Abstract
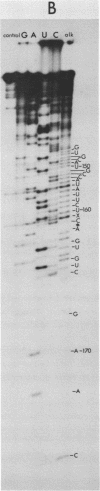
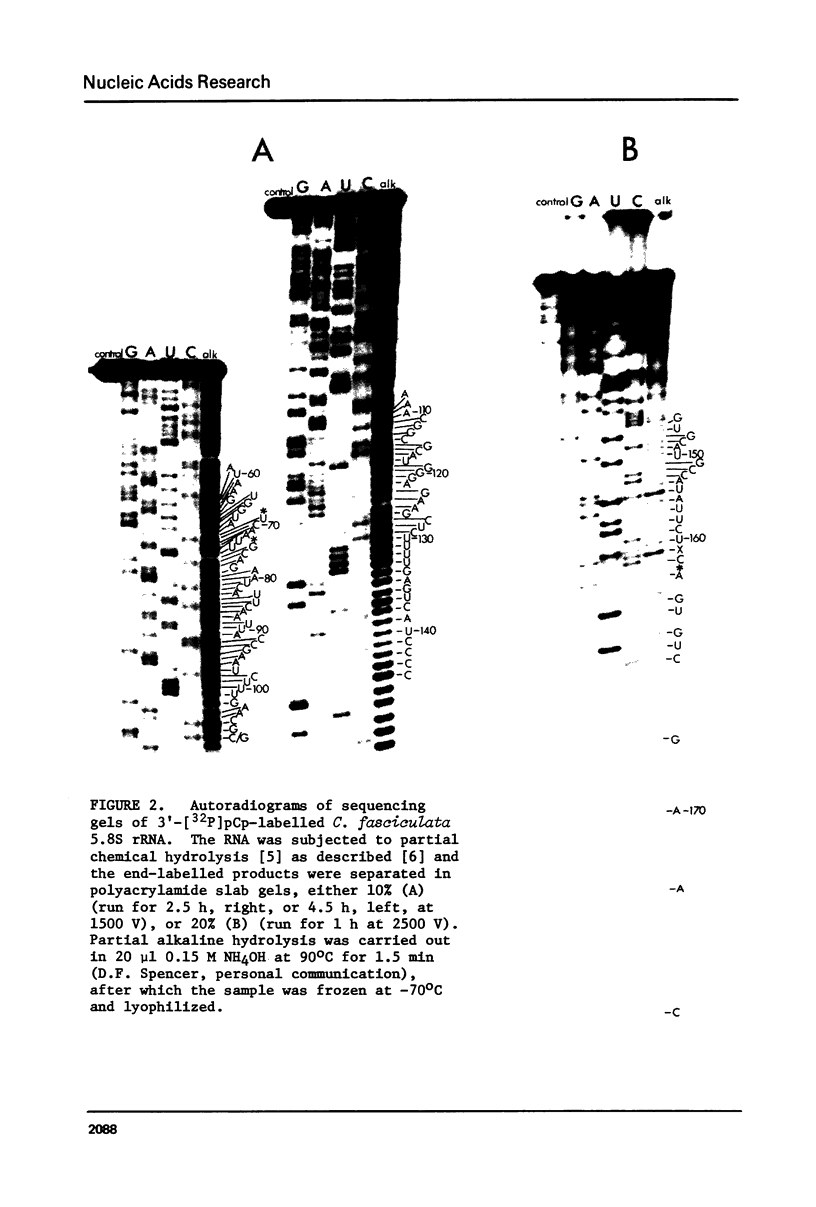
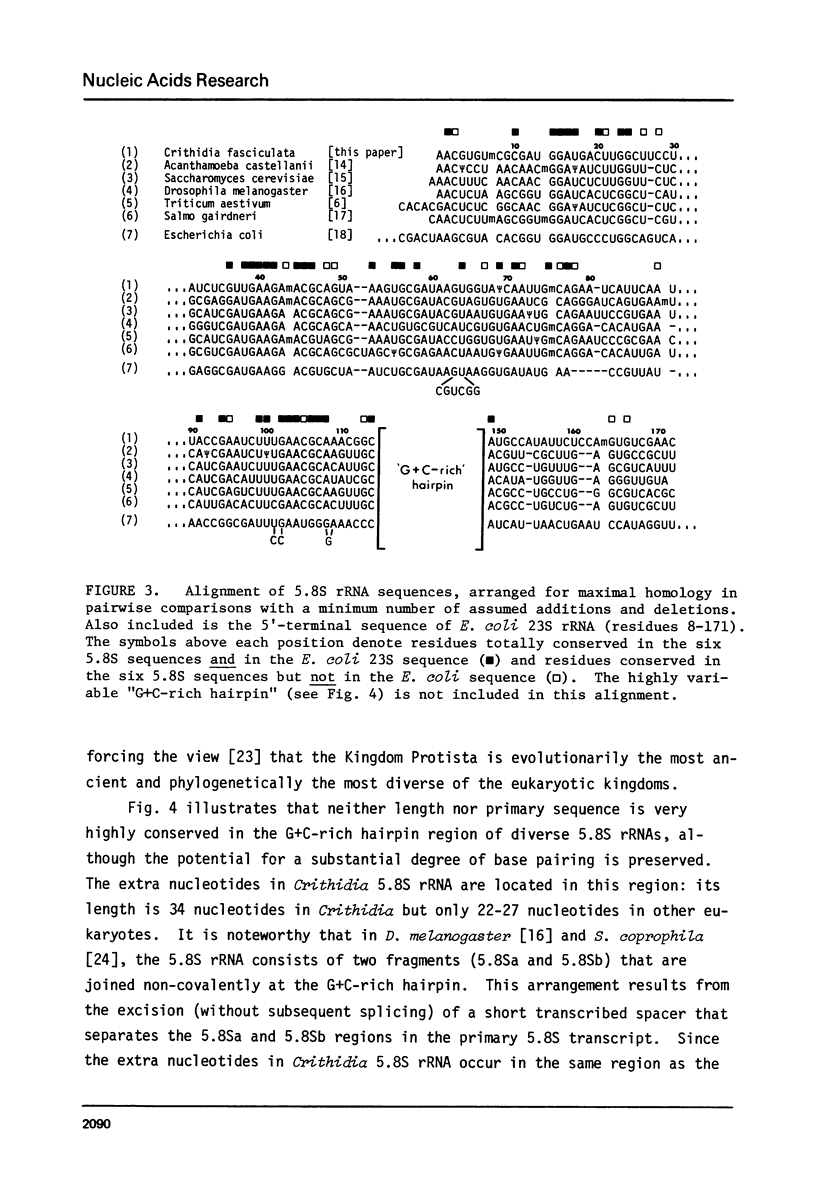
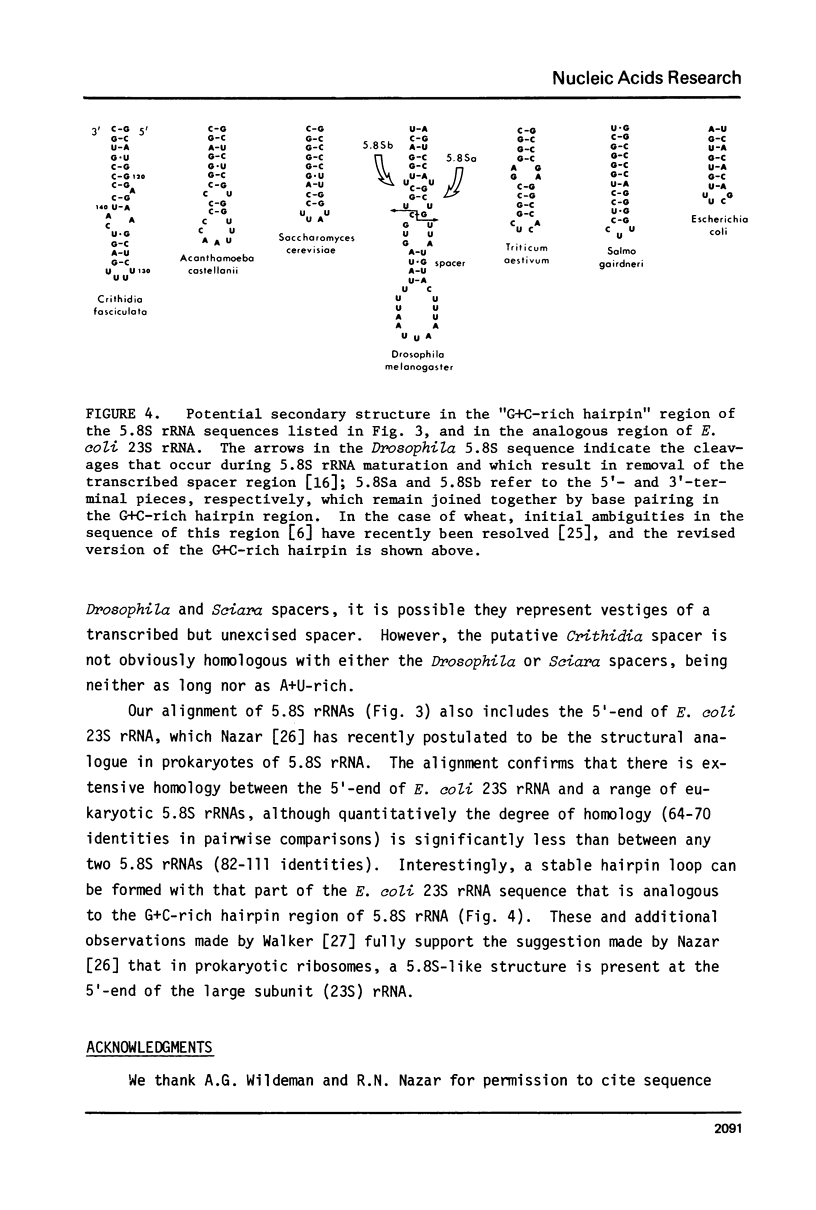
In Crithidia fasciculata, a trypanosomatid protozoan, the large ribosomal subunit contains five small RNA species (e, f, g, i, j) in addition to 5S rRNA [Gray, M.W. (1981) Mol. Cell. Biol. 1, 347-357]. The complete primary sequence of species i is shown here to be pAACGUGUmCGCGAUGGAUGACUUGGCUUCCUAUCUCGUUGA ... AGAmACGCAGUAAAGUGCGAUAAGUGGUApsiCAAUUGmCAGAAUCAUUCAAUUACCGAAUCUUUGAACGAAACGG ... CGCAUGGGAGAAGCUCUUUUGAGUCAUCCCCGUGCAUGCCAUAUUCUCCAmGUGUCGAA(C)OH. This sequence establishes that species i is a 5.8S rRNA, despite its exceptional length (171-172 nucleotides). The extra nucleotides in C. fasciculata 5.8S rRNA are located in a region whose primary sequence and length are highly variable among 5.8S rRNAs, but which is capable of forming a stable hairpin loop structure (the "G+C-rich hairpin"). The sequence of C. fasciculata 5.8S rRNA is no more closely related to that of another protozoan, Acanthamoeba castellanii, than it is to representative 5.8S rRNA sequences from the other eukaryotic kingdoms, emphasizing the deep phylogenetic divisions that seem to exist within the Kingdom Protista.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branlant C., Krol A., Machatt M. A., Ebel J. P. Structural study of ribosomal 23 S RNA from Escherichia coli. FEBS Lett. 1979 Nov 1;107(1):177–181. doi: 10.1016/0014-5793(79)80490-1. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann V. A. Collection of published 5S and 5.8S RNA sequences and their precursors. Nucleic Acids Res. 1981 Jan 10;9(1):r25–r42. doi: 10.1093/nar/9.1.213-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. W. The ribosomal RNA of the trypanosomatid protozoan Crithidia fasciculata: physical characteristics and methylated sequences. Can J Biochem. 1979 Jun;57(6):914–926. doi: 10.1139/o79-111. [DOI] [PubMed] [Google Scholar]
- Jordan B. R., Latil-Damotte M., Jourdan R. Coding and spacer sequences in the 5.8S-2S region of Sciara coprophila ribosomal DNA. Nucleic Acids Res. 1980 Aug 25;8(16):3565–3573. doi: 10.1093/nar/8.16.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay R. M., Gray M. W., Doolittle W. F. Nucleotide sequence of Crithidia fasciculata cytosol 5S ribosomal ribonucleic acid. Nucleic Acids Res. 1980 Nov 11;8(21):4911–4917. doi: 10.1093/nar/8.21.4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay R. M., Spencer D. F., Doolittle W. F., Gray M. W. Nucleotide sequences of wheat-embryo cytosol 5-S and 5.8-S ribosomal ribonucleic acids. Eur J Biochem. 1980 Dec;112(3):561–576. doi: 10.1111/j.1432-1033.1980.tb06122.x. [DOI] [PubMed] [Google Scholar]
- Nazar R. N. A 5.8 S rRNA-like sequence in prokaryotic 23 S rRNA. FEBS Lett. 1980 Oct 6;119(2):212–214. doi: 10.1016/0014-5793(80)80254-7. [DOI] [PubMed] [Google Scholar]
- Nazar R. N., Roy K. L. Nucleotide sequence of rainbow trout (Salmo gairdneri) ribosomal 5.8 S ribonucleic acid. J Biol Chem. 1978 Jan 25;253(2):395–399. [PubMed] [Google Scholar]
- Nazar R. N., Sitz T. O. Role of the 5'-terminal sequence in the RNA binding site of yeast 5.8 S rRNA. FEBS Lett. 1980 Jun 16;115(1):71–76. doi: 10.1016/0014-5793(80)80729-0. [DOI] [PubMed] [Google Scholar]
- Nishikawa K., Takemura S. Nucleotide sequence of 5 S RNA from Torulopsis utilis. FEBS Lett. 1974 Mar 15;40(1):106–109. doi: 10.1016/0014-5793(74)80904-x. [DOI] [PubMed] [Google Scholar]
- Nishimura S., Harada F., Narushima U., Seno T. Purification of methionine-, valine-, phenylalanine- and tyrosine-specific tRNA from Escherichia coli. Biochim Biophys Acta. 1967 Jun 20;142(1):133–148. doi: 10.1016/0005-2787(67)90522-9. [DOI] [PubMed] [Google Scholar]
- Pavlakis G. N., Jordan B. R., Wurst R. M., Vournakis J. N. Sequence and secondary structure of Drosophila melanogaster 5.8S and 2S rRNAs and of the processing site between them. Nucleic Acids Res. 1979 Dec 20;7(8):2213–2238. doi: 10.1093/nar/7.8.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogg H., Brambilla R., Keith G., Staehelin M. An improved method for the separation and quantitation of the modified nucleosides of transfer RNA. Nucleic Acids Res. 1976 Jan;3(1):285–295. doi: 10.1093/nar/3.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M. The nucleotide sequence of Saccharomyces cerevisiae 5.8 S ribosomal ribonucleic acid. J Biol Chem. 1973 Jun 10;248(11):3860–3875. [PubMed] [Google Scholar]
- SINGH H., LANE B. G. THE ALKALI-STABLE DINUCLEOTIDE SEQUENCES IN 18S+28S RIBONUCLEATES FROM WHEAT GERM. Can J Biochem. 1964 Jul;42:1011–1021. doi: 10.1139/o64-112. [DOI] [PubMed] [Google Scholar]
- Simoncsits A., Brownlee G. G., Brown R. S., Rubin J. R., Guilley H. New rapid gel sequencing method for RNA. Nature. 1977 Oct 27;269(5631):833–836. doi: 10.1038/269833a0. [DOI] [PubMed] [Google Scholar]
- Walker W. F. Proposed sequence homology between the 5'-end regions of prokaryotic 23 S rRNA and eukaryotic 28 S rRNA. Relevance to the hypothesis that 5.8 S rRNA is homologous to the 5'-end region of 23 S rRNA. FEBS Lett. 1981 Apr 20;126(2):150–151. doi: 10.1016/0014-5793(81)80228-1. [DOI] [PubMed] [Google Scholar]
- Whittaker R. H. New concepts of kingdoms or organisms. Evolutionary relations are better represented by new classifications than by the traditional two kingdoms. Science. 1969 Jan 10;163(3863):150–160. doi: 10.1126/science.163.3863.150. [DOI] [PubMed] [Google Scholar]
- Wrede P., Erdmann V. A. Escherichia coli 5S RNA binding proteins L18 and L25 interact with 5.8S RNA but not with 5S RNA from yeast ribosomes. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2706–2709. doi: 10.1073/pnas.74.7.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]