Abstract
Context:
The role of ovarian hormones in maintaining neuronal integrity and cognitive function is still debated. This study was undertaken to clarify the potential relationship between postmenopausal hormone use and the cholinergic system.
Objective:
We hypothesized that early initiated hormone therapy (HT) preserves the cholinergic system and that estrogen therapy (ET) would be associated with higher levels of acetylcholinesterase activity in the posterior cingulate cortex and hippocampus compared to estrogen plus progestin therapy (EPT) or no HT.
Design and Setting:
We conducted a cross-sectional study at a university teaching hospital.
Patients:
Fifty postmenopausal women (age, 65.2 ± 0.7 yr) with early long-term HT (n = 34; 13 ET and 21 EPT) or no HT (n = 16) participated in the study.
Interventions:
There were no interventions.
Main Outcome Measure:
We measured cholinergic activity (acetylcholinesterase) in the hippocampus and posterior cingulate brain regions as measured by N-[11C]methylpiperidin-4-yl propionate and positron emission tomography as a marker of cholinergic function.
Results:
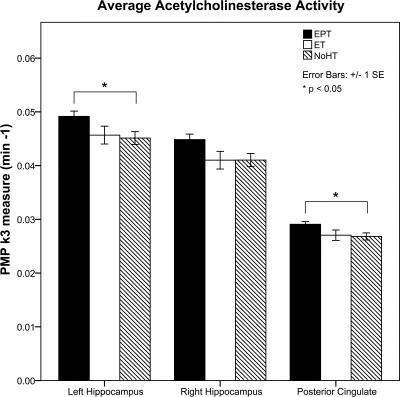
Significant effects of treatment on cholinergic activity measures were obtained in the left hippocampus (F = 3.56; P = 0.04), right hippocampus (F = 3.42; P = 0.04), and posterior cingulate (F = 3.76; P = 0.03). No significant effects were observed in a cortical control region. Post hoc testing identified greater cholinergic activity in the EPT group compared to the no-HT group in the left hippocampus (P = 0.048) and posterior cingulate (P = 0.045), with a nonstatistically significant trend in the right hippocampus (P = 0.073).
Conclusions:
A differential effect of postmenopausal ET and EPT on cholinergic neuronal integrity was identified in postmenopausal women. The findings are consistent with a preservation of cholinergic neuronal integrity in the EPT group.
The role of estrogen in maintaining neuronal integrity and cognitive function is of great significance. In women, evidence suggests that postmenopausal hormone therapy (HT) is associated with improved or preserved cognitive function; however, not all studies support this (for reviews, see Refs. 1 and 2). The Women's Health Initiative Memory Study (WHIMS) found an increased risk of dementia in hormone users (3) and a potential differential effect of hormone combinations (3–5). Specifically, the combined HT (conjugated equine estrogens plus medroxyprogesterone acetate) group demonstrated an increased risk of dementia (4), whereas the CEE group demonstrated a trend but no significant difference for greater dementia risk compared with controls (3). This contrasts with previous prospective observational studies suggesting that HT reduces the risk or delays the onset of Alzheimer's disease (AD) (6–8). The Women's Health Initiative Study of Cognitive Aging (WHISCA) also demonstrated different neuropsychological effects of the hormone preparations (9, 10): spatial processing improved with combination therapy and worsened with estrogen only.
To explain discrepancies between observational and randomized trials, it has been suggested that the timing of HT treatment (from menopause onset) may be an important factor in neuroprotection (11–13). Data from animal models have shown that early HT after ovariectomy, compared with delayed treatment, more effectively preserves hippocampal CA1 synaptic density in rats (14) and maintains cognitive function in rats and nonhuman primates (15, 16). In women, the cognitive effects of estrogen initiated early in menopause have not been well studied. A reassessment of randomized studies, evaluating treatment early or later in menopause, suggests that hormone use early, rather than later, in menopause may provide cognitive benefit (2). Furthermore, a follow-up study of women randomized to HT or placebo for 2–3 yr in early postmenopause found reduced risk of cognitive impairment in those who previously received HT (17). Likewise, early initiators of HT performed better than late initiators on the Mini-Mental State Examination (MMSE) and during an attentional task (18).
The source of such neuroprotective effects of HT on cognitive function is not fully understood; however, the cholinergic system, known to be critically involved in cognition, memory, and the aging process, is a major brain target for hormone activity (19, 20). Estrogen receptors are present throughout the cerebral cortex (21), as well as within the nuclei of the basal forebrain, a major source of cholinergic innervation (22). Estrogen provides trophic support to cholinergic cells and regulates various markers of cholinergic function, including choline acetyltransferase and acetylcholine release (22–28).
In the ovariectomized primate model, both short-term and long-term HT preserved cholinergic fibers (29–31). In women, manipulation of cholinergic activity can alter cognitive functioning. For instance, anticholinergic therapy after a medically induced menopause resulted in more false-positive errors in verbal recognition and reduced frontal functional magnetic resonance imaging (fMRI) activation (32). Furthermore, in most animal and human models, the detrimental cognitive effects of anticholinergics are attenuated by pretreatment or cotreatment with estrogen (33–36), although not in all (37).
Studies have attempted to link cholinergic system functioning to HT in women using neuroimaging techniques that allow noninvasive study of cholinergic synaptic densities. A study of postmenopausal estrogen therapy (ET) and brain muscarinic receptor density using single photon emission computed tomography (SPECT) and (R,R)[123I] I-QNB showed that long-term ET, compared with no ET, was associated with higher muscarinic receptor concentrations in the hippocampus, left striatum, frontal cortex, and thalamus. Furthermore, peripheral estradiol levels correlated with muscarinic receptor densities (38). In a previous pilot study, we examined the relationship between postmenopausal ET and the cholinergic system using SPECT and [123I]iodobenzovesamicol, labeling the presynaptic vesicular acetylcholine transporter, a measure of cholinergic terminal density. In that work, we showed that the length of HT use was positively associated with greater concentrations of cholinergic synaptic terminals in multiple cortical regions, and that the ET group had higher cholinergic synaptic concentrations in the posterior cingulate region (an associate cognitive region) than the estrogen plus progestin therapy (EPT) group. Most likely because of the small sample size, we were unable to identify a difference in cholinergic density between the HT and no-HT therapy groups (39).
The current cross-sectional study was undertaken to examine this question in greater detail and to clarify the potential relationship between postmenopausal hormone use and the cholinergic system. For this purpose, we quantified acetylcholinesterase (AChE) activity, a surrogate of cholinergic functional capacity, using the positron emission tomography (PET) radioligand N-[11C]methylpiperidin-4-yl propionate ([11C]PMP) (40–42). We studied healthy postmenopausal women treated with ET, EPT, or no HT. We studied women who initiated HT within 2 yr of menopause and hypothesized that initiating HT before the development of central neurovascular pathology would preserve neurochemical systems involved in cognition. We further expected that ET would be associated with higher levels of AChE concentrations in the posterior cingulate cortex and hippocampus compared with EPT or control groups.
Subjects and Methods
Subjects
As part of a comprehensive evaluation of menopausal HT, 50 healthy right-handed postmenopausal women, 60 yr or older, were recruited by advertisement (43). Menopause was defined as the absence of menstrual periods for 1 yr, the onset of severe symptoms after hysterectomy, or the time of hysterectomy with bilateral oophorectomy. Women included in the study either had never used hormones (n = 16) or had taken HT continuously for at least 10 yr (n = 34; 13 ET and 21 EPT). The hormone group began treatment within 2 yr of menopause and included both current hormone users and women who had recently stopped HT. All individuals on HT used an identical dose and preparation of estrogen: 0.625 mg/d CEE (Premarin; Wyeth Ayerst, Philadelphia, PA), with or without cyclic or continuous medroxyprogesterone acetate (Provera; Pfizer, New York, NY; or Prempro, Wyeth Pharmaceuticals, Philadelphia, PA).
Eleven of the 34 hormone-treated women were currently taking hormones (four of 13 women in the ET group and seven of 21 women in the EPT group). For those no longer taking hormones, mean time since hormone use ended was 2.2 ± 0.2 yr. All women in the ET group had undergone hysterectomy, including nine of 13 women (69.2%) with bilateral oophorectomy.
Subjects underwent an initial phone screen followed by a complete medical, psychiatric, and neurological history and physical exam in the Medical Clinical Research Unit. A neuropsychological battery of tests was given including: 1) Mini-Mental State Examination (44), a brief screening measure of dementia; 2) Shipley Institute of Living Scale (45), a short estimate of intellectual power; and 3) Geriatric Depression Rating Scale (46).
Screening laboratory tests included electrolytes, glucose, complete blood count, TSH, and estradiol. Exclusion criteria included acute or uncorrected medical illnesses, the use of centrally acting medications, intermittent estrogen use, phytoestrogen supplements, smoking within the last 5 yr, inability to tolerate scanning procedures, and contraindications to magnetic resonance imaging (MRI).
After a full description of the study, written informed consent was obtained. All procedures were approved by the University of Michigan's institutional review board and the radiation safety committee.
Scanning procedures and image processing
Subjects were positioned in the scanner gantry, and an iv line was placed in an antecubital vein. A light forehead restraint was used to minimize intrascan head movement. Small head movements during the emission scans were corrected using an automated computer algorithm (47).
AChE activity was defined with PET to calculate tracer kinetic estimates of the local hydrolysis rate of [11C]PMP. The [11C]PMP radioligand, an acetylcholine analog that is hydrolyzed by AChE (48), was prepared by N-[11C] methylation of piperidin-4-yl propionate in high radiochemical purity (42). Blood-brain barrier transport rate (K1R) and AChE activity measures (k3) were calculated using a kinetic modeling approach that does not require arterial plasma sampling (49, 50). The average injected radioactivity was 18.4 ± 1.5 mCi (mean ± sd).
Emission data were collected as a sequence of 17 image dynamic PET frames over an 80-min scanning period using a Siemens ECAT Exact HR+ scanner (Siemens, Knoxville, TN) operated in three-dimensional mode with septa retracted. Images were reconstructed using Fourier rebinning and the iterative OSEM routine (four iterations, 16 subsets) resulting in images with a full-width at half-maximum resolution of approximately 5.5 mm both in-plane and axially. Attenuation correction was performed using a 6-min transmission scan ([68Ge] source) obtained before the emission study, also with iterative reconstruction of the blank/transmission data followed by segmentation of the attenuation image and reprojection.
Anatomical MRI scans were acquired axially using a 3T whole-body MRI scanner (General Electric, Milwaukee, WI) equipped with a standard head coil. A T1-weighted coronal image set was acquired with a spoiled gradient recalled three-dimensional volumetric acquisition [repetition time = 9.6, echo time = 3.3, inversion recovery preparation = 200 msec, flip angle = 17°, bandwidth = 15.63, 24-cm field of view, 1.5-mm slice thickness, 106–110 slices, 256 × 256 matrix, and two excitations].
Parametric K1R and k3 images were coregistered to the subject's magnetic resonance (MR) images using SPM2 software (Wellcome Department of Cognitive Neurology, London, UK). Because spatial normalization of older brains to standard templates generated from young adults is not ideal, we used a minimal deformation template (MDT2) derived from 25 older normal subjects for the anatomical normalization of the PET and MR images, developed by the Imaging of Dementia and Aging Laboratory at the University of California, Davis (51). The quality of coregistration and normalization was confirmed for each subject individually by comparing the transformed MR and PET images to each other and the MDT2 template.
Analysis
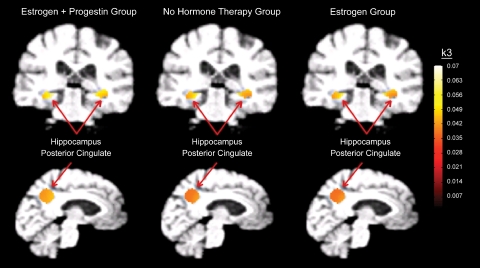
Volumes of interest (VOI) in the posterior cingulate and bilateral hippocampus were defined based on a priori hypotheses regarding hormonal effects on AChE functioning in the aging brain. Based on the results from a previous study (39), we extracted data from the posterior cingulate using a 20-mm diameter spherical region of interest with voxel coordinates x, y, z = 0, −50, and 27, respectively. For the hippocampal VOI, we used hippocampal templates created specifically for the MDT2 template [anatomical boundaries described in Sun et al. (51)]. Data for a control region not typically affected in the dementias was also examined to test the specificity of the findings. This VOI was generated using the gray matter cortical boundaries of the motor and premotor cortex [Brodmann areas (BA) 4 and 6] and medial frontal cortex (BA 8–11) using predefined templates applied in stereotactic space (52).
Possible differences in regional cholinergic activity indices between groups were examined by ANOVA or analysis of covariance (ANCOVA) with Tukey post hoc testing. Pearson correlations were analyzed to determine the relationship between regional cholinergic activity, age, and covariates of interest.
Results
Demographic and baseline information for study participants is described in Tables 1 and 2. The average age of participants was 65.2 ± 0.7 yr. The neuropsychological data showed normal-range IQ and absence of dementia and depression in all groups. There were no differences between the three groups in neuropsychological test results, age, or education. Post hoc two-group comparisons show that age at HT initiation and duration of hormone use differed between the ET and the combined EPT groups, with the ET group initiating HT earlier and maintaining in treatment longer. There were no statistical differences between current and past hormone users.
Table 1.
Demographic and baseline information for study participants
| Mean ± sd |
P t-Testa | Mean ± sd Never treated | P ANOVAb | |||
|---|---|---|---|---|---|---|
| All subjects | ET | EPT | ||||
| n | 50 | 13 | 21 | 16 | ||
| Age (yr) | 65.2 ± 4.8 | 66.2 ± 4.3 | 64.4 ± 4.8 | 0.30 | 65.6 ± 5.1 | 0.56 |
| Education (yr) | 16.6 ± 2.4 | 15.8 ± 2.3 | 17.3 ± 2.6 | 0.11 | 16.3 ± 2.0 | 0.20 |
| Age began HT | 47.7 ± 4.0 | 44.8 ± 3.5 | 49.4 ± 3.3 | 0.00 | ||
| Years on HT | 15.3 ± 5.6 | 18.6 ± 6.3 | 13.3 ± 4.1 | 0.01 | ||
| Mini-Mental State Examination | 28.6 ± 1.6 | 28.3 ± 1.8 | 28.8 ± 1.3 | 0.34 | 28.8 ± 1.9 | 0.65 |
| Shipley Estimated IQ | 114.3 ± 9.2 | 110.2 ± 9.7 | 113.7 ± 8.6 | 0.29 | 118.3 ± 8.3 | 0.06 |
| Geriatric Depression Rating Scale | 0.7 ± 0.9 | 1.1 ± 1.3 | 0.5 ± 0.8 | 0.10 | 0.6 ± 0.8 | 0.19 |
t-Test comparison between ET and EPT groups.
ANOVA comparison between all three treatment groups.
Table 2.
Demographic and baseline information for current and past HT users
| Current users (mean ± sd) | Past users (mean ± sd) | t-Testa (P) | |
|---|---|---|---|
| n | 11 | 23 | |
| Age (yr) | 63.7 ± 4.0 | 65.7 ± 4.9 | 0.24 |
| Education (yr) | 15.5 ± 2.7 | 17.3 ± 2.3 | 0.06 |
| Age began HT | 46.8 ± 4.5 | 48.1 ± 3.8 | 0.40 |
| Years on HT | 15.1 ± 6.4 | 15.4 ± 5.4 | 0.87 |
| Mini-Mental State Examination | 28.4 ± 1.8 | 28.7 ± 1.3 | 0.66 |
| Shipley Estimated IQ | 111.8 ± 10.6 | 112.7 ± 8.5 | 0.80 |
| Geriatric Depression Rating Scale | 0.6 ± 1.1 | 0.7 ± 1.0 | 0.72 |
| Time since HT was stopped (yr) | 2.2 ± 1.0 |
t-Test comparison between current and past hormone users.
Significant differences in cholinergic activity measures between the three groups were detected by ANOVA in the hippocampus and posterior cingulate (Table 3 and Fig. 1). Post hoc testing with Tukey revealed higher cholinergic activity in the EPT group compared with the no-HT group in the left hippocampus (P = 0.048) and posterior cingulate (P = 0.045), with a trend in the same direction for the right hippocampus (P = 0.073) (Fig. 2). No significant differences in cholinergic activity were observed for the reference region (Table 3). t-Test comparisons of current and former HT groups revealed no significant differences in any of these regions. In a correlational analysis that included all subjects, age was not related to regional cholinergic activity. Additionally, in the hormone group, there was no association identified between age of hormone initiation and regional cholinergic activity.
Table 3.
Average AChE activity with PMP PET
| Region | Mean ± sd |
P |
||||||
|---|---|---|---|---|---|---|---|---|
| All subjects PMP | ET PMP | EPT PMP | No-HT PMP | EPT vs. no-HTa | ET vs. no-HTa | ET vs. EPTb | ANOVAc | |
| n | 50 | 13 | 21 | 16 | ||||
| Left hippocampus | 0.047 ± 0.005 | 0.046 ± 0.006 | 0.049 ± 0.004 | 0.045 ± 0.005 | 0.048 | 0.954 | 0.121 | 0.036 |
| Right hippocampus | 0.043 ± 0.005 | 0.041 ± 0.006 | 0.045 ± 0.005 | 0.041 ± 0.005 | 0.073 | 1.000 | 0.123 | 0.041 |
| Posterior cingulate | 0.028 ± 0.002 | 0.027 ± 0.003 | 0.029 ± 0.002 | 0.027 ± 0.003 | 0.045 | 0.974 | 0.024 | 0.031 |
| Reference region | 0.034 ± 0.003 | 0.033 ± 0.005 | 0.035 ± 0.003 | 0.034 ± 0.002 | 0.430 | 0.872 | 0.086 | 0.205 |
PMP measure is k3 (min−1).
Tukey honestly significant difference post hoc test.
ANCOVA controlling for years on HT.
ANOVA comparison between all three treatment groups.
Fig. 1.
Brain images of AChE activity (k3 values, min−1) in the selected regions of interest.
Fig. 2.
ET, EPT, and no-HT group comparisons of AChE activity.
Given that there were differences in cholinergic activity between groups and differences in years of total HT between the two hormone groups (ET and EPT), ANCOVA was performed to see whether this measure of estrogen exposure accounted for the regional cholinergic differences. Therefore, a final ANCOVA analysis included all three subject groups and years on HT as covariate, with results maintaining significant effects of treatment in the posterior cingulate (P = 0.024).
Discussion
The present report describes relationships between early initiation, long-term use of HT, and cholinergic activity, as well as the differential effects of conjugated equine estrogens and combination conjugated equine estrogens/medroxyprogesterone acetate. We identified an 8–10% greater AChE activity in the hippocampus and posterior cingulate in the EPT group compared with the no-HT group, consistent with a preservation of cholinergic neuronal integrity in that sample.
There are a number of mechanisms through which gonadal steroids may maintain cognitive function. In animal models, estrogen has been shown to affect neuronal function by modulating neurotransmission, acting as a neuroprotectant and antioxidant, increasing neurite branching and synaptogenesis, increasing cerebral blood flow, regulating β-amyloid production, and affecting trophic factors (27, 53–59). Recently, it was reported that estradiol and progesterone are potent regulators of mitochondrial function in the brain (60). Estradiol interacts with IGF-I to activate neuronal survival pathways (61), and activation of either ERα or ERβ may promote neuroprotection (62). Furthermore, genomic studies suggest that polymorphisms of ERα increase the risk of cognitive impairment (63), and that an interaction may exist between ERα polymorphisms and ApoE alleles in conferring this increased risk (64). Thus, estrogen may impact brain function through multiple mechanisms.
In this study, we focused on the effect of HT on the cholinergic neurotransmitter system. We used AChE activity to measure cholinergic neuronal function (40–42). Our finding of a positive effect of hormone use on cholinergic activity is consistent with both human and animal studies. An effect of postmenopausal HT on cholinergic function is supported by data showing trophic effects of estrogen and progesterone on cortical cholinergic neurons in nonhuman primates (31) and prior studies showing similar effects in cultured hippocampal cells (28). More recently, in ovariectomized monkeys, long-term estrogen replacement (2 yr) preserved cholinergic fibers in the prefrontal cortex, whereas placebo treatment resulted in a significant decrease (29). In addition, PET studies in primates using [18F]fluorobenzyltrozamicol, a cholinergic radiotracer selective for the vesicular acetylcholine transporter, have shown a reduction in transporter concentrations (reflecting cholinergic presynaptic density) in the monkey striatum 3 yr after ovariectomy and sustained increases in binding after initiating ET (66). Furthermore, a SPECT study of human brain muscarinic receptor concentrations demonstrated increased receptor availability in the hippocampus with ET (38).
Contrary to our previous pilot study (39) in which we were unable to detect a difference between the HT and no-HT groups, here we identified increased cholinergic activity in the EPT group, compared with both the no-HT and ET groups. In the present study, the ET group had an onset of menopause (after hysterectomy and oophorectomy in all cases) that was earlier than that of the no-HT and EPT groups. This may have influenced cholinergic functional integrity by reducing natural exposure to gonadal steroids.
Additionally, a neurochemical explanation may relate to differing neural growth actions of estrogen and progesterone. Estrogen-inducible progesterone receptors are present in the hippocampus (68). Studies report contradictory effects of progesterone on brain areas critical for cognition (69). Some data suggest a beneficial effect of progesterone (70–72). Goodman et al. (72) showed that progesterone reduced neuronal vulnerability to excitotoxic, metabolic, and oxidative injuries. However, other studies report down-regulation of dendritic spine growth when estrogen is combined with progesterone (73, 74), reversal of estrogen-induced increases in neurotrophins by progesterone (73, 75), and induction of spatial memory deficits in rat and human models (76, 77). Likewise, studies demonstrate that the synthetic progestin medroxyprogesterone acetate does not provide neuroprotection from excitotoxicity (78) and impairs memory in ovariectomized rats (79).
The posterior cingulate cortex, noted in this study to have increased cholinergic density in the EPT group, has been identified as a brain region involved in very early stages of AD. This is an associative cortical region with connections to the hippocampal formation and other brain areas involved in cognitive processing (80–85). It has been implicated in both the encoding and retrieval of episodic memory (86–89) and is thought to link verbal and nonverbal information with prior knowledge (90–93). Profound reductions in glucose metabolism in this region have been identified in patients who presented with isolated memory impairments and later developed AD (94). Furthermore, decreased glucose metabolism in the posterior cingulate, as well as in the parietal, temporal, and frontal cortices, is present in established AD (95, 96).
In a study of functional connectivity of the hippocampus during short-term memory tasks in young and older populations with PET and a regional cerebral blood flow marker, the posterior cingulate was found to be differentially activated as a function of age. The younger group activated a neural network that included the prefrontal cortex, fusiform gyrus and posterior cingulate, whereas the older subjects activated more anterior regions, but not the posterior cingulate (97). Furthermore, an effect of estrogen in this and other cortical brain regions has been demonstrated during working memory tasks in a placebo-controlled fMRI study. Specifically, the administration of conjugated equine estrogens for 21 d in postmenopausal women was associated with increased prefrontal cortical and posterior cingulate activation during the retrieval component of a working memory task (98). These data suggest that the posterior cingulate cortex undergoes functional changes during the aging process that are influenced by estrogen and have cognitive implications.
The hippocampus, a region central to cognitive function, displayed higher cholinergic activity in hormone users in this study. In AD and mild cognitive impairment, hippocampal changes are prominent and include hypometabolism, morphological alterations, neuronal atrophy, and decreased volume (99–110). In women with AD, estradiol levels in the cerebral spinal fluid are positively correlated with glucose metabolism in the hippocampus (111). Furthermore, in healthy postmenopausal women, estrogen use has been associated with greater gray matter volumes in the hippocampus, amygdala, and multiple cortical areas (112, 113). This hippocampal effect has also been noted for postmenopausal women with susceptible ApoE genes and with HT users having higher hippocampal volumes compared with controls (114). Furthermore, the use of tamoxifen, a selective estrogen receptor modulator that lowers estrogen activity, has been associated with smaller hippocampal volumes compared with women on ET (115).
The timing of onset of HT may be an important factor in neuroprotection (11–13). Our findings, in subjects selected for early initiation of hormone use, show increased cholinergic activity in brain areas critical for cognition (hippocampus and posterior cingulate) and support this critical window concept. Data from the Research into Memory, Brain Function and Estrogen Replacement (REMEMBER) pilot also indicate that early HT initiation may benefit some cognitive domains (global cognition, attention and concentration, and verbal expression), whereas late initiation may be detrimental to cognition (18). Future studies need to examine the relationship between measures of cholinergic integrity and neuropsychological testing data of relevant cognitive domains.
Our study included women with both early initiation and long-term hormone use; therefore we are unable to separate their individual effects. Neural effects of long-term hormone use are less clear in the existing literature but point toward benefits primarily with use in the earlier menopausal years (113, 116, 117). The present cross-sectional study design was also limited by the lack of prospective randomization between the HT and no-HT groups, which may allow for selection biases, and by the lack of longitudinal follow-up to assess cognitive impact. However, effects of early initiation and long-term hormone use are difficult to study in randomized, longitudinal designs because of the length of treatment time required. In addition, this design relies on patient recall on initiation of use, which may not be entirely accurate. Although HT users generally tend to be healthier and more educated (65, 67, 118), our groups were matched for education level and had no major medical illnesses. Furthermore, our hormone group included both current and past hormone users; however, these groups were similar in demographics and the average time off of HT was short (2.2 yr).
In summary, a differential effect of ET and EPT on cholinergic neuronal integrity was identified in postmenopausal women. We found that HT, particularly EPT, influences the survival or plasticity of cholinergic cells in postmenopausal women. Further understanding of the actions of estrogens and progestins in the brain will facilitate the development of individualized HT regimens, appropriate alternatives to standard HT, and medications targeted to prevent cognitive aging.
Acknowledgments
We thank the Michigan Clinical Research Unit and the fMRI laboratory at the University of Michigan for their assistance. We thank Anne Tkaczyk for study recruitment and coordination. We also thank the investigators from the Jagust Lab at the University of California, Berkley, and the Imaging of Dementia and Aging Lab at the University of California, Davis, for providing the region of interest templates. We especially thank the participants of our study.
This work was supported by the National Center for Research Resources (Grants K23 RR17043 and UL1RR024896), and, for investigator support, by the National Institute for Child Health and Human Development (Grant 5T32HD007048), the National Institute on Aging and the Office for Research on Women's Health (Grant RO1AG027675), the University of Michigan's Postdoctoral Translational Scholars Program award, and the Phil F. Jenkins Foundation.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AChE
- Acetylcholinesterase
- AD
- Alzheimer's disease
- ANCOVA
- analysis of covariance
- [11C]PMP
- N-[11C]methylpiperidin-4-yl propionate
- EPT
- estrogen plus progestin therapy
- ET
- estrogen therapy
- fMRI
- functional magnetic resonance imaging
- HT
- hormone therapy
- MR
- magnetic resonance
- MRI
- MR imaging
- PET
- positron emission tomography
- SPECT
- single photon emission computed tomography
- VOI
- volume of interest.
References
- 1. Low LF, Anstey KJ. 2006. Hormone replacement therapy and cognitive performance in postmenopausal women—review by cognitive domain. Neurosci Biobehav Rev 30:66–84 [DOI] [PubMed] [Google Scholar]
- 2. Maki PM. 2005. A systematic review of clinical trials of hormone therapy on cognitive function: effects of age at initiation and progestin use. Ann NY Acad Sci 1052:182–197 [DOI] [PubMed] [Google Scholar]
- 3. Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH; Women's Health Initiative Memory Study 2004. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA 291:2947–2958 [DOI] [PubMed] [Google Scholar]
- 4. Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. 2003. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA 289:2651–2662 [DOI] [PubMed] [Google Scholar]
- 5. Coker LH, Espeland MA, Rapp SR, Legault C, Resnick SM, Hogan P, Gaussoin S, Dailey M, Shumaker SA. 2010. Postmenopausal hormone therapy and cognitive outcomes: the Women's Health Initiative Memory Study (WHIMS). J Steroid Biochem Mol Biol 118:304–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R. 1996. Effect of oestrogen during menopause on risk and age at onset of Alzheimer's disease. Lancet 348:429–432 [DOI] [PubMed] [Google Scholar]
- 7. Kawas C, Resnick S, Morrison A, Brookmeyer R, Corrada M, Zonderman A, Bacal C, Lingle DD, Metter E. 1997. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer's disease. The Baltimore Longitudinal Study of Aging. Neurology 48:1517–1521 [DOI] [PubMed] [Google Scholar]
- 8. Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JC, Cache County Memory Study Investigators 2002. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA 288:2123–2129 [DOI] [PubMed] [Google Scholar]
- 9. Resnick SM, Maki PM, Rapp SR, Espeland MA, Brunner R, Coker LH, Granek IA, Hogan P, Ockene JK, Shumaker SA. 2006. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J Clin Endocrinol Metab 91:1802–1810 [DOI] [PubMed] [Google Scholar]
- 10. Resnick SM, Espeland MA, An Y, Maki PM, Coker LH, Jackson R, Stefanick ML, Wallace R, Rapp SR; Women's Health Initiative Study of Cognitive Aging Investigators 2009. Effects of conjugated equine estrogens on cognition and affect in postmenopausal women with prior hysterectomy. J Clin Endocrinol Metab 94:4152–4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gibbs RB, Gabor R. 2003. Estrogen and cognition: applying preclinical findings to clinical perspectives. J Neurosci Res 74:637–643 [DOI] [PubMed] [Google Scholar]
- 12. Sherwin BB. 2005. Estrogen and memory in women: how can we reconcile the findings? Horm Behav 47:371–375 [DOI] [PubMed] [Google Scholar]
- 13. Maki PM. 2006. Hormone therapy and cognitive function: is there a critical period for benefit? Neuroscience 138:1027–1030 [DOI] [PubMed] [Google Scholar]
- 14. Silva I, Mello LE, Freymüller E, Haidar MA, Baracat EC. 2003. Onset of estrogen replacement has a critical effect on synaptic density of CA1 hippocampus in ovariectomized adult rates. Menopause 10:406–411 [DOI] [PubMed] [Google Scholar]
- 15. Gibbs RB. 2000. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging 21:107–116 [DOI] [PubMed] [Google Scholar]
- 16. Rapp PR, Morrison JH, Roberts JA. 2003. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci 23:5708–5714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bagger YZ, Tankó LB, Alexandersen P, Qin G, Christiansen C. 2005. Early postmenopausal hormone therapy may prevent cognitive impairment late in life. Menopause 12:12–17 [DOI] [PubMed] [Google Scholar]
- 18. MacLennan AH, Henderson VW, Paine BJ, Mathias J, Ramsay EN, Ryan P, Stocks NP, Taylor AW. 2006. Hormone therapy, timing of initiation, and cognition in women aged older than 60 years: the REMEMBER pilot study. Menopause 13:28–36 [DOI] [PubMed] [Google Scholar]
- 19. Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. 1982. The cholinergic hypothesis of geriatric memory dysfunction. Science 217:408–414 [DOI] [PubMed] [Google Scholar]
- 20. Perry EK, Johnson M, Kerwin JM, Piggott MA, Court JA, Shaw PJ, Ince PG, Brown A, Perry RH. 1992. Convergent cholinergic activities in aging and Alzheimer's disease. Neurobiol Aging 13:393–400 [DOI] [PubMed] [Google Scholar]
- 21. Osterlund MK, Gustafsson JA, Keller E, Hurd YL. 2000. Estrogen receptor β (ERβ) messenger ribonucleic acid (mRNA) expression within the human forebrain: distinct distribution pattern to ERα mRNA. J Clin Endocrinol Metab 85:3840–3846 [DOI] [PubMed] [Google Scholar]
- 22. Toran-Allerand CD. 1996. The estrogen/neurotrophin connection during neural development: is co-localization of estrogen receptors with the neurotrophins and their receptors biologically relevant? Dev Neurosci 18:36–48 [DOI] [PubMed] [Google Scholar]
- 23. McMillan PJ, Singer CA, Dorsa DM. 1996. The effects of ovariectomy and estrogen replacement on trkA and choline acetyltransferase mRNA expression in the basal forebrain of the adult female Sprague-Dawley rat. J Neurosci 16:1860–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gibbs RB, Hashash A, Johnson DA. 1997. Effects of estrogen on potassium-stimulated acetylcholine release in the hippocampus and overlying cortex of adult rats. Brain Res 749:143–146 [DOI] [PubMed] [Google Scholar]
- 25. Luine V, Park D, Joh T, Reis D, McEwen B. 1980. Immunochemical demonstration of increased choline acetyltransferase concentration in rat preoptic area after estradiol administration. Brain Res 191:273–277 [DOI] [PubMed] [Google Scholar]
- 26. Ping SE, Trieu J, Wlodek ME, Barrett GL. 2008. Effects of estrogen on basal forebrain cholinergic neurons and spatial learning. J Neurosci Res 86:1588–1598 [DOI] [PubMed] [Google Scholar]
- 27. Singh M, Meyer EM, Simpkins JW. 1995. The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid in cortical and hippocampal brain regions of female Sprague-Dawley rats. Endocrinology 136:2320–2324 [DOI] [PubMed] [Google Scholar]
- 28. McEwen BS, Alves SE, Bulloch K, Weiland NG. 1997. Ovarian steroids and the brain: implications for cognition and aging. Neurology 48(5 Suppl 7):S8–S15 [DOI] [PubMed] [Google Scholar]
- 29. Tinkler GP, Tobin JR, Voytko ML. 2004. Effects of two years of estrogen loss or replacement on nucleus basalis cholinergic neurons and cholinergic fibers to the dorsolateral prefrontal and inferior parietal cortex of monkeys. J Comp Neurol 469:507–521 [DOI] [PubMed] [Google Scholar]
- 30. Tinkler GP, Voytko ML. 2005. Estrogen modulates cognitive and cholinergic processes in surgically menopausal monkeys. Prog Neuropsychopharmacol Biol Psychiatry 29:423–431 [DOI] [PubMed] [Google Scholar]
- 31. Kritzer MF, Kohama SG. 1999. Ovarian hormones differentially influence immunoreactivity for dopamine β-hydroxylase, choline acetyltransferase, and serotonin in the dorsolateral prefrontal cortex of adult rhesus monkeys. J Comp Neurol 409:438–451 [DOI] [PubMed] [Google Scholar]
- 32. Craig MC, Fletcher PC, Daly EM, Rymer J, Brammer M, Giampietro V, Stahl D, Maki PM, Murphy DG. 2009. The interactive effect of the cholinergic system and acute ovarian suppression. Horm Behav 55:41–49 [DOI] [PubMed] [Google Scholar]
- 33. Dumas J, Hancur-Bucci C, Naylor M, Sites C, Newhouse P. 2006. Estrogen treatment effects on anticholinergic-induced cognitive dysfunction in normal postmenopausal women. Neuropsychopharmacology 31:2065–2078 [DOI] [PubMed] [Google Scholar]
- 34. Gibbs RB, Burke AM, Johnson DA. 1998. Estrogen replacement attenuates effects of scopolamine and lorazepam on memory acquisition and retention. Horm Behav 34:112–125 [DOI] [PubMed] [Google Scholar]
- 35. Dumas J, Hancur-Bucci C, Naylor M, Sites C, Newhouse P. 2008. Estradiol interacts with the cholinergic system to affect verbal memory in postmenopausal women: evidence for the critical period hypothesis. Horm Behav 53:159–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Acosta JI, Mayer L, Talboom JS, Zay C, Scheldrup M, Castillo J, Demers LM, Enders CK, Bimonte-Nelson HA. 2009. Premarin improves memory, prevents scopolamine-induced amnesia and increases number of basal forebrain choline acetyltransferase positive cells in middle-aged surgically menopausal rats. Horm Behav 55:454–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bartholomeusz CF, Wesnes KA, Kulkarni J, Vitetta L, Croft RJ, Nathan PJ. 2008. Estradiol treatment and its interaction with the cholinergic system: effects on cognitive function in healthy young women. Horm Behav 54:684–693 [DOI] [PubMed] [Google Scholar]
- 38. Norbury R, Travis MJ, Erlandsson K, Waddington W, Ell PJ, Murphy DG. 2007. Estrogen therapy and brain muscarinic receptor density in healthy females: a SPET study. Horm Behav 51:249–257 [DOI] [PubMed] [Google Scholar]
- 39. Smith YR, Minoshima S, Kuhl DE, Zubieta JK. 2001. Effects of long-term hormone replacement therapy on cholinergic synaptic concentrations in healthy postmenopausal women. J Clin Endocrinol Metab 86:679–684 [DOI] [PubMed] [Google Scholar]
- 40. Kuhl DE, Koeppe RA, Minoshima S, Snyder SE, Ficaro EP, Foster NL, Frey KA, Kilbourn MR. 1999. In vivo mapping of cerebral acetylcholinesterase activity in aging and Alzheimer's disease. Neurology 52:691–699 [DOI] [PubMed] [Google Scholar]
- 41. Kilbourn MR, Snyder SE, Sherman PS, Kuhl DE. 1996. In vivo studies of acetylcholinesterase activity using a labeled substrate, N-[C11]methylpiperidin-4-yl propionate ([C11]PMP). Synapse 22:123–131 [DOI] [PubMed] [Google Scholar]
- 42. Snyder SE, Tluczek L, Jewett DM, Nguyen TB, Kuhl DE, Kilbourn MR. 1998. Synthesis of 1-[C11]methylpiperidin-4-yl propionate ([C11]PMP for in vivo measurements of acetylcholinesterase activity. Nucl Med Biol 25:751–754 [DOI] [PubMed] [Google Scholar]
- 43. Berent-Spillson A, Persad CC, Love T, Tkaczyk A, Wang H, Reame NK, Frey KA, Zubieta JK, Smith YR. 2010. Early menopausal hormone use influences brain regions used for visual working memory. Menopause 17:692–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Folstein MF, Folstein SE, McHugh PR. 1975. “Mini-mental state”. J Psychiatr Res 12:189–198 [DOI] [PubMed] [Google Scholar]
- 45. Shipley WC. 1946. Institute of living scale. Los Angeles: Western Psychological Services [Google Scholar]
- 46. Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. 1982. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 17:37–49 [DOI] [PubMed] [Google Scholar]
- 47. Minoshima S, Koeppe RA, Mintun MA, Berger KL, Taylor SF, Frey KA, Kuhl DE. 1993. Automated detection of the intercommissural line for stereotactic localization of functional brain images. J Nucl Med 34:322–329 [PubMed] [Google Scholar]
- 48. Irie T, Fukushi K, Akimoto Y, Tamagami H, Nozaki T. 1994. Design and evaluation of radioactive acetylcholine analogs for mapping brain acetylcholinesterase (AChE) in vivo. Nucl Med Biol 21:801–808 [DOI] [PubMed] [Google Scholar]
- 49. Koeppe RA, Frey KA, Snyder SE, Meyer P, Kilbourn MR, Kuhl DE. 1999. Kinetic modeling of N-[11C]methylpiperidin-4-yl propionate: alternatives for analysis of an irreversible positron emission tomography tracer for measurement of acetylcholinesterase activity in human brain. J Cereb Blood Flow Metab 19:1150–1163 [DOI] [PubMed] [Google Scholar]
- 50. Nagatsuka Si S, Fukushi K, Shinotoh H, Namba H, Iyo M, Tanaka N, Aotsuka A, Ota T, Tanada S, Irie T. 2001. Kinetic analysis of [11C]MP4A using a high-radioactivity brain region that represents an integrated input function for measurement of cerebral acetylcholinesterase activity without arterial blood sampling. J Cereb Blood Flow Metab 21:1354–1366 [DOI] [PubMed] [Google Scholar]
- 51. Sun FT, Schriber RA, Greenia JM, He J, Gitcho A, Jagust WJ. 2007. Automated template-based PET region of interest analyses in the aging brain. NeuroImage 34:608–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Koeppe RA, Gilman S, Joshi A, Liu S, Little R, Junck L, Heumann M, Frey KA, Albin RL. 2005. 11C-DTBZ and 18F-FDG PET measures in differentiating dementias. J Nucl Med 46:936–944 [PubMed] [Google Scholar]
- 53. Toran-Allerand CD, Miranda RC, Bentham WD, Sohrabji F, Brown TJ, Hochberg RB, MacLusky NJ. 1992. Estrogen receptors colocalize with low-affinity nerve growth factor growth factor receptors in cholinergic neurons of the basal forebrain. Proc Natl Acad Sci USA 89:4668–4672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brinton RD, Proffitt P, Tran J, Luu R. 1997. Equilin, a principal component of the estrogen replacement therapy Premarin, increases the growth of cortical neurons via an NMDA receptor-mediated mechanism. Exp Neurol 147:211–220 [DOI] [PubMed] [Google Scholar]
- 55. Hao J, Janssen WG, Tang Y, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. 2003. Estrogen increases the number of spinophilin-immunoreactive spines in the hippocampus of young and aged female rhesus monkeys. J Comp Neurol 465:540–550 [DOI] [PubMed] [Google Scholar]
- 56. Petanceska SS, Nagy V, Frail D, Gandy S. 2000. Ovariectomy and 17β-estradiol modulate the levels of Alzheimer's amyloid β peptides in brain. Neurology 54:2212–2217 [DOI] [PubMed] [Google Scholar]
- 57. Schönknecht P, Pantel J, Klinga K, Jensen M, Hartmann T, Salbach B, Schröder J. 2001. Reduced cerebrospinal fluid estradiol levels are associated with increased amyloid levels in female patients with Alzheimer's disease. Neurosci Lett 307:122–124 [DOI] [PubMed] [Google Scholar]
- 58. Savaskan E, Olivieri G, Meier F, Ravid R, Müller-Spahn F. 2001. Hippocampal estrogen-receptor immunoreactivity is increased in Alzheimer's disease. Brain Research 908:113–119 [DOI] [PubMed] [Google Scholar]
- 59. Suzuki S, Brown CM, Wise PM. 2006. Mechanisms of neuroprotection by estrogen. Endocrine 29:209–215 [DOI] [PubMed] [Google Scholar]
- 60. Irwin RW, Yao J, Hamilton RT, Cadenas E, Brinton RD, Nilsen J. 2008. Progesterone and estrogen regulate oxidative metabolism in brain mitochondria. Endocrinology 149:3167–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Garcia-Segura LM, Diz-Chaves Y, Perez-Martin M, Darnaudery M. 2007. Estradiol, insulin-like growth factor-I and brain aging. Psychoneuroendocrinology 32(Suppl 1):S57–S61 Please confirm or correct revised journal name, supplement number, page range added to Ref. 61 [DOI] [PubMed] [Google Scholar]
- 62. Zhao L, Wu TW, Brinton RD. 2004. Estrogen receptor subtypes α and β contribute to neuroprotection and increased Bci-2 expression in primary hippocampal neurons. Brain Res 1010:22–34 [DOI] [PubMed] [Google Scholar]
- 63. Yaffe K, Lui LY, Grady D, Stone K, Morin P. 2002. Estrogen receptor 1 polymorphisms and risk of cognitive impairment in older women. Biol Psychiatry 51:677–682 [DOI] [PubMed] [Google Scholar]
- 64. Mattila KM, Axelman K, Rinne JO, Blomberg M, Lehtimäki T, Laippala P, Röyttä M, Viitanen M, Wahlund L, Winblad B, Lannfelt L. 2000. Interaction between estrogen receptor 1 and the ε4 allele of apolipoprotein E increases the risk of familial Alzheimer's disease in women. Neurosci Lett 282:45–48 [DOI] [PubMed] [Google Scholar]
- 65. Cauley JA, Cummings SR, Black DM, Mascioli SR, Seeley DG. 1990. Prevalence and determinants of estrogen replacement therapy in elderly women. Am J Obstet Gynecol 163:1438–1444 [DOI] [PubMed] [Google Scholar]
- 66. Voytko ML, Tinkler GP. 2004. Cognitive function and its neural mechanisms in nonhuman primate models of aging. Alzheimer's disease, and menopause. Front Biosci 9:1899–1914 [DOI] [PubMed] [Google Scholar]
- 67. Egeland GM, Kuller LH, Matthews KA, Kelsey SF, Cauley J, Guzick D. 1991. Premenopausal determinants of menopausal estrogen use. Prev Med 20:343–349 [DOI] [PubMed] [Google Scholar]
- 68. Parsons B, Rainbow TC, MacLusky NJ, McEwen BS. 1982. Progestin receptor levels in rat hypothalamic and limbic nuclei. J Neurosci 2:1446–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nilsen J, Brinton RD. 2002. Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology 143:205–212 [DOI] [PubMed] [Google Scholar]
- 70. Gonzalez Deniselle MC, Lopez Costa JJ, Gonzalez SL, Labombarda F, Garay L, Guennoun R, Schumacher M, De Nicola AF. 2002. Basis of progesterone protection in spinal cord neurodegeneration. J Steroid Biochem Mol Biol 83:199–209 [DOI] [PubMed] [Google Scholar]
- 71. Singh M. 2006. Progesterone-induced neuroprotection. Endocrine 29:271–274 [DOI] [PubMed] [Google Scholar]
- 72. Goodman Y, Bruce AJ, Cheng B, Mattson MP. 1996. Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury, and amyloid β-peptide toxicity in hippocampal neurons. J Neurochem 66:1836–1844 [DOI] [PubMed] [Google Scholar]
- 73. McEwen BS, Woolley CS. 1994. Estradiol and progesterone regulate neuronal structure and synaptic connectivity in adult as well as developing brain. Exp Gerontol 29:431–436 [DOI] [PubMed] [Google Scholar]
- 74. Woolley CS, McEwen BS. 1993. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol 336:293–306 [DOI] [PubMed] [Google Scholar]
- 75. Bimonte-Nelson HA, Nelson ME, Granholm AC. 2004. Progesterone counteracts estrogen-induced increase in neurotrophins in the aged female rat brain. NeuroReport 15:2659–2663 [DOI] [PubMed] [Google Scholar]
- 76. Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm AC. 2006. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur J Neurosci 24:229–242 [DOI] [PubMed] [Google Scholar]
- 77. Bimonte-Nelson HA, Singleton RS, Williams BJ, Granholm AC. 2004. Ovarian hormones and cognition in the aged female rat. II. Progesterone supplementation reverses the cognitive enhancing effect of ovariectomy. Behav Neurosci 118:707–714 [DOI] [PubMed] [Google Scholar]
- 78. Ciriza I, Carrero P, Frye CA, Garcia-Segura LM. 2006. Reduced metabolites mediate neuroprotective effects of progesterone in the adult rat hippocampus. The synthetic progestin medroxyprogesterone acetate (Provera) is not neuroprotective. J Neurobiol 66:916–928 [DOI] [PubMed] [Google Scholar]
- 79. Braden BB, Talboom JS, Crain ID, Simard AR, Lukas RJ, Prokai L, Scheldrup MR, Bowman BL, Bimonte-Nelson HA. 2010. Medroxyprogesterone acetate impairs memory and alters the GABAergic system in aged surgically menopausal rats. Neurobiol Learn Mem 93:444–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Baleydier C, Mauguiere F. 1980. The duality of the cingulate gyrus in monkey. Neuroanatomical study and functional hypothesis. Brain 103:525–554 [DOI] [PubMed] [Google Scholar]
- 81. Sutherland RJ, Whishaw IQ, Kolb B. 1988. Contributions of cingulate cortex to two forms of spatial learning and memory. J Neurosci 8:1863–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Meunier M, Destrade C. 1988. Electrolytic but not ibotenic acid lesions of the posterior cingulate cortex produce transitory facilitation of learning in mice. Behav Brain Res 27:161–172 [DOI] [PubMed] [Google Scholar]
- 83. Sif J, Meunier M, Messier C, Calas A, Destrade C. 1989. Quantitative 2-deoxyglucose study of a functional dissociation between anterior and posterior cingulate cortices in mice. Neurosci Lett 101:223–228 [DOI] [PubMed] [Google Scholar]
- 84. Vogt BA, Finch DM, Olson CR. 1992. Functional heterogeneity in cingulate cortex; the anterior executive and posterior evaluative regions. Cereb Cortex 2:435–443 [DOI] [PubMed] [Google Scholar]
- 85. Yukie M. 1995. Neural connections of auditory association cortex with the posterior cingulate cortex in the monkey. Neurosci Res 22:179–187 [DOI] [PubMed] [Google Scholar]
- 86. Desgranges B, Baron JC, Eustache F. 1998. The functional neuroanatomy of episodic memory: the role of the frontal lobes, the hippocampal formation, and other areas. NeuroImage 8:198–213 [DOI] [PubMed] [Google Scholar]
- 87. Shallice T, Fletcher P, Frith CD, Grasby P, Frackowiak RS, Dolan RJ. 1994. Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature 368:633–635 [DOI] [PubMed] [Google Scholar]
- 88. Petrides M, Alivisatos B, Evans AC. 1995. Functional activation of the human ventrolateral prefrontal cortex during mnemonic retrieval of verbal information. Proc Natl Acad Sci USA 92:5803–5807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Owen AM, Milner B, Petrides M, Evans AC. 1996. Memory for object features versus memory for object location: a positron-emission tomography study of encoding and retrieval processes. Proc Natl Acad Sci USA 93:9212–9217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Maguire EA, Frith CD, Morris RG. 1999. The functional neuroanatomy of comprehension and memory: the importance of prior knowledge. Brain 122:1839–1850 [DOI] [PubMed] [Google Scholar]
- 91. Henson RN, Rugg MD, Shallice T, Josephs O, Dolan RJ. 1999. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. J Neurosci 19:3962–3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Düzel E, Cabeza R, Picton TW, Yonelinas AP, Scheich H, Heinze HJ, Tulving E. 1999. Task-related and item-related brain processes of memory retrieval. Proc Natl Acad Sci USA 96:1794–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kim JJ, Andreasen NC, O'Leary DS, Wiser AK, Ponto LL, Watkins GL, Hichwa RD. 1999. Direct comparison of the neural substrates of recognition memory for words and faces. Brain 122:1069–1083 [DOI] [PubMed] [Google Scholar]
- 94. Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. 1997. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer's disease. Ann Neurol 42:85–94 [DOI] [PubMed] [Google Scholar]
- 95. Small GW, Kuhl DE, Riege WH, Fujikawa DG, Ashford JW, Metter EJ, Mazziotta JC. 1989. Cerebral glucose metabolic patterns in Alzheimer's disease. Effect of gender and age at dementia onset. Arch Gen Psychiatry 46:527–532 [DOI] [PubMed] [Google Scholar]
- 96. Kuhl DE, Minoshima S, Fessler JA, Frey KA, Foster NL, Ficaro EP, Wieland DM, Koeppe RA. 1996. In vivo mapping of cholinergic terminals in normal aging, Alzheimer's disease and Parkinson's disease. Ann Neurol 40:399–410 [DOI] [PubMed] [Google Scholar]
- 97. Della-Maggiore V, Sekuler AB, Grady CL, Bennett PJ, Sekuler R, McIntosh AR. 2000. Corticolimbic interactions associated with performance on a short-term memory task are modified by age. J Neurosci 20:8410–8416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Naftolin F, Palter SF, Marchione KE, Katz L, Shankweiler DP, Fletcher JM, Lacadie C, Keltz M, Gore JC. 1999. Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. JAMA 281:1197–1202 [DOI] [PubMed] [Google Scholar]
- 99. Mosconi L, Tsui WH, De Santi S, Li J, Rusinek H, Convit A, Li Y, Boppana M, de Leon MJ. 2005. Reduced hippocampal metabolism in MCI and AD: automated FDG-PET image analysis. Neurology 64:1860–1867 [DOI] [PubMed] [Google Scholar]
- 100. Ouchi Y, Nobezawa S, Okada H, Yoshikawa E, Futatsubashi M, Kaneko M. 1998. Altered glucose metabolism in the hippocampal head in memory impairment. Neurology 51:136–142 [DOI] [PubMed] [Google Scholar]
- 101. de Leon MJ, McRae T, Rusinek H, Convit A, De Santi S, Tarshish C, Golomb J, Volkow N, Daisley K, Orentreich N, McEwen B. 1997. Cortisol reduces hippocampal glucose metabolism in normal elderly but not in Alzheimer's disease. J Clin Endocrinol Metab 82:3251–3259 [DOI] [PubMed] [Google Scholar]
- 102. de Leon MJ, Convit A, Wolf OT, Tarshish CY, DeSanti S, Rusinek H, Tsui W, Kandil E, Scherer AJ, Roche A, Imossi A, Thorn E, Bobinski M, Caraos C, Lesbre P, Schlyer D, Poirier J, Reisberg B, Fowler J. 2001. Prediction of cognitive decline in normal elderly subjects with 2-[18F]fluoro-2-deoxy-D-glucose/positron emission tomography (FDG/PET). Proc Natl Acad Sci USA 98:10966–10971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. De Santi S, de Leon MJ, Rusinek H, Convit A, Tarshish CY, Roche A, Tsui WH, Kandil E, Boppana M, Daisley K, Wang GJ, Schlyer D, Fowler J. 2001. Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiol Aging 22:529–539 [DOI] [PubMed] [Google Scholar]
- 104. Nestor PJ, Fryer TD, Smielewski P, Hodges JR. 2003. Limbic hypometabolism in Alzheimer's disease and mild cognitive impairment. Ann Neurol 54:343–351 [DOI] [PubMed] [Google Scholar]
- 105. Gómez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT. 1996. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. J Neurosci 16:4491–4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Delacourte A, David JP, Sergeant N, Buée L, Wattez A, Vermersch P, Ghozali F, Fallet-Bianco C, Pasquier F, Lebert F, Petit H, Di Menza C. 1999. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer's disease. Neurology 52:1158–1165 [DOI] [PubMed] [Google Scholar]
- 107. Fox NC, Schott JM. 2004. Imaging cerebral atrophy: normal ageing to Alzheimer's disease. Lancet 363:392–394 [DOI] [PubMed] [Google Scholar]
- 108. de Leon MJ, Golomb J, George AE, Convit A, Tarshish CY, McRae T, De Santi S, Smith G, Ferris SH, Noz M. 1993. The radiologic prediction of Alzheimer disease: the atrophic hippocampal formation. AJNR 14:897–906 [PMC free article] [PubMed] [Google Scholar]
- 109. Jack CR, Jr, Petersen RC, O'Brien PC, Tangalos EG. 1992. MR-based hippocampal volumetry in the diagnosis of Alzheimer's disease. Neurology 42:183–188 [DOI] [PubMed] [Google Scholar]
- 110. Jack CR, Jr, Petersen RC, Xu YC, O'Brien PC, Smith GE, Ivnik RJ, Boeve BF, Waring SC, Tangalos EG, Kokmen E. 1999. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology 52:1397–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Schönknecht P, Henze M, Hunt A, Klinga K, Haberkorn U, Schröder J. 2003. Hippocampal glucose metabolism is associated with cerebrospinal fluid estrogen levels in postmenopausal women with Alzheimer's disease. Psychiatry Res 124:125–127 [PubMed] [Google Scholar]
- 112. Eberling JL, Wu C, Haan MN, Mungas D, Buonocore M, Jagust WJ. 2003. Preliminary evidence that estrogen protects against age-related hippocampal atrophy. Neurobiol Aging 24:725–732 [DOI] [PubMed] [Google Scholar]
- 113. Boccardi M, Ghidoni R, Govoni S, Testa C, Benussi L, Bonetti M, Binetti G, Frisoni GB. 2006. Effects of hormone therapy on brain morphology of healthy postmenopausal women: a voxel-based morphometry study. Menopause 13:584–591 [DOI] [PubMed] [Google Scholar]
- 114. Hu L, Yue Y, Zuo PP, Jin ZY, Feng F, You H, Li ML, Ge QS. 2006. Evaluation of neuroprotective effects of long-term low dose hormone replacement therapy on postmenopausal women brain hippocampus using magnetic resonance scanner. Chin Med Sci J 21:214–218 [PubMed] [Google Scholar]
- 115. Eberling JL, Wu C, Tong-Turnbeaugh R, Jagust WJ. 2004. Estrogen- and tamoxifen-associated effects on brain structure and function. NeuroImage 21:364–371 [DOI] [PubMed] [Google Scholar]
- 116. Lord C, Buss C, Lupien SJ, Pruessner JC. 2008. Hippocampal volumes are larger in postmenopausal women using estrogen therapy compared to past users, never users and men: a possible window of opportunity effect. Neurobiol Aging 29:95–101 [DOI] [PubMed] [Google Scholar]
- 117. Erickson KI, Colcombe SJ, Elavsky S, McAuley E, Korol DL, Scalf PE, Kramer AF. 2007. Interactive effects of fitness and hormone treatment on brain health in postmenopausal women. Neurobiol Aging 28:179–185 [DOI] [PubMed] [Google Scholar]
- 118. Barrett-Connor E, Kritz-Silverstein D. 1993. Estrogen replacement therapy and cognitive function in older women. JAMA 269:2637–2641 [PubMed] [Google Scholar]