Abstract
Context:
Animal models suggest that the osteoblast-stimulating actions of PTH are mediated by acute suppression of sclerostin, an inhibitor of the anabolic Wnt pathway. The immediate physiological changes in serum sclerostin in response to PTH infusion have not been reported in human studies.
Objective:
We sought to determine the acute physiological effects of PTH infusion on serum sclerostin and bone turnover markers in healthy adult men.
Design, Setting, and Participants:
Fifty-three healthy adult men underwent an 18-h iv infusion of human PTH(1-34) at a dose of 0.55 U/kg · h.
Outcomes:
Serum levels of ionized calcium, sclerostin, and markers of bone formation (osteocalcin and amino-terminal propeptide of type I procollagen) and bone resorption (C-telopeptide and N-telopeptide) were obtained at 0, 6, 12, and 18 h.
Results:
Serum ionized calcium, C-telopeptide, and N-telopeptide increased, and osteocalcin and amino-terminal propeptide of type I procollagen fell linearly throughout the PTH infusion (P < 0.001 for all). Average ± sem sclerostin levels declined from 936 ± 65 to 813 ± 63 pg/ml at 6 h (P < 0.001) and remained stably suppressed for the duration of the PTH infusion. There were no significant correlations between change in sclerostin and change in bone markers.
Conclusions:
Serum sclerostin declined in response to acute PTH infusion within 6 h in healthy adult men. The early plateau in sclerostin suppression may indicate that maximal stimulation of the Wnt pathway is achieved quickly after exposure to PTH. Our findings support the hypothesis that PTH may mediate its anabolic effects in part via suppression of sclerostin.
Parathyroid hormone (PTH) has both anabolic and catabolic skeletal effects, although the molecular mechanisms that underlie these functions are incompletely defined. Moreover, the manner of PTH administration influences the balance of the anabolic and catabolic effects in bone. Specifically, intermittent PTH administration leads to marked increases in bone mass (1), whereas continuous exposure to PTH is associated with low bone mass (2).
Recently, it has been proposed that sclerostin, an antagonist of the Wnt pathway, may mediate, at least in part, the anabolic effects of PTH (3–8). Sclerostin is a 22.5-kDa secreted cysteine-knot protein encoded by SOST and is expressed almost exclusively in osteocytes (9). Sclerostin is a negative regulator of bone formation that inhibits the canonical Wnt/β-catenin signaling pathway (10), and loss-of-function mutations in SOST lead to the clinical disorders of sclerosteosis (OMIM 269500) and van Buchem's disease (OMIM 239100), both characterized by high bone mass phenotypes.
Animal models of PTH administration suggest that both intermittent administration and continuous infusion suppress SOST expression (3, 4, 6). In human studies, there are conflicting data on the effect of chronic intermittent sc PTH therapy on serum sclerostin (11–13). However, the immediate physiological changes in sclerostin production in response to PTH infusion have not been reported in human studies.
In this study, we sought to determine the acute physiological effects of PTH infusion on serum sclerostin and bone markers of formation and resorption in healthy adult men.
Subjects and Methods
Study subjects
Fifty-eight men between the ages of 20 and 45 yr were recruited for a previously reported study (14). All men were healthy, had no history of bone-modifying disorders, and were not taking any drugs known to affect bone turnover markers. The present analysis is restricted to 53 men for whom serum samples were available. The study was approved by the Institutional Review Board of Partners HealthCare Systems, and all subjects provided written informed consent.
PTH infusion
Subjects were admitted to the Mallinckrodt General Clinical Research Center for a 24-h iv infusion of human PTH(1-34) (Bachem, Torrance, CA) at a dose of 0.55 U/kg · h. Whole-blood ionized calcium levels and serum levels of sclerostin, N-telopeptide (NTX), C-telopeptide (CTX), osteocalcin (OC), and amino-terminal propeptide of type I procollagen (P1NP) were measured every 6 h during the PTH infusion. If blood ionized calcium levels exceeded 1.50 mmol/liter, infusions were discontinued. Because the ionized calcium levels exceeded 1.5 mmol/liter before the 24-h time point in a majority of subjects, only the markers drawn at 0, 6, 12, and 18 h were measured.
Measurements
Serum sclerostin was measured using a sandwich ELISA (Biomedica Gruppe, Wien, Austria) with a detection limit of 200 pg/ml and intra- and interassay coefficients of variation of 4–6 and 5–7%, respectively. Serum NTX was measured using a competitive inhibition enzyme immunoassay (Osteomark; Ostex International, Seattle, WA) with a sensitivity of 1 nm bone collagen equivalent and intra- and interassay coefficients of variation of 6 and 9%, respectively. Serum CTX was measured using a double-antibody ELISA (Serum Crosslaps; Nordic Biosciences Diagnostics A/S, Herlev, Denmark) with intra- and interassay coefficients of variation of 4.7 and 13.5%, respectively. Serum OC was measured using a double-antibody immunoradiometric assay (Nichols Institute, San Juan Capistrano, CA) with a sensitivity of 0.5 ng/ml and intra- and interassay coefficients of variation of 2–4 and 3–6%, respectively. Serum P1NP was measured using a RIA (Orion Diagnostica, Espoo, Finland) with a sensitivity of 2 ng/ml and intra- and interassay coefficients of variation of 5–14 and 2–3%, respectively.
Statistics
All data are summarized by mean ± sem unless otherwise specified. Changes in ionized calcium, sclerostin, and bone turnover markers during PTH infusion were analyzed with repeated-measures ANOVA. Relationships between variables of interest were assessed with Pearson correlation. Analyses were performed with SAS version 9.2 (SAS Institute Inc., Cary, NC). Resulting values of P < 0.05 (two-sided) were considered statistically significant.
Results
The baseline clinical characteristics of the study subjects were unremarkable (Table 1).
Table 1.
Baseline characteristics
| Mean ± sd | |
|---|---|
| n | 53 |
| Age (yr) | 30 ± 8 |
| Weight (kg) | 77 ± 14 |
| Calcium (mg/dl) | 9.5 ± 0.4 |
| Creatinine (mg/dl) | 1.0 ± 0.1 |
| Alkaline phosphatase (U/liter) | 75 ± 16 |
| Testosterone (ng/dl) | 478 ± 156 |
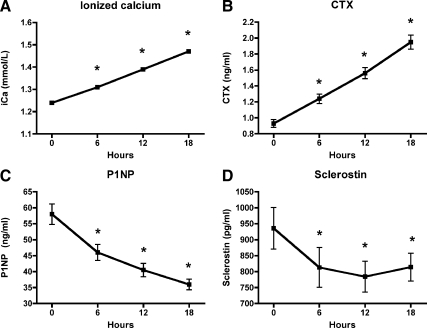
We observed linear changes in ionized calcium and bone turnover markers during the 18-h PTH infusion (Fig. 1, A–C). As has been previously described (14), serum markers of bone resorption increased linearly throughout acute PTH infusion (at 0 and 18 h, CTX was 0.93 ± 0.05 and 1.95 ± 0.09 ng/ml, P < 0.001; NTX was 18.1 ± 0.7 and 31.6 ± 1.5 nmol/liter, P < 0.001). In contrast, serum markers of bone formation declined linearly (at 0 and 18 h, P1NP was 58.0 ± 3.2 and 36.0 ± 1.7 ng/ml, P < 0.001; OC was 13.6 ± 0.6 and 10.2 ± 0.5 ng/ml, P < 0.001).
Fig. 1.
Changes in ionized calcium (A), CTX (B), P1NP (C), and sclerostin (D) during PTH infusion. Ionized calcium and CTX increased linearly whereas P1NP decreased linearly during PTH infusion (P < 0.001 for all). Sclerostin declined by 6 h and was maintained at a reduced level throughout the infusion (P = 0.007).
Serum sclerostin declined by 6 h in response to acute PTH infusion (from 936 ± 65 to 813 ± 62 pg/ml, P < 0.001; Fig. 1D), and was maintained at a reduced level throughout the duration of the infusion (P = 0.007). This early plateau effect is in contrast to the linear changes observed in bone turnover markers. We did not observe any significant correlation between baseline sclerostin levels and baseline bone formation or resorption markers. Furthermore, there was no association between change in sclerostin levels and change in any of the bone turnover markers.
Discussion
Ever since its identification a decade ago, sclerostin has been postulated to have several roles in the regulation of the skeleton. In this study, we found that serum sclerostin declines acutely in response to PTH infusion in healthy adult men. These results are consistent with the hypothesis that the anabolic actions of PTH may be in part mediated by sclerostin.
Several lines of evidence suggest that PTH plays an important role in regulating sclerostin. Transgenic mice expressing a constitutively active PTH-1 receptor exclusively in osteocytes exhibit reduced expression of sclerostin and increased Wnt signaling as well as increased bone mass and bone remodeling (7). In vivo models of PTH(1-34) and PTH(1–84) administration suggest that both intermittent and continuous infusion suppress SOST mRNA expression and greatly reduce sclerostin protein levels (3, 4, 6). Although a mouse model of continuous PTH infusion suggested that SOST expression declines linearly over 40 h (3), we found in this clinical study that an early plateau in serum sclerostin suppression is achieved in less than 6 h of PTH infusion. This difference may be due to discrepancies in PTH-dosing equivalencies or may suggest distinctions between sclerostin levels in the bone marrow space vs. the circulation. Alternatively, the difference in sclerostin's response to PTH may reflect inherent differences in the mouse and the human.
In clinical studies of postmenopausal women, there are conflicting data on the effect of intermittent sc PTH therapy on serum sclerostin (11–13). One study found that serum sclerostin levels decreased after 3 wk of teriparatide [human PTH(1-34)] treatment (sclerostin was measured 4 h after teriparatide administration at baseline and 3 wk) (11). Similar reductions were observed in marrow plasma sclerostin levels that were obtained in a subset of women, although the results were not statistically significant due to an underpowered sample size (11). In contrast, two studies of chronic teriparatide treatment did not find any significant change in serum sclerostin levels over 6- or 18-month periods (12, 13). The differences between these studies may lie in the measured time point; animal studies suggest that intermittent PTH injections cause transient suppression in sclerostin expression, which nadirs at 4–6 h (3, 4). In at least one of the studies showing no association (12), serum sclerostin was measured only 24 h after the last teriparatide injection. Our study of continuous PTH infusion demonstrates that acute exposure to PTH leads to a rapid decline in sclerostin levels. These findings are consistent with the observation of reduced circulating sclerostin levels in patients with chronic elevations of PTH from primary hyperparathyroidism (15). Furthermore, our results suggest that maximal stimulation of the anabolic Wnt pathway via sclerostin suppression is achieved quickly after exposure to PTH, whereas markers of bone resorption continue to increase linearly over time. One possible hypothesis is that intermittent teriparatide therapy capitalizes on this early anabolic effect while minimizing the sustained linear increases in bone resorption seen with either prolonged PTH administration or primary hyperparathyroidism.
The mechanism by which PTH suppresses sclerostin is an area of active investigation. Osteocyte-specific SOST overexpression is able to overcome the high bone mass phenotype seen in osteocyte-specific constitutively active PTH-1 receptor, suggesting that sclerostin functions downstream of PTH (8). It is postulated that PTH may suppress sclerostin via inhibition of the myocyte enhancer factor-2 (MEF2) transcription factors, which control the SOST enhancer element. Mutations within this enhancer element were initially identified as the defects responsible for Van Buchem's disease, a clinical disorder characterized by low sclerostin levels and high bone mass (16). It was subsequently found that the MEF2 response element located within the enhancer is required to activate SOST transcription (5). Furthermore, PTH greatly suppressed the transcriptional activity of the SOST bone enhancer in vitro, which appeared to be mediated by MEF2 transcription factors (5). Additional in vivo and clinical studies are required to determine whether PTH-induced bone formation is dependent on MEF2 transcription factors.
Several limitations of this study deserve mention. We studied only men, and thus results cannot be generalized to women. We cannot rule out the possibility that physiological diurnal variation in sclerostin levels may be impacting our results during the PTH infusion. In addition, the infusion model leads to PTH levels that exceed physiological levels or those achieved with intermittent PTH treatment, and it is possible that sclerostin's response may vary by dose. As has been noted in previous studies (17–19), acute PTH infusion led to short-term suppression of bone formation markers, in contrast to primary hyperparathyroidism and chronic intermittent PTH treatment. Although it is unclear how the bone marker response may change with prolonged PTH exposure, the finding of reduced sclerostin levels in primary hyperparathyroidism (15) suggests that the declines in sclerostin that we observed with 18 h infusion are likely sustained. Lastly, our study relies on measurements of circulating sclerostin, whereas sclerostin is thought to exert primarily paracrine effects. Although circulating levels of sclerostin have been strongly correlated with marrow plasma levels in other studies (11, 20), it is possible that peripheral levels are not sensitive enough to detect changes that may be occurring at the level of the bone.
Conclusions
In summary, we observed an acute and early decline in serum sclerostin in response to PTH that was maintained throughout a continuous infusion. Our data support the hypothesis that PTH may mediate its anabolic effects in part via suppression of sclerostin. Given the key role that sclerostin plays in modulating bone formation, additional studies should focus on better defining the interaction between PTH and sclerostin in bone metabolism.
Acknowledgments
We thank the nurses and staff of the Mallinckrodt General Clinical Research Center for the care of the study volunteers and Dr. Patrick Sluss of the Massachusetts General Hospital Clinical Laboratory Research Core.
This work was supported by National Institutes of Health Grants K23-RR16310 and Massachusetts General Hospital Clinical Research Center Grant RR-1066.
Disclosure Summary: The authors report no disclosures.
Footnotes
- CTX
- C-telopeptide
- MEF2
- myocyte enhancer factor-2
- NTX
- N-telopeptide
- OC
- osteocalcin
- P1NP
- amino-terminal propeptide of type I procollagen.
References
- 1. Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. 2001. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441 [DOI] [PubMed] [Google Scholar]
- 2. Silverberg SJ, Shane E, de la Cruz L, Dempster DW, Feldman F, Seldin D, Jacobs TP, Siris ES, Cafferty M, Parisien MV, Lindsay R, Clemens TL, Bilezikian JP. 1989. Skeletal disease in primary hyperparathyroidism. J Bone Miner Res 4:283–291 [DOI] [PubMed] [Google Scholar]
- 3. Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O'Brien CA, Manolagas SC, Jilka RL. 2005. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology 146:4577–4583 [DOI] [PubMed] [Google Scholar]
- 4. Keller H, Kneissel M. 2005. SOST is a target gene for PTH in bone. Bone 37:148–158 [DOI] [PubMed] [Google Scholar]
- 5. Leupin O, Kramer I, Collette NM, Loots GG, Natt F, Kneissel M, Keller H. 2007. Control of the SOST bone enhancer by PTH using MEF2 transcription factors. J Bone Miner Res 22:1957–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Silvestrini G, Ballanti P, Leopizzi M, Sebastiani M, Berni S, Di Vito M, Bonucci E. 2007. Effects of intermittent parathyroid hormone (PTH) administration on SOST mRNA and protein in rat bone. J Mol Histol 38:261–269 [DOI] [PubMed] [Google Scholar]
- 7. O'Brien CA, Plotkin LI, Galli C, Goellner JJ, Gortazar AR, Allen MR, Robling AG, Bouxsein M, Schipani E, Turner CH, Jilka RL, Weinstein RS, Manolagas SC, Bellido T. 2008. Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS ONE 3:e2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rhee Y, Allen MR, Condon K, Lezcano V, Ronda AC, Galli C, Olivos N, Passeri G, O'Brien CA, Bivi N, Plotkin LI, Bellido T. 2011. PTH receptor signaling in osteocytes governs periosteal bone formation and intra-cortical remodeling. J Bone Miner Res 26:1035–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Bezooijen RL, Roelen BA, Visser A, van der Wee-Pals L, de Wilt E, Karperien M, Hamersma H, Papapoulos SE, ten Dijke P, Löwik CW. 2004. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med 199:805–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D. 2005. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem 280:19883–19887 [DOI] [PubMed] [Google Scholar]
- 11. Drake MT, Srinivasan B, Mödder UI, Peterson JM, McCready LK, Riggs BL, Dwyer D, Stolina M, Kostenuik P, Khosla S. 2010. Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab 95:5056–5062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Polyzos SA, Anastasilakis AD, Bratengeier C, Woloszczuk W, Papatheodorou A, Terpos E. 11 January 2011. Serum sclerostin levels positively correlate with lumbar spinal bone mineral density in postmenopausal women-the six-month effect of risedronate and teriparatide. Osteoporosis Int 10.1007/s00198-010-1525-6 [DOI] [PubMed] [Google Scholar]
- 13. Gatti D, Viapiana O, Idolazzi L, Fracassi E, Rossini M, Adami S. 2011. The waning of teriparatide effect on bone formation markers in postmenopausal osteoporosis is associated with increasing serum levels of DKK1. J Clin Endocrinol Metab 96:1555–1559 [DOI] [PubMed] [Google Scholar]
- 14. Lee H, Finkelstein JS, Miller M, Comeaux SJ, Cohen RI, Leder BZ. 2006. Effects of selective testosterone and estradiol withdrawal on skeletal sensitivity to parathyroid hormone in men. J Clin Endocrinol Metab 91:1069–1075 [DOI] [PubMed] [Google Scholar]
- 15. van Lierop AH, Witteveen JE, Hamdy NA, Papapoulos SE. 2010. Patients with primary hyperparathyroidism have lower circulating sclerostin levels than euparathyroid controls. Eur J Endocrinol 163:833–837 [DOI] [PubMed] [Google Scholar]
- 16. Loots GG, Kneissel M, Keller H, Baptist M, Chang J, Collette NM, Ovcharenko D, Plajzer-Frick I, Rubin EM. 2005. Genomic deletion of a long-range bone enhancer misregulates sclerostin in Van Buchem disease. Genome Res 15:928–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Joborn C, Ljunghall S, Larsson K, Lindh E, Naessén T, Wide L, Akerström G, Rastad J. 1991. Skeletal responsiveness to parathyroid hormone in healthy females: relationship to menopause and oestrogen replacement. Clin Endocrinol (Oxf) 34:335–339 [DOI] [PubMed] [Google Scholar]
- 18. Cosman F, Shen V, Xie F, Seibel M, Ratcliffe A, Lindsay R. 1993. Estrogen protection against bone resorbing effects of parathyroid hormone infusion. Assessment by use of biochemical markers. Ann Intern Med 118:337–343 [DOI] [PubMed] [Google Scholar]
- 19. Leder BZ, Smith MR, Fallon MA, Lee ML, Finkelstein JS. 2001. Effects of gonadal steroid suppression on skeletal sensitivity to parathyroid hormone in men. J Clin Endocrinol Metab 86:511–516 [DOI] [PubMed] [Google Scholar]
- 20. Mödder UI, Roforth MM, Hoey K, McCready LK, Peterson JM, Monroe DG, Oursler MJ, Khosla S. 2011. Effects of estrogen on osteoprogenitor cells and cytokines/bone-regulatory factors in postmenopausal women. Bone 49:202–207 [DOI] [PMC free article] [PubMed] [Google Scholar]