrenergic mediation of hypoglycemia-associated autonomic failure. Diabetes 60:602–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Verberne AJ, Sartor DM. 2010. Rostroventrolateral medullary neurons modulate glucose homeostasis in the rat. Am J Physiol Endocrinol Metab 299:E802–E807 [DOI] [PubMed] [Google Scholar]