Abstract
Vascular endothelial growth factor (VEGF) is an angiogenic factor that also functions as an autocrine growth factor for VEGFR-2+ melanomas. In multiple studies VEGFR-2 was detected by immunostaining in 78 to 89% of human melanoma cells, suggesting most melanoma patients would benefit from anti-VEGF therapy. Here, we evaluated 167 human melanoma specimens in a tissue microarray (TMA) to verify the presence of VEGFR-2, but found disparities in staining with commercial antibodies A-3 and 55B11. Antibody A-3 stained melanoma cells in 79% of specimens, consistent with published results, but we noted extensive non-specific staining of other cells such as smooth muscle and histiocytes. In contrast, antibody 55B11 stained melanoma cells in only 7% (95% CI: 3.3 – 11.5) of specimens. As an internal positive control for VEGFR-2 detection, vascular endothelial cells stained with antibody 55B11 in all specimens. We compared VEGFR-2+ and VEGFR-2neg melanoma cell lines by immunoblotting and immunohistochemistry after siRNA knockdown and transient over expression of VEGFR-2 to validate antibody specificity. Immunoblotting revealed A-3 primarily cross-reacted with several proteins in both cell lines and these were unaffected by siRNA knockdown of VEGFR-2. In contrast, 55B11 staining of VEGFR-2+ cells was mostly eliminated by siRNA knockdown of VEGFR-2, and increased in VEGFR-2neg melanoma cell lines following transfection to express ectopic VEGFR-2. Our results show relatively few melanoma cells (<10%) express detectable levels of VEGFR-2, therefore the majority of melanoma patients are unlikely to benefit from anti-proliferative effects of anti-VEGF therapy.
Keywords: siRNA, immunohistochemistry, Tissue Microarray, autocrine, bevacizumab
Introduction
Melanoma is an increasingly common cancer and a major cause of cancer-related death.1, 2 Given its low response rate to existing therapies, there is a need for new approaches to combat systemic disease. One such approach is to disrupt angiogenesis. Vascular endothelial growth factor (VEGF) plays a key role in stimulating angiogenesis by binding VEGF receptors (VEGFR) on endothelial cells. In addition to the potential clinical value of blocking angiogenesis, anti-VEGF therapies may inhibit melanoma cell proliferation directly by interrupting autocrine signaling that involves activation of VEGF receptors on the melanoma cells themselves.3
There are three primary VEGF receptors,4, 5 of which VEGFR-2 is the dominant effector of VEGF function on most cells and is the most relevant in the metastatic melanoma microenvironment.6 Activation of VEGFR-2, a receptor tyrosine kinase, contributes to cell proliferation, survival, migration, and a host of other functions.7, 8 Multiple studies have reported that human metastatic melanoma cells themselves express VEGFR-2 in 78–89% of tumors.9–13 In addition, VEGFR-2 is expressed in about half of human melanoma cell lines.3 When considering the clinical translation of our in vitro findings on autocrine VEGF/VEGFR-2 signaling, we examined VEGFR-2 expression in melanoma specimens and were surprised to find only 7% (instead of 78–89%) of metastatic melanomas were positive for VEGFR-2. We have explored this discrepancy by testing commercial antibodies for VEGFR-2. A commercial antibody with demonstrated specificity for VEGFR-2 shows that VEGFR-2 expression is relatively rare in metastatic melanoma specimens.
Materials and Methods
Cell Culture
Melanoma cell lines used in this study were derived from tumors and cultured as described previously.3, 14–16
Immunoblotting
Cells from melanoma lines were harvested and extracts prepared as described previously.3, 15 Proteins (20 ug/lane) were resolved by SDS-PAGE using 4–12 % gradient gels and transferred to low fluorescence Immobilon-P (Millipore). Membranes were blocked in Odyssey blocking buffer (Licor, Lincoln, NE) and probed with antibodies in Odyssey blocking buffer with 0.1% Tween 20. Fluorescent-conjugated secondary antibodies were detected using the Licor Odyssey infrared imaging system and the images were quantitated using Odyssey application software Version 3.0.
Antibodies
Anti-VEGFR-2 antibodies were purchased from Santa Cruz (clone A-3, catalog #sc-6251) and Cell Signaling (clone 55B11; catalog #2479). Beta-Actin Antibody was purchased from VWR. Anti-mouse IgG, IRDye conjugated purified immunoglobulin from goat and anti-rabbit IgG, IRDye conjugated purified immunoglobulin from goat were purchased from LiCor Biosciences.
Transfection experiments
Small interfering RNA (siRNA) oligonucleotides that target VEGFR-2 (ON-TARGETplus SMARTpool) and a nontargeting siRNA pool were purchased from Millipore and transfected with the Amaxa nucleofector kit, as described previously.3 A plasmid encoding full-length VEGFR-2 was purchased from Origene (cat. #SC118716) and transiently transfected with the Amaxa nucleofector kit as described above. Cells were aliquoted for harvesting into lysates for immunoblotting as described above or made into cytospins with 100,000 cells per cytospin. Cytospin slides were formalin fixed for 10 min and air-dried for 15 min. Immunohistochemical staining was performed on the cytospins under the same staining conditions appropriate to each antibody, as described below for melanoma tissue specimens.
Tissue MicroArrays (TMAs)
TMAs were constructed from tissue cores of 1.0 mm diameter from the tumor areas and transferred into a recipient paraffin block using a semiautomated tissue array instrument (TMArrayer, Pathology Devices, Westminster, MD). Quadruplicate 1-mm-diameter tissue cores were taken from each specimen. Eight composite TMA blocks containing 18–22 specimens each were created. Sequential 4 μm sections from the TMA blocks were cut for H&E staining and immunohistochemical staining. TMAs containing the 43 metastatic melanoma specimens, shown in Figure 1 and summarized in Table 1, were prepared from 42 patients. Of the 42 patients, 15 were female and 27 were male. Patients' ages varied from 46 to 84, with a mean age of 61±15. The location of the melanoma metastases was lymph nodes (13 samples), skin and soft tissue (26 samples) and small intestine (3 samples).
Figure 1.
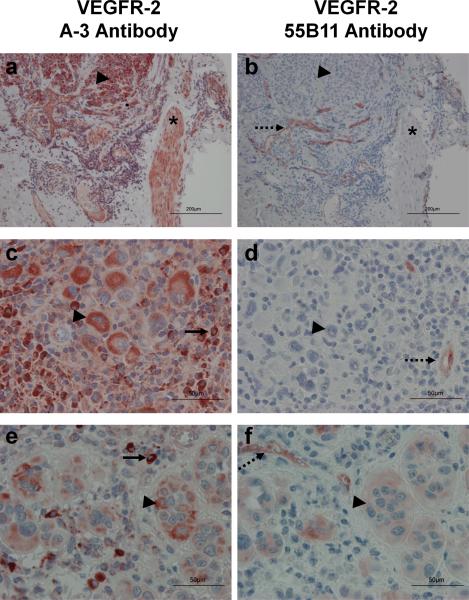
Immunohistochemistry of melanoma TMAs. TMAs were processed and stained as described under materials and methods with antibody A-3 (panels a, c, and e), or antibody 55B11 (panels b, d, and f). Endothelial cells serve as an internal positive control for VEGFR-2 (dashed arrows). Smooth muscle cells (*), histiocytes (solid arrows), and melanoma cells (arrowheads) are indicated. (Magnification, a and b: X100, scale bar = 200 um; c, d, e and f: X400 and scale bar = 50 um).
Table 1.
Immunohistochemical staining of melanoma cells on Tissue Microarrays (TMAs) comparing VEGFR-2 Antibody A-3 versus VEGFR-2 Antibody 55B11
| VEGFR-2 | A-3 Antibody | 55B11 Antibody |
|---|---|---|
| Positive | 34 (79%) | 4 (9%) |
| + | 24 | 4 |
| + − ++ | 5 | 0 |
| ++ | 4 | 0 |
| ++ − +++ | 1 | 0 |
| +++ | 0 | 0 |
| Negative | 9 (21%) | 39 (91%) |
|
| ||
| Total number tumor specimens | 43 | 43 |
Immunohistochemistry and Scoring of Staining
Immunohistochemistry with human anti-VEGFR-2 (A-3 or 55B11) used VECTASTAIN Elite ABC (peroxidase; Vector Laboratories, Burlingame, CA). TMA tissue sections were deparaffinized in xylene and rehydrated by sequential incubation in ethanol/water solutions. Antigen retrieval was performed at: pH=6 at 95°C for 20 min using the citrate-based Vector antigen unmasking solution (Cat. No. H-3300) for antibody A-3 and pH=9, at 125°C for 30 sec using the High pH Vector antigen unmasking solution (Cat. No. H-3301) for antibody 55B11 in a PASCAL pressure cooker system (Dako, Carpinteria, CA). In other experiments, staining was done with each antibody following identical antigen retrieval protocol. Samples were blocked with serum for 20 min and incubated 0.5% H2O2 in PBS for 10 min to reduce endogenous peroxidase activity. Sections were incubated with antibody A-3 (1:200) or antibody 55B11 (1:300) in a moist chamber at 4°C overnight, then with biotinylated secondary antibody for 30 min and with streptavidin-peroxidase complex for 30 min. Sections were developed with Vector AEC substrate kit, rinsed with water, counterstained with Hemotoxylin (Vector Laboratories, Burlingame, CA) and cover slip was applied with aqueous mounting medium (VectaMount, Vector Laboratories, Burlingame, CA). Images were obtained using an Olympus BX51 microscope coupled to an Olympus BP70 digital camera (Olympus America Inc, Center Valley, PA), and software Image ProPlus 4.5 for Windows. Intensity of the staining was scored by a pathologist. The intensity was scored as: 0, negative staining; +/−, weak staining; +, mild staining; ++, moderate staining; and +++, intense staining. Staining distribution was also noted as rare; when staining was present in rare cells, focal; when staining was present in some areas, or diffuse; when staining was present in >80% of the tissue section.
Human Subjects
All of the research involving human subjects was approved by the University of Virginia's IRB (Human Investigation Committee, HIC 5202 and HIC 10598) in accordance with assurances filed with and approved by the Department of Health and Human Services.
Results
Immunohistochemistry of melanoma tissue microarrays for VEGFR-2
To estimate the frequency of VEGFR-2 expression on human melanoma cells, we stained matched TMAs containing 43 metastatic melanoma samples with commercially-available antibodies: A-311–13 and 55B113. The results are summarized in Table 1, and representative microscopic images are shown in Figure 1. Endothelial cells, which serve as an internal positive control for staining of endogenous VEGFR-2 (reviewed in17 and18), stained with antibody 55B11, but showed no staining or very weak staining with antibody A-3 (Figure 1). On the other hand, antibody A-3 showed strong staining of vascular smooth muscle (panel a) and histiocytes (panel c) whereas antibody 55B11 did not stain either histiocytes or smooth muscle cells (panels b and d). For clarity, higher magnification images of melanoma cells stained positive for VEGFR-2 with antibodies A-3 and 55B11 are shown in Figure 1, panels e and f. Antibody A-3 stained melanoma cells in 79% of the specimens, but antibody 55B11 only stained 9% (Table 1). The staining intensity on melanoma cells with antibody A-3 varied from 1+ to 3+ whereas the positive samples with antibody 55B11 only showed 1+ staining. These results revealed disparities in staining by these commercial antibodies and prompted a broader census of melanomas and more thorough examination of antibody specificity.
To analyze VEGFR-2 expression on melanoma cells among melanoma metastases, we used antibody 55B11 to stain additional TMA blocks for a total of 167 melanoma samples from 133 patients (58 female, 75 male, ages 23 – 90; mean 59±16). The tumor samples on the TMAs included 9 primary cutaneous and 158 metastatic melanomas. Metastatic melanomas were located in lymph node (57), skin and soft tissue (93), small intestine (7), and peritoneum (1). Melanoma cell morphology was epithelioid in 133 samples, spindle in 7, and mixed (epithelioid and spindle) in 27.
Melanoma cells from only 11 of the 167 specimens immunostained for VEGFR-2 (7%; 95% exact binomial confidence interval: 3.3, 11.5). These 11 specimens were from 11 different patients (11/133 = 8%) and all had only 1+ staining (Table 2). The mean age and sex of the 11 patients with VEGFR-2+ melanomas, as well as the location and histology of the melanomas are listed in Table 2. Our conclusion was that VEGFR-2 is rarely expressed by melanoma cells in tumors, and only at relatively low levels. This contrasts with previous reports that a large majority of melanomas express VEGFR-2.
Table 2.
VEGFR-2 immunohistochemical staining of melanoma specimens and demographics of patients positive for VEGFR-2 from 8 TMAs using VEGFR-2 55B11 Antibody
| VEGFR-2 (55B11 Antibody) | Number (%) |
|---|---|
| Positive | 11 (7%) |
| Rare + | 1 |
| Focal + | 2 |
| +/− | 3 |
| + | 5 |
| Negative | 156 (93%) |
|
| |
| Total patient tumor specimens | 167 |
| Demographics of 11 patients with melanomas positive for VEGR-2 | |
|---|---|
| Age (mean±SD) | 55.58±15.68 |
|
| |
| Sex | |
| Male | 5 |
| Female | 6 |
|
| |
| Melanoma Location | |
| Primary | 1 |
| Metastatic | 10 |
| Lymph Node | 4 |
| Skin/Soft Tissue | 5 |
| Small Intestine | 1 |
|
| |
| Histology | |
| Epithelioid | 10 |
| Spindle | 1 |
| Mixed | 0 |
Immunoblotting melanoma cell extracts for VEGFR-2
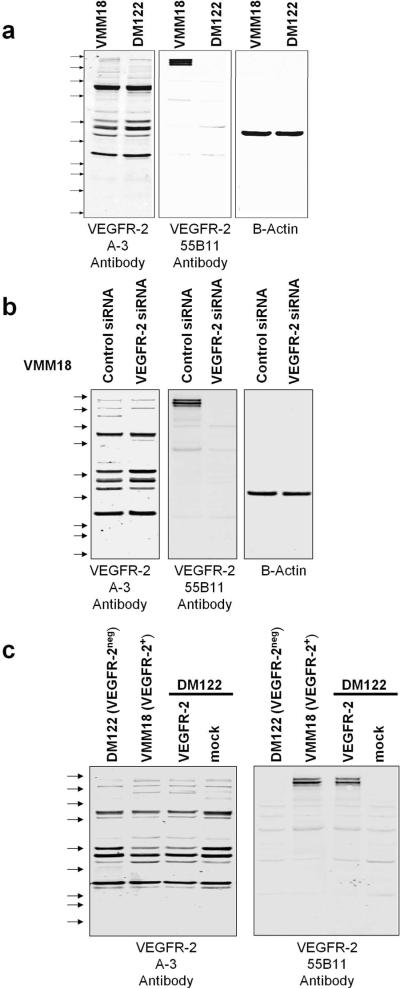
To validate the specificity of the anti-VEGFR-2 antibodies, we performed immunoblotting with A-3 and 55B11. Extracts of melanoma cell lines that either express or do not express VEGFR-2 (VMM18 and DM122,3 respectively) were analyzed by immunoblotting (Figure 2a). There was no obvious difference between VMM18 and DM122 cells in the overall pattern of bands prominently stained by antibody A-3. Antibody A-3 does stain a band corresponding to the size of VEGFR-2 in the VMM18 extracts. However, most of the immunostaining intensity with antibody A-3 was in other bands between 25 and 100 kDa that were common to VMM18 and DM122 cells. In contrast, with antibody 55B11 a dominant triplet of proteins (210–230 kDa) corresponding to VEGFR-2 was immunostained in extracts of VMM18 and these bands were not seen with DM122 cells.3
Figure 2.
Immunoblotting VEGFR-2 in melanoma cell extracts. a), Triplicate samples of 20 ug protein of VMM18 (VEGFR-2+) and DM122 (VEGFR-2neg) melanoma cell line extracts were resolved on the same gel and transferred to Immobilon. Separate sections of the filter were immunoblotted with antibodies A-3, 55B11, or Beta Actin as loading control. The positions of Precision Plus Kaleidoscope standards are shown by the arrows. Top to bottom, they are 250, 150, 100, 75, 50, 37, 25, 20, 15, and 10 kDa. b) VMM18 cells were transfected with control siRNA or siRNA to VEGFR-2 and aliquots of 20 ug protein were resolved on the same gel and analyzed by immunoblotting with antibodies A-3, 55B11, and Beta Actin as loading control. c) DM122 cells were mock transfected or transfected with a plasmid encoding VEGFR-2 and aliquots of 20 ug protein were resolved and analyzed by immunoblotting with antibodies A-3 and 55B11.
We transfected VMM18 cells with siRNA to knockdown endogenous VEGFR-2. Nonspecific siRNA was transfected into a separate sample of VMM18 cells as a control. Cell extracts were prepared and split into 3 aliquots that were resolved on the same gel, transferred onto a filter that was cut into strips and these were immunoblotted with different antibodies. Immunoblotting showed knockdown of VEGFR-2 dramatically reduced staining of the 210–230 kDa bands with antibody 55B11, whereas immunoblotting of the several bands by antibody A-3 was not affected (Figure 2b). This indicated that the major bands stained by antibody A-3 were not fragments of VEGFR-2 and instead were cross-reactive proteins endogenous to the melanoma cells. This experiment was independently replicated with identical results. We concluded that antibody 55B11 primarily stained VEGFR-2 whereas antibody A-3 primarily reacted with several other proteins.
We transiently transfected VEGFR-2neg DM122 melanoma cells with a plasmid to express full-length VEGFR-2. Cell extracts were prepared and split into 3 aliquots that were resolved on the same gel and immunoblotted with the A-3 and 55B11 VEGFR-2 antibodies (Figure 2c). Blotting for beta-actin was used as a loading control (not shown). Immunoblotting revealed multiple bands with the A-3 antibody with 95% of the total staining intensity in bands <150 kDa. However, immunoblotting with the 55B11 antibody showed a pair of bands at approximately 210 and 230 kDa corresponding to VEGFR-2, accounting for > 90% of the total staining intensity. These data demonstrate that the 55B11 antibody has high specificity for VEGFR-2, whereas the A-3 antibody predominantly reacts with other endogenous protein in melanoma cells.
Immunohistochemistry of melanoma cell extracts for VEGFR-2
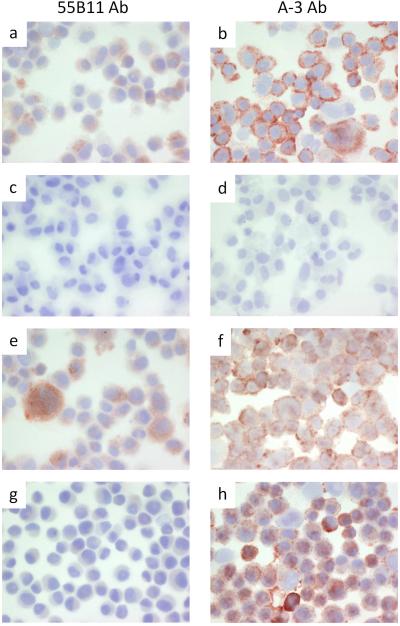
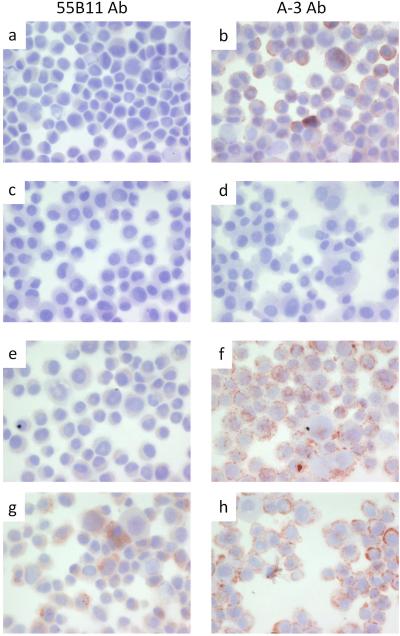
We compared immunostaining of the VEGFR-2+ VMM18 melanoma cell line transiently transfected with siRNA to knockdown VEGFR-2 (Figure 3) and the DM122 VEGFR-2-null melanoma cell line expressing ectopic full-length VEGFR-2 (Figure 4). Cytospins were prepared to deposit the cells, which were stained with the 55B11 antibody (Figures 3 and 4, panels a, e, and g) vs. the A-3 antibody (Figures 3 and 4, panels b, f, and h). As negative controls, samples were stained the same, but omitting incubation with primary antibody (Figures 3 and 4, panels c and d). Untreated cells were used as additional control (Figures 3 and 4, panels a and b). In Figure 3, VMM18 cells transfected with the non-specific control siRNA stained positive with both the 55B11 and A-3 antibodies, as expected (Figure 3, panels e and f). VMM18 cells transfected with siRNA to target VEGFR-2 were no longer stained with the 55B11 antibody (Figure 3, panel g) but remained positive for staining with the A-3 antibody (Figure 3, panel h). In Figure 4, the VEGFR-2 negative DM122 cells that were mock transfected (panels e and f) did not stain with the 55B11 antibody, but did stain positive with the A-3 antibody. However, DM122 cells expressing full-length VEGFR-2 showed a difference from control and now stained positive with the 55B11 antibody. In contrast, there was no difference in staining with the A-3 antibody between DM122 cells (Figure 4, panels g and h). Thus, immunohistochemical staining of the melanoma cell lines (Figures 3 and 4) matched the immunoblotting results (Figure 2b and 2c) and showed that only staining with 55B11 antibody and not staining with the A-3 antibody was affected by either knockdown or over expression of VEGFR-2.
Figure 3.
Immunohistochemistry of VMM18 melanoma cells. Cytospins were processed and stained as described under materials and methods with antibody 55B11 (panels a, c, e, and g), or antibody A-3 (panels b, d, f, and h). VMM18 melanoma cells alone (positive control) are shown in panels a and b. VMM18 melanoma cells without primary antibody (negative control) are shown in panels c and d. VMM18 melanoma cells transfected with non-specific control siRNA are shown in panels e and f. VMM18 melanoma cells transfected with siRNA to VEGFR-2 are shown in panels g and h.
Figure 4.
Immunohistochemistry of DM122 melanoma cells. Cytospins were processed and stained as described under materials and methods with antibody 55B11 (panels a, c, e, and g), or antibody A-3 (panels b, d, f, and h). DM122 melanoma cells alone (positive control) are shown in panels a and b. DM122 melanoma cells without primary antibody (negative control) are shown in panels c and d. DM122 melanoma cells mock transfected are shown in panels e and f. DM122 melanoma cells transfected with plasmid encoding VEGFR-2 are shown in panels g and h.
Discussion
We set out to examine VEGFR-2 expression in melanomas by immunohistochemistry because of our interest in using the anti-VEGF antibody bevacizumab as a clinical therapeutic agent.19 We found VEGFR-2+ melanoma cells in only 7% human melanoma specimens, an unexpected result that challenges multiple reports in the literature. Previous reports of VEGFR-2 expression in a majority of human metastatic melanomas used a commercial antibody, but did not demonstrate its specificity.11–13 The discrepancy between previous reports and our present studies can be attributed to differences in the commercially-available antibodies used to detect VEGFR-2. Here we used both immunoblotting and immunohistochemistry of different melanoma cell lines plus knockdown of VEGFR-2 by siRNA and knock-in with full-length VEGFR-2 to validate the specificity of antibody 55B11 for VEGFR-2. Our conclusion is that prior reports overestimated the expression of VEGFR-2 in melanoma cells because the A-3 antibody used in immunohistochemistry cross-reacted with other proteins. This situation of non-specific staining for biomarker proteins is not unprecedented. For example, two commonly used monoclonal antibodies to Foxp3 give significantly different staining patterns with formalin-fixed, paraffin-embedded sections.20 Additionally, two commonly used VEGFR-3 antibodies showed different immunoblotting results with more than 60 cell lines and different staining patterns in more than 450 tissues.21
Based on the results of our TMA analysis, only very few melanoma cells in human tumors express VEGFR-2, and only at a relatively low level. While previous studies indicated that a majority of human melanomas express VEGFR-2, it also had been reported that up to 50% of melanomas do not express VEGFR-2.22 Additionally, efforts to utilize VEGFR-2 as a target in melanoma have had limited success. Inhibition of VEGFR-2 had no significant influence on growth and metastasis of B16 melanoma.23 Furthermore, Sorafenib, a small molecule inhibitor which targets both B-Raf and VEGFR-2, has shown promise in other cancers known to be driven by VEGF,24 but has been relatively ineffective as a single agent in melanoma. These studies cast doubt on the utility of VEGFR-2 as an effective target in melanoma. Nevertheless, identification of a VEGFR-2 positive subset of melanomas still presents an opportunity to personalize therapy using bevacizumab and other tyrosine kinase inhibitors that specifically target VEGFR-2 dependent signaling. However, in patients unselected for VEGFR-2 expression by the melanoma cells, the potential value of anti-VEGF therapy in melanoma is likely to be mediated primarily by blocking angiogenesis, rather than by inhibiting autocrine growth signaling.
Acknowledgements
The authors declare no conflicts. We thank the members of the Slingluff laboratory for helpful discussions and Mark Smolkin for help with statistical analysis. We thank Dr. Jochen Schaefer and the UVA Biorepository and Tissue Research Facility for their assistance in generating the TMAs. This work was partly supported by grant GM56362 to Dr. David L. Brautigan from USPHS NCI. Kerrington R. Molhoek, Ph.D. was supported by the American Cancer Society, California Division Campaign for Research 2007 Postdoctoral Fellowship. Support was also provided by the NIH/NCI grant R01 CA57653 to Dr. Craig L. Slingluff, Jr. and the University of Virginia Cancer Center Support Grant (NIH/NCI P30 CA44579). Partial support was also provided by a gift from the Commonwealth Foundation for Cancer Research and by the James and Rebecca Craig Foundation.
References
- 1.Diepgen TL, Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002;146(Suppl 61):1–6. doi: 10.1046/j.1365-2133.146.s61.2.x. [DOI] [PubMed] [Google Scholar]
- 2.Lachiewicz AM, Berwick M, Wiggins CL, Thomas NE. Survival differences between patients with scalp or neck melanoma and those with melanoma of other sites in the Surveillance, Epidemiology, and End Results (SEER) program. Arch Dermatol. 2008;144(4):515–21. doi: 10.1001/archderm.144.4.515. [DOI] [PubMed] [Google Scholar]
- 3.Molhoek KR, Griesemann H, Shu J, Gershenwald JE, Brautigan DL, Slingluff CL., Jr. Human melanoma cytolysis by combined inhibition of mammalian target of rapamycin and vascular endothelial growth factor/vascular endothelial growth factor receptor-2. Cancer Res. 2008;68(11):4392–7. doi: 10.1158/0008-5472.CAN-07-5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer RD, Mohammadi M, Rahimi N. A single amino acid substitution in the activation loop defines the decoy characteristic of VEGFR-1/FLT-1. J Biol Chem. 2006;281(2):867–75. doi: 10.1074/jbc.M506454200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wissmann C, Detmar M. Pathways targeting tumor lymphangiogenesis. Clin Cancer Res. 2006;12(23):6865–8. doi: 10.1158/1078-0432.CCR-06-1800. [DOI] [PubMed] [Google Scholar]
- 6.Rawlings NG, Simko E, Bebchuk T, Caldwell SJ, Singh B. Localization of integrin alpha(v)beta3 and vascular endothelial growth factor receptor-2 (KDR/Flk-1) in cutaneous and oral melanomas of dog. Histol Histopathol. 2003;18(3):819–26. doi: 10.14670/HH-18.819. [DOI] [PubMed] [Google Scholar]
- 7.Kim DW, Lu B, Hallahan DE. Receptor tyrosine kinase inhibitors as anti-angiogenic agents. Curr Opin Investig Drugs. 2004;5(6):597–604. [PubMed] [Google Scholar]
- 8.Brunelleschi S, Penengo L, Santoro MM, Gaudino G. Receptor tyrosine kinases as target for anti-cancer therapy. Curr Pharm Des. 2002;8(22):1959–72. doi: 10.2174/1381612023393530. [DOI] [PubMed] [Google Scholar]
- 9.Salven P, Heikkila P, Joensuu H. Enhanced expression of vascular endothelial growth factor in metastatic melanoma. Br J Cancer. 1997;76(7):930–4. doi: 10.1038/bjc.1997.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gitay-Goren H, Halaban R, Neufeld G. Human melanoma cells but not normal melanocytes express vascular endothelial growth factor receptors. Biochem Biophys Res Commun. 1993;190(3):702–8. doi: 10.1006/bbrc.1993.1106. [DOI] [PubMed] [Google Scholar]
- 11.Straume O, Akslen LA. Expresson of vascular endothelial growth factor, its receptors (FLT-1, KDR) and TSP-1 related to microvessel density and patient outcome in vertical growth phase melanomas. Am J Pathol. 2001;159(1):223–35. doi: 10.1016/S0002-9440(10)61688-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pisacane AM, Risio M. VEGF and VEGFR-2 immunohistochemistry in human melanocytic naevi and cutaneous melanomas. Melanoma Res. 2005;15(1):39–43. doi: 10.1097/00008390-200502000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Mehnert JM, McCarthy MM, Jilaveanu L, Flaherty KT, Aziz S, Camp RL, Rimm DL, Kluger HM. Quantitative expression of VEGF, VEGF-R1, VEGF-R2, and VEGF-R3 in melanoma tissue microarrays. Hum Pathol. 41(3):375–84. doi: 10.1016/j.humpath.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamshchikov GV, Mullins DW, Chang CC, Ogino T, Thompson L, Presley J, Galavotti H, Aquila W, Deacon D, Ross W, Patterson JW, Engelhard VH, et al. Sequential immune escape and shifting of T cell responses in a long-term survivor of melanoma. J Immunol. 2005;174(11):6863–71. doi: 10.4049/jimmunol.174.11.6863. [DOI] [PubMed] [Google Scholar]
- 15.Molhoek KR, Brautigan DL, Slingluff CL., Jr. Synergistic inhibition of human melanoma proliferation by combination treatment with B-Raf inhibitor BAY43-9006 and mTOR inhibitor Rapamycin. J Transl Med. 2005;3:39. doi: 10.1186/1479-5876-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darrow TL, Slingluff CL, Jr., Seigler HF. The role of HLA class I antigens in recognition of melanoma cells by tumor-specific cytotoxic T lymphocytes. Evidence for shared tumor antigens. J Immunol. 1989;142(9):3329–35. [PubMed] [Google Scholar]
- 17.Hristov M, Erl W, Weber PC. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol. 2003;23(7):1185–9. doi: 10.1161/01.ATV.0000073832.49290.B5. [DOI] [PubMed] [Google Scholar]
- 18.Holmes K, Roberts OL, Thomas AM, Cross MJ. Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal. 2007;19(10):2003–12. doi: 10.1016/j.cellsig.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3(5):391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 20.Woo YL, Sterling J, Crawford R, van der Burg SH, Coleman N, Stanley M. FOXP3 immunohistochemistry on formalin-fixed paraffin-embedded tissue: poor correlation between different antibodies. J Clin Pathol. 2008;61(8):969–71. doi: 10.1136/jcp.2008.056200. [DOI] [PubMed] [Google Scholar]
- 21.Petrova TV, Bono P, Holnthoner W, Chesnes J, Pytowski B, Sihto H, Laakkonen P, Heikkila P, Joensuu H, Alitalo K. VEGFR-3 expression is restricted to blood and lymphatic vessels in solid tumors. Cancer Cell. 2008;13(6):554–6. doi: 10.1016/j.ccr.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 22.Brychtova S, Bezdekova M, Brychta T, Tichy M. The role of vascular endothelial growth factors and their receptors in malignant melanomas. Neoplasma. 2008;55(4):273–9. [PubMed] [Google Scholar]
- 23.Gille J, Heidenreich R, Pinter A, Schmitz J, Boehme B, Hicklin DJ, Henschler R, Breier G. Simultaneous blockade of VEGFR-1 and VEGFR-2 activation is necessary to efficiently inhibit experimental melanoma growth and metastasis formation. Int J Cancer. 2007;120(9):1899–908. doi: 10.1002/ijc.22531. [DOI] [PubMed] [Google Scholar]
- 24.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]