Abstract
Aggression frequently coincides with specific dimensions of emotionality, such as impulsivity, risk-taking, and drug abuse. Serotonergic (5-HTergic) neurotransmission contributes to the regulation of numerous neurobiological functions, and is thought to play a key role in modulating aggressive responses. The current study uses selectively-bred High (bHR) and Low (bLR) Responder rats that exhibit differences in emotionality and behavioral control, with bHRs exhibiting heightened novelty-induced exploration, impulsivity, and increased sensitivity to drugs of abuse, and with bLRs characterized by exaggerated depressive- and anxiety-like behaviors. Based on this behavioral profile we hypothesized that bHR rats exhibit increased aggression along with changes in testosterone and corticosterone secretion characteristic of aggression, and that these changes are accompanied by alterations in the expression of key genes that regulate 5-HTergic neurotransmission (Tph2 and Sert) as well as in the activation of 5-HTergic cell groups following aggressive encounter. Our data demonstrate that when compared to bLR rats, bHRs express increased baseline Tph2 and Sert in select brainstem nuclei, and when tested on the resident-intruder test they exhibited: 1) increased aggressive behavior; 2) potentiated corticosterone and testosterone secretion; and 3) diminished intrusion-induced c-fos expression in select 5-HTergic brainstem cell groups. The most prominent gene expression differences occurred in the B9 cell group, pontomesencephalic reticular formation, median raphe, and the gigantocellular nucleus pars α. These data are consistent with the notion that altered 5-HT neurotransmission contributes to bHRs’ heightened aggression. Furthermore, they indicate that a specific subset of brainstem 5-HTergic cell groups contributes to the regulation of intrusion-elicited behavioral responses.
Keywords: emotionality, brainstem, TPH2, SERT, c-fos, resident-intruder test
1. Introduction
Serotonergic (5-HTergic) neurotransmission contributes to the regulation of virtually every neurobiological function, including sensory, motor, homeostatic, cognitive, and higher order executive functions. Furthermore, dysregulated 5-HTergic neurotransmission contributes to diverse neuropsychiatric illnesses, including major depression, bipolar disorder, suicide, sleep disorders, conduct disorders, Tourette’s syndrome, and psychosis (Arango et al., 2002; Asberg et al., 1976; de Boer and Koolhaas, 2005; Lopez-Figueroa et al., 2004; Parsey et al., 2006; Rodrigo-Angulo et al., 2000; Thase, 1999). Despite such widespread functional roles, 5-HTergic neurons number only in the tens of thousands in the mammalian brain, yet these cells are highly complex, sending collateralized projections to multiple targets (Jacobs and Azmitia, 1992; Steinbusch, 1984). Emerging evidence points to functional and structural heterogeneity in the organization of these cell groups. (Abrams et al., 2004; Clark et al., 2006; Jones and Light, 1992; Lowry, 2002;Lowry et al., 2008; Waselus et al., 2006). For example, 5-HTergic neurons within the caudal dorsal raphe nucleus (DRC) project to limbic targets, including septum and the bed nucleus of the stria terminalis (Waselus et al., 2006), are thought to regulate mood and affect, and are potentially involved in anxiety, depression, and suicide (Lowry et al., 2008). On the other hand, 5-HTergic neurons located more rostrally and ventrally project to the basal ganglia (Waselus et al., 2006), have been implicated in motor control and cognitive regulation, and may play a role in Tourette’s syndrome and obsessive compulsive disorder (Lowry et al., 2008). Together these observations suggest the existence of parallel 5-HTergic circuits that mediate specific behaviors and physiological states.
One such behavior is aggression, which has been linked with altered 5-HTergic tone in numerous clinical studies and rodent models (Davidson et al., 2000; Kruk et al., 1998; Miczek et al., 2007; Nelson and Trainor, 2007). Interestingly, while there is a well-established link between aggression and 5-HT, the exact nature of this relationship is complex. Distinct types of aggressive displays (e.g. pathological aggression versus territorial aggression) have been associated with distinct neurochemical changes (either diminished or potentiated 5-HTergic transmission (Nelson and Trainor, 2007)). Moreover, individual susceptibility to aggression is influenced by a variety of biological factors as well as behavioral traits.
We recently developed two contrasting rodent lines -- High Responder (bHR) and Low Responder (bLR) rats that were selectively-bred for their propensity to explore a novel environment. Compared to their bLR counterparts, bHR rats exhibit heightened novelty exploration, enhanced impulsivity and reward drive, as well as decreased depressive- and anxiety-like behaviors (Clinton et al., 2007; Flagel et al., 2010; Perez et al., 2009; Stead et al., 2006). These behavioral responses are reminiscent of human externalizing neuropsychiatric disorders, which also include pathological aggression (Flagel et al., 2010). Thus, in the current study we hypothesized that bHR rats exhibit heightened aggressive behavior that is correlated with altered endocrine and gene expression changes within the brainstem 5-HTergic cell groups.
2. Results
2.1 Behavior on the Resident-Intruder Test
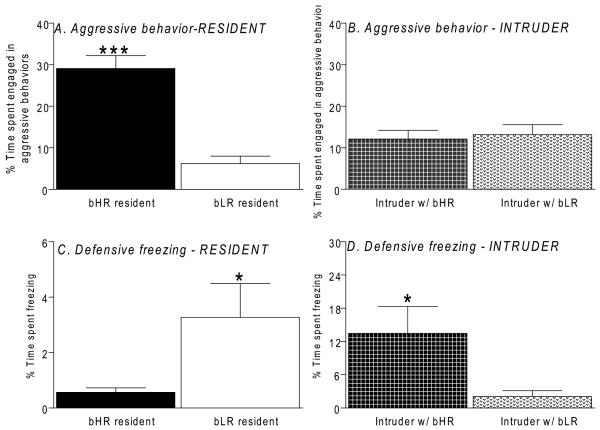
When exposed to the intruder, bHR residents spent markedly more time engaged in aggressive behaviors compared to bLR residents (F1, 33 = 34.83, p<0.0001; Fig. 1A). In contrast, resident bLRs spent significantly more time in defensive freezing behavior (F1, 33 = 4.18, p<0.05; Fig. 1C). No difference in aggressive behavior was detected between intruder animals paired either with bHR or bLR rats (Fig. 1B); however, time spent in defensive freezing was significantly higher in intruder rats paired with bHRs (F1,33 = 4.69, p<0.05; Fig. 1D). Overall, bLR residents spent the vast majority of their time engaged in other (non-aggressive) behaviors, which was significantly greater than their bHR counterparts (F1, 33 = 7.67, p<0.01). Details about specific aggressive and non-aggressive behaviors are shown in Table 1.
Figure 1.
Behavioral differences in bHR/bLR rats on the resident-intruder test. Selectively-bred HR resident male rats exhibited increased aggressive behavior compared to their bLR counterparts (A; p < 0.001); intruder male rats showed similarly low levels of aggressive behavior when exposed to either bHR or bLR residents (B; p > 0.05). bLR residents exhibited increased defensive behaviors (C; p < 0.05). Intruder rats exposed to bHR residents exhibited markedly increased defensive freezing compared to those interacting with bLR residents (D; p < 0.05). * – p < 0.05, *** – p < 0.001 compared to bLR rats.
Table 1.
Behavioral profiles of bHR/bLR rats on the resident intruder test.
| HR Resident | LR Resident | p-value | |||
|---|---|---|---|---|---|
| mean | SEM | mean | SEM | ||
| DEFENSIVE BEHAVIOR TOTAL | 0.56 | 0.18 | 3.37 | 1.58 | <0.05 |
| Aggressive sniff | 12.05 | 1.47 | 5.15 | 1.72 | <0.005 |
| Aggressive groom/bite | 5.02 | 1.76 | 0.32 | 0.19 | <0.05 |
| Boxing | 12.06 | 1.80 | 0.71 | 0.23 | <0.0001 |
| AGGRESSIVE BEHAVIOR TOTAL | 29.12 | 3.07 | 6.18 | 1.82 | <0.0001 |
| Eat/Groom/Drink | 2.95 | 0.84 | 10.76 | 2.81 | <0.01 |
| Rear | 31.31 | 3.21 | 30.38 | 3.26 | 0.84 |
| Run | 35.99 | 2.93 | 44.70 | 3.56 | 0.07 |
| Rest | 0.03 | 0.01 | 0.02 | 0.02 | 0.86 |
| OTHER BEHAVIOR TOTAL | 70.27 | 3.08 | 85.86 | 5.05 | <0.01 |
Values represent percentage of time spent performing each behavior during the 10-minute intruder encounter. Data are presented as mean ± SEM.
2.2 Testosterone and Corticosterone Levels
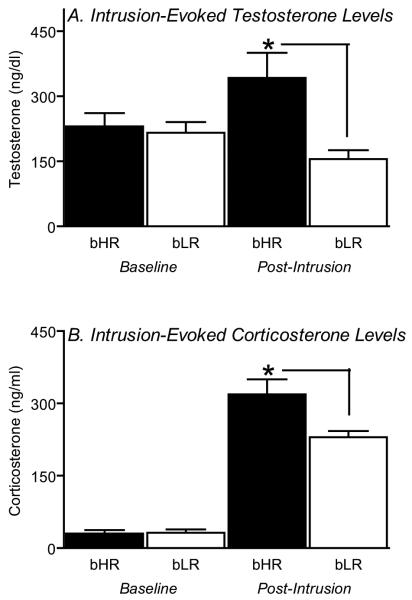
Increased aggressive behavior in bHR resident rats was accompanied by a significant increase in circulating testosterone levels (Fig. 2A). In contrast, bLR residents did not increase their secretion of testosterone; consequently, post-intrusion testosterone levels were more than two-fold greater in bHR vs. bLR rats (F1, 38 = 9.03, p<0.01; Fig. 2A).
Figure 2.
Total testosterone and corticosterone levels in bHR and bLR rats. Selectively-bred bHR, but not bLR, resident rats increased their total testosterone levels in response to intruder exposure (A; p < 0.01). Intruder exposure elicited an increase in circulating corticosterone levels in both types of rats, but this response was significantly greater in bHRs (B; p < 0.05). * – p < 0.05 vs. bLR.
Exposure to the intruder elicited increased secretion of corticosterone in both bLR and bHR rats (main effect of intrusion compared to baseline, F1, 44 = 181.21, p<0.0001). However, this response was significantly greater in bHR animals (main effect of phenotype F1, 44 = 4.52, p<0.05 and significant phenotype × intrusion interaction F1, 44 = 4.91, p<0.05; Fig. 2B).
2.3 Characterization of Raphe Cell Groups
Examination of Sert autoradiograms revealed distinct clusters of 5-HTergic cell groups throughout the brainstem. Such neurochemical parcellation agreed with cytoarchitectonic differences, as was apparent when Sert signal was digitally projected onto adjacent cresyl violet-stained tissue sections (Supplementary Fig. 1).
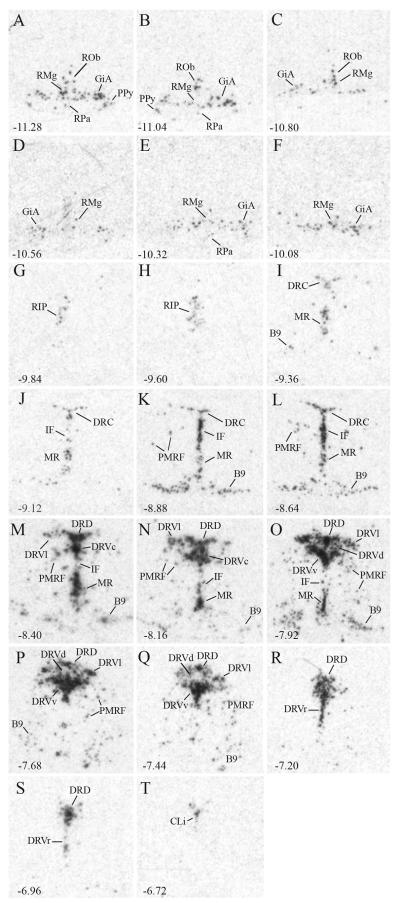
Using a combination of neurochemical and cytoarchitectonic criteria, we examined tissue sections at 240 μm intervals and identified distinct 5-HTergic cell groups throughout the brainstem (Fig. 3). We observed differences in density, intensity, and distribution of Sert signal associated with different cell groups. These differences also corresponded with alterations in cell size, cell shape, and packing density that were apparent in cresyl violet-stained material. Tissue processed for Tph2 immunocytochemistry revealed the same parcellation of 5-HT-ergic cell groups as revealed by Sert ISH, and similar differences in cell size, shape and density as suggested by cresyl violet stain (Supplementary Fig. 2).
Figure 3.
Serotonergic cell groups in the rat brainstem. Cell groups were parcelated based on a combination of neurochemical and cytoarchitectonic criteria (see text and Supplementary Fig. 1 for additional details). Sert expression across the rostro-caudal extent of the brainstem was used to delineate cell group boundaries. Data are presented caudal (A) to rostral (T) from tissue sections 240 μm apart, and numbers in the lower left of each panel correspond to distances from bregma in mm. Note differences in shape, packing density, and signal intensity among different cell groups and along the rostro-caudal extent. Abbreviations: B9 – B9 cell group; CLi – caudal linear raphe; DRC – caudal dorsal raphe; DRD – dorsal division of the dorsal raphe; DRVc – ventral division of the dorsal raphe, caudal portion; DRVd – ventral division of the dorsal raphe, dorsal portion; DRVl – ventral division of the dorsal raphe, lateral portion; DRVr – ventral division of the dorsal raphe, rostral portion; DRVv – ventral division of the dorsal raphe, ventral portion; GiA – gigantocellular nucleus pars α; IF – interfascicular raphe; MR – median raphe; PMRF – pontomesencephalic reticular formation; PPy – parapyramidal cell group; RIP – raphe interpositus; RMg – raphe magnus; ROb – raphe obscurus; RPa – raphe pallidus.
Caudal 5-HTergic cell groups were located within the rostral medulla and caudal pons, while rostral groups were located within rostral pons and the midbrain (Fig. 3). Intensity and area occupied by the Sert signal was clearly greater within the more rostral cell groups (compare panels K-R to panels A-H in Figure 3 and Supplemental Figure 2). Among these rostral cell groups, dorsal raphe nucleus had the most complex organization and consisted of caudal (DRC), dorsal (DRD) and ventral divisions. Its ventral division was further parcelated into caudal (DRVc), rostral (DRVr), ventral (DRVv), dorsal (DRVd), and lateral (DRVl) subdivisions. Intrafascicular raphe (IF) was located along the midline extending from the dorsal to ventral extent of the medial longitudinal fasciculus. Median raphe (MR) contained dense signal and was likewise located along the midline, but ventral to IF. In addition, laterally-displaced Sert signal was detected within the pontomesencephalic reticular formation (PMRF), as well as more ventrally in and around the medial lemniscus, corresponding to the B9 cell group.
More caudally, much of the Sert signal was confined to the ventromedial medulla and the gigantocellular nucleus pars α (GiA), as well as the caudal raphe nuclei, including: obscurus (ROb), magnus (RMg), and pallidus (RPa). More laterally, Sert was expressed ventrally and laterally to the pyramidal tract in the medulla-pons within the parapyramidal cell group (PPy).
2.4 Tph2 and Sert Expression
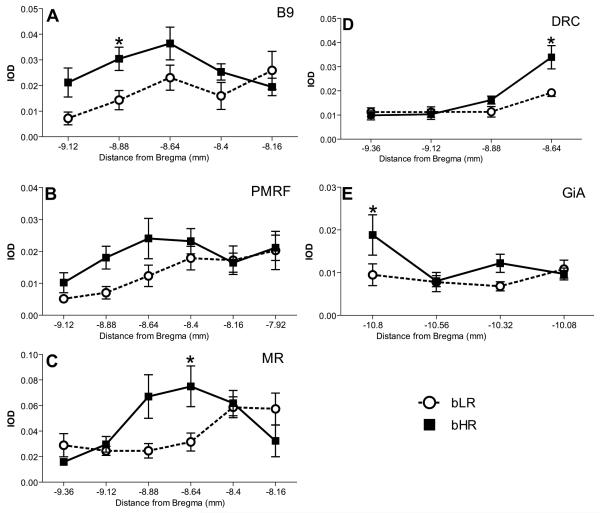
Our initial observations revealed greater signal for Tph2 and Sert in bHR rats as compared to bLRs (Fig. 4). Subsequent analysis of Tph2 gene expression revealed a significant main effect of bHR/bLR phenotype within: the B9 cell group (F1,51= 8.47, p<0.01; Fig. 5A) and the PMRF (F1,78= 8.47, p<0.05; Fig. 5B), GiA (F1,36= 4.67, p<0.05; Fig. 5E). An interaction between phenotype and the rostro-caudal anatomical position was observed within MR (F8,94 = 8.47, p<0.01; Fig. 5C); in DRC a main effect of phenotype (bHR/bLR) along with an interaction between phenotype and rostrocaudal position was detected: (F1,41= 6.64, p<0.05 and F3,41= 5.00, p<0.01, respectively; Fig. 5D). These differences appeared to be anatomically segregated, so that greater Tph2 expression levels were detected in bHR rats within the middle and middle-caudal portions of B9, PMRF, MR, and GiA (Fig. 4; Fig. 5A-C, E). Within DRC, bHR rats similarly exhibited elevated Tph2 expression, but at the rostral pole of this cell group (Fig. 4; Fig 5D).
Figure 4.
Examples of Tph2 (A, B) and Sert (C, D) expression differences between bLR (A, C) and bHR (B, D) rats. Images were taken from the adjacent sections of the same bLR and bHR rats at −8.64 mm from Bregma. Note an increase in signal in the bHR images as compared to bLR. These differences reached significance in the labeled cell groups at this anatomical level (see subsequent figures and text). Abbreviations same as in Fig. 3
Figure 5.
Greater Tph2 expression in bHR versus bLR rats. Significant bHR/bLR differences in Tph2 expression were detected in the: B9 cell group (A), pontomesencephalic reticular formation (PMRF; B), median raphe (MR; C), caudal dorsal raphe (DRC; D), and gigantocellular nucleus pars α (GiA; E). These expression differences were influenced by the rostro-caudal position, so that within B9, PMRF, and MR expression differences were observed caudally. In contrast, within DRC expression differences were detected near its rostral pole. * – p < 0.05 vs. bLR post- hoc comparison at the indicated level.
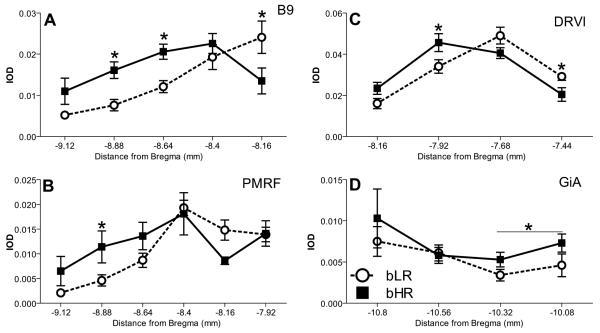
Analysis of Sert expression revealed bHR/bLR group differences within B9, PMRF, DRVl, and GiA. These differences were dependent on the rostro-caudal position within each nucleus as evidenced by a significant interaction between phenotype and rostrocaudal position within all regions illustrated in Fig. 5, including the B9 cell group (F4,75= 5.95, p<0.001; Fig. 6A); PMRF (F1,40= 8.53, p<0.01; −9.12 mm to −8.64 mm from Bregma), but not more rostrally (F1,47=1.70, p=0.2; −8.4 mm to −7.92 mm from Bregma; Fig. 6B); DRVl (F3,61=6.38, p<0.001; Fig. 6C); and GiA where Sert levels significantly greater in bHR rats rostrally (F3,23= 5.34, p<0.05; −10.32 mm to −10.08 mm from Bregma), but not caudally (F1,19= 5.34, p>0.5; −0.56 mm to −10.80 mm from Bregma; Fig. 6D). These differences were similar to those observed for Tph2, which was also increased in bHRs within some of the same cell groups, including: B9, PMRF, and GiA.
Figure 6.
Greater Sert expression in bHR versus bLR rats. Significant differences in Sert expression were detected within: the B9 cell group (A); pontomesencephalic reticular formation (PMRF; B); ventral division of the dorsal raphe, lateral portion (DRVl; C); and gigantocellular nucleus pars α (GiA; D). As with Tph2 these differences were more pronounced caudally within B9 and PMRF. * – p < 0.05 vs. bLR post-hoc comparison at the indicated level.
2.5 C-fos Expression
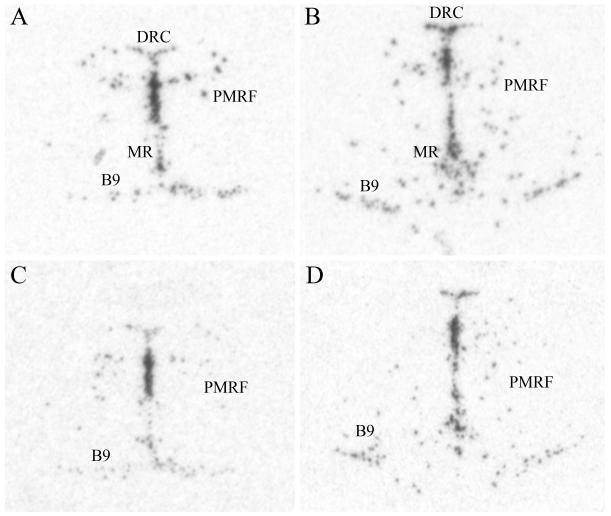
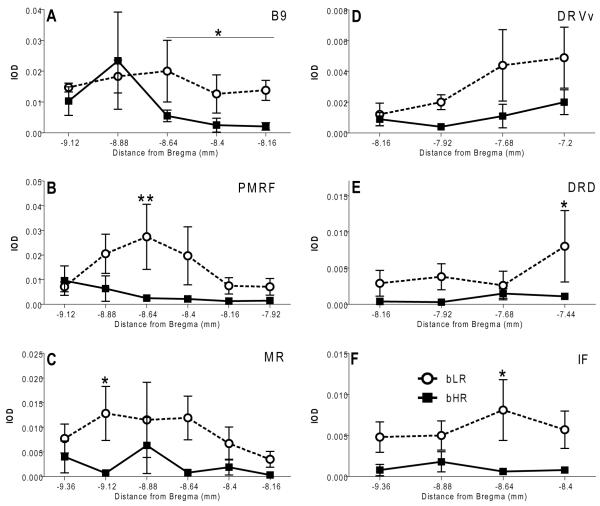
We used c-fos expression as an indicator of the activation of brainstem cell groups by the intruder exposure. Within bLR brains we observed distinct c-fos expression within IF, PMRF, and B9 that was absent in the bHRs (Fig. 7 A-C versus D-F). Statistical analyses confirmed this observation, regarding increased post-intrusion c-fos mRNA levels in bLR rats as compared to bHRs in multiple cell groups (Fig. 8). Prominent differences were detected within B9 (F1,22=6.45, p<0.05; levels −8.64 mm, −8.4 mm, and −8.16 mm from Bregma) and PMRF (F1,47= 8.94, p<0.01; Fig. 8A, B), two areas in which we also detected bHR/bLR differences in Tph2 and Sert expression (Figs. 5A, B and 6A, B). Similar differences were also observed in MR (F1,44= 7.47, p<0.05; Fig. 8C), DRVv (F1,27= 6.41, p<0.05; Fig. 8D), DRD (F1,29= 5.23, p<0.05; Fig. 8E), and IF (F1,32= 13.22, p<0.01; Fig. 8F).
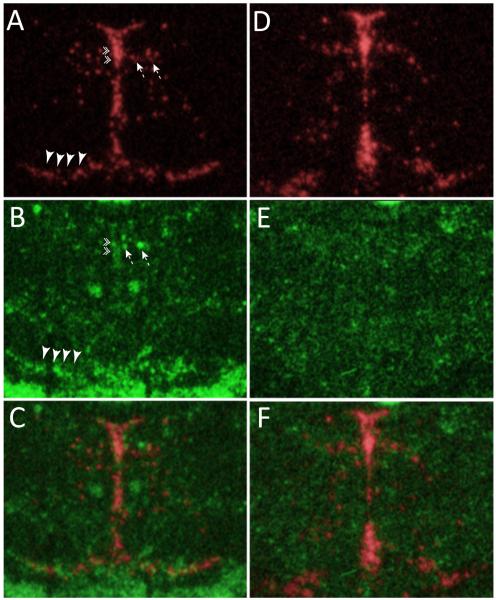
Figure 7.
Sert and c-fos expression. Sert (A, D) and c-fos (B, E) autoradiograms were digitally inverted and pseudocolored (Sert – red, c-fos – green). Sert and c-fos images from adjacent sections were digitally overlaid and linked (C, F). Sert signal was then used to identify different serotonergic cell groups, and the boundaries of these cell groups were then projected onto the adjacent c-fos image for quantification. Data in panels A-C are from a bLR animal, while those in D-F are from a bHR rat. Note increased c-fos expression in the bLR brain within: intrafascicular raphe (double arrowheads), pontine mesencephalic reticular formation (dashed arrows), and the B9 cell group (large arrowheads).
Figure 8.
Increased intruder-induced activation of serotonergic cell groups in bLR rats. Following the intruder exposure, c-fos expression was significantly increased within several serotonergic cell groups in bLR residents relatively to bHRs. Similar to Tph2 and Sert, in three cell groups these expression differences were influenced by the rostro-caudal position and were most prominent rostrally within: the B9 cell group (A), ventral portion of the ventral dorsal raphe (D), and the dorsal subdivision of the dorsal raphe (E). In contrast, significant bHR/bLR differences in c-fos expression were detected within the middle-caudal portion of the pontomesencephalic reticular formation (B). Similarly increased expression of c-fos in bLR residents was also observed in the median (MR; C) and intrafascicular (IF; F) raphe. * – p<0.05 vs. bHR.
3. Discussion
Our data demonstrate that compared to bLRs, bHRs exhibit: 1) enhanced aggression when facing an intruder; 2) potentiated intruder-induced corticosterone and testosterone secretion; 3) increased baseline Tph2 and Sert gene expression; and 4) decreased intrusion-evoked c-fos mRNA in a subset of 5-HTergic cell groups. While a number of brainstem 5-HTergic cell populations were examined, gene expression differences were restricted to a subset of groups (B9, PMRF, MR, GiA) suggesting that these areas may play a specific role in shaping intrusion-evoked behavioral and endocrine responses. It also seems feasible that a different collection of 5-HT cell groups mediate other actions of 5-HT (e.g. homeostasis, sensory processing, executive function, etc.)
3.1 Behavioral and Endocrine Differences
The bHR/bLR aggression differences were not associated with divergent behavior of the intruders themselves, since bHR and bLR opponents exhibited similarly low levels of aggression. Rather, our findings point to inherent bHR/bLR differences in predisposition toward aggression, and complement earlier work showing increased impulsivity, stronger reward drive, and decreased depression and anxiety behaviors (all of which co-occur with increased aggression) in bHR versus bLR rats (Flagel et al., 2010; Stead et al., 2006). bHRs’ collection of behavioral traits closely resembles human externalizing disorders, including substance abuse, conduct disorders, and pathological aggression (Dawe et al., 2004). Intruder exposure also elicited a testosterone surge in bHRs but not bLRs, which is consistent with human studies linking elevated plasma testosterone levels and enhanced violence (Ehrenkranz et al., 1974; Kruk et al., 1998). Interestingly, testosterone and its metabolites interact with 5-HT within forebrain areas, and may inhibit or facilitate aggression depending on the endocrine milieu and anatomical location (Simon et al., 1998).
Intruder exposure induced corticosterone secretion in both groups with a significantly greater response in bHRs. These findings are consistent with other reports of increased corticosterone secretion following social encounters (File and Seth, 2003). While elevated corticosterone levels often correlate with aggression in rodents (Hayden-Hixson and Ferris, 1991) and humans (van Bokhoven et al., 2005), this relationship is complicated and the literature is not entirely in agreement (Summers and Winberg, 2006). This heterogeneity may represent inherent individual differences in emotionality, risk-taking, and impulsivity. For example, stress exposure elicits greater cortisol secretion in risk-seeking individuals, suggesting a rewarding effect of stress (Filaire et al., 2007). Likewise, mild stress in bHRs elicits a greater corticosterone response compared to bLRs (Kabbaj et al., 2000). Since corticosterone has rewarding properties (Piazza et al., 1993), and bHR rats have an increased reward drive, it is feasible that aggressive behavior has rewarding properties for the bHR residents.
3.2 Gene Expression Differences
A large literature in humans and animals has linked altered 5-HT transmission and aggression (de Boer and Koolhaas, 2005). Since bHRs exhibited increased intrusion-induced aggression, testosterone and corticosterone secretion, we hypothesized that these differences are accompanied by baseline differences in the expression of key 5-HTergic genes, and in turn, basal differences in 5-HTergic tone. Because emerging evidence points to anatomical and functional heterogeneity within brainstem 5-HTergic cell groups (Clark et al., 2006; Lowry et al., 2008), we initially characterized the location and borders of 5-HTergic cell groups in our material using a combination of neurochemical and cytoarchitectonic criteria. The fact that bLR/bHR differences were not only confined to a subset of cell groups but were detected at precise rostro-caudal positions within these groups (see below) lends further support to the notion of functional and anatomical heterogeneity as well as specificity within these regions.
Although 5-HT levels at the synapse are determined by several factors, activities of Tph2 and Sert (which regulate synthesis and reuptake, respectively) are critical for the setting of overall 5-HTergic tone (Cooper et al., 2003). At baseline, bHRs exhibited higher Tph2 and Sert mRNA within three areas: B9, PMRF, and GiA. bHRs also showed increased Tph2 in MR and DRC, and increased Sert in DRVl. Although Tph2 and Sert expression were assayed immediately following homecage intrusion, it is unlikely that observed bHR/bLR differences represent behavior-induced effects. Stimulus-evoked mRNA alterations for non-immediate early genes (such as Tph2 and Sert) usually require several hours to appear (Curran and Morgan, 1995). Other reports demonstrate behaviorally- or pharmacologically-induced Tph2 and/or Sert alterations after a delay of several hours or days, rather than five minutes as in our study (Oliva et al., 2005; Semple-Rowland et al., 1996). Therefore, bHRs’ observed Tph2 and Sert increases likely represent greater 5-HT synthesis and turnover, at least within a subset of 5-HT innervated areas (Kim et al., 2005).
Our study also measured expression of c-fos, a reliable and robust marker of neuronal activation (Curran and Morgan, 1995), following intruder exposure. We found diminished c-fos mRNA expression in bHR versus bLR rats within a specific subset of 5-HTergic cell groups. There were prominent c-fos differences within cell groups with significant Tph2 and/or Sert alterations (B9, PMRF, and MR), as well as within three additional groups: DMVv, DRD, and IF.
Together these mRNA differences suggest that four cell groups – B9, PMRF, MR, and GiA are key regulators of 5-HT transmission in shaping individual differences in aggressive behavior. It is feasible that baseline bHR/bLR differences in Tph2 and Sert impact their inborn predisposition to aggression and contribute more broadly to their distinct behavioral profiles. Furthermore, c-fos differences within the same cell groups suggest altered recruitment of these regions in response to homecage intrusion. Since these c-fos changes occurred within the same brainstem cell groups showing Tph2 and Sert differences, they suggest that the baseline and intrusion-evoked expression alterations are functionally related. However, since our autoradiogram-based ISH method lacks cellular resolution, we cannot be sure whether or not the c-fos differences are confined to 5-HTergic neurons. Numerous studies have characterized non-5-HTergic neurons within the raphe that powerfully modulate 5-HTergic neurons, such as GABA-mediated inhibition of cell firing and transmitter release (Hurley et al., 2003; Melander et al., 1986). We are currently refining a dual-label ISH methodology for quantifying c-fos within neurochemically defined raphe neurons, as well as with laser-capture microdissection coupled with high-throughput gene expression assays (Kerman et al., 2006a). With these tools in-hand, future studies will focus on identifying the neurochemical content of neurons that express intrusion-evoked c-fos in bHRs versus bLRs. Furthermore, these gene expression differences may depend on aggressive experience of individual animals, because Caramaschi and colleagues have demonstrated that in mice selected for short attack latencies serotonin levels decrease in the prefrontal cortex with repeated resident-intruder fighting experience (Caramaschi et al., 2008).
While the resident-intruder paradigm is typically used to assess aggression, it is notable that this stimulus potentially evokes a wide range of behavioral responses, including fear and anxiety. Therefore, our observed c-fos differences may reflect increased behavioral inhibition (e.g. fear, anxiety) in bLRs facing intrusion. Our corticosterone data demonstrate that the intruder encounter is stressful, and previous reports show that bLRs exhibit high anxiety in stressful situations (Stead et al., 2006). Thus, these c-fos differences may reflect bLRs’ anxiety during the novel social encounter, as evidenced by their increased defensive freezing. Future work will be required to fully explore these possibilities.
Previous studies have implicated these 5-HTergic brainstem cell groups in the regulation of behavioral responses that may be elicited by intrusion and other types of stressors. For example, anatomical studies show that 5-HTergic neurons within B9, DRC, and MR project to septum and hippocampus, which both play prominent roles in regulating affect and stress responsivity (Lowry et al., 2008; Waselus et al., 2006). 5-HTergic neurons within GiA project to the spinal cord to innervate motoneurons, sympathetic preganglionic neurons, and the dorsal horn (Kerman et al., 2003; Kerman et al., 2006b; Kerman, 2008; Mason, 2001). This region also contains high levels of μ and κ opioid receptors, and is thought to mediate stress-induced motor, autonomic, and analgesic responses characteristic of ‘fight-or-flight’ (Mansour et al., 1988;Mason, 2001). Although B9 and PMRF contain over 20% of the total number of serotonergic neurons in the brain, second only to the dorsal raphe (Lowry et al., 2008), their function is unknown. Anatomical studies in several species demonstrated that B9 neurons project to the hypothalamus, cortex, hippocampus, septum, MR, and basal forebrain (Vertes and Martin, 1988). Less is known about connectivity of PMRF neurons, with a single report of its projection to the basal forebrain (Vertes and Martin, 1988). Together these observations suggest that B9 and PMRF participate in regulating vigilance, affect, hormonal stress responses, and homeostasis. Since homecage intrusion engages all of these neurobiological systems, it is feasible that B9 and PMRF play a prominent role in shaping aggression. In addition, most of the bHR/bLR gene expression differences in these regions were detected within their caudal portions, suggesting rostro-caudal functional topography of these groups.
3.3 Conclusions
Together our data indicate that bHR animals represent a novel rodent model of aggressive behavior that co-occurs with increased novelty-seeking, impulsivity, and propensity for drug self-administration. bHRs exhibit basally altered expression of genes that regulate synaptic 5-HT levels, and diminished intrusion-evoked c-fos in brainstem nuclei that are enriched in their content of 5-HT cells. These gene expression differences were consistently detected in a subset of 5-HTergic cell groups, suggesting existence of a dedicated 5-HTergic circuit that regulates aggressive behavior. Various nodes of this circuit likely modulate distinct facets of aggression, including affective, endocrine, and physiological responses, although future work is required to investigate these possibilities.
4. Experimental Procedure
4.1 Animals
Adult male bHR (total n = 40) and bLR (total n = 40) rats were acquired from our in-house breeding colony where we have maintained these lines for several generations (Stead et al., 2006). bHR and bLR rats used in these studies were from the 21st and 22nd generations of our colony. In addition, a group of adult Sprague-Dawley male rats (n=60) was purchased from Charles River (Wilmington, MA) and allowed to acclimate to our housing facilities for at least 1 week prior to any behavioral testing. All rats were housed in a dedicated animal facility with 12:12 light-dark cycle (lights on at 6 a.m.). Rat chow and water were available ad libitum. All experiments were approved by the University of Michigan University Committee on Use and Care of Animals, and were conducted in accordance with the National Institute of Health (NIH) guidelines on laboratory animal use and care dictated by the National Research Council in 1996. All work was carried out in accordance with European Commission (EC) Directive 86/609/EEC for animal experiments and the Horizontal Legislation on the Protection of Animals Used for Scientific Purposes (http://ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm).
4.2 Experimental Design
Four different experimental groups were employed, with each group consisting of 10 bHR and 10 bLR male rats. Animals in Group 1 were tested on the resident-intruder test (described below) only; animals in Group 2 were used for replication of behavioral testing and collection of trunk blood for endocrine assays (total testosterone and corticosterone); Group 3 animals were exposed to female rats for 14 days, but were not presented with intruders. These animals were euthanized on day 15, and their trunk blood was used for the determination of baseline testosterone and corticosterone levels. Group 4 animals were tested in the resident-intruder paradigm, and their brains were used for in situ hybridization (ISH) studies.
4.3 Behavioral Testing
Male bHR/bLR rats were housed with a female mating partner of the same selectively-bred line (i.e. male bHR with female bHR, and male bLR with female bLR) for 14 days. On the day of testing (day 15), the female partner was removed and each resident was presented for 10 min. with a size-matched commercially-purchased male Sprague-Dawley rat classified as the intruder. Each intruder rat was only used once in the resident-intruder paradigm. This social encounter was videotaped, and was subsequently scored (Observer 5.0.20, Noldus Information Technologies, The Netherlands) by a blinded observer for aggressive behaviors: sniffing, boxing, and biting; defensive behavior: freezing; and other behaviors: rearing, running, and eating/drinking in resident and intruder rats. This resident-intruder paradigm has been validated by numerous laboratories as a reliable tool for the characterization of aggression in rodents (Bannai et al., 2007; de Almeida et al., 2008; de Boer and Koolhaas, 2005; van Erp and Miczek, 2007).
4.4 Hormone Assays
Five minutes following resident-intruder testing (15 minutes after beginning of testing) bHR/bLR resident animals were sacrificed by rapid decapitation. Brains were removed, snap frozen and stored at −80° C for later in situ hybridization (ISH) studies (see next section). Trunk blood samples were collected in EDTA-coated tubes to assess testosterone and corticosterone levels. Blood samples were separated by centrifugation (1,000g × 10 min at 4°C), and plasma was removed, frozen and stored at −80°C. Testosterone and corticosterone levels were measured using commercially available radioimmunoassay kits (MP Biomedicals, Solon, OH) according to manufacturer instructions. The sensitivity of the assays was 12.5 ng/ml, and intra- and inter-assay coefficients of variation were less than 5%.
4.5 Tissue Processing
Brains were cryostat sectioned at 12 μm, immediately thaw-mounted onto Fisherbrand Superfrost Plus Microscope Slides (Fisher Scientific, http://www.fishersci.com/), and subsequently prepared for ISH. The expression of Sert, Tph2, and c-fos mRNAs was assessed at 240 μm intervals throughout rostrocaudal extent of the brainstem. Tph2 and Sert were selected for analysis because of their critical roles in regulating 5-HT synthesis and synaptic reuptake, respectively. We examined expression of the immediate early gene c-fos as an indicator of regional activation following exposure to the resident-intruder paradigm. At each anatomical level, adjacent slides were processed for each of the 3 transcripts, with an additional slide stained with cresyl violet.
Our ISH protocol has been previously described (Kabbaj et al., 2000). Briefly, sections were fixed in 4% paraformaldehyde at room temperature for 1 hour. The slides were then washed 5 min. × 3 in 2X SSC (300mM NaCl/30mM sodium citrate, pH 7.2). Next, the slides were placed in a solution containing acetic anhydride (0.25%) in triethanolamine (0.1 M), pH 8.0, for 10 min at room temperature, rinsed in distilled water, and dehydrated through graded ethanol washes (50%, 75%, 85%, 95%, and 100%). After air drying, adjacent tissue sections were hybridized overnight at 55°C with a 35S-labeled cRNA probes (see below). Following hybridization, coverslips were removed and the slides were washed with 2X SSC and incubated for 1 hour in RNaseA at 37°C, followed by multiple washes in increasingly stringent SSC solutions. Slides were then rinsed in distilled H2O, dehydrated through graded ethanol washes, air-dried, and apposed to Kodak XAR film (Eastman Kodak, Rochester, NY). Slides processed for Tph2 and Sert were exposed to film for 48 hours, while slides processed for c-fos were exposed to film for 7 days; these development times were chosen since they were found to be within the linear portion of the development curve for each probe for the brain regions of interest.
The following cRNA riboprobes were used: Sert (alias Slc6a4; NCBI reference sequence: X63253, pos. – 655-1,234), Tph2 (alias Ntph; NCBI reference sequence – AY098915, pos. – 1,550-2,580), and c-fos (alias Fos; NCBI reference sequence – X06769, pos. – 585-1,368). Specificity of labeling was confirmed by the absence of signal following hybridization with sense riboprobes generated for the same positions of the target mRNAs. Autoradiograms were developed and digitized using a ScanMaker 1000XL Pro flatbed scanner (Microtek, Carson, CA) with LaserSoft Imaging software (AG, Kiel, Germany) at 1,600 dpi. Digitized images were analyzed using ImageJ Analysis Software for PC from NIH. Optical density measurements were corrected for background, and then multiplied by the area sampled in μm2 to produce an integrated optical density (IOD) measurement.
4.6 Quantification of in Situ Hybridization Data
Adjacent cresyl violet-stained sections were digitized at 1,600 dpi using Super CoolScan 4000 ED slide scanner (Nikon, http://www.nikon.com/). Parcellation of the brainstem 5-HT cell groups was carried out by using Sert autoradiograms and adjacent cresyl violet stained sections, which were digitally overlaid using Adobe Photoshop CS2 (http://www.adobe.com/). This method afforded the use of neurochemical and cytoarchitectonic criteria for the delineation of separate 5-HTergic cell groups. We generated a detailed map of the brainstem 5-HTergic cell groups, and then used it to guide quantification of Tph2 and Sert expression.
For quantification of c-fos expression, Sert and c-fos autoradiograms from adjacent tissue sections were digitally aligned in Photoshop and exported to ImageJ 1.41o (http://rsb.info.nih.gov/ij). Sert ISH signal was then used to delineate the boundaries of 5-HTergic cell groups, which were projected onto c-fos autoradiograms from adjacent tissue sections. Background adjusted signal and integrated optical density (IOD) were quantified for each probe within each cell group.
4.7 Statistical Analyses
Data were analyzed using either a one-way ANOVA with (bLR/bHR) phenotype as the independent variable (for analysis of behavioral data), or a two-way ANOVA with phenotype and either rostro-caudal position (for analysis of ISH data) or intruder exposure status (for endocrine assays) as independent variables. Significant main effects or interactions were followed up by post-hoc comparisons using Fisher’s Exact Test. Data were analyzed using SPSS 17.0 for Windows (SPSS Institute, http://www.spss.com/) and are presented as mean ± SEM. For all tests α=0.05.
Supplementary Material
*Highlights.
Highlights for Kerman, Clinton et al “High Novelty-Seeking Predicts Aggression and Gene Expression Differences within Defined Serotonergic Cell Groups”:
We examined aggression in two rat lines bred for high versus low novelty-seeking.
As predicted, high novelty-seeking rats exhibited exaggerated aggressive behavior.
Gene expression studies point to altered serotonin levels leading to aggression.
Aggression-related serotonin differences occur in specific brainstem nuclei.
Acknowledgements
This work was supported by NIMH 5K99MH081927 (IAK), NARSAD Young Investigator award (IAK), NIMH 1K99MH085859 (SMC), Office of Naval Research N00014-02-1-0879 (HA and SJW), and NIDA PPG 5P01DA021633-02 (HA and SJW). The authors would like to thank Sue Miller for excellent technical assistance as well as Dr. Peter Blandino, Dr. Shelly Flagel, Dr. Brian Mickey, and Ms. Rebecca Simmons for their critical reading of an earlier version of the manuscript. None of the funding sources had roles in study design; data collection, analysis and interpretation, manuscript preparation, or the decision to submit the work for publication. There are no biomedical financial interests or conflicts of interest for any of the authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams JK, Johnson PL, Hollis JH, Lowry CA. Anatomic and functional topography of the dorsal raphe nucleus. Ann N Y Acad Sci. 2004;1018:46–57. doi: 10.1196/annals.1296.005. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Mann JJ. Serotonin brain circuits involved in major depression and suicide. Progress in Brain Research. 2002;136:443–53. doi: 10.1016/s0079-6123(02)36037-0. [DOI] [PubMed] [Google Scholar]
- Asberg M, Thoren P, Traskman L, Bertilsson L, Ringberger V. “Serotonin depression”--a biochemical subgroup within the affective disorders? Science. 1976;191:478–80. doi: 10.1126/science.1246632. [DOI] [PubMed] [Google Scholar]
- Bannai M, Fish EW, Faccidomo S, Miczek KA. Anti-aggressive effects of agonists at 5-HT1B receptors in the dorsal raphe nucleus of mice. Psychopharmacology (Berl) 2007;193:295–304. doi: 10.1007/s00213-007-0780-5. [DOI] [PubMed] [Google Scholar]
- Caramaschi D, de Boer SF, de Vries H, Koolhaas JM. Development of violence in mice through repeated victory along with changes in prefrontal cortex neurochemistry. Behav Brain Res. 2008;189:263–72. doi: 10.1016/j.bbr.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Clark MS, McDevitt RA, Neumaier JF. Quantitative mapping of tryptophan hydroxylase-2, 5-HT1A, 5-HT1B, and serotonin transporter expression across the anteroposterior axis of the rat dorsal and median raphe nuclei. J Comp Neurol. 2006;498:611–23. doi: 10.1002/cne.21073. [DOI] [PubMed] [Google Scholar]
- Clinton SM, Vazquez DM, Kabbaj M, Kabbaj MH, Watson SJ, Akil H. Individual differences in novelty-seeking and emotional reactivity correlate with variation in maternal behavior. Horm Behav. 2007;51:655–64. doi: 10.1016/j.yhbeh.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JR, Bloom FE, Roth RH. The Biochemical Basis of Neuropharmacology. Oxford University Press; New York: 2003. [Google Scholar]
- Curran T, Morgan JI. Fos: an immediate-early transcription factor in neurons. J Neurobiol. 1995;26:403–12. doi: 10.1002/neu.480260312. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation--a possible prelude to violence. Science. 2000;289:591–4. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Dawe S, Gullo MJ, Loxton NJ. Reward drive and rash impulsiveness as dimensions of impulsivity: implications for substance misuse. Addict Behav. 2004;29:1389–405. doi: 10.1016/j.addbeh.2004.06.004. [DOI] [PubMed] [Google Scholar]
- de Almeida RM, Benini Q, Betat JS, Hipolide DC, Miczek KA, Svensson AI. Heightened aggression after chronic flunitrazepam in male rats: potential links to cortical and caudate-putamen-binding sites. Psychopharmacology (Berl) 2008;197:309–18. doi: 10.1007/s00213-007-1031-5. [DOI] [PubMed] [Google Scholar]
- de Boer SF, Koolhaas JM. 5-HT1A and 5-HT1B receptor agonists and aggression: a pharmacological challenge of the serotonin deficiency hypothesis. Eur J Pharmacol. 2005;526:125–39. doi: 10.1016/j.ejphar.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Ehrenkranz J, Bliss E, Sheard MH. Plasma testosterone: correlation with aggressive behavior and social dominance in man. Psychosom Med. 1974;36:469–75. doi: 10.1097/00006842-197411000-00002. [DOI] [PubMed] [Google Scholar]
- Filaire E, Alix D, Rouveix M, Le Scanff C. Motivation, stress, anxiety, and cortisol responses in elite paragliders. Percept Mot Skills. 2007;104:1271–81. doi: 10.2466/pms.104.4.1271-1281. [DOI] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, Phillips PE, Akil H. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hixson DM, Ferris CF. Cortisol exerts site-, context- and dose-dependent effects on agonistic responding in hamsters. J Neuroendocrinol. 1991;3:613–22. doi: 10.1111/j.1365-2826.1991.tb00326.x. [DOI] [PubMed] [Google Scholar]
- Hurley RW, Banfor P, Hammond DL. Spinal pharmacology of antinociception produced by microinjection of mu or delta opioid receptor agonists in the ventromedial medulla of the rat. Neuroscience. 2003;118:789–96. doi: 10.1016/s0306-4522(03)00041-1. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jones SL, Light AR. Serotoninergic medullary raphespinal projection to the lumbar spinal cord in the rat: a retrograde immunohistochemical study. J Comp Neurol. 1992;322:599–610. doi: 10.1002/cne.903220413. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci. 2000;20:6983–8. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerman IA, Enquist LW, Watson SJ, Yates BJ. Brainstem substrates of sympatho-motor circuitry identified using trans-synaptic tracing with pseudorabies virus recombinants. J Neurosci. 2003;23:4657–66. doi: 10.1523/JNEUROSCI.23-11-04657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerman IA, Buck BJ, Evans SJ, Akil H, Watson SJ. Combining laser capture microdissection with quantitative real-time PCR: Effects of tissue manipulation on RNA quality and gene expression. J Neurosci Methods. 2006a;153:71–85. doi: 10.1016/j.jneumeth.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Kerman IA, Shabrang C, Taylor L, Akil H, Watson SJ. Relationship of presympathetic-premotor neurons to the serotonergic transmitter system in the rat brainstem. J Comp Neurol. 2006b;499:882–96. doi: 10.1002/cne.21129. [DOI] [PubMed] [Google Scholar]
- Kerman IA. Organization of brain somatomotor-sympathetic circuits. Exp Brain Res. 2008;187:1–16. doi: 10.1007/s00221-008-1337-5. [DOI] [PubMed] [Google Scholar]
- Kim DK, Tolliver TJ, Huang SJ, Martin BJ, Andrews AM, Wichems C, Holmes A, Lesch KP, Murphy DL. Altered serotonin synthesis, turnover and dynamic regulation in multiple brain regions of mice lacking the serotonin transporter. Neuropharmacology. 2005;49:798–810. doi: 10.1016/j.neuropharm.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Kruk MR, Westphal KG, Van Erp AM, van Asperen J, Cave BJ, Slater E, de Koning J, Haller J. The hypothalamus: cross-roads of endocrine and behavioural regulation in grooming and aggression. Neurosci Biobehav Rev. 1998;23:163–77. doi: 10.1016/s0149-7634(98)00018-9. [DOI] [PubMed] [Google Scholar]
- Lopez-Figueroa AL, Norton CS, Lopez-Figueroa MO, Armellini-Dodel D, Burke S, Akil H, Lopez JF, Watson SJ. Serotonin 5-HT1A, 5-HT1B, and 5-HT2A receptor mRNA expression in subjects with major depression, bipolar disorder, and schizophrenia. Biol Psychiatry. 2004;55:225–33. doi: 10.1016/j.biopsych.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Lowry CA. Functional subsets of serotonergic neurones: implications for control of the hypothalamic-pituitary-adrenal axis. Journal of Neuroendocrinology. 2002;14:911–23. doi: 10.1046/j.1365-2826.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Evans AK, Gasser PJ, Hale MW, Staub DR, Shekhar A. Topographic organization and chemoarchitecture of the dorsal raphe nucleus and the median raphe nucleus. In: Monti JM, Pandi-Perumal SR, Jacobs BL, Nutt DJ, editors. Serotonin and Sleep: Molecular, Functional and Clinical Aspects. Birkhäuser Verlag; Switzerland: 2008. [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Anatomy of CNS opioid receptors. Trends Neurosci. 1988;11:308–14. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- Mason P. Contributions of the medullary raphe and ventromedial reticular region to pain modulation and other homeostatic functions. Annual Review of Neuroscience. 2001;24:737–77. doi: 10.1146/annurev.neuro.24.1.737. [DOI] [PubMed] [Google Scholar]
- Melander T, Hokfelt T, Rokaeus A, Cuello AC, Oertel WH, Verhofstad A, Goldstein M. Coexistence of galanin-like immunoreactivity with catecholamines, 5-hydroxytryptamine, GABA and neuropeptides in the rat CNS. Journal of Neuroscience. 1986;6:3640–54. doi: 10.1523/JNEUROSCI.06-12-03640.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Faccidomo SP, Fish EW, DeBold JF. Neurochemistry and Molecular Neurobiology of Aggressive Behavior. In: Lajtha A, Blaustein JD, editors. Neurobiology Behavioral Neurochemistry, Neuroendocrinology and Molecular Neurobiology. 2007. [Google Scholar]
- Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8:536–46. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- Oliva JM, Uriguen L, Perez-Rial S, Manzanares J. Time course of opioid and cannabinoid gene transcription alterations induced by repeated administration with fluoxetine in the rat brain. Neuropharmacology. 2005;49:618–26. doi: 10.1016/j.neuropharm.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Hastings RS, Oquendo MA, Huang YY, Simpson N, Arcement J, Huang Y, Ogden RT, Van Heertum RL, Arango V, Mann JJ. Lower serotonin transporter binding potential in the human brain during major depressive episodes. Am J Psychiatry. 2006;163:52–8. doi: 10.1176/appi.ajp.163.1.52. [DOI] [PubMed] [Google Scholar]
- Perez JA, Clinton SM, Turner CA, Watson SJ, Akil H. A new role for FGF2 as an endogenous inhibitor of anxiety. J Neurosci. 2009;29:6379–87. doi: 10.1523/JNEUROSCI.4829-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deroche V, Deminiere JM, Maccari S, Le Moal M, Simon H. Corticosterone in the range of stress-induced levels possesses reinforcing properties: implications for sensation-seeking behaviors. Proc Natl Acad Sci U S A. 1993;90:11738–42. doi: 10.1073/pnas.90.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo-Angulo ML, Rodriguez-Veiga E, Reinoso-Suarez F. Serotonergic connections to the ventral oral pontine reticular nucleus: implication in paradoxical sleep modulation. J Comp Neurol. 2000;418:93–105. doi: 10.1002/(sici)1096-9861(20000228)418:1<93::aid-cne7>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Semple-Rowland SL, Mahatme A, Rowland NE. Effects of dexfenfluramine or 5,7-dihydroxytryptamine on tryptophan hydroxylase and serotonin transporter mRNAS in rat dorsal raphe. Brain Res Mol Brain Res. 1996;41:121–7. doi: 10.1016/0169-328x(96)00076-9. [DOI] [PubMed] [Google Scholar]
- Simon NG, Cologer-Clifford A, Lu SF, McKenna SE, Hu S. Testosterone and its metabolites modulate 5HT1A and 5HT1B agonist effects on intermale aggression. Neurosci Biobehav Rev. 1998;23:325–36. doi: 10.1016/s0149-7634(98)00034-7. [DOI] [PubMed] [Google Scholar]
- Stead JD, Clinton S, Neal C, Schneider J, Jama A, Miller S, Vazquez DM, Watson SJ, Akil H. Selective breeding for divergence in novelty-seeking traits: heritability and enrichment in spontaneous anxiety-related behaviors. Behav Genet. 2006;36:697–712. doi: 10.1007/s10519-006-9058-7. [DOI] [PubMed] [Google Scholar]
- Steinbusch HWM. Serotonin-immunoreactive neurons and their projections in the CNS. In: Björklund A, Hökfelt T, Kuhar MJ, editors. Handbook of Chemical Neuroanatomy, Vol. 3, Classical Transmitter and Transmitter Receptors in the CNS. Elsevier; Amsterdam: 1984. pp. 68–125. Part II. [Google Scholar]
- Summers CH, Winberg S. Interactions between the neural regulation of stress and aggression. J Exp Biol. 2006;209:4581–9. doi: 10.1242/jeb.02565. [DOI] [PubMed] [Google Scholar]
- Thase ME. Antidepressant treatment of the depressed patient with insomnia. J Clin Psychiatry. 1999;60(Suppl 17):28–31. discussion 46-8. [PubMed] [Google Scholar]
- van Bokhoven I, Van Goozen SH, van Engeland H, Schaal B, Arseneault L, Seguin JR, Nagin DS, Vitaro F, Tremblay RE. Salivary cortisol and aggression in a population-based longitudinal study of adolescent males. J Neural Transm. 2005;112:1083–96. doi: 10.1007/s00702-004-0253-5. [DOI] [PubMed] [Google Scholar]
- van Erp AM, Miczek KA. Increased accumbal dopamine during daily alcohol consumption and subsequent aggressive behavior in rats. Psychopharmacology (Berl) 2007;191:679–88. doi: 10.1007/s00213-006-0637-3. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Martin GF. Autoradiographic analysis of ascending projections from the pontine and mesencephalic reticular formation and the median raphe nucleus in the rat. J Comp Neurol. 1988;275:511–41. doi: 10.1002/cne.902750404. [DOI] [PubMed] [Google Scholar]
- Waselus M, Galvez JP, Valentino RJ, Van Bockstaele EJ. Differential projections of dorsal raphe nucleus neurons to the lateral septum and striatum. J Chem Neuroanat. 2006;31:233–42. doi: 10.1016/j.jchemneu.2006.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.