Abstract
Context:
Multiple endocrine neoplasia type 1 (MEN1) is caused by mutations in the menin (MEN1) gene. The mechanism(s) by which MEN1 mutations lead to pituitary tumor formation remain(s) unknown.
Objective:
The aim of the study was to identify the pediatric MEN1-associated pituitary tumor transcriptome.
Patients and Methods:
A patient harboring a MEN1 mutation (c.525C>G; p.H139D) who presented with an early-onset mammosomatotroph pituitary adenoma was studied. Microarray analysis was performed in the tumor sample and compared with the profile observed in normal pituitaries and in a sporadic mammosomatotropinoma. Validation of the microarray results was performed using quantitative real-time PCR and immunohistochemical analysis for selected genes.
Results:
In the MEN1-associated pituitary adenoma, 59 and 24 genes were found to be significantly up- and down-regulated, respectively. The up-regulated genes included those involved in cell growth and maintenance, apoptosis, growth arrest, and tumorigenesis. Moreover, we observed decreased expression in genes neuroendocrine in nature and related to growth or apoptosis. Only 21 of the 59 genes differentially expressed in the MEN1-associated adenoma showed a similar expression profile to that seen in the sporadic mammosomatotropinoma; for some genes an opposite expression profile was observed.
Conclusions:
We identified changes in the transcriptome that occur in pituitary GH- and PRL-producing cells after the loss of menin expression; some of the gene changes are necessary for tumor evolution, and others may be tertiary. Nevertheless, the rare overlap between the expression profiles of the MEN1 tumor vs. that of its sporadic counterpart suggests that these tumors evolve along different molecular pathways.
Multiple endocrine neoplasia type 1 (MEN1), an autosomal dominant syndrome associated with tumors of the parathyroids, pancreas, duodenum, anterior pituitary, and other tissues (1–3), is caused by germline mutations in the MEN1 gene in approximately 70% of the familial cases (4–6). The product of the MEN1 gene (located on chromosome 11q13), menin, is a tumor suppressor protein with functions that are still not fully understood (7). MEN1 is regarded as a disease of mostly young adults (8). Anterior pituitary tumors in MEN1 have an age and sex distribution and hormone profile similar to those of pituitary tumors in the general population (9); typically these tumors present at an average age of 30 to 35 yr (10). In 2000, we described the earliest (at the time) presentation of any tumor in MEN1 in a 5-yr-old boy with a mammosomatotroph pituitary macroadenoma. The patient harbored a germline MEN1 gene mutation (c.525C>G; p.H139D) (11).
To search for genes that could be involved in the unexpectedly early development of this aggressive pituitary tumor, we analyzed its transcriptome. The data were compared with those of normal pituitary tissues and the transcriptome of a sporadic but invasive mammosomatotroph adenoma. The array data for selected genes were confirmed by real-time quantitative PCR (qPCR) and/or immunohistochemistry.
Patients and Methods
Patients and samples
We studied a patient with MEN1 who was diagnosed with a mammosomatotroph pituitary adenoma at age 5 yr; he harbored a heterozygous germline mutation in the MEN1 gene (c.525C>G; p.H139D) (11). A family history of MEN1 was apparent (father and two aunts with hyperparathyroidism, one of them also with prolactinoma), and all affected relatives harbored the same H139D mutation (11). The tumor was an invasive macroadenoma with erosion of the sella floor and compression of the optic chiasm. Prolactin (PRL) and IGF-I levels were markedly elevated, and the patient was initially treated with bromocriptine. On therapy, the tumor increased in size, so subsequently the patient underwent transsphenoidal surgery in 2000. Complete excision of the tumor was not possible because of extensive invasion of the left cavernous sinus, and in the postoperative period, the disease was temporarily controlled with the use of somatostatin analog (octreotide) associated with cabergoline (11). In 2001, fractionated radiotherapy was performed. Two years later, the patient developed GH deficiency, and recombinant GH replacement therapy was instituted for 1 yr. Thyroid hormone replacement was also initiated at that time and is currently maintained. The patient completed puberty on gonadal hormone replacement, and he is still being treated with testosterone. To date, the patient has not developed clinically significant hyperparathyroidism or hypergastrinemia.
For comparison, we studied an invasive sporadic mammosomatotroph pituitary tumor from a patient with acromegaly, whose symptoms started at the age of 20 yr. This patient was treated with dopamine agonists, and after 9 yr, transsphenoidal surgery was performed because of an increase in the size of the tumor, despite medical treatment. This tumor did not harbor a MEN1 coding sequence mutation.
Tissues from the two patients' pituitary tumors were collected at surgery under research protocols approved by the Institutional Review Boards and have been described previously (11, 12). Both the MEN1-associated adenoma and the sporadic pituitary adenoma stained positively for PRL and GH and negatively for other pituitary hormones, consistent with a mammosomatotroph lineage. The MEN1-associated adenoma displayed loss of heterozygosity for the MEN1 gene (11). Mutations in the GNAS gene were ruled out in both tumor samples. The patient with the MEN1 tumor was sequenced for germline AIP gene mutations, and there were none.
RNA extraction and amplification
Total RNA was isolated from frozen tissues using TRIZol Reagent (Invitrogen, Carlsbad, CA) and was further purified using the RNeasy maxi-kits (QIAGEN, Inc., Valencia, CA) according to the manufacturer's instructions. The quality of the RNA was evaluated by spectrophotometry and agarose gel electrophoresis.
Normal pituitary RNA for amplification and microarray analysis was obtained commercially (BD Biosciences Clontech, Palo Alto, CA) and comprised a pool of poly A+ RNA from 88 male/female (sudden death) Caucasians, ages 16–88 yr. Total tumor RNA (5 μg) and poly A+ selected (0.2 μg) RNA from pooled normal pituitaries were subject to linear RNA amplification using commercial reagents (MessageAMP aRNA Kit; Ambion, Austin, TX) in a single round of amplification. In a 10-μl reverse transcription reaction, RNA was transcribed by priming with a T7-(dT)24 primer. Second strand synthesis was initiated by RNase digestion. The purified double-stranded cDNA served as a template for in vitro transcription to generate amplified RNA as directed by the manufacturer.
Sample labeling and hybridization
Amplified RNA (3 μg) was used for each experiment. Labeling was performed by random hexamer-primed reverse transcription reactions incorporating either green fluorescent cyanine 3-dUTP or red fluorescent cyanine 5-dUTP using the MICROMAX direct cDNA microarray system (NEN PerkinElmer Life Sciences Inc., Boston, MA). After labeling, samples (specimens and normal) were combined, purified, and concentrated with Microcon YM30 columns (Millipore Corp., Bedford, MA). After denaturation, probes were cohybridized at 65 C for 16 h to a microarray glass slide that was made at the National Cancer Institute (NCI, Bethesda, MD) core facility, as we have previously published (13). Slides were then washed in 0.5× standard saline citrate (SSC), 0.01% sodium dodecyl sulfate for 10 min; 0.06× SSC, 0.01 SSC for 5 min; and scanned on a dual-laser microarray scanner (GenePix 4000; Axon Instruments Inc., Foster City, CA) as described previously (13). The experiments were repeated at least twice and in each case included reciprocal labeling of fluorochromes. The images were analyzed by GenePix Pro 3.0 (Axon Instruments). Data were imported into the NCI Center for Information Technology database (NCI-CIT μArray) for subsequent analysis.
Microarray data analysis
Scanned hybridization images were inspected for artifacts and other defects; all spots with defective hybridization were then excluded. The Cye3 and Cye5 fluorescent intensities and background values were obtained for individual spots using the GenePix 4000 microarray software (Axon Instruments). Signal intensity for each spot was determined according to the following formula: [mean spot intensity] − [median background intensities], as previously described (13).
Criteria for representation in the analysis included individual spots with signal intensities of at least 1000 pixels in at least one channel (after background subtraction), and a significant cutoff signal:background ratio of 2.0 in both channels. To minimize intersample variation in the efficiency of Cye5 and Cye3 labeling, individual slides were normalized as previously described (13). For the MEN1-associated adenoma, individual spot ratios that fulfilled the above criteria were determined from two experiments that were in each case subject to reciprocal labeling. Significance was assigned if the ratio in at least three of the four arrays lay outside of the cutoff values. The sporadic adenoma was subject to a similar analysis, except that values were determined from a single experiment with reciprocal labeling. The mean values of individual spots fulfilling all of the above criteria were then calculated.
Immunohistochemical analysis
Immunohistochemistry for cFos, Ptx2, and Gsα (the product of the GNAS gene) was performed by the avidin-biotin-immunoperoxidase technique. Briefly, sections were dewaxed in xylene and rehydrated through graded alcohols, followed by three 5-min washes in PBS. Sections were quenched for 10 min in 3% hydrogen peroxide to remove any endogenous peroxidase activity, then washed three times in PBS. Antigen retrieval was performed by boiling in 10 mm sodium citrate (pH 6) for 10 min in a pressure cooker. Slides were allowed to cool for 1 h to room temperature and were then washed in distilled water. Tissues were blocked in 10% normal serum for 30 min, followed by incubation with primary antibodies at 4 C overnight. The anti-cFos polyclonal antibody, sc-52-G (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), polyclonal anti-Ptx2 antibody, sc-8747 (Santa Cruz Biotechnology, Inc.), and polyclonal anti-Gsα (LF129) were used at 1:100 dilution (∼2 μg/ml) in separate trials. After washing, sections were incubated in biotinylated secondary antibodies (1:400) for 30 min. The signal was detected and amplified using the ABC peroxidase method (Vector, Burlingame, CA) and visualized with 3,3′-diaminobenzidine. Controls for the immunohistochemistry procedure were performed by omission of primary antibody in the incubation and substitution with blocking solution and processed in parallel with the experimental groups. Sections were counterstained with hematoxylin or methyl green, briefly washed in distilled water before dehydration in alcohols, xylene, and mounted with Permount and coverslips.
Real-time qPCR
qPCR for expression of the genes FOS, GNAS, GADD45A, NTS, and AKAP9 was performed in both the MEN1-associated and sporadic pituitary tumor samples, using a 20-μl working master mix containing 1.0 μl of the cDNA template in 1× TaqMan universal Master Mix (Applied Biosystems, Foster City, CA) and 200 nm final concentration of the primers and the probe (Applied Biosystems). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as an endogenous control. The reaction was run in an Applied Biosystems 7900H Real-time PCR Sequence Detection System using the following parameters: 50 C for 2 min, 95 C for 10 min, 40 cycles of 95 C (denature) for 15 sec, with 60 C for 1 min (annealing, extension). For controls, normal human pituitary RNA was commercially obtained from BD Bioscience Clontech (Palo Alto, CA). Each qPCR was processed in duplicate experiments. Relative quantification was performed using the 2−ΔΔCT method (14).
Results
Differential gene expression in the MEN1-associated pituitary adenoma
We used cDNA microarray analysis to first compare global changes in gene expression from the MEN1-associated pituitary adenoma vs. pooled postmortem normal pituitaries. Selected genes had to be up- or down-regulated at least 2-fold in at least three of four arrays. Using these stringent criteria, 83 genes were identified: 59 genes were overexpressed, and 24 genes were underexpressed in the adenoma (Table 1).
Table 1.
All genes with significant expression differences in the MEN1-associated pituitary adenoma
| Overexpressed |
Underexpressed |
||
|---|---|---|---|
| Gene | Fold increase | Gene | Fold decrease |
| CBX1 | 2.02 | CGA | 0.03 |
| SPCS1 | 2.20 | FSHB | 0.06 |
| KPNA2 | 2.34 | SH3GL2 | 0.09 |
| HTATSF1 | 2.40 | DIO2 | 0.11 |
| YWHAH | 2.50 | GPC4 | 0.17 |
| C20orf3 | 2.54 | PITX1 | 0.18 |
| EEF1B2 | 2.55 | CGB5 | 0.19 |
| ENTPD4 | 2.71 | COL6A1 | 0.19 |
| ACTB | 2.73 | GPC3 | 0.20 |
| SCG5 | 2.79 | CDH1 | 0.20 |
| SRF | 2.85 | NNAT | 0.21 |
| NME1 | 2.86 | HP | 0.22 |
| EPHX2 | 2.91 | SPARCL1 | 0.22 |
| ZNF189 | 2.93 | MGP | 0.25 |
| ITM2B | 2.98 | HSPG2 | 0.27 |
| TPD52 | 3.00 | CD74 | 0.28 |
| FOS | 3.02 | HLA-DRB5 | 0.30 |
| MLLT11 | 3.11 | IGFBP4 | 0.32 |
| RABEPK | 3.12 | MDK | 0.36 |
| RFK | 3.34 | HLA-DRB3 | 0.39 |
| DDOST | 3.34 | APOD | 0.42 |
| RBM3 | 3.40 | HLA-DRA | 0.43 |
| SGCE | 3.42 | PGRMC1 | 0.45 |
| RCN1 | 3.43 | GSTM2 | 0.60 |
| KIF5B | 3.44 | ||
| DUSP6 | 3.47 | ||
| GNAS | 3.51 | ||
| BMS1 | 3.66 | ||
| ARPC5 L | 3.68 | ||
| ZNF177 | 3.70 | ||
| CELF2 | 3.74 | ||
| SPINT2 | 3.76 | ||
| IMPA2 | 3.90 | ||
| ARG2 | 3.91 | ||
| IRF9 | 3.92 | ||
| SHC1 | 3.95 | ||
| MIDN | 4.21 | ||
| CD63 | 4.30 | ||
| LEPROT | 4.33 | ||
| SLC38A1 | 4.47 | ||
| GNG3 | 4.51 | ||
| THBS4 | 4.59 | ||
| PBXIP1 | 5.05 | ||
| B2M | 5.06 | ||
| GADD45A | 5.53 | ||
| IL13RA2 | 5.91 | ||
| PIP5K1B | 6.02 | ||
| SLC15A2 | 6.05 | ||
| SGK1 | 6.74 | ||
| FOSB | 6.80 | ||
| ERP29 | 7.09 | ||
| CTSH | 7.15 | ||
| DCLK1 | 7.73 | ||
| NTS | 8.72 | ||
| HLA-B | 9.10 | ||
| THBS2 | 9.58 | ||
| DUSP5 | 10.57 | ||
| VGF | 11.08 | ||
| PENK | 11.78 | ||
The protein products for some of the genes found to be overexpressed in our study are involved in cell growth and maintenance, for example serum response factor (SRF), Gsα (GNAS) and FBJ viral homolog B (FOSB) (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). Others, such as serum/glucocorticoid regulated kinase 1 (SGK-1), dual specificity phosphatases 5 and 6 (DUSP5 and DUSP6) and growth arrest in response to DNA damage (GADD45A), are involved in apoptosis, whereas tumor protein D52 (TPD52), SHC transforming protein 1 (SHC1) and the v-fos FBJ viral homolog (FOS) are involved in tumorigenesis. Except for GNAS, dysregulation of the highlighted genes in this tumor type has not been reported before. It should be noted that this tumor was not carrying a somatic mutation in GNAS (11).
The underexpressed genes are listed in Table 1 and Supplemental Table 2. In some cases decreased expression most likely reflects the derived pituitary lineage of the specimen (somatolactotroph) vs. total anterior pituitary gland. Thus, it is perhaps not surprising that we observed decreased expression of the glycoprotein hormones α-subunit (CGA), β-subunit of FSH (FSHB), and the progesterone receptor membrane component 1 (PGRMC1).
Significant underexpression of glypicans 3 and 4 (GPC3 and GPC4) and of the pituitary developmental gene PITX1 was also apparent. Interestingly, previous studies of Ptx-1 expression in pituitary adenomas, representing all of the major subtypes, have reported this gene to be ubiquitously expressed at levels similar to that seen in normal pituitary (15, 16). Other genes of note that were underrepresented include the tumor suppressor gene cadherin 1 (CDH1), neuronatin (NNAT) and the IGF binding protein 4 (IGFBP4) gene. For NNAT, a previous report has shown expression in all major pituitary tumor subtypes with the exception of prolactinomas (17).
Genes differentially expressed between the MEN1-associated and the sporadic pituitary adenoma
To identify common and unique genes subject to dysregulation in the familial pituitary adenoma vs. those seen in its sporadic counterpart, we compared these tumor forms by microarray analysis. Applying the cutoff criteria described above, 21 genes were identified in common and are shown in Table 2. In the majority of cases, down-regulation was concordant between the two tumor forms, as exemplified by decreased expression of PITX1 and of the GPC4 and GPC3 genes. However, for some genes overexpression was apparent in the familial adenoma (FOS, C20orf3, and HTATSF1), whereas decreased expression was observed in the sporadic counterpart.
Table 2.
Genes displaying significant expression differences in both the MEN1-associated and sporadic pituitary adenomas
| MEN1 tumor | Sporadic tumor | Map | Gene | Gene identity |
|---|---|---|---|---|
| Fold increase | ||||
| 2.40 | 0.30 | Xq26.1-q27.2 | HTATSF1 | HIV-1 Tat specific factor 1 |
| 2.54 | 0.29 | 20p11.22-p11.21 | C20orf3 | Chromosome 20 open reading frame 3 |
| 3.02 | 0.26 | 14q24.3 | FOS | FBJ murine osteosarcoma viral oncogene homolog |
| 3.44 | 0.86 | 10pter-q22.1 | KIF5B | Kinesin family member 5B |
| 7.73 | 0.43 | 13q13 | DCLK1 | Doublecortin-like kinase 1 |
| Fold decrease | ||||
| 0.03 | 0.01 | 6q12-q21 | CGA | Glycoprotein hormones, α polypeptide |
| 0.06 | 0.04 | 11p13 | FSHB | FSH, β polypeptide |
| 0.17 | 0.13 | Xq26.1 | GPC4 | Glypican 4 |
| 0.18 | 0.25 | 5q31 | PITX1 | Paired-like homeodomain 1 |
| 0.19 | 0.01 | 19q13.32 | CGB5 | Chorionic gonadotropin, β polypeptide 5 |
| 0.19 | 0.25 | 21q22.3 | COL6A1 | Collagen, type VI, α 1 |
| 0.20 | 0.33 | Xq26.1 | GPC3 | Glypican 3 |
| 0.21 | 0.44 | 20q11.2-q12 | NNAT | Neuronatin |
| 0.22 | 0.14 | 4q22.1 | SPARCL1 | SPARC-like 1 (mast9, hevin) |
| 0.25 | 0.03 | 12p13.1-p12.3 | MGP | Matrix Gla protein |
| 0.27 | 0.09 | 1p36.1-p35 | HSPG2 | Heparan sulfate proteoglycan 2 |
| 0.28 | 0.18 | 5q32 | CD74 | CD74 molecule, major histocompatibility complex, class II invariant chain |
| 0.30 | 0.07 | 6p21.3 | HLA-DRB5 | Major histocompatibility complex, class II, DR β 5 |
| 0.39 | 0.17 | 6p21.3 | HLA-DRB3 | Major histocompatibility complex, class II, DR β 3 |
| 0.42 | 0.20 | 3q26.2-qter | APOD | Apolipoprotein D |
| 0.43 | 0.13 | 6p21.3 | HLA-DRA | Major histocompatibility complex, class II, DR α |
We next searched for genes subject to dysregulation in the familial tumor that were not aberrantly expressed in the sporadic adenoma; 61 genes were identified, 54 genes were overexpressed, and seven genes were underexpressed (Table 3). Although some of the overexpressed genes were highlighted in our initial analysis relative to normal pituitary tissue (such as SGK-1, SRF, TPD52, GNAS, and FOSB, which we discussed above), comparison with the sporadic mammosomatotropinoma identified a unique gene set (Table 3). Similarly, down-regulated genes unique to the MEN1 adenoma were also identified: CDH1, IGFBP4, and SH3GL2.
Table 3.
Genes displaying significant expression differences in the MEN1-associated pituitary adenoma only
| Map | Gene | Gene identity | |
|---|---|---|---|
| Fold increase | |||
| 2.02 | 17q | CBX1 | Chromobox homolog 1 (HP1 β homolog Drosophila) |
| 2.20 | 3p21.31 | SPCS1 | Signal peptidase complex subunit 1 homolog (Saccharomyces cerevisiae) |
| 2.34 | 17q23.1-q23.3 | KPNA2 | Karyopherin α 2 (RAG cohort 1, importin α 1) |
| 2.50 | 22q12.3 | YWHAH | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, η polypeptide |
| 2.55 | 2q33-q34 | EEF1B2 | Eukaryotic translation elongation factor 1 β 2 |
| 2.71 | 8p21.2 | ENTPD4 | Ectonucleoside triphosphate diphosphohydrolase 4 |
| 2.73 | 7p15-p12 | ACTB | Actin, β |
| 2.79 | 15q13-q14 | SCG5 | Secretogranin V (7B2 protein) |
| 2.85 | 6p21.1 | SRF | Serum response factor (c-fos serum response element-binding transcription factor) |
| 2.86 | 17q21.3 | NME1 | Nonmetastatic cells 1, protein (NM23A) expressed in |
| 2.91 | 8p21-p12 | EPHX2 | Epoxide hydrolase 2, cytoplasmic |
| 2.93 | 9q22-q31 | ZNF189 | Zinc finger protein 189 |
| 2.98 | 13q14.3 | ITM2B | Integral membrane protein 2B |
| 3.00 | 8q21 | TPD52 | Tumor protein D52 |
| 3.11 | 1q21 | MLLT11 | Myeloid/lymphoid or mixed-lineage leukemia (trithorax homolog, Drosophila); translocated to, 11 |
| 3.12 | 9q34.11 | RABEPK | Rab9 effector protein with kelch motifs |
| 3.34 | 9q21.13 | RFK | Riboflavin kinase |
| 3.34 | 1p36.1 | DDOST | Dolichyl-diphosphooligosaccharide-protein glycosyltransferase |
| 3.40 | Xp11.2 | RBM3 | RNA binding motif (RNP1, RRM) protein 3 |
| 3.42 | 7q21-q22 | SGCE | Sarcoglycan, ϵ |
| 3.43 | 11p13 | RCN1 | Reticulocalbin 1, EF-hand calcium binding domain |
| 3.47 | 12q22-q23 | DUSP6 | Dual specificity phosphatase 6 |
| 3.51 | 20q13.2-q13.3 | GNAS | GNAS complex locus |
| 3.66 | 10q11.21 | BMS1 | BMS1 homolog, ribosome assembly protein (yeast) |
| 3.68 | 9q34.11 | ARPC5 L | Actin-related protein 2/3 complex, subunit 5-like |
| 3.70 | 19p13.2 | ZNF177 | Zinc finger protein 177 |
| 3.74 | 10p13 | CELF2 | CUGBP, Elav-like family member 2 |
| 3.76 | 19q13.1 | SPINT2 | Serine peptidase inhibitor, Kunitz type, 2 |
| 3.90 | 18p11.2 | IMPA2 | Inositol(myo)-1(or 4)-monophosphatase 2 |
| 3.91 | 14q24.1-q24.3 | ARG2 | Arginase, type II |
| 3.92 | 14q11.2 | IRF9 | Interferon regulatory factor 9 |
| 3.95 | 1q21 | SHC1 | SHC (Src homology 2 domain containing) transforming protein 1 |
| 4.21 | 19p13.3 | MIDN | Midnolin |
| 4.30 | 12q12-q13 | CD63 | CD63 molecule |
| 4.33 | 1p31.3 | LEPROT | Leptin receptor overlapping transcript |
| 4.47 | 12q12 | SLC38A1 | Solute carrier family 38, member 1 |
| 4.51 | 11p11 | GNG3 | Guanine nucleotide binding protein (G protein), γ 3 |
| 4.59 | 5q13 | THBS4 | Thrombospondin 4 |
| 5.05 | 1q21.3 | PBXIP1 | Pre-B-cell leukemia homeobox interacting protein 1 |
| 5.06 | 15q21-q22.2 | B2M | β-2-microglobulin |
| 5.53 | 1p31.2-p31.1 | GADD45A | Growth arrest and DNA-damage-inducible, α |
| 5.91 | Xq13.1-q28 | IL13RA2 | Interleukin 13 receptor, α 2 |
| 6.02 | 9q13 | PIP5K1B | Phosphatidylinositol-4-phosphate 5-kinase, type I, β |
| 6.05 | 3q13.33 | SLC15A2 | Solute carrier family 15 (H+/peptide transporter), member 2 |
| 6.74 | 6q23 | SGK1 | Serum/glucocorticoid regulated kinase 1 |
| 6.80 | 19q13.32 | FOSB | FBJ murine osteosarcoma viral oncogene homolog B |
| 7.09 | 12q24.13 | ERP29 | Endoplasmic reticulum protein 29 |
| 7.15 | 15q24-q25 | CTSH | Cathepsin H |
| 8.72 | 12q21 | NTS | Neurotensin |
| 9.10 | 6p21.3 | HLA-B | Major histocompatibility complex, class I, B |
| 9.58 | 6q27 | THBS2 | Thrombospondin 2 |
| 10.57 | 10q25 | DUSP5 | Dual specificity phosphatase 5 |
| 11.08 | 7q22 | VGF | VGF nerve growth factor inducible |
| 11.78 | 8q23-q24 | PENK | Proenkephalin |
| Fold decrease | |||
| 0.09 | 9p22 | SH3GL2 | SH3-domain GRB2-like 2 |
| 0.20 | 16q22.1 | CDH1 | Cadherin 1, type 1, E-cadherin (epithelial) |
| 0.22 | 16q22.1 | HP | Haptoglobin |
| 0.32 | 17q12-q21.1 | IGFBP4 | IGF binding protein 4 |
| 0.36 | 11p11.2 | MDK | Midkine (neurite growth-promoting factor 2) |
| 0.45 | Xq22-q24 | PGRMC1 | Progesterone receptor membrane component 1 |
| 0.60 | 1p13.3 | GSTM2 | Glutathione S-transferase μ 2 (muscle) |
Validation by RNA expression analysis
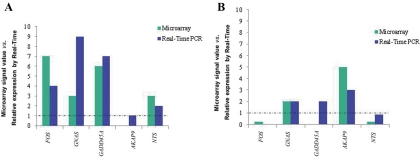
For validation of the microarray results, the genes FOS, GNAS, GADD45A, and NTS, which appeared to be overexpressed by microarray analysis in the MEN1-associated pituitary adenoma, were further studied using qPCR. For all these genes, qPCR agreed with the microarray results (Fig. 1). Protein kinase A anchor protein 9 (AKAP9) gene was neither up- nor down-regulated in the MEN1-associated adenoma, relative to normal pituitaries, in both microarray and qPCR analysis (Fig. 1A).
Fig. 1.
Microarray signal values and relative expression measured by real-time PCR for the genes FOS, GNAS, GADD45A, AKAP9, and NTS. Dotted lines indicate the expression of the corresponding genes in normal pituitaries. A, Studies performed in the MEN1-associated pituitary tumor, showing concordance with the expression pattern observed in microarray analysis, for all genes evaluated. B, Results from the sporadic pituitary adenoma. Except for GADD45A, the pattern of gene expression observed in microarray was confirmed by real-time PCR.
For the sporadic mammosomatotropinoma, the genes GNAS and AKAP9, which appeared to be overexpressed in the microarray analysis, also showed increased relative expression measured by qPCR (Fig. 1B). Similarly, the underexpressed genes FOS and NTS showed decreased relative expression by qPCR. For GADD45A in the sporadic tumor, the expression measured by qPCR was increased and not consistent with the microarray analysis (Fig. 1B).
Immunohistochemistry
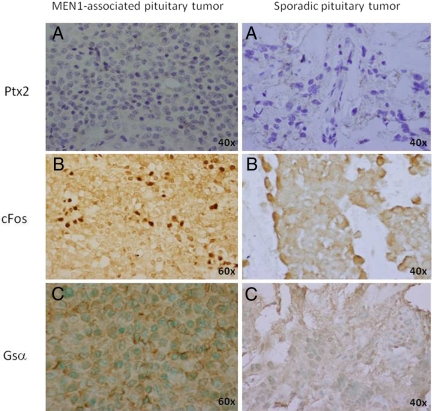
Pituitary homeobox factor 2 (Ptx-2), which is normally expressed in the developing and mature pituitary (18), stained negatively in both the MEN1-associated and the sporadic pituitary tumor (Fig. 2A), as predicted by the transcriptomic and expression analyses. The MEN1 adenoma demonstrated a predominantly granular, nuclear staining for cFos, whereas staining in the sporadic mammosomatotropinoma was negative (Fig. 2B). Staining for Gsα was positive in the MEN1-associated (cytoplasmic/membranous pattern) and negative in the sporadic pituitary tumor (Fig. 2C).
Fig. 2.
Immunohistochemical analysis for Ptx2, cFos, and Gsα proteins. A, Immunostaining was negative for Ptx2 in both the MEN1-associated and the sporadic pituitary tumor. In contrast, cFos (B) and Gsα (C) were expressed in the MEN1-associated but negative in the sporadic pituitary tumor.
Discussion
The pleomorphic MEN1 syndrome, which affects several endocrine tissues, results from germline mutations in the putative tumor suppressor gene MEN1 (19). Over 1300 mutations have been reported so far, and pituitary adenomas occur in approximately 40% of cases (9, 20), an observation consistent with the data obtained from mouse models; heterozygote mice (with one menin allele inactivated) develop pituitary tumors in a frequency of 37% (21, 22). MEN1-associated pituitary tumors are more aggressive than sporadic pituitary tumors, and the median age at presentation is 34 yr for prolactinomas (23). In a series of 324 MEN1 patients, 136 presented with pituitary adenomas, with the age of onset ranging between 18 and 83 yr (9). In 2000, we described the youngest presentation of a pituitary tumor in MEN1, in a 5-yr-old boy with a GH- and PRL-cosecreting macroadenoma (11). Because of this early presentation and the rarity of this event even among patients with MEN1, we hypothesized that this tumor had a unique gene profile. We employed microarray analysis, determined the tumor's transcriptome, and compared it to those of postmortem normal pituitaries and to that from a sporadic mammosomatotropinoma.
The analysis provided support for the hypothesis that this unusual tumor had a relatively unique transcriptome. For some of the genes found overexpressed in the MEN1-associated tumor, a role in tumorigenesis in other tumor types has been established, including TPD52 [increased expression in pancreatic and other tumor types (24–26), and FOS that in vivo transforms chondroblasts and osteoblasts (27–29)]. The SHC1 gene also showed significant increase in expression. SHC1 gene amplification and increased transcript levels have been described previously in hepatocellular carcinoma (30). In addition, several genes involved in cell growth and maintenance showed increased expression, including GNAS, FOSB, and SRF, compared with normal pituitary tissues. In sporadic somatotroph adenomas, increased expression of GNAS in tumors that were gsp− (relative to their gsp+ counterpart) has been reported previously (31). Thus, our findings of increased levels of GNAS transcript, as well as positivity for Gsα protein by immunohistochemistry, in this gsp− macroadenoma are consistent with the previous report.
The SRF gene product is a transcription factor that binds a serum response element (SRE) associated with a variety of genes that include FOS and FOSB and numerous other genes involved in cell growth and differentiation (32). Interestingly, both of these downstream targets, FOS and FOSB, showed increased expression in the derived microarray profile, and their expression may reflect increased levels of SRF within this tumor. Indeed, normal pituitary tissue and pituitary tumors in general do not express cFos (27–29, 32).
In the MEN1 adenoma, there was increased expression of gene transcripts involved in growth arrest and apoptotic pathways. Of particular note, we saw increased expression of the serum and glucocorticoid-induced protein kinase gene (SGK-1) and the DUSP5 and DUSP6 genes. SGK-1 is a multifunctional kinase, and in many cell types endogenous SGK-1 steady-state protein levels are very low but can be acutely up-regulated in response to glucocorticoid receptor activation, serum stimulation, and cell stress (33). In breast epithelial and cancer cell lines, glucocorticoid receptor-mediated transcriptional activation of SGK-1 is associated with promotion of cell survival (34).
In contrast, DUSP5 and DUSP6 are involved in growth suppressive pathways through inactivation of growth-stimulating signals (35). DUSP6 is down-regulated in the majority of invasive pancreatic carcinoma, and enforced expression induces apoptosis (35). Similarly, DUSP5 has a potential role in deactivation of MAPK or stress-activated protein kinases and is a direct target of p53 (36). In common with SGK-1, the DUSP5 gene is also induced in response to serum stimulation (37). It is, therefore, possible that the increased coexpression of these pro- and antiapoptotic mediators may reflect an appropriate response to factors that are themselves inappropriately expressed. If this were the case, then serum-responsive expression of both the proapoptotic (DUSP5 and DUSP6) and antiapoptotic (SGK-1) genes may reflect the commonality of their regulatory mechanism. Interestingly and in this context, our profile also showed increased transcript expression of the serum response element-binding protein SRF. Although our studies show increased expression of pro- and antiapoptotic mediators, their relative contribution toward unregulated growth will most likely be influenced by their particular cellular specificities and integrity of downstream mediators in their respective pathways.
A significantly smaller number of genes in the familial adenoma were underexpressed; however, for several of these genes, decreased expression reflected their cellular origin relative to our reference tissue-total normal pituitary gland. Thus, decreased expression of α- and β-subunits of the glycoprotein (α-SU) hormone and FSH, respectively, and of progesterone receptor membrane component 1 (PGRMC1) in somatolactotrophs is not unexpected; it supports the validity of our data and was described before in GH-secreting adenomas (38). Candidate gene approaches have described ubiquitous expression of both NNAT (17) and the panpituitary transcriptional regulator Ptx-1 (PITX-1) in all major pituitary adenoma subtypes, except prolactinomas (15, 16). PITX-1 was found overexpressed in GH-secreting adenomas in a previous study (39). Our analysis showed both of these genes to be underexpressed; by immunostaining, PITX-2 (Ptx-2) was also decreased, although not significantly so in the transcriptomic analysis.
The glypicans (GPC1–6) are a family of heparan sulfate proteoglycans, and loss-of-function mutations of GPC3 cause Simpson-Golabi-Behmel syndrome in humans (40). Patients with Simpson-Golabi-Behmel syndrome often develop embryonal tumors (neuroblastoma, Wilm's tumor); and loss of GPC3 expression, which has been reported in several tumor types, including ovarian (41), breast (42), and malignant mesothelioma cells (43), is thought to play a role in growth inhibition and programmed cell death. Our analysis highlights, for the first time, reduced expression of two members of this proteoglycan family in pituitary tumorigenesis.
Although the expression profiles derived from the familial and sporadic macroadenomas showed some degree of commonality with respect to the genes subject to dysregulation (for example, in both there was decreased expression of the genes GPC, NNAT, and PITX1), we also identified an apparent unique set of genes in the MEN1 adenoma subject to dysregulation. For example, ERP29, which is thought to play a role in reduction of oxidative damage, was found to be overexpressed in the MEN1-associated tumor, but not in the sporadic tumor. In a recent study, overexpression of this gene was seen in a nonfunctioning pituitary adenoma (44). Overexpression of SGK-1, FOS, and FOSB is also apparent in the familial tumor but not in its sporadic counterpart. In agreement, staining for the protein c-Fos was positive only in the MEN1-associated adenoma. Because these genes are serum and/or SRF responsive, it is worth noting that the familial, but not the sporadic adenoma, also overexpresses the SRF gene.
The MEN1 adenoma also displayed a unique profile of transcripts underexpressed that included, for example, CDH1 and IGFBP4. Loss of E-cadherin (CDH1) function supports tumor-cell migration, invasion, and metastatic dissemination and has been reported in multiple tumor types (45). The role of IGFBP4 in tumor biology is poorly understood; however, some studies suggest that this binding protein exerts growth inhibitory (46) and antiapoptotic effects (47).
To our knowledge no previous studies have employed microarray analysis to identify genes subject to dysregulation in tumor material associated with MEN1. In our study, some genes with altered expression may most likely reflect loss of menin within the familial adenoma. Numerous studies establish menin as a bona fide tumor suppressor that elicits its action through multiple pathways, including those involved in the inhibition of cell proliferation (14–17), DNA repair and apoptosis (18), and repression of telomerase expression (19). Although the exact mechanisms by which menin exerts these effects are still unknown (7), a number of protein partners have been identified (48). For one of these partners, JunD, menin deprivation can switch this transcription factor from growth suppressor to growth promoter (49). Our data may point to some of the genes that respond to this switch from suppressor to growth-promoting pathways.
In summary, we have studied gene expression of a rare MEN1 adenoma. Many of the identified genes are known to play a role in tumor progression in other tissues and have not been implicated in pituitary tumorigenesis. These data are useful for the study of MEN1 tumors and pituitary lesions in general and provide insight into molecular pathways that may be exploited therapeutically for the treatment of aggressive pituitary adenomas.
Acknowledgments
This work was supported by the National Institutes of Health Intramural Programs of the Eunice Kennedy Shriver National Institute of Child Health & Human Development and the National Cancer Institute; and also, in part, by a grant from the University of Keele (to W.E.F.) and by a grant from Conselho Nacional de Desenvolvimento Científico e Tecnológico and the University of Brasilia, Brazil (to M.F.A.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AKAP9
- Anchor protein 9
- CDH1
- cadherin 1
- DUSP
- dual specificity phosphatase
- FOS
- FBJ viral homolog
- GADD45A
- growth arrest in response to DNA damage
- GNAS
- Gsα
- GPC3
- glypican 3
- IGFBP
- IGF binding protein
- MEN1
- multiple endocrine neoplasia type 1
- NNAT
- neuronatin
- PGRCM1
- progesterone receptor membrane component 1
- Ptx-2
- pituitary homeobox factor 2
- qPCR
- quantitative PCR
- SGK-1
- serum/glucocorticoid regulated kinase 1
- SHC1
- SHC transforming protein 1
- SRF
- serum response factor
- SSC
- standard saline citrate
- TPD52
- tumor protein D52.
References
- 1. Wermer P. 1954. Genetic aspects of adenomatosis of endocrine glands. Am J Med 16:363–371 [DOI] [PubMed] [Google Scholar]
- 2. Ballard HS, Fame B, Hartsock RJ. 1964. Familial multiple endocrine adenoma-peptic ulcer complex. Medicine (Baltimore) 43:481–516 [PubMed] [Google Scholar]
- 3. Brandi ML, Gagel RF, Angeli A, Bilezikian JP, Beck-Peccoz P, Bordi C, Conte-Devolx B, Falchetti A, Gheri RG, Libroia A, Lips CJ, Lombardi G, Mannelli M, Pacini F, Ponder BA, Raue F, Skogseid B, Tamburrano G, Thakker RV, Thompson NW, Tomassetti P, Tonelli F, Wells AS, Jr, Marx SJ. 2001. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab 86:5658–5671 [DOI] [PubMed] [Google Scholar]
- 4. Chandrasekharappa SC, Guru SC, Manickam P, Olufemi SE, Collins FS, Emmert-Buck MR, Debelenko LV, Zhuang Z, Lubensky IA, Liotta LA, Crabtree JS, Wang Y, Roe BA, Weisemann J, Boguski MS, Agarwal SK, Kester MB, Kim YS, Heppner C, Dong Q, Spiegel AM, Burns AL, Marx SJ. 1997. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science 276:404–407 [DOI] [PubMed] [Google Scholar]
- 5. Ellard S, Hattersley AT, Brewer CM, Vaidya B. 2005. Detection of an MEN1 gene mutation depends on clinical features and supports current referral criteria for diagnostic molecular genetic testing. Clin Endocrinol (Oxf) 62:169–175 [DOI] [PubMed] [Google Scholar]
- 6. Klein RD, Salih S, Bessoni J, Bale AE. 2005. Clinical testing for multiple endocrine neoplasia type 1 in a DNA diagnostic laboratory. Genet Med 7:131–138 [DOI] [PubMed] [Google Scholar]
- 7. Balogh K, Patócs A, Hunyady L, Rácz K. 2010. Menin dynamics and functional insight: take your partners. Mol Cell Endocrinol 326:80–84 [DOI] [PubMed] [Google Scholar]
- 8. Marx SJ, Agarwal SK, Kester MB, Heppner C, Kim YS, Skarulis MC, James LA, Goldsmith PK, Saggar SK, Park SY, Spiegel AM, Burns AL, Debelenko LV, Zhuang Z, Lubensky IA, Liotta LA, Emmert-Buck MR, Guru SC, Manickam P, Crabtree J, Erdos MR, Collins FS, Chandrasekharappa SC. 1999. Multiple endocrine neoplasia type 1: clinical and genetic features of the hereditary endocrine neoplasias. Recent Prog Horm Res 54:397–438; discussion 438–439 [PubMed] [Google Scholar]
- 9. Vergès B, Boureille F, Goudet P, Murat A, Beckers A, Sassolas G, Cougard P, Chambe B, Montvernay C, Calender A. 2002. Pituitary disease in MEN type 1 (MEN1): data from the France-Belgium MEN1 multicenter study. J Clin Endocrinol Metab 87:457–465 [DOI] [PubMed] [Google Scholar]
- 10. Agarwal SK, Ozawa A, Mateo CM, Marx SJ. 2009. The MEN1 gene and pituitary tumours. Horm Res 71(Suppl 2):131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stratakis CA, Schussheim DH, Freedman SM, Keil MF, Pack SD, Agarwal SK, Skarulis MC, Weil RJ, Lubensky IA, Zhuang Z, Oldfield EH, Marx SJ. 2000. Pituitary macroadenoma in a 5-year-old: an early expression of multiple endocrine neoplasia type 1. J Clin Endocrinol Metab 85:4776–4780 [DOI] [PubMed] [Google Scholar]
- 12. Farrell WE, Simpson DJ, Bicknell J, Magnay JL, Kyrodimou E, Thakker RV, Clayton RN. 1999. Sequence analysis and transcript expression of the MEN1 gene in sporadic pituitary tumours. Br J Cancer 80:44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bourdeau I, Antonini SR, Lacroix A, Kirschner LS, Matyakhina L, Lorang D, Libutti SK, Stratakis CA. 2004. Gene array analysis of macronodular adrenal hyperplasia confirms clinical heterogeneity and identifies several candidate genes as molecular mediators. Oncogene 23:1575–1585 [DOI] [PubMed] [Google Scholar]
- 14. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 15. Pellegrini-Bouiller I, Manrique C, Gunz G, Grino M, Zamora AJ, Figarella-Branger D, Grisoli F, Jaquet P, Enjalbert A. 1999. Expression of the members of the Ptx family of transcription factors in human pituitary adenomas. J Clin Endocrinol Metab 84:2212–2220 [DOI] [PubMed] [Google Scholar]
- 16. Tahara S, Kurotani R, Sanno N, Takumi I, Yoshimura S, Osamura RY, Teramoto A. 2000. Expression of pituitary homeo box 1 (Ptx1) in human non-neoplastic pituitaries and pituitary adenomas. Mod Pathol 13:1097–1108 [DOI] [PubMed] [Google Scholar]
- 17. Usui H, Morii K, Tanaka R, Tamura T, Washiyama K, Ichikawa T, Kumanishi T. 1997. cDNA cloning and mRNA expression analysis of the human neuronatin. High level expression in human pituitary gland and pituitary adenomas. J Mol Neurosci 9:55–60 [DOI] [PubMed] [Google Scholar]
- 18. Gage PJ, Camper SA. 1997. Pituitary homeobox 2, a novel member of the bicoid-related family of homeobox genes, is a potential regulator of anterior structure formation. Hum Mol Genet 6:457–464 [DOI] [PubMed] [Google Scholar]
- 19. Thakker RV. 1998. Multiple endocrine neoplasia—syndromes of the twentieth century. J Clin Endocrinol Metab 83:2617–2620 [DOI] [PubMed] [Google Scholar]
- 20. Daly AF, Jaffrain-Rea ML, Beckers A. 2005. Clinical and genetic features of familial pituitary adenomas. Horm Metab Res 37:347–354 [DOI] [PubMed] [Google Scholar]
- 21. Bertolino P, Tong WM, Galendo D, Wang ZQ, Zhang CX. 2003. Heterozygous Men1 mutant mice develop a range of endocrine tumors mimicking multiple endocrine neoplasia type 1. Mol Endocrinol 17:1880–1892 [DOI] [PubMed] [Google Scholar]
- 22. Crabtree JS, Scacheri PC, Ward JM, Garrett-Beal L, Emmert-Buck MR, Edgemon KA, Lorang D, Libutti SK, Chandrasekharappa SC, Marx SJ, Spiegel AM, Collins FS. 2001. A mouse model of multiple endocrine neoplasia, type 1, develops multiple endocrine tumors. Proc Natl Acad Sci USA 98:1118–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Machens A, Schaaf L, Karges W, Frank-Raue K, Bartsch DK, Rothmund M, Schneyer U, Goretzki P, Raue F, Dralle H. 2007. Age-related penetrance of endocrine tumours in multiple endocrine neoplasia type 1 (MEN1): a multicentre study of 258 gene carriers. Clin Endocrinol (Oxf) 67:613–622 [DOI] [PubMed] [Google Scholar]
- 24. Byrne JA, Mattei MG, Basset P. 1996. Definition of the tumor protein D52 (TPD52) gene family through cloning of D52 homologues in human (hD53) and mouse (mD52). Genomics 35:523–532 [DOI] [PubMed] [Google Scholar]
- 25. Byrne JA, Tomasetto C, Garnier JM, Rouyer N, Mattei MG, Bellocq JP, Rio MC, Basset P. 1995. A screening method to identify genes commonly overexpressed in carcinomas and the identification of a novel complementary DNA sequence. Cancer Res 55:2896–2903 [PubMed] [Google Scholar]
- 26. Chen SL, Maroulakou IG, Green JE, Romano-Spica V, Modi W, Lautenberger J, Bhat NK. 1996. Isolation and characterization of a novel gene expressed in multiple cancers. Oncogene 12:741–751 [PubMed] [Google Scholar]
- 27. Grigoriadis AE, Schellander K, Wang ZQ, Wagner EF. 1993. Osteoblasts are target cells for transformation in c-fos transgenic mice. J Cell Biol 122:685–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rüther U, Komitowski D, Schubert FR, Wagner EF. 1989. c-fos expression induces bone tumors in transgenic mice. Oncogene 4:861–865 [PubMed] [Google Scholar]
- 29. Wang ZQ, Grigoriadis AE, Möhle-Steinlein U, Wagner EF. 1991. A novel target cell for c-fos-induced oncogenesis: development of chondrogenic tumours in embryonic stem cell chimeras. EMBO J 10:2437–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wong N, Chan A, Lee SW, Lam E, To KF, Lai PB, Li XN, Liew CT, Johnson PJ. 2003. Positional mapping for amplified DNA sequences on 1q21-q22 in hepatocellular carcinoma indicates candidate genes over-expression. J Hepatol 38:298–306 [DOI] [PubMed] [Google Scholar]
- 31. Barlier A, Pellegrini-Bouiller I, Gunz G, Zamora AJ, Jaquet P, Enjalbert A. 1999. Impact of gsp oncogene on the expression of genes coding for Gsα, Pit-1, Gi2α, and somatostatin receptor 2 in human somatotroph adenomas: involvement in octreotide sensitivity. J Clin Endocrinol Metab 84:2759–2765 [DOI] [PubMed] [Google Scholar]
- 32. Chai J, Tarnawski AS. 2002. Serum response factor: discovery, biochemistry, biological roles and implications for tissue injury healing. J Physiol Pharmacol 53:147–157 [PubMed] [Google Scholar]
- 33. Lang F, Cohen P. 2001. Regulation and physiological roles of serum- and glucocorticoid-induced protein kinase isoforms. Sci STKE 2001:re17. [DOI] [PubMed] [Google Scholar]
- 34. Brickley DR, Mikosz CA, Hagan CR, Conzen SD. 2002. Ubiquitin modification of serum and glucocorticoid-induced protein kinase-1 (SGK-1). J Biol Chem 277:43064–43070 [DOI] [PubMed] [Google Scholar]
- 35. Furukawa T, Sunamura M, Motoi F, Matsuno S, Horii A. 2003. Potential tumor suppressive pathway involving DUSP6/MKP-3 in pancreatic cancer. Am J Pathol 162:1807–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ueda K, Arakawa H, Nakamura Y. 2003. Dual-specificity phosphatase 5 (DUSP5) as a direct transcriptional target of tumor suppressor p53. Oncogene 22:5586–5591 [DOI] [PubMed] [Google Scholar]
- 37. Ishibashi T, Bottaro DP, Michieli P, Kelley CA, Aaronson SA. 1994. A novel dual specificity phosphatase induced by serum stimulation and heat shock. J Biol Chem 269:29897–29902 [PubMed] [Google Scholar]
- 38. Morris DG, Musat M, Czirják S, Hanzély Z, Lillington DM, Korbonits M, Grossman AB. 2005. Differential gene expression in pituitary adenomas by oligonucleotide array analysis. Eur J Endocrinol 153:143–151 [DOI] [PubMed] [Google Scholar]
- 39. Evans CO, Young AN, Brown MR, Brat DJ, Parks JS, Neish AS, Oyesiku NM. 2001. Novel patterns of gene expression in pituitary adenomas identified by complementary deoxyribonucleic acid microarrays and quantitative reverse transcription-polymerase chain reaction. J Clin Endocrinol Metab 86:3097–3107 [DOI] [PubMed] [Google Scholar]
- 40. Pilia G, Hughes-Benzie RM, MacKenzie A, Baybayan P, Chen EY, Huber R, Neri G, Cao A, Forabosco A, Schlessinger D. 1996. Mutations in GPC3, a glypican gene, cause the Simpson-Golabi-Behmel overgrowth syndrome. Nat Genet 12:241–247 [DOI] [PubMed] [Google Scholar]
- 41. Lin H, Huber R, Schlessinger D, Morin PJ. 1999. Frequent silencing of the GPC3 gene in ovarian cancer cell lines. Cancer Res 59:807–810 [PubMed] [Google Scholar]
- 42. Xiang YY, Ladeda V, Filmus J. 2001. Glypican-3 expression is silenced in human breast cancer. Oncogene 20:7408–7412 [DOI] [PubMed] [Google Scholar]
- 43. Murthy SS, Shen T, De Rienzo A, Lee WC, Ferriola PC, Jhanwar SC, Mossman BT, Filmus J, Testa JR. 2000. Expression of GPC3, an X-linked recessive overgrowth gene, is silenced in malignant mesothelioma. Oncogene 19:410–416 [DOI] [PubMed] [Google Scholar]
- 44. Zhan X, Desiderio DM. 2010. Signaling pathway networks mined from human pituitary adenoma proteomics data. BMC Med Genomics 3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cavallaro U, Christofori G. 2004. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer 4:118–132 [DOI] [PubMed] [Google Scholar]
- 46. Drivdahl RH, Sprenger C, Trimm K, Plymate SR. 2001. Inhibition of growth and increased expression of insulin-like growth factor-binding protein-3 (IGFBP-3) and -6 in prostate cancer cells stably transfected with antisense IGFBP-4 complementary deoxyribonucleic acid. Endocrinology 142:1990–1998 [DOI] [PubMed] [Google Scholar]
- 47. Vertosick FT, Jr, Selker RG, Arena VC. 1991. Survival of patients with well-differentiated astrocytomas diagnosed in the era of computed tomography. Neurosurgery 28:496–501 [DOI] [PubMed] [Google Scholar]
- 48. Yang Y, Hua X. 2007. In search of tumor suppressing functions of menin. Mol Cell Endocrinol 265–266:34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Agarwal SK, Novotny EA, Crabtree JS, Weitzman JB, Yaniv M, Burns AL, Chandrasekharappa SC, Collins FS, Spiegel AM, Marx SJ. 2003. Transcription factor JunD, deprived of menin, switches from growth suppressor to growth promoter. Proc Natl Acad Sci USA 100:10770–10775 [DOI] [PMC free article] [PubMed] [Google Scholar]