Abstract
The apical sodium dependent bile acid transporter (ASBT –SLC10A2) mediates intestinal, renal and cholangiocyte bile acid reclamation. Transcriptional regulation of ASBT is well-described, while information on post-transcriptional regulation is limited. Prior studies suggested that ontogeny of ASBT is controlled in part by changes in mRNA stability. We studied the role that HuR and TTP play in regulating the expression of mRNA that contains the 3’UTR of rat ASBT. The 3’ UTR was incorporated into an SV-40 driven luciferase reporter (rASBT3-luciferase) for rapid screening of regulatory effects. Silencing HuR reduced luciferase reporter activity, while silencing TTP enhanced luciferase activity. Conversely, overexpression of HuR enhanced rASBT3-luciferase reporter activity. The same 3’UTR fragments of rat ASBT were incorporated into a beta-globin coding mRNA construct for analysis of mRNA stability (rASBT3-ßglobin). mRNA half-life was progressively shortened by the incorporation of increasing sized fragments of the 3’UTR. Silencing HuR shortened the half-life of rASBT3-ßglobin containing 0.3 kb of the rat ASBT 3’UTR. Gel shift assays revealed binding of HuR and TTP to rat ASBT 3’UTR. Endogenously expressed human ASBT mRNA half-lives and steady-state protein levels in Caco-2 cells were repressed when HuR was silenced but was enhanced when TTP was silenced. Developmental changes in HuR and TTP protein abundance correlated with previously characterized ontogenic changes in rat ileal and renal ASBT expression.
Conclusion
These studies not only show that ASBT expression is controlled at the level of mRNA stability via its 3’UTR, but also identify HuR and TTP as two key trans-acting factors that are involved in exerting counterregulatory effects on ASBT mRNA stability.
Keywords: gene expression, ileum, intestine, ontogeny, RNA binding protein
The apical sodium dependent bile acid transporter (ASBT) is the major carrier protein involved in the ileal reabsorption of bile acids (1, 2). ASBT also transports bile acids across the apical membrane of renal proximal convoluted tubule cells and cholangiocytes. Ileal transport plays a critical role in the enterohepatic circulation of bile salts. Bile acids are essential for normal liver function, in particular for maintenance of bile flow. In addition, they are essential for intestinal absorption of fat and fat-soluble vitamins. ASBT mediated ileal bile acid transport leads to physiologically relevant signaling to the gallbladder and liver via ileal secretion of fibroblast growth factor-19 (3, 4). Both deficiency and surplus of bile acids can lead to liver-based pathologic processes. As such, a number of mechanisms exist permitting tight regulation of bile acid homeostasis, thereby preventing disease (5).
The regulation of ASBT expression is complex and has been the subject of many recent investigations. Mechanisms of transcriptional control of ASBT expression have been elucidated over the past 10 years (1). More recent studies have implicated post-transcriptional processes in regulating ASBT expression (6-8). Normal ontogeny of ileal ASBT expression in the rat is biphasic, with fetal expression, postnatal repression and induction at weaning (9). Postnatal repression of ASBT expression may provide a critical signal to enhance hepatic synthesis of bile acids thereby expanding the bile acid pool size. Descriptive analyses of the ontogeny of ASBT in rat ileum and kidney suggest that ASBT expression may be controlled in part by regulated changes in mRNA stability (10, 11). During normal development in the rat ileum there is a greater than 100-fold increase in steady-state ASBT mRNA levels, while there is only a 10-fold difference in ASBT transcription as assessed by nuclear run-on assays (10, 11). In preweaning kidney steady-state ASBT mRNA levels are 10-fold higher than in the ileum, yet transcription rates are similar. These findings suggest that mRNA stabilization contributes to the steady-state accumulation of ASBT mRNA in the adult ileum. Moreover, they also support that differential stabilization of ASBT mRNA plays a critical role in controlling ASBT expression in a tissue-specific manner. Currently, there are no data that describe a molecular mechanism for the regulation of ASBT expression via changes in mRNA stability and thus the following investigations were undertaken.
EXPERIMENTAL PROCEDURES
Cell lines, siRNA, expression constructs and antibodies
Rat IEC-6 intestinal epithelial cells and human Caco-2 colon epithelial cells (American Type Culture Collection, Manassas, VA) were maintained in Ham’s F-12 medium containing 10% fetal calf serum. The anti-HuR, anti-TTP, anti-Auf-1, anti-KSRP antibodies, HuR and TTP small interfering RNAs (siRNA) and control siRNA (siScr) were purchased from Santa Cruz Biotechnologies, Inc. (Santa Cruz, CA). The plasmid construct pcDNA3.1/mHuRcoding-3’UTR/Flag contains a wild-type (wt) HuR gene and was a generous gift from Dr. Beth S. Lee (Ohio State University, Columbus, Ohio) (12). The anti-human ASBT antibody was a generous gift from Dr. Paul Dawson (Wake Forest University School of Medicine, Winston Salem, NC)(13). The anti-β-actin antibody was purchased from Sigma (St. Louis, MO).
Generation of recombinant plasmid constructs
Recombinant plasmids were prepared using standard techniques with details provided in supplemental methods. The full length rat and human ASBT 3’ untranslated regions (UTR) were subcloned into pCRII-TOPO (pCRII-rASBT3’/3.1 and pCRII-hASBT3’/2.1 kb 3’UTR, respectively). Three fragments of the rat ASBT 3’UTR 1155-4270 [(3.1 kb, the full 3’UTR region), 2434-4128 (1.7 kb) and 2114-2434 (0.3 kb) (GenBank NM_017222) (Figure 1)] were subcloned into either a pGL3 vector for luciferase assays or into a ß-globin reporter for mRNA half-life determinations.
Figure 1. Rat and human apical sodium dependent bile acid transporter 3’ untranslated RNA.
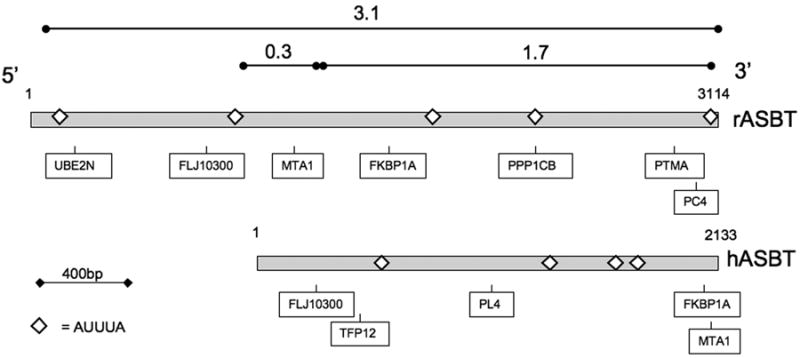
The rat (rASBT) and human (hASBT) 3’UTR are depicted by gray rectangles with the position of AUUUA sites indicated by open diamonds. Additional predicted potential HuR binding sites are shown (UBE2N = Ubiquitin-conjugated enzyme E2N, MTA1 = metastasis associated 1, FKBP1A = FK506 binding protein 1A, PPP1CB = Protein phosphatase 1, catalytic subunit ß, PTMA = prothymosin alpha, PC4 = RNA polymerase II transcription cofactor 4, from (37)). The composition of the fragments of rASBT 3’UTR used for in vitro analyses is indicated by lines with circles at the ends.
Transient transfection and dual luciferase assay
IEC-6 or Caco-2 cells (5 × 105/well) were transfected with 3 μg of the rASBT3’-luciferase construct of interest and 0.1 μg of a quantification control plasmid pRL-TK containing a thymidine kinase promoter-driven Renilla luciferase gene (Promega, Madison, WI). Transfection and luciferase activity measurements were performed as previously described (14). Results are expressed as relative light units (RLU), the ratio of firefly to Renilla luciferase activities (15).
Northern blot analysis and mRNA half-life assay
IEC-6 cells (5 × 106) were transfected with 30 μg of rASBT3-β-globin hybrid constructs plus or minus 10 μM siHuR for 48 hours before mRNA half-life assays. In addition to withdrawal of serum from the medium, Transcription and translation of logarithmically growing cells were terminated by withdrawal of serum and addition of 10 μg/ml actinomycin D and 10 μg/ml cycloheximide. Total cellular RNA was extracted at different times using TRIzol reagent (Invitrogen, Carlsbad, CA). Twenty micrograms of total cellular RNA were analyzed by Northern blotting (16)with 32P-labeled ß-globin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA (16, 17). Signal was quantified using a Phosphoimager Molecular Imager FX (Bio-Rad, Hercules, CA). mRNA half-life was determined by linear regression of a natural log transformation against time.
In vitro transcription and labeling of RNA oligonucleotides
The 32P-labeled RNA probes of the 0.3 kb rASBT 3’UTR were synthesized by in vitro transcription of 0.5 μg of SalI-nicked plasmid constructs pSPT18-rASBT3’/0.3 using T7 RNA polymerase and an SP6/T7 in vitro transcription kit (Roche, Nutley, NJ) (16). Transcripts were purified using native polyacrylamide gel electrophoresis (16). RNA oligonucleotides (Invitrogen, Carlsbad, CA) were 3’-end labeled with 32P using Ambion’s protocol (Ambion Inc., Austin, TX). Unincorporated label was removed using a NucAway spin column (Ambion Inc., Austin, TX).
Preparation of cellular and tissue homogenates
IEC-6 and Caco-2 cells (5 × 106) were untreated or transfected with 30 μg of a wt HuR plasmid construct pcDNA3.1/mHuRcoding-3’UTR/Flag or 10 μM of siHuR or control siRNA for 48 hours prior to harvesting for homogenate preparation. For developmental studies, rat ileal epithelium and kidney cells were derived from 1 and 4 week old Sprague-Dawley rats (Taconic, Hudson, NY). The terminal ileum was defined as the distal 30% of the length of the small bowel. Tissues were homogenized with an Omni-Mixer homogenizer (Omni International, Kennesaw, GA) before protein extraction. Cellular and tissue homogenates were prepared by four cycling of freezing and thawing of cells in a lysis buffer containing 20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM beta-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin, and 1 mM PMSF, with the latter added immediately before use. The supernatant containing protein homogenate was obtained by centrifugation at 16,000 g for 15 min at 4°C.
Gel shift assay
Twenty micrograms of IEC-6 cytoplasmic protein were incubated with 2 × 104 cpm of 32P-labeled transcript for 30 min at 37°C, followed by treatments to minimize non-specific protein bindings as described previously (18). Samples were electrophoresed in 7% native polyacrylamide gel with 0.5X TBE (Tries borate-EDTA) running buffer. Dried gels were exposed to film at -80°C for 24 h.
Western blot analysis
Fifty micrograms of IEC-6 or Caco-2 proteins were electrophoresed in 12% polyacrylamide gels and analyzed by Western blotting (18). Membranes were stripped with Restore™ Western Blot Stripping Buffer (Bio-Rad, Hercules, CA) for sequential probing.
Animal care
Animals were housed, fed, and handled according to the NIH Guide for the Care and Use of Laboratory Animals and under a protocol approved by Institutional Animal Care and Use Committee of the University of Pittsburgh.
Statistical analysis
Statistical analysis was performed using In-Stat software (GraphPad Software, Inc., San Diego, CA). Unless otherwise stated, means were compared using the Tukey-Kramer multiple comparisons test, all values were mean ± SD, and a value of P<0.05 was considered statistically significant.
RESULTS
The Rat ASBT 3’UTR Destabilizes mRNA
The 3’UTR was divided based upon convenient restriction sites to begin to dissect out the relevant cis-elements. Three different sized fragments of the 3’UTR were incorporated into two different reporter constructs, one for rapid assessment on reporter protein expression (rASBT3’-luciferase) and one for kinetic analysis of mRNA half-life (rASBT3’-ßglobin) (Figure 1). Construct veracity was confirmed by sequence analysis and northern blotting (Supplemental Figures 1 and 2). There was a step-wise decrease in luciferase reporter activity in rASBT3’-luciferase constructs containing increasing sized fragments of the rat ASBT 3’UTR (Figure 2A). In order to determine whether the effect on luciferase reporter activity was mediated by changes in mRNA stability or translation, mRNA half-life was assessed for the various rASBT-ßglobin constructs. Half-life was progressively shorter in constructs containing larger fragments of the rat ASBT 3’UTR (Figures 2B and Supplemental Figure 3). Basal half-life of the ß-globin core construct was 36 hours and decreased to approximately 50 minutes in constructs that incorporated the full 3’ UTR of rat ASBT. The observed profound destabilizing effect from the 3’UTR of rASBT mRNA well accounts for the repression of luciferase activity by the rat ASBT 3’UTR. As an initial investigation of the potential RNA binding proteins mediating this effect, gel shift assays were performed using the entire rat or human ASBT 3’UTR (Figure 2C and Supplemental Figure 4). Four well characterized RNA binding proteins were investigated to initiate analysis of the mechanisms involved in regulating ASBT mRNA stability; gel shift was observed with extracts from IEC-6 (for rat) or Caco-2 (for human) cells and antibodies directed against HuR and TTP but not Auf-1 or KSRP (Figure 2C and Supplemental Figure 4). All four of the RNA binding proteins are expressed in ileum and kidney cells and tissues (Figure 2D).
Figure 2. Effect of rASBT 3’UTR on mRNA stability.
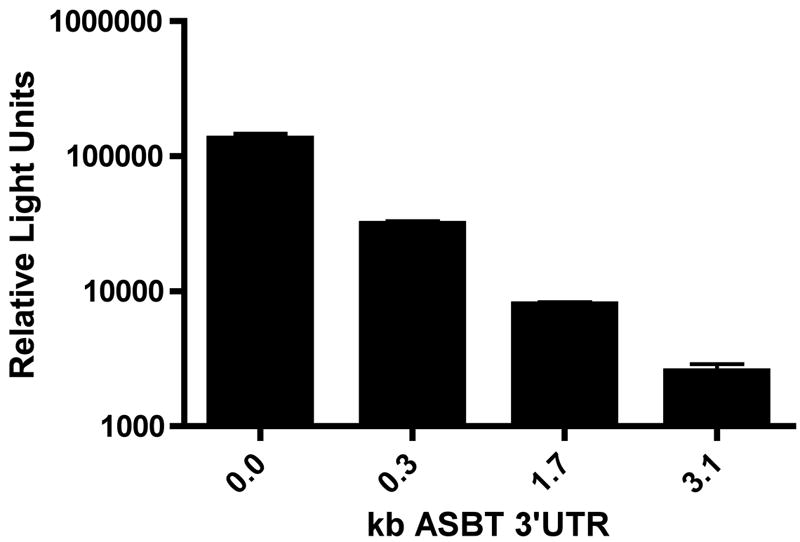
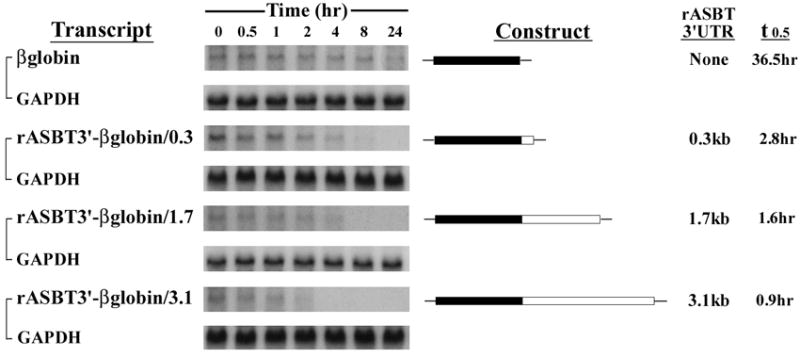
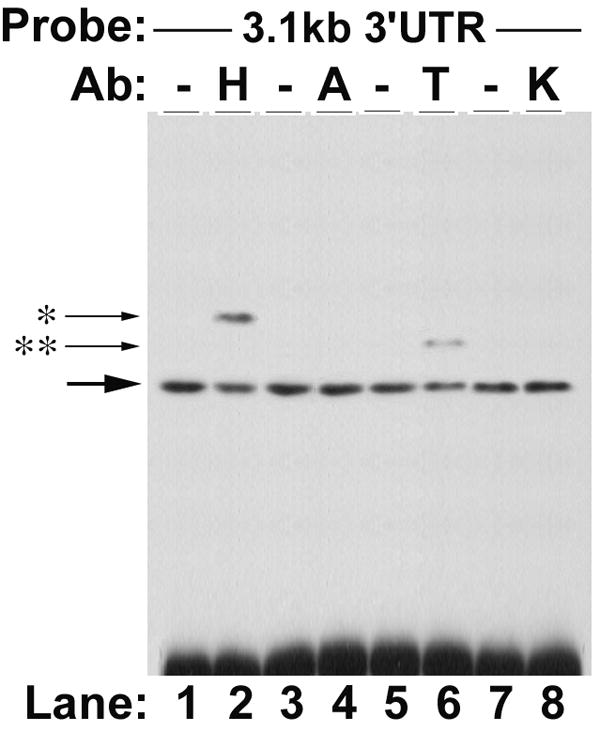
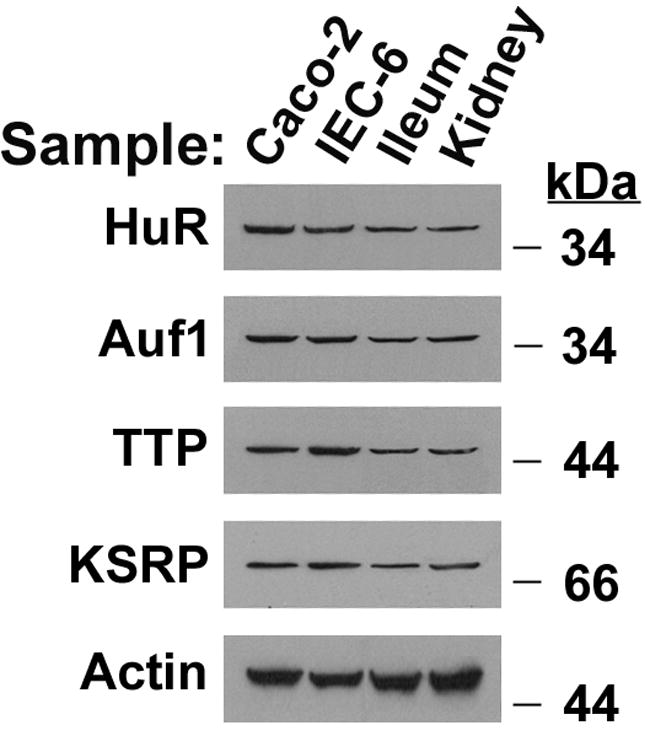
2A. rASBT3’-luciferase Assay. Various rASBT3’-luciferase constructs were generated from an SV-40 driven luciferase reporter. Constructs were transfected into IEC-6 cells and luciferase activity was assessed. Luciferase activity is reduced as larger fragments of rASBT 3’UTR are incorporated. Bars represent the mean of nine measurement in three separate sets of experiments. Error bars represent standard of deviation. The y-axis is logarithmic. 2B. rASBT3’-ßglobin Assay. Various rASBT3’-ßglobin constructs were generated from a c-fos promoter driven ß-globin coding region containing reporter construct. Constructs were transfected into IEC-6 cells and RNA transcription was terminated by serum removal and the addition of actinomycin D and cycloheximide. At the specified time points RNA was extracted and analyzed by Northern blot for globin (top row for each pair) and glyceraldehyde 3-phosphate dehydrogenase (bottom row). Half life was determined by natural log transformation (Supplemental Figure 3). Results are from a single experiment. Globin mRNA abundance reduced with time in all experiments, with a step-wise increase in the rate of reduction as the size of the incorporated rASBT 3’UTR increased. Similar results were obtained when cycloheximide was omitted (data not shown). 2C. Gel-shift analysis of potential RNA binding proteins. The full length 3.1 kb rat ASBT 3’UTR was radiolabelled, incubated with IEC-6 cell extracts and supershifting was assessed with antibodies against HuR (H), Auf-1 (A), TTP (T) and KSRP (K). Supershift was noted in both studies with HuR and TTP. 2D. Western blot of ileum and kidney. 50 mcg of homogenates of Caco-2 (lane 1), IEC-6 (lane 2), adult rat ileum (lane 3) and adult rat kidney (lane 4) were blotted for the RNA binding proteins, HuR, Auf1, TTP and KSRP. All 4 proteins were expressed.
HuR Stabilizes and TTP Destabilizes RNA containing the Rat ASBT 3’UTR
The effect of alterations in HuR expression were first studied using the rASBT3’-luciferase constructs. HuR loss of function was accomplished using HuR siRNA, while gain of function was achieved with a wild type expression vector. Alterations in HuR expression had no effect on the basal luciferase activity of the reporter construct (Figure 3A). Luciferase activity of all of the rASBT3’-luciferase constructs was significantly increased when HuR was overexpressed, while it was significantly reduced when HuR was knocked down by siRNA (Figure 3A). In contrast, silencing TTP had the opposite effect on the luciferase activity for the rASBT3’-0.3kb construct (Figure 3B). Luciferase activity was markedly increased when TTP was silenced, while it was reduced when HuR was silenced.
Figure 3. Effect of HuR and TTP on rat ASBT 3’UTR mediated changes in mRNA stability.
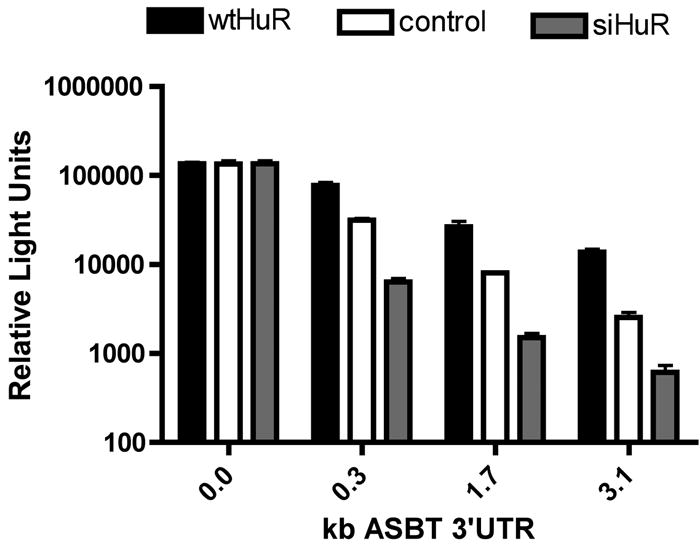
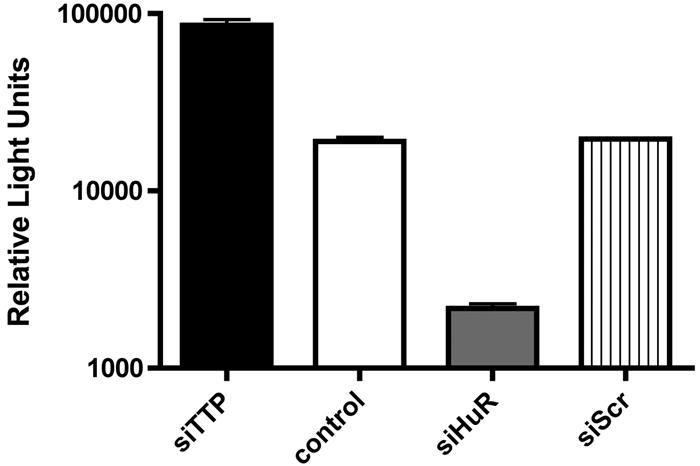
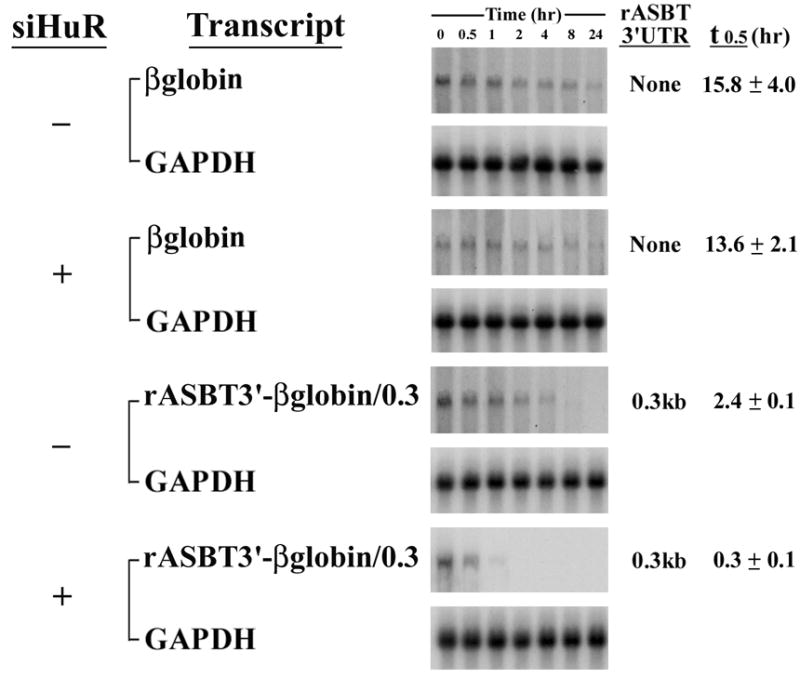
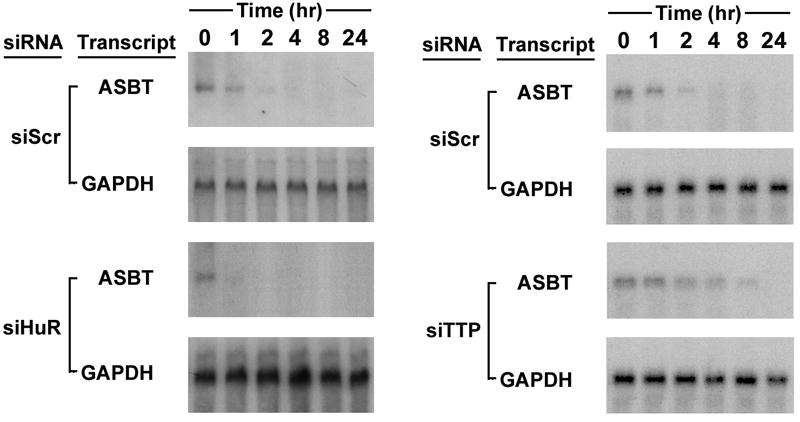
3A-B. rASBT3’-luciferase Assay. 3A. The rASBT3’-luciferase constructs were transfected into IEC-6 cells and luciferase activity was assessed in control cells, those with siRNA mediated reduction in HuR expression (siHuR) or those with overexpression of wild type mouse HuR (wtHuR). Overexpression of HuR enhances luciferase activity, while silencing induces the opposite effect in all constructs except the basal SV-40 driven construct that does not contain rASBT 3’UTR. Bars represent the mean of nine measurement in three separate sets of experiments. Error bars represent standard of deviation. The y-axis is logarithmic. 3B. The rASBT3’-0.3kb luciferase construct was transfected into IEC-6 cells that were treated with siTTP, siHuR, scrambled control (siScr) or vehicle (control). Luciferase activity was markedly increased when TTP was silenced, while it was reduced when HuR was silenced. 3C. rASBT3’-ßglobin Assay. The ß-globin control reporter construct and one containing 0.3kb of rASBT 3’UTR were transfected into Caco-2 cells treated with either scrambled anti-sense RNA or HuR siRNA. At the specified time points after RNA transcription was terminated RNA was extracted and analyzed by Northern blotting for either ß-globin or glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Natural log transformation of the data was used to derive half-lives. Silencing HuR had no effect on the control reporter but reduced the rASBT 3’UTR mRNA’s half-life from 2.4 to 0.3 hours. 3D. Effect of HuR and TTP on endogenous Caco-2 ASBT mRNA stability. Caco-2 cells were treated with either siHuR (panel A) or siTTP (panel B). Scrambled antisense (siScr) was used as a control. 48 hours after this transfection, RNA synthesis was terminated using Actinomycin D and cycloheximide. RNA was prepared from cells at timed intervals after RNA synthesis was terminated. In control cells, ASBT mRNA was detectable 2 to 4 hours after RNA synthesis was terminated, while in siHuR treated cells ASBT mRNA could barely be detected at 1 hour and in siTTP cells ASBT mRNA was detectable at 8 hours.
The specific effect of HuR silencing was assessed using a rASBT-ßglobin construct which incorporated 0.3 kb of the rASBT 3’UTR. This short construct was chosen because the half-life of the longer constructs was too brief for this analysis. Silencing HuR did not alter the half-life of the basal globin reporter construct, but did lead to a more than 75% reduction in the half life of the construct containing 0.3 kb of the rat ASBT 3’UTR (Figure 3C). The effects of silencing HuR and TTP on endogenous ASBT mRNA stability were examined in Caco-2 cells, since they have been previously shown to express all of these genes (19). Silencing HuR diminished the stability of ASBT, while the opposite effect was seen with TTP (Figure 3D). These counterregulatory effects translated into changes in steady-state ASBT protein expression in Caco-2 cells (Figures 4A and 4B). There was a positive correlation between HuR and ASBT protein expression (Figure 4A), while an inverse relationship was observed between TTP and ASBT (Figure 4B).
Figure 4. Effect of HuR and TTP on ASBT protein expression.
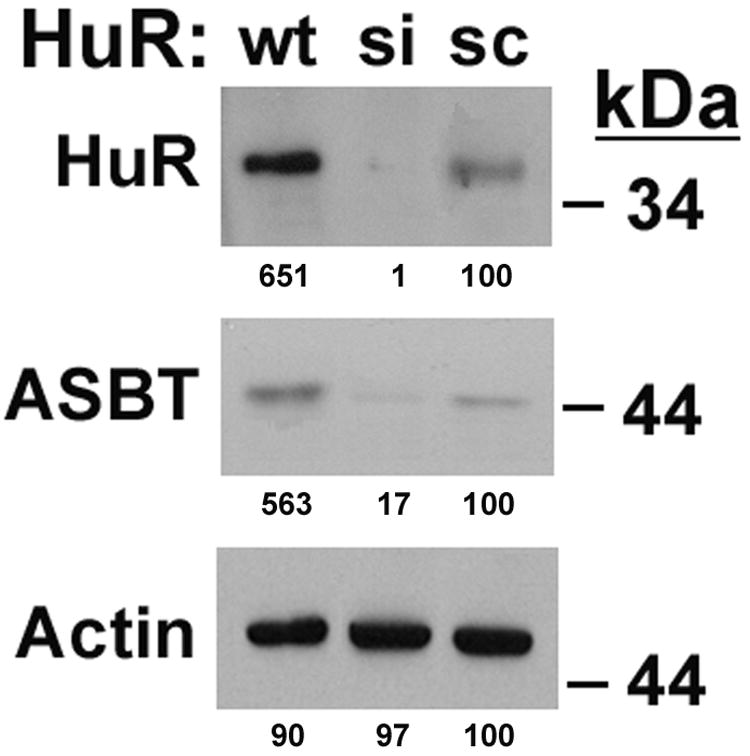
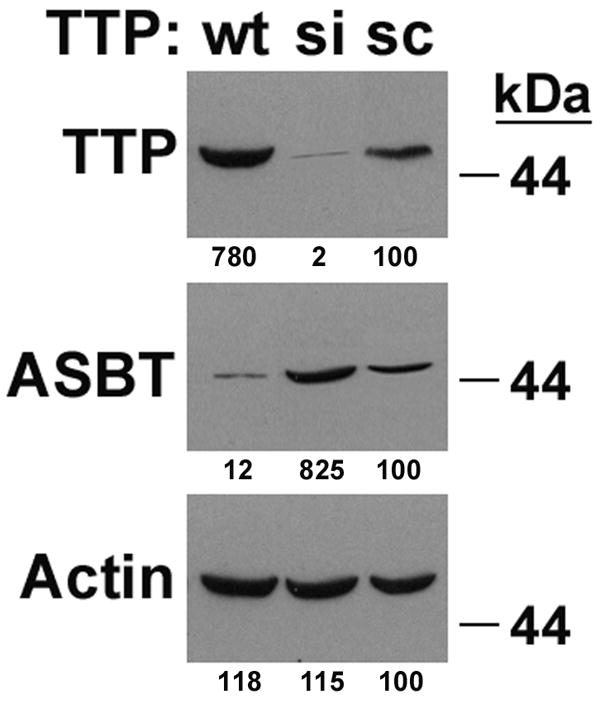
Loss or gain of function of HuR (4A) or TTP (4B) was accomplished using siRNA constructs (si) or by overexpression of wild type constructs (wt). Control cells were transfected with a scrambled antisense construct (sc). Western blot analysis reveals the expected changes in expression of HuR and TTP with the si or wt constructs. Silencing HuR leads to reduced ASBT expression, while HuR overexpression is associated with increased ASBT abundance. The exact opposite effects are observed with changes in TTP expression. The numbers under each band represents densitometry for that band – treatment with sc is set at 100% for each blot.
HuR and TTP are Developmentally Regulated in the Rat Ileum in a Pattern Consistent with ASBT Ontogeny
HuR and TTP expression in the developing rat ileum and kidney were assessed by Western blot and gel shift assays (Figures 5A and 5B). In rat ileum, HuR expression was minimal or absent in preweaning (postnatal day 7) samples, while TTP was minimal or absent in postweaning (postnatal day 28) samples (Figure 5A and Supplemental Figure 5). The pattern of expression correlated with that observed for ASBT during the same time period. In contrast, the expression of both HuR and ASBT was unchanged during the same time period in the kidney (Figure 5A and Supplemental Figure 6). There was a less substantial decrease in TTP during rat kidney ontogeny. These changes were mirrored in analysis of the gel shift patterns using extracts derived from developing ileum and kidney (Figure 5B). Thus, ASBT mRNA levels during development are proportional to the levels of HuR, but are inversely proportional to the levels of TTP.
Figure 5. Ontogeny of HuR, TTP and ASBT in rat ileum and kidney.
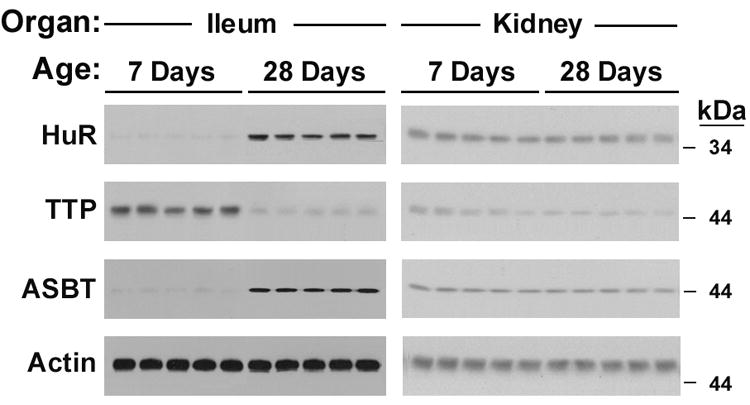
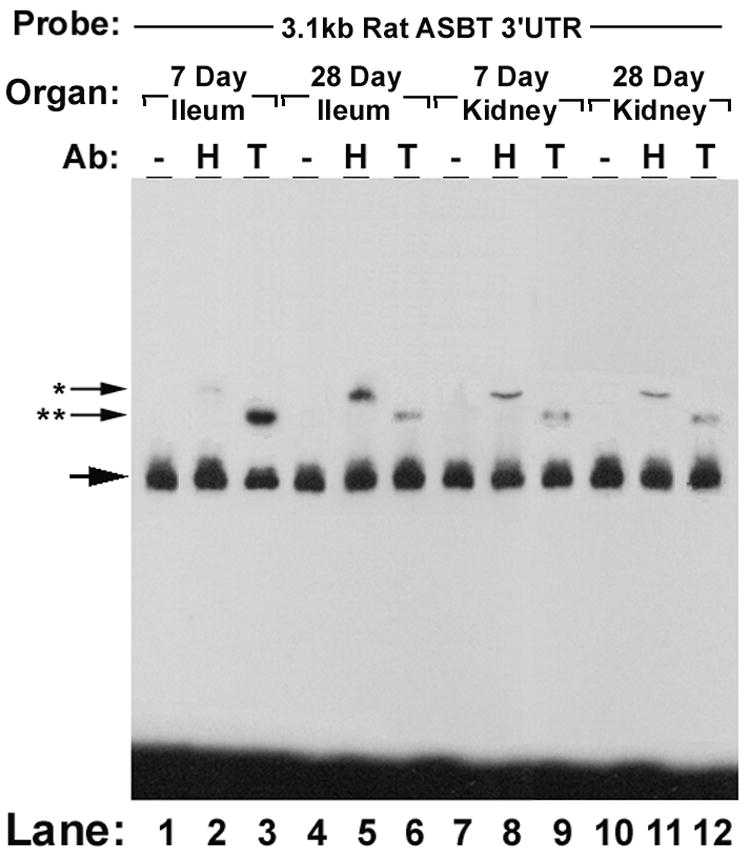
5A - Western blot analysis. Western blot analysis of 5 separate samples of ileal and renal homogenates from 7 and 28 day old rats. Blots were probed for HuR, TTP, ASBT and actin (loading control). In ileum at one week HuR is low and TTP is high (when ASBT is relatively unstable) and at four weeks HuR is high and TTP is low (when ASBT is relatively stable). 5B – Gel shift analysis. Supershift assays of the rat ASBT 3.1 kb 3’UTR with extracts from rat ileum and kidney at age 7 days (preweaning) and 28 days (postweaning). Supershifting is seen in all samples with a suggestion of a greater amount of HuR in postweaning ileum and greater amount of TTP in preweaning ileum.
DISCUSSION
The biologic significance and mechanism(s) of changes in mRNA stability in the intestine are relatively poorly understood, while changes in RNA stability in the liver impact on a variety of biologically and clinically relevant processes (20-24). As in the case of ASBT, regulation by changes in mRNA stability has been implicated primarily on the basis of finding discrepancy between steady-state mRNA levels and transcription rates (as measured by nuclear run-on assays) and/or by the finding of inducible changes in mRNA half-lives in vitro or in vivo (25-27). LDL receptor mRNA is stabilized by several RNA binding proteins (21). Liver regeneration is controlled in part by Apobec-1 complementation factor mediated changes in IL-6 mRNA stability (23). Cyclooxygenase-2 (Cox-2) mRNA half-lives are increased by chenodeoxcholic acid or ceramide in a rat intestinal epithelial cell line (28).
Hu antigen R protein (HuR) is expressed in both liver and intestine and has been shown to regulate a wide range of biologically important processes (20, 22, 29-31). HuR is a 32-kDa member of the Hu/ELAV (embryonic lethal abnormal vision)-like family of proteins. Its expression is considered to be ubiquitous. HuR binding to mRNA species can have two distinct and interrelated effects; it enhances mRNA stability and promotes mRNA translation (30). Relevant effects of HuR on gene expression have been shown for cyclin A, cyclin B1, Cox-2, TNF-α, connexins, beta catenin, and methionine adenosyltransferase to name a few (20, 22, 32-34). It is plausible that HuR plays an important role in regulation of gap junctions in the liver and in liver regeneration. The RNA binding protein tristetraprolin (TTP) has been shown to be counterregulatory for the effects of HuR in colon carcinogenesis and in Caco-2 cells (34). In light of their expression in the intestine and known effects on mRNA stability we have investigated the roles that HuR and TTP play in regulating ASBT expression.
Our studies reveal novel roles for HuR and TTP in the regulation of the bile acid transporter, ASBT, at the level of mRNA turnover. HuR stabilizes ASBT mRNA leading to enhanced expression, while TTP induces the opposite effect. The 3’UTR of the rat ASBT mRNA can confer instability onto an otherwise stable ß-globin mRNA. Even a fragment of 3’UTR as small as 300 bp destabilizes RNA reporter constructs. Examination of the rat and human ASBT 3’ UTR reveals multiple copies of AUUUA motif and other U-rich regions that are commonly found in a large number of AU-rich or GU-rich RNA destabilizing elements (35, 36). These sequence features also serve as potential HuR binding sites. Additional distinct potential HuR binding sites are predicted to be present in both the rat and human ASBT 3’UTR ((37) and Figure 1). Presumably, HuR binding to some of these potential sites promotes increased stability of ASBT mRNA by preventing the binding of RNA-destabilizing proteins such as AUF1, TTP, and CUG-BP1 (38-41). Identification of the specific cis-elements that mediate the effects of these RNA-binding proteins will require further experimental investigations which will characterize this aspect of the molecular regulation of ASBT expression.
The physiologic significance of HuR and TTP on ASBT mediated bile acid transport in vivo is suggested by our studies. The ontogenic patterns of HuR and TTP expression in the rat ileum and kidney correlate very well with prior examination of developmental-stage specific ASBT expression, which suggests that mRNA stability played a key role in mediating this regulation (10, 11). Our studies reveal distinct ontogenic changes in both HuR and TTP expression that occurs at weaning in rat ileum. ASBT mRNA levels change during development in a manner that parallels the levels of HuR, but are inversely proportional to the levels of TTP. There are dramatic changes in the cell structure and function of the intestine at weaning in the rat (42-44). The molecular mechanisms that control these changes are not well understood. It is intriguing that there is a coordinate and mirror image pattern of expression of HuR and TTP in the ileum that follows this pattern of developmental change at weaning.
Genes with tightly controlled regulation often have counter-regulatory molecules. Similar coordinate changes in HuR and TTP have been described in colon carcinogenesis (34). Whether HuR and TTP are part of the global change in gene expression or a regulator of this change needs to be assessed. The latter is a distinct possibility in light of the critical importance of HuR in normal intestinal homeostasis (45) and its correlative role in cancer progression (46, 47). Global deletion of HuR is embryonic lethal, while postnatal deletion yields severe intestinal disease (45). The effects of postnatal but preweaning HuR deletion will need to be assessed to determine the role that HuR plays in intestinal development, especially as it relates to changes in ASBT expression.
Supplementary Material
Acknowledgments
Financial Support: This work was supported in part by grants to BLS (NIH NIDDK DK 54165) and to ABS (NIH GM46454 and the Houston Endowments).
Abbreviations
- ASBT
apical sodium dependent bile acid transporter
- r
rat
- UTR
untranslated region
- HuR
Hu Antigen R
- TTP
tristetraprolin
- IEC
intestinal epithelial cell
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- TK
thymidine kinase
Contributor Information
Frank Chen, Email: Frank.Chen@chp.edu.
Ann-Bin Shyu, Email: Ann-Bin.Shyu@uth.tmc.edu.
Benjamin L. Shneider, Email: Benjamin.Shneider@chp.edu.
References
- 1.Dawson PA, Shneider B, Hofmann AF. Bile formation and the enterohepatic circulation of bile acids. In: Johnson LR, Barrett K, Ghishan F, Merchant J, Said H, Wood J, editors. Physiology of the Gastrointestinal Tract. 4. Elsevier; 2006. pp. 1437–1462. [Google Scholar]
- 2.Dawson PA, Lan T, Rao A. Bile acid transporters. J Lipid Res. 2009;50:2340–2357. doi: 10.1194/jlr.R900012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, Suino-Powell K, Xu HE, et al. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;331:1621–1624. doi: 10.1126/science.1198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones S. Mini-review: endocrine actions of fibroblast growth factor 19. Mol Pharm. 2008;5:42–48. doi: 10.1021/mp700105z. [DOI] [PubMed] [Google Scholar]
- 5.Kosters A, Karpen SJ. Bile acid transporters in health and disease. Xenobiotica. 2008;38:1043–1071. doi: 10.1080/00498250802040584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Annaba F, Sarwar Z, Kumar P, Saksena S, Turner JR, Dudeja PK, Gill RK, et al. Modulation of ileal bile acid transporter (ASBT) activity by depletion of plasma membrane cholesterol: association with lipid rafts. Am J Physiol Gastrointest Liver Physiol. 2008;294:G489–497. doi: 10.1152/ajpgi.00237.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarwar Z, Annaba F, Dwivedi A, Saksena S, Gill RK, Alrefai WA. Modulation of ileal apical Na+-dependent bile acid transporter ASBT by protein kinase C. Am J Physiol Gastrointest Liver Physiol. 2009;297:G532–538. doi: 10.1152/ajpgi.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia X, Roundtree M, Merikhi A, Lu X, Shentu S, Lesage G. Degradation of the apical sodium-dependent bile acid transporter by the ubiquitin-proteasome pathway in cholangiocytes. J Biol Chem. 2004;279:44931–44937. doi: 10.1074/jbc.M400969200. [DOI] [PubMed] [Google Scholar]
- 9.Shneider B, Setchell KDR, Crossman M. Fetal and perinatal expression of ileal and renal sodium-dependent bile acid transport in the rat. Pediatr Res. 1997;42:189–194. doi: 10.1203/00006450-199708000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Shneider BL, Dawson PA, Christie DM, Hardikar W, Wong MH, Suchy FJ. Cloning and molecular characterization of the ontogeny of a rat ileal sodium-dependent bile acid transporter. J Clin Invest. 1995;95:745–754. doi: 10.1172/JCI117722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christie DM, Dawson PA, Thevananther S, Shneider BL. Comparative analysis of the ontogeny of a sodium-dependent bile acid transporter in rat kidney and ileum. Am J Physiol. 1996;271:G377–G385. doi: 10.1152/ajpgi.1996.271.2.G377. [DOI] [PubMed] [Google Scholar]
- 12.Ayupova DA, Singh M, Leonard EC, Basile DP, Lee BS. Expression of the RNA-stabilizing protein HuR in ischemia-reperfusion injury of rat kidney. Am J Physiol Renal Physiol. 2009;297:F95–F105. doi: 10.1152/ajprenal.90632.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung D, Fantin AC, Scheurer U, Fried M, Kullak-Ublick GA. Human ileal bile acid transporter gene ASBT (SLC10A2) is transactivated by the glucocorticoid receptor. Gut. 2004;53:78–84. doi: 10.1136/gut.53.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinha J, Chen F, Miloh T, Burns RC, Yu Z, Shneider BL. beta-Klotho and FGF-15/19 inhibit the apical sodium-dependent bile acid transporter in enterocytes and cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 2008;295:G996–G1003. doi: 10.1152/ajpgi.90343.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen F, Ma L, Al-Ansari N, Shneider B. The role of AP-1 in the transcriptional regulation of the rat apical sodium-dependent bile acid transporter. J Biol Chem. 2001;276:38703–38714. doi: 10.1074/jbc.M104511200. [DOI] [PubMed] [Google Scholar]
- 16.Chen FY, Amara FM, Wright JA. Mammalian ribonucleotide reductase R1 mRNA stability under normal and phorbol ester stimulating conditions: involvement of a cis-trans interaction at the 3’ untranslated region. EMBO J. 1993;12:3977–3986. doi: 10.1002/j.1460-2075.1993.tb06075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shyu A, Greenberg M, Belasco J. The c-fos transcript is targeted for rapid decay by two distinct mRNA degradation pathways. Genes Dev. 1989;3:60–72. doi: 10.1101/gad.3.1.60. [DOI] [PubMed] [Google Scholar]
- 18.Chen F, Ma L, Sartor B, Li F, Xiong H, Sun A, Shneider B. Inflammatory-mediated repression of the rat ileal sodium-dependent bile acid transporter by c-fos nuclear translocation. Gastroenterology. 2002;123:2005–2016. doi: 10.1053/gast.2002.37055. [DOI] [PubMed] [Google Scholar]
- 19.Neimark E, Chen F, Li X, Magid MS, Alasio TM, Frankenberg T, Sinha J, et al. c-Fos is a critical mediator of inflammatory-mediated repression of the apical sodium-dependent bile acid transporter. Gastroenterology. 2006;131:554–567. doi: 10.1053/j.gastro.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Ale-Agha N, Galban S, Sobieroy C, Abdelmohsen K, Gorospe M, Sies H, Klotz LO. HuR regulates gap junctional intercellular communication by controlling beta-catenin levels and adherens junction integrity. Hepatology. 2009;50:1567–1576. doi: 10.1002/hep.23146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, Chen W, Zhou Y, Abidi P, Sharpe O, Robinson WH, Kraemer FB, et al. Identification of mRNA binding proteins that regulate the stability of LDL receptor mRNA through AU-rich elements. J Lipid Res. 2009;50:820–831. doi: 10.1194/jlr.M800375-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vazquez-Chantada M, Fernandez-Ramos D, Embade N, Martinez-Lopez N, Varela-Rey M, Woodhoo A, Luka Z, et al. HuR/methyl-HuR and AUF1 regulate the MAT expressed during liver proliferation, differentiation, and carcinogenesis. Gastroenterology. 2010;138:1943–1953. doi: 10.1053/j.gastro.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blanc V, Sessa KJ, Kennedy S, Luo J, Davidson NO. Apobec-1 complementation factor modulates liver regeneration by post-transcriptional regulation of interleukin-6 mRNA stability. J Biol Chem. 2010;285:19184–19192. doi: 10.1074/jbc.M110.115147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerr TA, Korenblat KM, Davidson NO. MicroRNAs and liver disease. Transl Res. 2011;157:241–252. doi: 10.1016/j.trsl.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Z, Massey J, Via D. Differential regulation of cyclooxygenase-2 (COX-2) mRNA stability by interleukin-1 beta (IL-1 beta) and tumor necrosis factor-alpha (TNF-alpha) in human in vitro differentiated macrophages. Biochem Pharmacol. 2000;59:187–194. doi: 10.1016/s0006-2952(99)00312-3. [DOI] [PubMed] [Google Scholar]
- 26.Aronov S, Marx R, Ginzburg I. Identification of 3’UTR region implicated in tau mRNA stabilization in neuronal cells. J Mol Neurosci. 1999;12:131–145. doi: 10.1007/BF02736927. [DOI] [PubMed] [Google Scholar]
- 27.Nair A, Menon K. Regulatory role of the 3’ untranslated region of luteinizing hormone receptor: effect on mRNA stability. FEBS Lett. 2000;471:39–44. doi: 10.1016/s0014-5793(00)01365-x. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z, Sheng H, Shao J, Beauchamp RD, DuBois RN. Posttranscriptional regulation of cyclooxygenase-2 in rat intestinal epithelial cells. Neoplasia. 2000;2:523–530. doi: 10.1038/sj.neo.7900117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez de Silanes I, Lal A, Gorospe M. HuR: post-transcriptional paths to malignancy. RNA Biol. 2005;2:11–13. doi: 10.4161/rna.2.1.1552. [DOI] [PubMed] [Google Scholar]
- 30.Cherry J, Karschner V, Jones H, Pekala PH. HuR, an RNA-binding protein, involved in the control of cellular differentiation. In Vivo. 2006;20:17–23. [PubMed] [Google Scholar]
- 31.Kim HH, Gorospe M. Phosphorylated HuR shuttles in cycles. Cell Cycle. 2008;7:3124–3126. doi: 10.4161/cc.7.20.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang W, Caldwell MC, Lin S, Furneaux H, Gorospe M. HuR regulates cyclin A and cyclin B1 mRNA stability during cell proliferation. EMBO J. 2000;19:2340–2350. doi: 10.1093/emboj/19.10.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nabors LB, Suswam E, Huang Y, Yang X, Johnson MJ, King PH. Tumor necrosis factor alpha induces angiogenic factor up-regulation in malignant glioma cells: a role for RNA stabilization and HuR. Cancer Res. 2003;63:4181–4187. [PubMed] [Google Scholar]
- 34.Young LE, Sanduja S, Bemis-Standoli K, Pena EA, Price RL, Dixon DA. The mRNA binding proteins HuR and tristetraprolin regulate cyclooxygenase 2 expression during colon carcinogenesis. Gastroenterology. 2009;136:1669–1679. doi: 10.1053/j.gastro.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barreau C, Paillard L, Osborne HB. AU-rich elements and associated factors: are there unifying principles? Nucleic Acids Res. 2005;33:7138–7150. doi: 10.1093/nar/gki1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vlasova IA, Bohjanen PR. Posttranscriptional regulation of gene networks by GU-rich elements and CELF proteins. RNA Biol. 2008;5:201–207. doi: 10.4161/rna.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez de Silanes I, Zhan M, Lal A, Yang X, Gorospe M. Identification of a target RNA motif for RNA-binding protein HuR. Proc Natl Acad Sci U S A. 2004;101:2987–2992. doi: 10.1073/pnas.0306453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raineri I, Wegmueller D, Gross B, Certa U, Moroni C. Roles of AUF1 isoforms, HuR and BRF1 in ARE-dependent mRNA turnover studied by RNA interference. Nucleic Acids Res. 2004;32:1279–1288. doi: 10.1093/nar/gkh282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katsanou V, Papadaki O, Milatos S, Blackshear PJ, Anderson P, Kollias G, Kontoyiannis DL. HuR as a negative posttranscriptional modulator in inflammation. Mol Cell. 2005;19:777–789. doi: 10.1016/j.molcel.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 40.Lal A, Mazan-Mamczarz K, Kawai T, Yang X, Martindale JL, Gorospe M. Concurrent versus individual binding of HuR and AUF1 to common labile target mRNAs. EMBO J. 2004;23:3092–3102. doi: 10.1038/sj.emboj.7600305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hau HH, Walsh RJ, Ogilvie RL, Williams DA, Reilly CS, Bohjanen PR. Tristetraprolin recruits functional mRNA decay complexes to ARE sequences. J Cell Biochem. 2007;100:1477–1492. doi: 10.1002/jcb.21130. [DOI] [PubMed] [Google Scholar]
- 42.Leeper LL, Henning SJ. Development and tissue distribution of sucrase-isomaltase mRNA in rats. Am J Physiol. 1990;258:G52–58. doi: 10.1152/ajpgi.1990.258.1.G52. [DOI] [PubMed] [Google Scholar]
- 43.Wilson JM, Whitney JA, Neutra MR. Biogenesis of the apical endosome-lysosome complex during differentiation of absorptive epithelial cells in rat ileum. J Cell Sci. 1991;100(Pt 1):133–143. doi: 10.1242/jcs.100.1.133. [DOI] [PubMed] [Google Scholar]
- 44.Duluc I, Freund JN, Leberquier C, Kedinger M. Fetal endoderm primarily holds the temporal and positional information required for mammalian intestinal development. J Cell Biol. 1994;126:211–221. doi: 10.1083/jcb.126.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghosh M, Aguila HL, Michaud J, Ai Y, Wu MT, Hemmes A, Ristimaki A, et al. Essential role of the RNA-binding protein HuR in progenitor cell survival in mice. J Clin Invest. 2009;119:3530–3543. doi: 10.1172/JCI38263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heinonen M, Bono P, Narko K, Chang SH, Lundin J, Joensuu H, Furneaux H, et al. Cytoplasmic HuR expression is a prognostic factor in invasive ductal breast carcinoma. Cancer Res. 2005;65:2157–2161. doi: 10.1158/0008-5472.CAN-04-3765. [DOI] [PubMed] [Google Scholar]
- 47.Mrena J, Wiksten JP, Thiel A, Kokkola A, Pohjola L, Lundin J, Nordling S, et al. Cyclooxygenase-2 is an independent prognostic factor in gastric cancer and its expression is regulated by the messenger RNA stability factor HuR. Clin Cancer Res. 2005;11:7362–7368. doi: 10.1158/1078-0432.CCR-05-0764. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


