Abstract
Background and Aim
We investigated the dietary and gender influences on the expression and functionality of cholangiocyte bile salt transporters and development of biliary hyperplasia in cholesterol gallstone-susceptible C57L/J and resistant AKR/J mice.
Methods
C57L and AKR mice were fed chow, a lithogenic diet, or a cholic acid-containing diet for 14 days. Expression of cholangiocyte bile salt transporter proteins ASBT (SLC10A2), ILBP (FABP6), and MRP3 (ABCC3) were studied by Western blot analysis. Taurocholate uptake studies were performed using microperfusion of isolated bile duct units. The pre- and post-perfusion taurocholate concentrations were analyzed by high performance liquid chromatography. Biliary proliferation in liver sections was scored.
Results
The lithogenic diet induced ductular proliferation in C57L mice. On chow, SLC10A2 and ABCC3 were overexpressed in male and female C57L compared to AKR mice. A lithogenic diet reduced the expressions of FABP6 in both male and female C57L mice, SLC10A2 in female C57L mice, and ABCC3 in male C57L mice. These alterations in transporter expressions were not associated with changes in taurocholate uptake. The lithogenic diet induced biliary hyperplasia and reduced bile salt transporter expressions in C57L mice.
Conclusions
Although bile salt uptake was not increased in the bile duct unit, we speculate that the biliary hyperplasia on the lithogenic diet may lead to an increase in intrahepatic bile salt recycling during cholesterol cholelithogenesis.
Keywords: bile salt transporters, cholangiocytes, cholehepatic shunting, cholelithiasis, cholesterol, proliferation
Introduction
Cholelithiasis affects an estimated 20–30% of Western populations and hence is a significant and costly health problem.1 Two inbred mouse strains C57L (cholesterol gallstone–susceptible) and AKR (cholesterol gallstone–resistant) are important animal models for studying the genetics and pathophysiology of cholesterol gallstone disease.2 C57L mice are characterized phenotypically by higher bile flow, higher bile salt, phospholipid, and especially cholesterol secretion rates, and larger bile salt pool sizes compared to AKR mice.3 On a lithogenic diet (containing 0.5% cholic acid, 1% cholesterol, and 15% dairy fat), C57L mice develop sustained hypersecretion of cholesterol compared to bile salt plus phospholipid, which leads to lithogenic supersaturation of gallbladder bile and rapid cholesterol crystal nucleation, and gallstone formation with C57L males displaying a higher prevalence compared to females.3 There were no gender differences in the prevalence of cholesterol gallstones in the AKR strain; therefore, the bile salt transport system in the female AKR mice were not studied. The possibility that intrahepatic recycling of bile salts might augment differences in cholesterol contents of bile between the two strains is an appealing supposition.
Several bile salt transporters have been identified on large cholangiocytes. These include the apical sodium dependent bile salt transporter (SLC10A2) also known as apical or ileal sodium-dependent bile acid transporter (ASBT or IBAT) located on the apical membrane4,5 and mapped to mouse chromosome 8;6 fatty-acid- binding protein subclass 6 (FABP6) also known as ileal lipid (bile acid) binding protein (ILBP or IBABP) in the cytosol7,8 and mapped to mouse chromosome 11;9 and putatively the multiple-drug-resistance-related protein isoform 3 (ABCC3 also known as MRP3), expressed on the basolateral membrane domain as evidenced by human10 and rat11,12 studies. On the basolateral and apical domains of hepatocytes, the two principal bile salt transporters are the sodium taurocholate cotransporter (SLC10A1 also known as NTCP) and the bile salt export pump (ABCB11 also known as BSEP).13,14 In the normal intrahepatic biliary tree, cholangiocytes are morphologically and functionally heterogeneous with water and solute transport occurring primarily in large cholangiocytes (16).
Based on previous observations of inducible biliary hyperplasia and increased circulating bile salt pool size,15,16 we hypothesized that increases in bile salt secretion rate in C57L compared to AKR mice might be promoted, in part, by augmented intrahepatic bile salt recycling via increased transporter expression or an increase in absorptive surface area of large cholangiocytes. A putative increase in bile salt secretion rate may in turn lead to enhanced cholesterol secretion, which would augment cholesterol supersaturation of bile. Here we examined the influence of a standard lithogenic diet, an identical diet containing cholic acid alone without cholesterol, plus gender and mouse strain on the induction of biliary hyperplasia as well as the expression and functionality of bile salt transporters on large cholangiocytes.
Methods
Animals
Male and female C57L and male AKR mice, 9 to 11 weeks of age, were purchased from The Jackson Laboratories (Bar Harbor, ME, USA). Animals were housed in a temperature-controlled environment of 20 to 22°C, with normal light–dark cycles of 12 h each. Animals were permitted free access to water, and were fed standard chow, or the lithogenic diet as described below. For histologic studies, male/female C57L mice and male AKR mice, were fed either regular chow or the lithogenic diet containing 0.5% cholic acid, 1% cholesterol, and 15% dairy fat for 14 days. For transporter protein expression and functionality studies, male/female C57L mice and male AKR mice were fed regular chow or the lithogenic diet for 14 days.
Isolation of cholangiocytes
Cholangiocyte isolation followed the method of Alpini et al.17 Briefly, fasting mice were anesthetized with sodium pentobarbital (50 mg/kg intraperitoneal [i.p.]). A laparotomy was performed and the peritoneal cavity was entered, and the portal vein was cannulated with a 20-gauge intravenous (i.v.) catheter. To remove blood cells prior to harvesting, livers were perfused via the portal vein with a preoxygenated sterile solution of 0.9% NaCl. The livers were then placed in a preoxygenated Hepes-buffered saline (HBS) buffer (20 mM Hepes, 150 mM NaCl [pH 7.4]); hepatocytes were removed first by gentle mechanical disruption of Glisson’s capsule. The portal tract segments were then digested with an enzyme solution containing RPMI-1640 supplemented with 0.03% collagenase XI, 0.02% DNAase, and 0.03% hyaluronidase (Sigma-Aldrich, St. Louis, MO, USA) for 10 min at 37°C. The remaining portal tract segments were washed with preoxygenated HBS buffer solution, and further microdissection was performed at higher magnification to remove residual hepatocytes. The biliary tree segments were redigested with 0.02% DNAase, 0.03% collagenase type XI, and 0.05% hyaluronidase for 10 min at 37°C, and further microdissected under higher magnification.
Western blot analysis
Freshly isolated portal tract units were homogenized on ice in a Teflon homogenizer in buffer solution containing protease-inhibitors (pepstatin A 0.2 ug/uL and phenylmethylsulphonyl fluoride 0.4 uM; leupeptin 0.5 ug/uL, Tris 2.5 mM, and EDTA 0.13 mM [pH 7.4]), then sedimented at a relative centrifugal force (RCF) of 2000 g for 10 min. The resulting pellet was discarded and the supernatant was sedimented again at an RCF of 2000 g for 10 min. Protein concentrations were determined using the Bradford method (Bio-Rad, Richmond, CA, USA) with bovine serum albumin as the reference standard.
For Western blot analysis of SLC10A2, FABP6, and ABCC3 transporters, 100 µg of protein homogenate were heated to 95°C for 5 min, and cooled on ice before the samples were loaded onto NuPage 4–12% Bis-Tris mini-gel (Invitrogen, Carlsbad, CA, USA) for electrophoresis. The gel was then transferred overnight to nitrocellulose membranes. The blots were then washed at room temperature with phosphate-buffered saline containing 0.1% Tween 20 and 5% non-fat dry milk for 1 h, followed by incubation with primary antibodies for SLC10A2, FABP6, and ABCC3 for 2 h. The SLC10A2 antibody was obtained from Research Genetics (Invitrogen) using immunization of rabbits with peptide synthesized according to the published sequence of Schneider et al.4 The FABP6 antibody was monoclonal generously donated by Dr. Luis Agellon (University of Alberta, Edmonton, AB, Canada). The ABCC3 antibody was a polyclonal antibody used in previous studies,18 kindly provided by Dr. Hiroshi Suzuki (University of Tokyo, Tokyo, Japan). The primary SLC10A2 antibody was incubated at 1 : 100 dilution, FABP6 antibody at 1 : 14 000 dilution, and ABCC3 antibody at 1 : 1000 dilution. Following these procedures, the membrane was incubated with 0.2-µg/mL goat anti-rabbit horseradish peroxidase–conjugated secondary antibody (Biosource International, Camarillo, CA, USA).
Protein size was estimated using prestained molecular weight standards (Superscript II RT protein ladder; Invitrogen). Immunoreactive bands were revealed with enhanced chemiluminescence light substrate (Amersham Biosciences, Piscataway, NJ, USA). The density of each band was read with a densitometeter (Biorad Life Sciences, Hercules, CA, USA) and quantified using Geldoc densitometer software (Biorad).
Histopathologic examination
Immediately after sacrifice, livers or fragments of liver tissue from experimental and control mice were placed in 10% buffered formalin. The formalin fixed tissue was embedded in paraffin, and 5-µm thick sections were stained with hematoxylin–eosin. Sections were reviewed by a hepatopathologist (J.N.G.) who was blinded to the mouse strain, gender, and diet. Bile ducts were quantified by counting the number of bile duct images per 100 portal tracts. Bile duct density greater than a normal of 1 per portal tract was considered to be in a proliferative phase.
Isolation of bile duct units and bile salt uptake studies
Bile salt uptake by cholangiocytes was studied using microperfusion of freshly isolated bile duct units (IBDU) employing previously published methods.19 IBDU are formed by large cholangiocytes; bile ductules are formed by small cholangiocytes. In brief, mice were anesthetized with pentobarbital sodium (50 mg/kg i.p.). A laparotomy was performed and the liver was perfused with sterile 0.9% NaCl solution through the portal vein at 4°C. Subsequently, liquid Trypan blue agar (2 to 3 mL) was injected into the portal vein. The liver was then removed and immersed in preoxygenated HBS buffer solution at 4°C. After the hepatic capsule and surface hepatocytes were removed, intrahepatic bile ducts were dissociated under a dissecting microscope using the portal vein (now filled with Trypan blue agar) as reference marker. The dissociated bile duct was digested by shaking at 37°C for 10 min in an enzyme solution as used for cholangiocyte isolation (vide supra). Further microdissection was performed at higher magnification to remove residual hepatocytes, fragments of portal veins and hepatic arteries, and excess connective tissue
The IBDUs were then perfused using a microperfusion apparatus previously described19 with perfusate containing Krebs–Ringer bicarbonate buffer (KRB) of the following composition: 120 mM NaCl, 5.9 mM KCl, 1.2 mM Na2HPO4, 5 mM glucose, 1.25 mM CaCl2, 1 mM MgSO4, and 25 mM NaHCO3. Purified sodium taurocholate was then added to the KRB solution to achieve a final concentration of 50 mM at 7.4 pH. The perfusion rate was 114 nL/min for 60 min in the microperfusion apparatus.20 Pre- and post-perfusates from the perfusion studies were collected and analyzed for taurocholate concentration using high performance liquid chromatography.
Statistical analysis
Data were analyzed using Student’s t-test. All results were expressed as mean ± standard error. P-values of less than 0.05 were considered to be significant.
Results
Histologic studies
Figure 1 displays representative liver histology of AKR and C57L on chow (Fig. 1a,b) and lithogenic diets (Fig. 1c,d), respectively. In the C57L strain, ductular proliferation became significantly more obvious than on the lithogenic diet with 31 ± 3 ductules per 100 portal tracts compared to 6 ± 2 ductules per 100 portal tracts on the chow diet (P < 0.01). The ductular proliferation on the cholic acid diet was comparable to the lithogenic diet with 26 ± 4 ductules per 100 portal tracts compared to the same control (P < 0.01). In the AKR strain, ductular proliferation, although increased on the lithogenic and cholic acid diets, 21 ± 5 and 12 ± 2 respectively, was not significantly different compared to chow-fed animals (10 ± 1).
Figure 1.
Representative histopathologic sections of mouse livers. (a) Liver of an AKR mouse on chow; (b) liver of an AKR mouse on the lithogenic diet; (c) liver of an C57L mouse on chow; (d) liver of an C57L mouse on the lithogenic diet. Bile ducts (BD) and hepatic arteries (HA) are highlighted by means of arrows, and the portal vein (PV) is labeled intralumenally.
Bile salt transporter protein expression
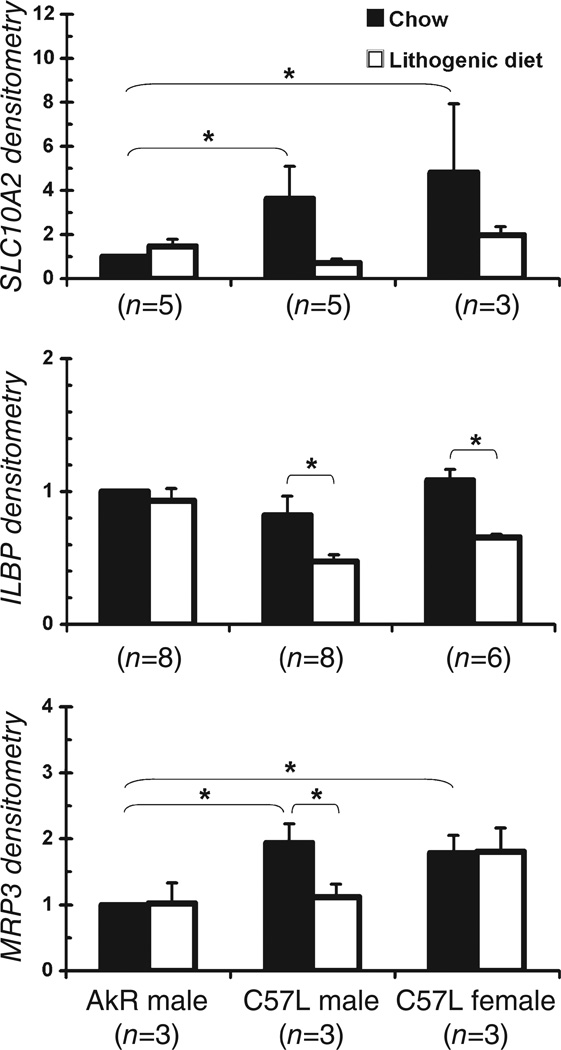
Figure 2 shows representative Western blots of SLC10A2, FABP6, and ABCC3. Densitometric analysis for SLC10A2, FABP6, and ABCC3 expressions were then normalized to actin, which was highly variable as displayed. Figure 3 illustrates that the actin-normalized relative protein expression of apical membrane transporter SLC10A2 was higher for male C57L mice on chow (3.6 ± 1.2, P = 0.05) compared to the AKR strain. On the lithogenic diet, the relative SLC10A2 expression in the AKR strain and C57L males (0.63 ± 0.1, P = 0.06) did not change.
Figure 2.
Western blot analysis of bile salt transporters apical sodium dependent bile salt transporter (SLC10A2), fatty-acid-binding protein subclass 6 (FABP6), and multiple-drug-resistance-related protein isoform 3 (ABCC3) expression with actin levels as controls. Lane 1, AKR male mouse on chow (n = 21); lane 2, AKR male mouse on the lithogenic diet (n = 21); lane 3, C57L male mouse on chow (n = 25); lane 4, C57L male mouse on the lithogenic diet (n = 25); lane 5, C57L female mouse on chow (n = 28); lane 6, C57L female mouse on the lithogenic diet (n = 28).
Figure 3.
Densitometric analysis of apical sodium dependent bile salt transporter (SLC10A2), fatty-acid-binding protein subclass 6 (FABP6), and multiple-drug-resistance-related protein isoform 3 (ABCC3) expression in AKR male mice, C57L male mice, and C57L female mice on chow and the lithogenic diet, normalized for actin. Asterisk indicates significant differences between bars (P < 0.05).
As shown in Figure 3, the expression of the cytosolic transporter FABP6 was similar for male and female C57L mice compared to male AKR mice on chow. While ingesting the lithogenic diet, the expression of FABP6 was downregulated in both male C57L mice (0.47 ± 0.05 vs 1, P = 0.04), and in female C57L mice (0.66 ± 0.02, P < 0.01). On the lithogenic diet, the relative FABP6 expression in male AKR mice remained constant.
As displayed in Figure 3, the relative expression of basolateral membrane transporter ABCC3 was higher in male C57L mice (1.9 ± 0.3, P = 0.03) and female C57L mice (1.8 ± 0.3, P = 0.04) compared to AKR mice on chow. In the case of the C57L strain, although expression was downregulated in male mice on the lithogenic diet (1.1 ± 0.2, P = 0.03), it remained unchanged in the female mice (1.8 ± 0.4). In the AKR strain, there were no changes in ABCC3 expression on the lithogenic diet. The relative expression levels of all three transporters in male AKR mice on the lithogenic diet, and male/female C57L mice on chow or lithogenic diet are summarized in Table 1.
Table 1.
Gender and dietary influences on cholangiocyte bile salt transporters in AKR and C57L mice
| Bile salt transporter | Male AKR lithogenic diet | Male C57L chow | Male C57L lithogenic diet | Female C57L chow | Female C57L lithogenic diet |
|---|---|---|---|---|---|
| SCL10A2 (ASBT) | ↔ | ↑ | ↔ | ↑ | ↔ |
| FABP6 (ILBP) | ↔ | ↔ | ↓ | ↔ | ↓ |
| ABCC3 (MRP3) | ↔ | ↑ | ↓ | ↑ | ↔ |
Bile salt uptake by bile duct units
Taurocholate uptake studies using IBDUs were performed for male AKR and C57L mice on chow and after 2 weeks of lithogenic diet feeding. As shown in Fig. 4, taurocholate uptake by IBDUs from mice on chow was 2.0 ± 0.4 nmol/min/mm length of bile duct unit for the AKR strain (n = 3) and 3.2 ± 1.2 nmol/min/mm for the C57L strain (n = 6). After 2 weeks of lithogenic diet feeding, sodium taurocholate uptake remained unchanged in both strains at 2.2 ± 1.1 nmol/min/mm for AKR (n = 4), and at 1.5 ± 0.4 nmol/min/mm for C57L (n = 3), all displaying nonsignificant differences between strains and diet.
Figure 4.
Taurocholate uptake using microperfusion of freshly isolated bile duct unit (IBDU) in C57L male mice on chow (n = 6) and the lithogenic diet (n = 6) compared to AKR male mice on chow (n = 6) and the lithogenic diet (n = 6). No significant differences per mm length are evident for IBDUs isolated from mice on chow or lithogenic diet.
Discussion
Our study aimed at determining indirectly the contribution, if any, of intrahepatic recycling (cholehepatic shunting) of bile salt via the transport system on large cholangiocytes to cholesterol gallstone formation using quantitative histologic assessment of the biliary tree as well as molecular and functional studies of bile salt transporters in ex vivo cholangiocyte units. We hypothesized that the differences in cholesterol contents of bile between the cholesterol gallstone–susceptible C57L mice and the cholesterol gallstone–resistant AKR mice is due to increased intrahepatic recycling of bile salts in the C57L strain. Our data showed (i) enhanced biliary hyperplasia in the cholesterol gallstone–susceptible C57L mice on the lithogenic diet; (ii) higher expression levels of membrane bile salt transport proteins SLC10A2 and the exchanger ABCC3 in C57L mice compared to AKR mice on chow; (iii) the lithogenic diet decreased expression levels of FABP6 and ABCC3 in male C57L mice, and SLC10A2 and FABP6 proteins in female C57L mice; and (iv) similar taurocholate uptake levels per length of isolated duct units in male C57L and AKR mice on chow and the lithogenic diet. These data strongly support the concept that the lithogenic diet–induced biliary hyperplasia but decreased expression of bile salt transporters all without significantly influencing their functionality per unit length as assayed in ex vivo sodium taurocholate uptake studies. The increase in the absorptive surface area of biliary epithelia resulting from the marked proliferation of bile ducts in C57L mice on the lithogenic diet may predict increases in cholangiocytic bile salt absorption; hence an increase in intrahepatic recycling of bile salts.
Previous studies have shown that bile acid feeding induced biliary hyperplasia in rats and increased cholehepatic recycling of the bile salt pool due to increases in SLC10A2 protein expression and bile salt transport activity.15,16 The increase in the density of intrahepatic bile ducts was attributed to increased concentration and secretion of bile salts in hepatic bile induced by bile acid feeding.20 In addition, in rats fed bile acids, Alpini et al.15 demonstrated by DNA synthetic rates and ductual secretory activities that the increased proliferative activity of cholangiocytes involved large and not small cholangiocytes. The results of the present study confirm previous findings in mice that a cholic acid–enriched diet (i.e. same diet as the lithogenic diet minus cholesterol) induces increased bile flow and biliary lipid secretion rates,21 and in our study, enhanced biliary ductular proliferation. The ductular proliferation observed with cholic acid feeding as well as the lithogenic diet strongly suggest that the cholesterol component is not responsible for this effect.
Bile salts are the natural ligands for the nuclear farnesoid X receptor (FXR). Bile salt responsiveness of the mouse SLC10A2 gene has been shown to be mediated indirectly by FXR-dependent activation of the short heterodimer partner SHP1 and subsequent inhibition of liver receptor homologue-1 (LRH-1) activity.22 Prior to this study, there were conflicting reports of positive, negative, and no feedback regulation of SLC10A2 expression by bile salt, with variable experimental methodologies and animal species.7,23–31 ASBT expression has been quantified at the level of protein7,27–31 and mRNA,7,26,27,31 and demonstrates how the contradictory nature of the literature reflects the difficulties of in vivo assessment of a transport system that is part of a highly integrated and tightly regulated metabolic pathway.
The bile salt transport system of large cholangiocytes responsible for cholehepatic shunting of bile salts consists of confirmed transporters—the apical transporter SLC10A2 and cytosolic transporter FABP6—and when this study was completed, ABCC3 was believed to be the putative basolateral transporter exchanger.11–13 Recently, an heteromeric organic solute transporter (OST) α/β was characterized and found to be a more likely basolateral true transporter for bile salts in ileocytes and cholangiocytes.32,33 The expression of OSTα-OSTβ proteins were not investigated in the present study since it was only described in the little skate and not in mammals32 when the present work was performed. The basolateral ABCC3 transporter could, of course, be an ancillary anion exchanger for bile salt, with OSTα-OSTβ as the principal transporter. Future investigations are needed to assess the lithogenic diet responsiveness of the OSTα-OSTβ basolateral transporter to fully define its perturbations in cholangiocyte bile salt transport. The gallstone susceptible C57L strain appeared to have higher levels of membrane transporters on chow compared to the gallstone-resistant AKR mice. Feeding the lithogenic diet, which contains both cholic acid and cholesterol, resulted in an overall reduction in the expression of all three transport proteins in cholangiocytes from C57L mice, but no net change in cholangiocytes from AKR mice. Our study of the cholangiocyte bile salt transporters in the C57L mice has clearly demonstrated exquisite sensitivity to the lithogenic diet in the absence of any significant alteration in normalized bile salt uptake in the microperfused bile duct system.
These results taken together revealed that the lithogenic diet induced more biliary hyperplasia in the cholesterol gallstone–susceptible C57L mice compared to gallstone-resistant AKR mice.3 Decreased expression levels or little change of bile salt transport proteins on the lithogenic diet in both male and female C57L mice, but their relative constancy in the AKR mice suggests more efficient enterohepatic cycling and FXR activation in C57L mice. The changes in expression levels of bile salt transporters did not translate into alterations in bile salt uptake, that is, normalized per mm bile duct length, as measured by microperfusion of freshly isolated bile duct units. This observation may in part relate to the concept that the function of membrane transporters is not merely regulated by the total number of transporters but also their functional state and cellular localization. Moreover, the functional state of other bile salt transporters in the transport system may also be crucial. Furthermore, as in the ileum, the cytosolic transporter FABP6 of cholangiocytes appears to be critical in preventing the direct exposure of intracellular organelles to potential bile salt toxicity. Therefore, the functional state of FABP6, which was uniformly decreased in expression in both mouse strains by the lithogenic diet, may also influence the uptake of bile salt by cholangiocytes.
The lithogenic mechanisms in gallstone-susceptible C57L mice are believed to be a combination of imbalance of lipid hemostasis and inflammatory responses to environmental stimuli. Our study focuses on the role of lipid hemostasis imbalance, principally, bile salt hypersecretion in C57L mice, and its role in cholesterol gallstone formation. Our results suggest that there may be increased overall cholehepatic bile salt recycling, thereby contributing to cholesterol supersaturation.
Finally, taking all our results together, we argue that an increase in the absorptive surface area of IBDUs due to the marked proliferation of bile ducts in C57L mice on the lithogenic diet may predict increases in cholangiocytic bile salt absorption. As found in this work, although equivalent levels of bile salt uptake per mm by individual bile duct units occurred, the proliferation density of transporters on the lithogenic diet, especially in C57L mice, should be capable of augmenting cholehepatic shunting. Therefore we can tentatively conclude from our studies that the lithogenic diet, which induces more biliary hyperplasia in C57L mice compared with AKR mice, should support increased overall cholehepatic bile salt shunting within the intrahepatic biliary tree despite constant bile salt absorption per unit length of ductules. This may contribute to enhanced bile salt shunting and hepatic resecretion, and possibly may augment cholesterol supersaturation of bile in the cholesterol gallstone–susceptible C57L murine strain.
Acknowledgments
Grant support of this study was provided in part by American Liver Foundation Fellowship and Faculty-Fellowship Transition Award to Dr. Julia J. Liu, USPHS (NIH) grants, DK 36588, DK 52911, and DK 24031, and DK 57993 (NFL). We would like to thank Dr Aiyu Gong (Mayo Clinic Foundation) for her outstanding technical assistance with the bile duct isolation and perfusion experiments.
Footnotes
Work Attributed to: Division of Gastroenterology, Brigham and Women’s Hospital.
References
- 1.Everhart JE, Khare M, Hill M, Maurer KR. Prevalence and ethnic differences in gallbladder disease in the United states. Gastroenterology. 1999;117:632–639. doi: 10.1016/s0016-5085(99)70456-7. [DOI] [PubMed] [Google Scholar]
- 2.Khanuja B, Cheah YC, Hunt M, et al. Lith1, a major gene affecting cholesterol gallstone formation among inbred strains of mice. Proc. Natl. Acad. Sci. USA. 1995;92:7729–7733. doi: 10.1073/pnas.92.17.7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang DQ, Lammert F, Paigen B, Carey MC. Phenotypic characterization of lith genes that determine susceptibility to cholesterol cholelithiasis in inbred mice. Pathophysiology of biliary lipid secretion. J. Lipid Res. 1999;40:2066–2079. [PubMed] [Google Scholar]
- 4.Shneider BL, Dawson PA, Christie DM, Hardikar W, Wong MH, Suchy FJ. Cloning and molecular characterization of the ontogeny of a rat ileal sodium-dependent bile acid transporter. J. Clin. Invest. 1995;95:745–754. doi: 10.1172/JCI117722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lazaridis KN, Pham L, Tietz P, et al. Rat cholangiocytes absorb bile acids at their apical domain via the ileal sodium-dependent bile acid transporter. J. Clin. Invest. 1997;100:2714–2721. doi: 10.1172/JCI119816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lammert F, Paigen B, Carey MC. Localization of the ileal sodium-bile salt cotransporter gene (Slc10a2) to mouse chromosome 8. Mamm. Genome. 1998;9:173–174. doi: 10.1007/s003359900715. [DOI] [PubMed] [Google Scholar]
- 7.Sauer P, Stiehl A, Fitscher BA, et al. Downregulation of ileal bile acid absorption in bile-duct-ligated rats. J. Hepatol. 2000;33:2–8. doi: 10.1016/s0168-8278(00)80152-x. [DOI] [PubMed] [Google Scholar]
- 8.Kramer W, Sauber K, Baringhaus KH, et al. Identification of the bile acid-binding site of the ileal lipid-binding protein by photoaffinity labeling, matrix-assisted laser desorption ionization-mass spectrometry, and NMR structure. J. Biol. Chem. 2001;276:7291–7301. doi: 10.1074/jbc.M006877200. [DOI] [PubMed] [Google Scholar]
- 9.Birkenmeier EH, Rowe LB, Crossman MW, Gordon JI. Ileal lipid-binding protein (Illbp) gene maps to mouse chromosome 11. Mamm. Genome. 1994;5:805–806. doi: 10.1007/BF00292019. [DOI] [PubMed] [Google Scholar]
- 10.Kool M, de Haas M, Scheffer GL, et al. Analysis of expression of cMOAT (MRP2), MRP3, MRP4, and MRP5, homologues of the multidrug resistance-associated protein gene (MRP1), in human cancer cell lines. Cancer Res. 1997;57:3537–3547. [PubMed] [Google Scholar]
- 11.Hirohashi T, Suzuki H, Sugiyama Y. Characterization of the transport properties of cloned rat multidrug resistance-associated protein 3 (MRP3) J. Biol. Chem. 1999;274:15181–15185. doi: 10.1074/jbc.274.21.15181. [DOI] [PubMed] [Google Scholar]
- 12.Hirohashi T, Suzuki H, Takikawa H, Sugiyama Y. ATP-dependent transport of bile salts by rat multidrug resistance-associated protein 3 (Mrp3) J. Biol. Chem. 2000;275:2905–2910. doi: 10.1074/jbc.275.4.2905. [DOI] [PubMed] [Google Scholar]
- 13.Muller O, Schalla C, Scheibner J, Stange EF, Fuchs M. Expression of liver plasma membrane transporters in gallstone-susceptible and gallstone-resistant mice. Biochem. J. 2002;361:673–679. doi: 10.1042/0264-6021:3610673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolters H, Elzinga BM, Baller JF, et al. Effects of bile salt flux variations on the expression of hepatic bile salt transporters in vivo in mice. J. Hepatol. 2002;37:556–563. doi: 10.1016/s0168-8278(02)00247-7. [DOI] [PubMed] [Google Scholar]
- 15.Alpini G, Glaser S, Robertson W, et al. Bile acids stimulate proliferative and secretory events in large but not small cholangiocytes. Am. J. Physiol. 1997;273:G518–G529. doi: 10.1152/ajpgi.1997.273.2.G518. [DOI] [PubMed] [Google Scholar]
- 16.Alpini G, Ueno Y, Glaser SS, et al. Bile acid feeding increased proliferative activity and apical bile acid transporter expression in both small and large rat cholangiocytes. Hepatology. 2001;34:868–876. doi: 10.1053/jhep.2001.28884. [DOI] [PubMed] [Google Scholar]
- 17.Alpini G, Roberts S, Kuntz SM, et al. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology. 1996;110:1636–1643. doi: 10.1053/gast.1996.v110.pm8613073. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa K, Suzuki H, Hirohashi T, et al. Characterization of inducible nature of MRP3 in rat liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;278:G438–G446. doi: 10.1152/ajpgi.2000.278.3.G438. [DOI] [PubMed] [Google Scholar]
- 19.Masyuk AI, Gong AY, Kip S, Burke MJ, LaRusso NF. Perfused rat intrahepatic bile ducts secrete and absorb water, solute, and ions. Gastroenterology. 2000;119:1672–1680. doi: 10.1053/gast.2000.20248. [DOI] [PubMed] [Google Scholar]
- 20.Alpini G, Glaser SS, Ueno Y, et al. Bile acid feeding induces cholangiocyte proliferation and secretion: evidence for bile acid-regulated ductal secretion. Gastroenterology. 1999;116:179–186. doi: 10.1016/s0016-5085(99)70242-8. [DOI] [PubMed] [Google Scholar]
- 21.Wang DQ, Lammert F, Cohen DE, Paigen B, Carey MC. Cholic acid aids absorption, biliary secretion, and phase transitions of cholesterol in murine cholelithogenesis. Am. J. Physiol. 1999;276:G751–G760. doi: 10.1152/ajpgi.1999.276.3.G751. [DOI] [PubMed] [Google Scholar]
- 22.Chen F, Ma L, Dawson PA, et al. Liver receptor homologue-1 mediates species- and cell line-specific bile acid-dependent negative feedback regulation of the apical sodium-dependent bile acid transporter. J. Biol. Chem. 2003;278:19909–19916. doi: 10.1074/jbc.M207903200. [DOI] [PubMed] [Google Scholar]
- 23.Lillienau J, Crombie DL, Munoz J, Longmire-Cook SJ, Hagey LR, Hofmann AF. Negative feedback regulation of the ileal bile acid transport system in rodents. Gastroenterology. 1993;104:38–46. doi: 10.1016/0016-5085(93)90833-x. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JV, Paul JM, Dumaswala R, Heubi JE. Downregulation of taurocholate transport by ileal BBM and liver BLM in biliary-diverted rats. Am. J. Physiol. 1994;267:G501–G507. doi: 10.1152/ajpgi.1994.267.4.G501. [DOI] [PubMed] [Google Scholar]
- 25.Dumaswala R, Berkowitz D, Heubi JE. Adaptive response of the enterohepatic circulation of bile acids to extrahepatic cholestasis. Hepatology. 1996;23:623–629. doi: 10.1002/hep.510230330. [DOI] [PubMed] [Google Scholar]
- 26.Torchia EC, Cheema SK, Agellon LB. Coordinate regulation of bile acid biosynthetic and recovery pathways. Biochem. Biophys. Res. Commun. 1996;225:128–133. doi: 10.1006/bbrc.1996.1141. [DOI] [PubMed] [Google Scholar]
- 27.Stravitz RT, Sanyal AJ, Pandak WM, Vlahcevic ZR, Beets JW, Dawson PA. Induction of sodium-dependent bile acid transporter messenger RNA, protein, and activity in rat ileum by cholic acid. Gastroenterology. 1997;113:1599–1608. doi: 10.1053/gast.1997.v113.pm9352862. [DOI] [PubMed] [Google Scholar]
- 28.Arrese M, Trauner M, Sacchiero RJ, Crossman MW, Shneider BL. Neither intestinal sequestration of bile acids nor common bile duct ligation modulate the expression and function of the rat ileal bile acid transporter. Hepatology. 1998;28:1081–1087. doi: 10.1002/hep.510280424. [DOI] [PubMed] [Google Scholar]
- 29.Xu G, Shneider BL, Shefer S, et al. Ileal bile acid transport regulates bile acid pool, synthesis, and plasma cholesterol levels differently in cholesterol-fed rats and rabbits. J. Lipid Res. 2000;41:298–304. [PubMed] [Google Scholar]
- 30.Xu G, Salen G, Shneider BL, et al. Cholecystectomy prevents expansion of the bile acid pool and inhibition of cholesterol 7alpha-hydroxylase in rabbits fed cholesterol. J. Lipid Res. 2001;42:1438–1443. [PubMed] [Google Scholar]
- 31.Lee J, Azzaroli F, Wang L, et al. Adaptive regulation of bile salt transporters in kidney and liver in obstructive cholestasis in the rat. Gastroenterology. 2001;121:1473–1484. doi: 10.1053/gast.2001.29608. [DOI] [PubMed] [Google Scholar]
- 32.Dawson PA, Hubbert M, Haywood J, et al. The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J. Biol. Chem. 2005;280:6960–6968. doi: 10.1074/jbc.M412752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ballatori N, Christian WV, Lee JY, et al. OSTalpha-OSTbeta: a major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia. Hepatology. 2005;42:1270–1279. doi: 10.1002/hep.20961. [DOI] [PubMed] [Google Scholar]