Abstract
Proteins in the milk release biologically active peptides upon enzymatic digestion. In the present study, we report the identification of novel monocyte/macrophage chemotactic peptides derived from enzymatically digested bovine β-casein, a casein family member that is a major constituent of the milk. β-casein fragments generated by actinase E showed potent chemotactic activity for human and mouse monocytes/macrophages, but not neutrophils, T lymphocytes or dendritic cells. The fragment-induced migration of human monocytes was inhibited by pertussis toxin and was not desensitized by a variety of known chemoattractants, suggesting that the digests activate a unique G protein-coupled receptor(s). The digests were further fractionated and purified to yield 3 small peptides. One peptide Q1 designated as “β-casochemotide-1” with the amino acid sequence of YPVEP (f114-118 of β-casein) induced high levels of macrophage chemotaxis. It also promoted calcium mobilization in macrophages, another indication of cell activation. Our study suggests that biologically active peptides released by actinase-digested milk β-casein may promote innate host immune responses by inducing macrophage migration and activation.
Keywords: β-casein, monocyte, macrophage, chemotaxis, peptide, β-casochemotide
1. Introduction
Milk proteins have been well known for their potential value as additives to many functional foods and beverages to enhance health benefits [1]. As the most abundant proteins in the milk, caseins have been considered as an excellent source of essential amino acids not synthesized in human. Since the discovery of opioid peptides, β-casomorphins [2], various peptides derived from bovine casein digests have been reported to possess biological activities such as anti-hypertension [3] and immunoenhancement [4, 5]. In particular, the immunomodulatory activities of casein digests have been well studied on immune cells for mitogenesis [6], cytotoxicity [7], immunoglobulin production [8] and augmentation of phagocytosis [9]. So far, the best characterized immunomodulatory peptide derived from bovine milk caseins is casein phosphopeptide (CPP) from β-casein (f1-28). CPP displays mitogenic activity on mouse spleen and rabbit Peyer’s patch cells [8] and stimulates their IgA production [8]. Moreover, CPP reduced allergic symptoms elicited by IgE in NC/Jic Jcl mice [10]. Since bovine β-casein contains other peptides in addition to CPP, further isolation and identification of biologically active fragments may yield products of pharmacologic and therapeutic importance.
Leukocyte infiltration induced by chemotactic factors in tissues is an essential event in host defense against microbial infection, resulting in inflammation. For instance, in the gut, macrophage recruitment is crucial for the elimination of invading pathogens [11]. In this study, we explored the possibility that β-casein yields peptide fragments which may promote monocyte/macrophage chemotaxis thus supporting the beneficial effects of milk products on host innate immune system. Here we report the identification of a pentapeptide from the actinase E digests of bovine milk β-casein that chemoattracts and activates human and mouse monocytes/macrophages by using unique Gαi-protein coupled receptor(s).
2. Materials and methods
2.1. Chemicals
β-casein (β-CN) and the synthetic formyl peptide fMLF were purchased from Sigma (St. Louis, MO, USA). Pepsin (EC 3.4.23.1; from porcine gastric mucosa) and proteinase K (EC 3.4.21.14; from Tritirachium album) were purchased from Boehringer Mannheim GmbH (Mannheim, Germany). Trypsin (EC 3.4.21.4; from porcine pancreas), α-chymotrypsin (EC 3.4.21.1; from bovine pancreas), thermolysin (EC 3.4.24.4; from Bacillus thermoproteolyticus) and papain (EC 3.4.22.2; from Carica papaya) were obtained from Wako Pure Chemical Co., Ltd. (Osaka, Japan). Actinase E (from Actinomyces spp.) was obtained from Kaken Pharmaceutical Co., Ltd. (Tokyo, Japan).
2.2. Cells
The murine macrophage-like cell line, J774-1 was obtained from the Institute for Development of Aging and Cancer, Tohoku University (Sendai, Miyagi, Japan). The cells were cultured and maintained in RPMI-1640 supplemented with 10% (v/v) fetal calf serum (FCS), 100 IU/ml penicillin and 100 µg/ml streptomycin. Human monocytes were purified by elutriation to yield over 90 % pure preparations from the human PBMC isolated from leukopacks through the courtesy of Transfusion Medicine Department, National Institute of Health Clinical Center (Bethesda, MD). Human neutrophils and T lymphocytes were also purified from PBMC by the method described previous report [12, 13]. Human mature dendritic cells were generated from monocytes as described previously [13].
2.3. Digestion of β-CN with proteases
β-CN was digested with various proteases. The optimal digestion conditions of buffer, pH and temperature were chosen as described in our previous report [3]. Ten mg of β-CN were dissolved in 10 ml optimal buffer for each enzyme and digested with pepsin or chymotrypsin at 25°C for 24 h or with other proteases at 37°C for 24 h (ratio of protein substrate to enzyme, 100:5, wt/wt). After digestion, each sample was heated at 98°C for 10 min to inactivate the protease. Part of the digests were ultrafiltrated to obtain fractions containing polypeptides of less than 3 kD. The protein content in digests was adjusted to 1.0 mg/ml by the Folin-Lowry method [14].
2.4. Chemotaxis assay
Chemotaxis was assessed using a 48-well chamber (Neuro Probe, Inc., MD, USA) technique, as previously described [15]. The cells were suspended at a concentration of 2 × 106 cells/ml for monocytes or 1 × 106 cells/ml for J774-1 cells in RPMI 1640 complete medium supplemented with 1% bovine serum albumin (BSA). A polyvinylpyrrolidone-free (PVPF) polycarbonate membrane (8 µm pore size, 25 × 80 mm, Neuro Probe) was assembled into the chemotaxis chamber in which the lower compartment contained 28 µL of the test sample. The cell suspension was placed in the upper compartment in a volume of 50 µL. After incubation at 37°C in humidified incubator with 5% CO2 for 1.5 h (for monocytes) or 2 h (for J774-1 cells), the membrane was recovered. Cells on the upper surface of the membrane were removed and washed with a phosphate buffer saline (PBS), and then stained with Field's Stain Solution (Muto Pure Chem. Ltd., Tokyo, Japan). The number of migrated cells was calculated in the high power field (HPF) of light microscope (× 200). The results are expressed as the mean number of migrated cells (± SD) in 1 HPF in 10 replicates and in some cases presented as chemotaxis index representing the fold increase in the number of migrating cells in response to stimuli, over the spontaneous cell migration in response to medium. β-casein without enzyme digestion and N-formyl-methionyl-leucyl-phenylalanine (fMLF) were used as controls.
2.5. SDS-PAGE
The digested β-CN samples were boiled in a SDS-PAGE loading buffer (Wako, Osaka, Japan) and analyzed with 20% (vol/vol) SDS-PAGE and stained with Coomassie Brilliant Blue (Quick CBB, Wako Pure Chemicals, Osaka, Japan).
2.6. Fractionation and purification of chemotactic peptides from the β-CN digests
Actinase digested β-CN were fractionated by reverse phase chromatography using a Sep-pak C18 cartridge (Waters Co., Ltd., MA, USA) with a stepwise elution of 30% to 90% (v/v) acetonitrile (ACN). Each fraction was lyophilized after the removal of ACN. Chemotactic activity was measured for lyophilized samples dissolved in a RPMI 1640 complete medium at a final concentration of 100 µg protein/ml. The 30% ACN fraction was used for further purification of chemotactic peptides. Fraction containing one mg of polypeptides was dissolved in 1 ml of 20 mM phosphate buffer and the chemotactic peptides were fractionated by anion exchange chromatography on HiTrap™ Q HP (Amersham Pharmacia Biotech AB, Uppsala, Sweden) in the Bio Logic chromatography system (Bio-Rad, CA, USA) by a linear gradient elution from 0 to 1 M of NaCl in 20 mM Tris-HCl buffer (pH 8.2). Each 0.25 mL of the eluate was monitored at 214 nm and was tested for macrophage chemotaxis. The active fraction (designated as Q fraction) in the anion exchange chromatography was further purified by HPLC using L-7000 series (Hitachi Ltd., Tokyo, Japan) equipped with a Mightysil RP-18GP (5 mm, 4.6 × 150 mm; KANTO CHEMICAL Co., Inc., Tokyo, Japan). Elution was performed with a linear gradient from 0% to 60% ACN containing 0.05% (v/v) TFA at 40°C with a flow rate of 0.5 ml/min, with detection at 214 nm. The fractionated samples were examined for the purity by re-chromatography and collected in eluate with 30 % ACN by Sep-pak C18 cartridge followed by examination of chemotactic activity.
2.7. Calcium mobilization
Calcium mobilization was assayed by incubating monocytes in loading buffer containing 138 mM NaCl, 6mM KCl, 1 mM CaCl2, 10 mM HEPES (pH 7.4), 5 mM glucose, 0.1 % BSA with 5 mM fura 2 (Sigma) at 37°C for 30 min. The dye-loaded cells were washed and resuspended in fresh loading buffer. The cells were then transferred into quartz cuvettes (2 × 106 cells in 2mL), which were placed in a luminescence spectrometer LS50 B (Perkin-Elmer Limited, Beaconsfield, U.K.). The digests (100 µg/mL) or fMLF (10−3 M) were added to the cuvettes at indicated time points. The ratio of fluorescence at 340 and 380 nm wavelength was calculated using the FL WinLab program (Perkin-Elmer). Calcium mobilization in mouse macrophages induced by Q1 peptide was detected in individually gated cells by a MRC-1024 confocal laser microscope (Bio-Rad, Richmond, CA, USA). The cultured cells (1 × 106 cells) on glass bottom culture dishes were pre-incubated in a drop of 100 µl RPMI-1640 complete medium containing 4 mM Fluo-3 AM (Dojindo Laboratories, Kumamoto, Japan) at 37°C for 30 min. The dye-loaded cells were washed and stimulated with different concentrations (10−5 to 10−3 M) of the peptide. The fluorescence intensity was measured on the gated cells.
2.8. Identification of chemotactic peptides
The chemotactic peptides were sequenced by automated Edman degradation using a protein sequencer (Procise 490; Perkin Elmer Co. Ltd., Applied Biosystem Division, Foster, CA) with a PTH-C18 column (2.1 mm × 220 mm; Perkin Elmer). Chemicals used in the 140C Microgradient Delivery System included 2 types of solvent (Perkin Elmer): A3 (3.5% [vol/vol] aqueous tetrahydrofuran) and B (ACN and isopropanol). The molecular mass of the peptides was analyzed by FAB-mass spectrometry with glycerol as a matrix under a positive charged mode at a low resolution of 8 kV. Final assignment of the peptide sequence was determined by comparing the sequence and molecular mass with the amino acid sequence of β-CN.
2.9. Statistics
All biological assays were performed at least three times and the results presented are from representative experiments. The significance between the test samples and the control was analyzed by student’s t test.
3. Results
3.1. Augmentation of chemotactic activity of β-CN by protease digestion
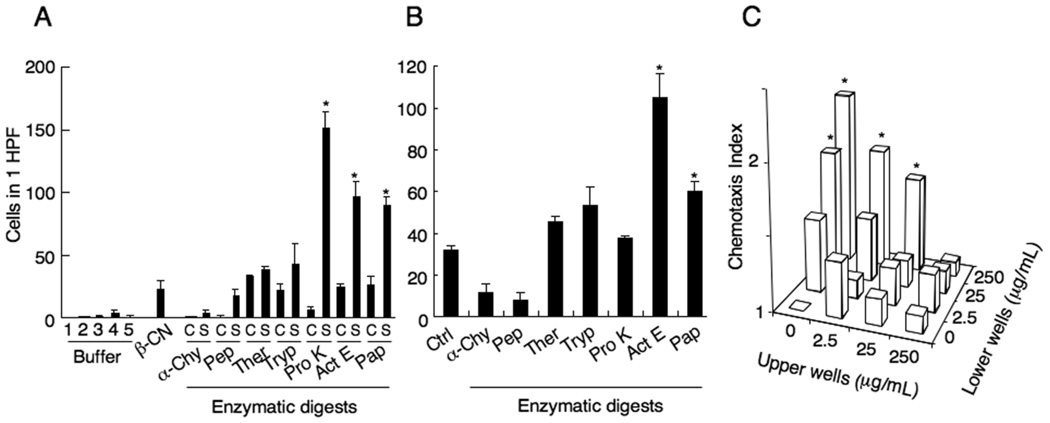
Seven β-CN digests were evaluated for chemotactic activity on mouse macrophages. Figure 1A shows that before protease digestion, intact β-CN exhibited a moderate chemotactic activity for mouse macrophages. However, a significantly increased chemotactic activity was observed after digestion with proteinase K, actinase E or papain, with 6.8, 4.3 or 4.0 fold higher than undigested β-CN, respectively. These digests were then tested on human monocytes. The digests by actinase E or Papain induced significant monocyte migration with more potent activity observed with actinase E digests (Fig. 1B). To confirm whether monocyte/macrophage migration induced by actinase E digests of β-CN was based on chemotaxis or chemokinesis, checkerboard analyses were conducted. The results showed that macrophage migrated only when higher concentrations of the digests were present in the lower wells of the chemotaxis chamber (Fig. 1C). There was no increased cell migration when higher concentrations of the digests were present in the upper wells. A low level of cell migration was observed when equal concentrations of the digests were present in both upper and lower wells. These results suggest that enzymatic digestion of β-CN generate fragments that directed migration of human monocytes and mouse macrophages.
Fig. 1.
Chemotactic activity of bovine β-CN enzymatic digests. (A) Enhanced mouse macrophage chemotactic activity of bovine β-CN enzymatic digests. The enzymatic reaction mixtures with (S) or without (C) β-CN were incubated at 37°C for 24 h. Buffer 1; for α-chymotrypsin, Buffer 2; for pepsin, Buffer 3; for protease K, Buffer 4; for thermolysin, trypsin and actinase E, Buffer 5; for papain. β-CN; β-casein, α-Chy; α-chymotrypsin, Pep; pepsin, Ther; thermolysin, Tryp; trypsin, Pro K; protease K, Act E; actinase E, Pap; papain. Macrophage chemotaxis was tested at a concentration of 500µg/mL β-CN. (B) Migration of human monocytes in response to enzymatic digests of β-CN. Each sample was then heated at 98°C for 10 min and tested for macrophage chemotaxis at a concentration of 500 µg/mL. The number of migrated macrophages was counted in high power fields (HPF) under microscopy (×200). The results are expressed as the mean number of migrated cells (± SD) in 1 HPF in 10 replicates. *p<0.01 as compared to cell migration in response to non-digested β-CN. (C) Checkerboard analysis of actinase E digests-induced monocyte migration. The cells were suspended at a concentration of 4 × 106 cells/mL in RPMI 1640. Different concentraions of the digests were placed in the upper and/or lower compartment of the chemotaxis chamber. The cells were placed in the upper wells. The results are expressed as the chemotaxis index. *p<0.01 as compared to cell migration in response to control.
We then examined the cell specificity of the chemotactic activity of β-CN digests. As shown in Figure 2A, human macrophages generated from monocytes stimulated with LPS responded well to the digests at levels comparable to monocytes. On the other hand, the digests did not show any chemotactic activity on human neutrophils (Fig. 2B), T lymphocytes (Fig. 2C) and mature dendritic cells (Fig. 2D). These results indicate that the β-CN digests exert preferential activity on mouse and human monocytes/macrophages.
Fig. 2.
Migration of immune cells in response to actinase E digests of β-CN. (A) Migration of human monocytes (a) and macrophages (b) in response to actinase E digests of β-CN. Macrophages were generated from monocytes by the 3 day-stimulation of LPS (100 ng/mL). Effect of actinase E digests of β-CN on the migration of human neutrophils (B), T lymphocytes (C) and mature dendritic cells (D). The separation of neutrophils and T lymphocytes, and the generation of mature dendritic from monocytes were done by the methods described in Materials and Methods. fMLF (10−7 M) and RANTES (25 ng/mL) were used as positive controls for neutrophils and T lymphocytes, respectively. The results were presented as chemotaxis index. *p<0.01 compared to cell response to medium control.
3.2. Involvement of a G protein-coupled receptor (GPCR)
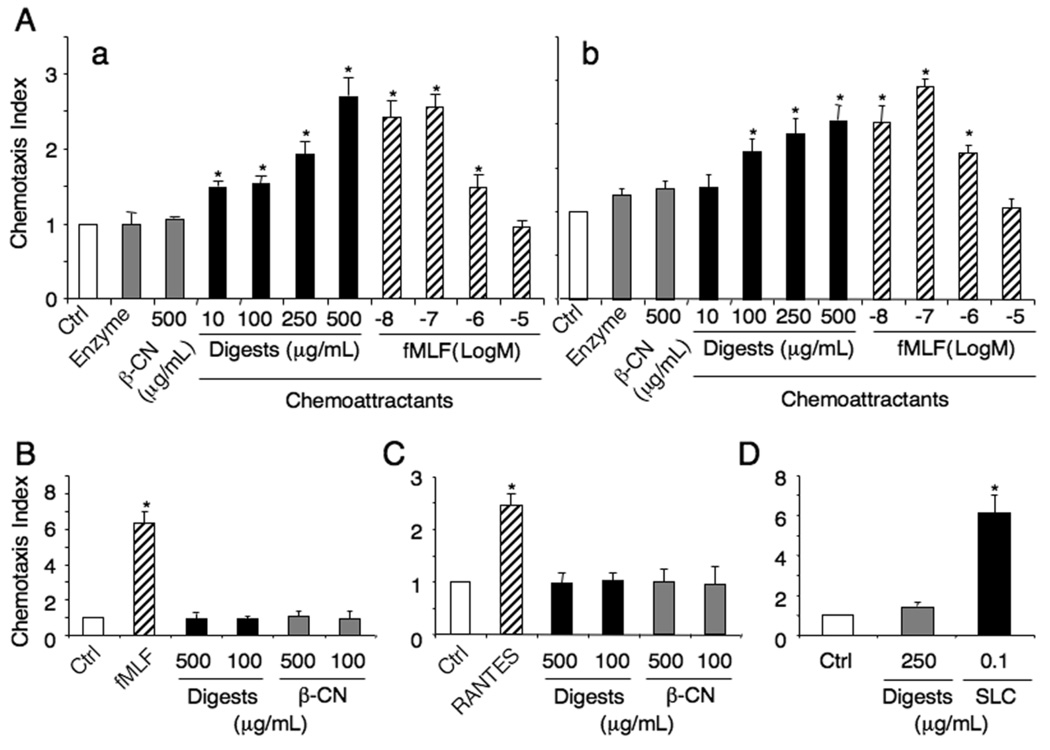
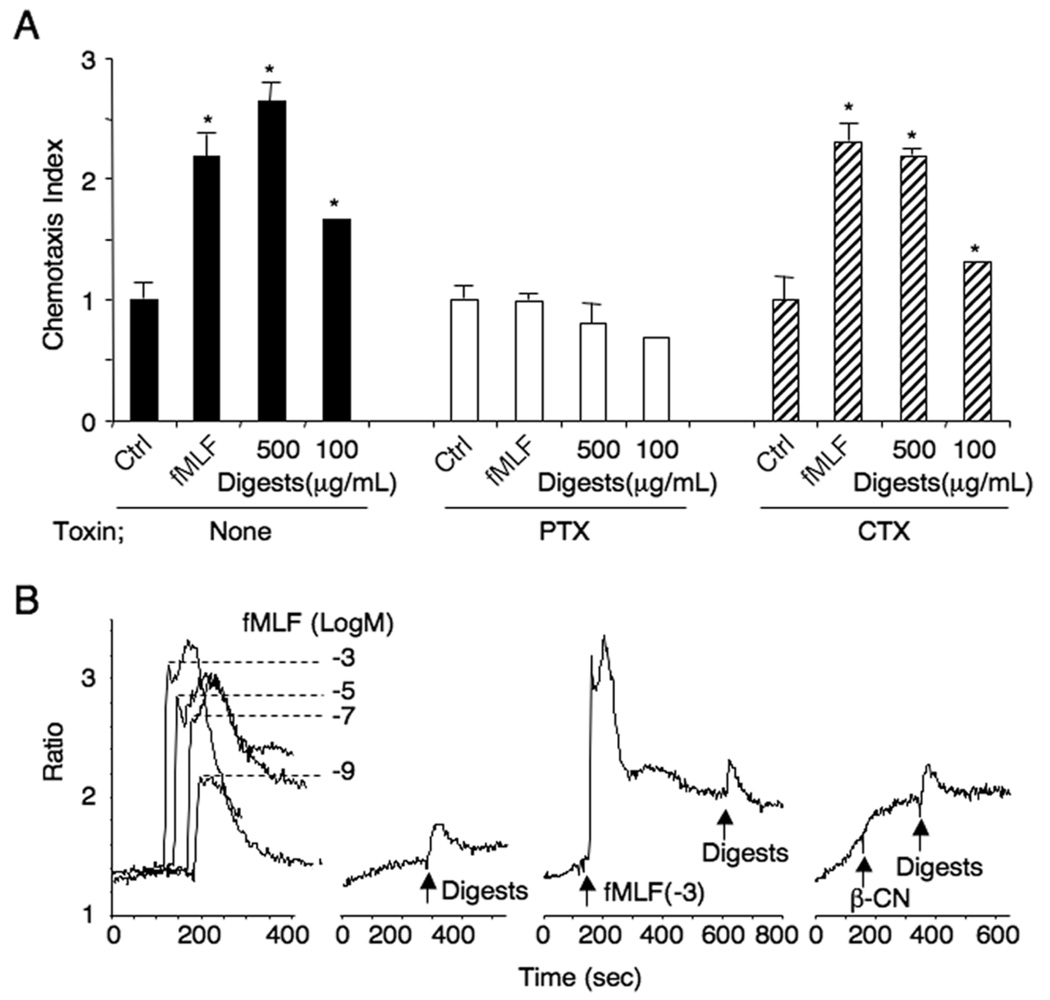
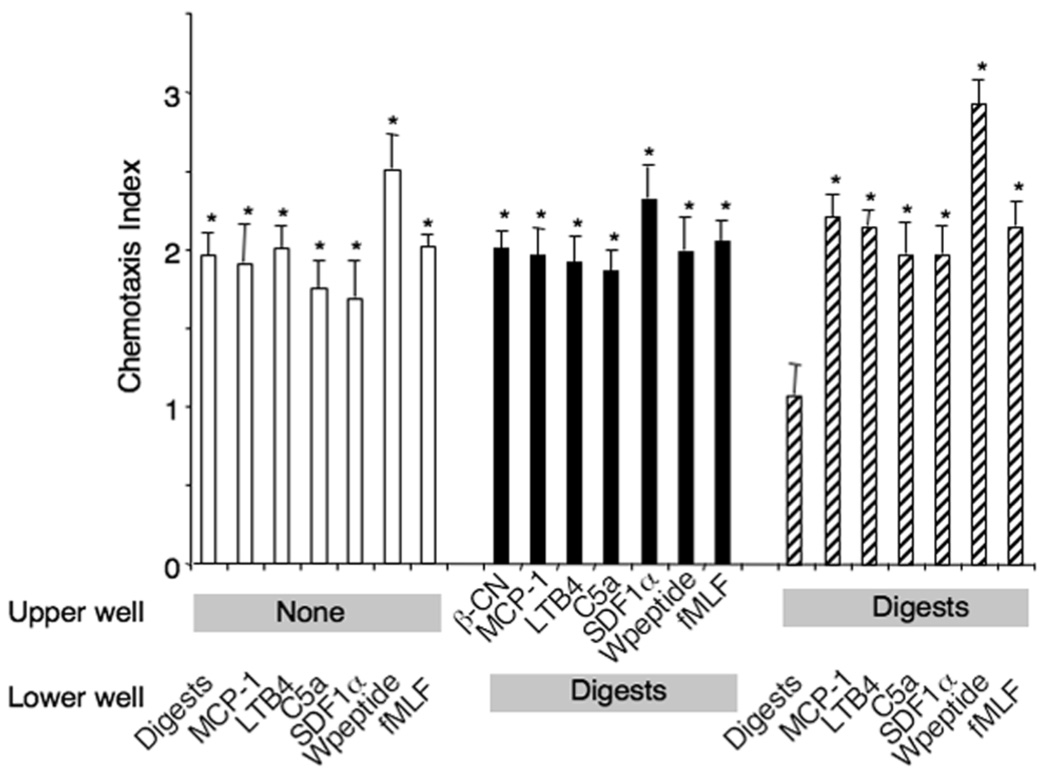
The migration of human monocytes to the β-CN digests was completely inhibited by pretreatment of the cells with pertussis toxin, but not by cholera toxin (Fig. 3A), suggesting that a Gαi protein-coupled receptor (GPCR) was involved in the chemotaxis response of cells to the digests. To characterize the identity of the receptor(s) used by the digests, cross-desensitization was performed using a variety of chemoattractants. Ca2+ flux induced by the digests in human monocytes was not desensitized by the bacterial chemotactic peptide, fMLF in a wide range of concentrations (Fig. 3B), excluding the possibility that the β-CN digests share receptors used by fMLF. The monocyte chemotaxis induced by the digests also was not desensitized by a variety of other known chemotactic agents including W peptide, another ligand for multiple fMLF receptors, stromal cell-derived factor 1α (SDF-1α), activated complement component 5 (C5a), leukotriene B4 (LTB4) and monocyte chemoattractant protein-1 (MCP-1) (Fig. 4). These results suggest that the β-CN digests induce monocyte chemotaxis via the activation of unique GPCR(s).
Fig. 3.
Characteristics of monocyte migration to actinase E digests of β-CN. (A) Effect of toxins on the migration of monocytes to actinase E digests of β-CN. The monocytes were pretreated with 100 ng/mL of pertussis toxin (PTX) or cholera toxin (CTX) at 37°C for 0.5 h prior to use for chemotaxis assay. fMLF (10−7 M) was used as a positive control. *p<0.01 compared to cell response to medium control. (B) Ca2+ mobilization in monocytes induced by actinase E digests of β-CN. The ratio of fluorescence at 340 and 380 nm wavelength was calculated using the FLWinLab program. Desensitization of the digests (100 µg/mL)-induced Ca2+ flux by fMLF (10−3 M) or β-CN (100 µg/mL) in monocytes was measured by sequentially stimulating the cells with both agonists.
Fig. 4.
Chemotactic activity of actinase E digests of β-CN to monocytes was not desensitized with chemoattractans. Desensitization of the digests-induced chemotaxis by several chemoattractants was measured in chemotaxis chamber in which lower wells placed with the digests (100µg/mL) and upper wells with the cells and chemoattractants. The concentrations of chemoattractants in upper wells were as follows; digests, 100µg/mL; MCP-1, 100ng/mL; LTB4, 1ng/mL; C5a, 1ng/mL; SDF1α, 100ng/mL; W peptide, 10−7 M; fMLP, 10−7 M. Each chemoattractant-induced migration was also tested in the presence of the digests in upper wells. *p<0.01 compared to cell response to medium control.
3.3. Low molecular weight chemotactic activity
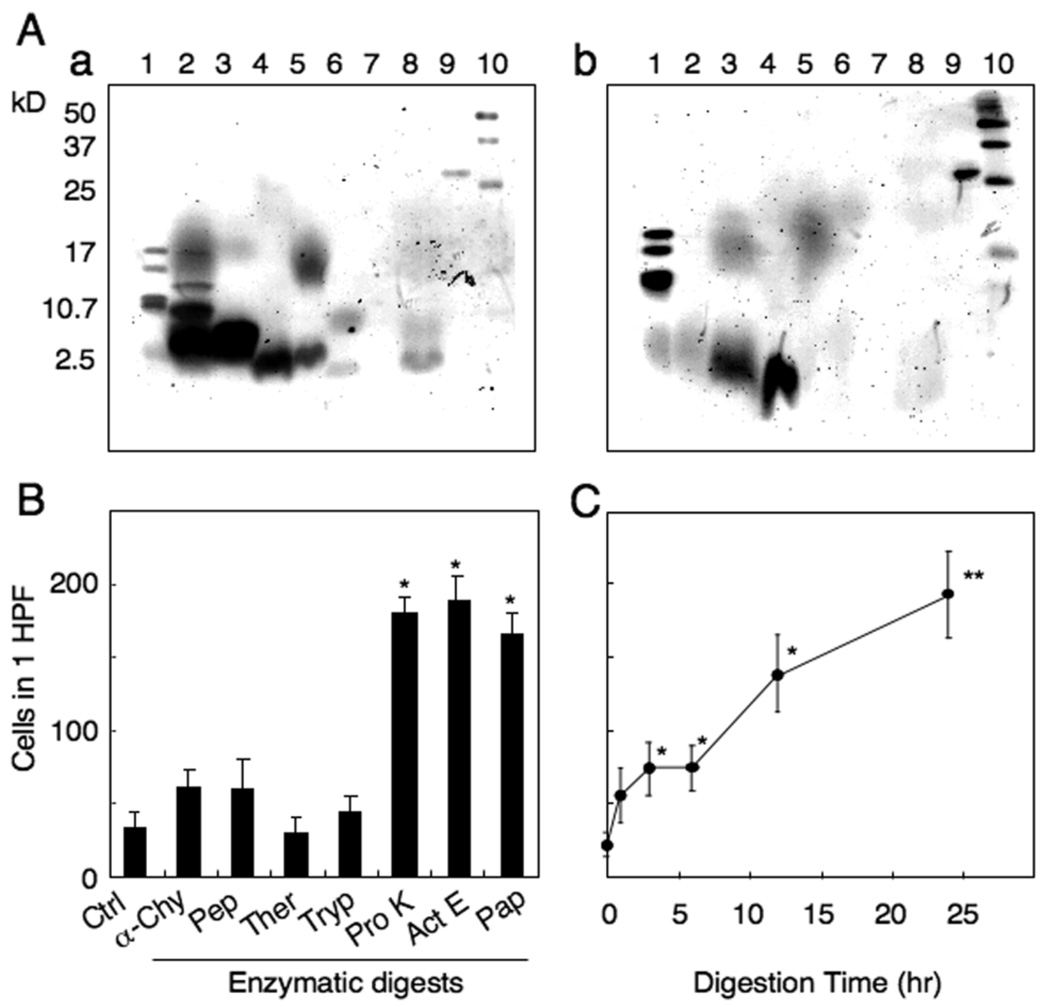
To characterize the nature of the chemotactic activity contained in the β-CN digests, the whole enzymatic digests and the digests ultrafiltrated into below the cut off of 3 kD were subjected to 20% SDS-PAGE to visualize peptide fragments (Fig. 5A). β-CN was well digested with proteinase K, actinase E or papain. No bands under 3 kD were visible in actinase E digests.
Fig. 5.
Chemotactic activity of low molecular digests of β-CN. (A) SDS-PAGE of enzymatic digests of β-CN. a: Whole enzymatic digests, b: Enzymatic digests of β-CN after ultrafiltrated to yield low molecular weight (<3 kD). Lanes 1,10; molecular weight marker, 2; digests with α-chymotrypsin, 3; pepsin, 4; thermolysin, 5; trypsin, 6; protease K, 7; actinase E, 8; papain, 9; non-digested β-CN. (B) Mouse macrophage chemotactic activity contained in the low molecular weight (<3 kD) fractions of β-casein. The results are expressed as the mean number (± SD) of migrated macrophages in 10 HPF. *p<0.01 compared to cell response to control. (C) β-CN was digested with actinase E for 1, 3, 6, 12 and 24 hr. The low molecular weight fractions (<3 kD) from each digest were tested for macrophage chemotaxis. *p<0.05, *p<0.01 compared to cell response to medium control.
We then tested the chemotactic activity contained in fragments of under 3 kD. The digests with proteinase K, actinase E or papain showed more potent activity as compared with digests of other enzymes (Fig. 5B). The highest chemotactic activity was detected in actinase E digests and a 3 hr digestion was sufficient to generate significant activity which was further enhanced when the digestion time was prolonged (Fig. 5C). Therefore, the 24 hr actinase digests were used for fractionation and purification of chemotactic peptides.
3.4. Fractionation and purification of chemotactic peptides
The low molecular weight actinase E digests of β-CN was first fractionated by hydrophobic chromatography using a Sep-pak C18 cartridge with a stepwise elution of 30%, 60% and 90% ACN (Table 1). After ultrafiltration, the permeate under 3 kD maintained similar chemotactic activity for macrophages as the whole β-CN digests. No activity was detected in non-adsorbed fractions and eluates with Milli Q water. Significant chemotactic activity was observed in the eluates with 30% or 60% ACN, while the 90% ACN eluates contained weaker activity. Because the yield in the 60% ACN eluates was lower than in the 30% ACN eluates, the 30% ACN eluates were used for further purification.
Table 1.
Yields and chemotactic activity of chemoattractants on the fractionation
| Fractionation | Yield (%) (protein) |
Chemotactic activity (cells in 1 HPF) mean ± SD |
|---|---|---|
| Whole | 100.0 | 135 ± 24 |
| Ultrafiltration | ||
| (Permeate) | 86.5 | 134 ± 14 |
| Non-adsorbed fraction | 30.4 | 0 |
| Adsorbed fraction | ||
| (eluates) | ||
| MilliQ | 28.8 | 0 |
| 30% ACN | 25.9 | 106 ± 7 |
| 60% ACN | 1.0 | 189 ± 14 |
| 90% ACN | 0.4 | 21 ± 3 |
The number of migrated mouse macrophages was calculated in the high power field (HPF) of light microscope (× 200). The results are expressed as the mean number of migrated cells (± SD) in 1 HPF in 10 replicates.
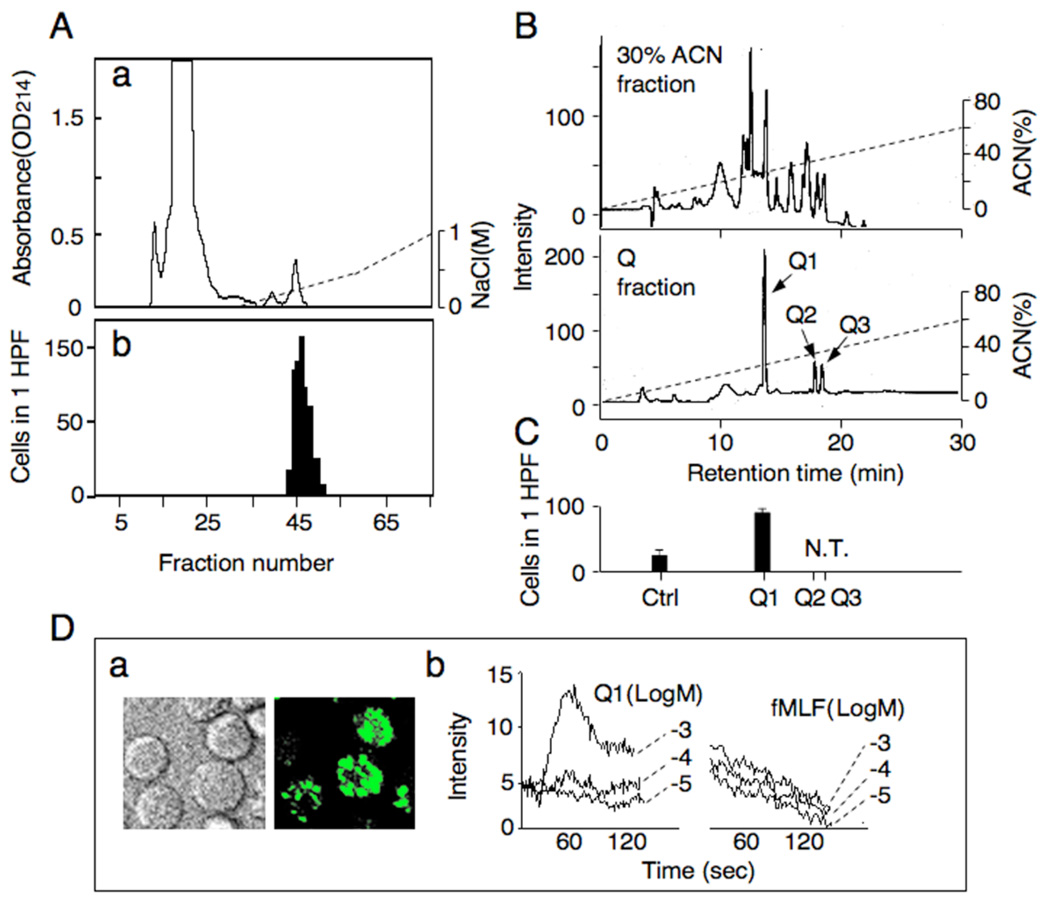
Eluates subjected to cation-exchange chromatography did not yield chemotactic fractions (data not shown). We therefore used anion-exchange chromatography. Two peptide peaks appeared and the macrophage chemotactic activity was detected in the second peak corresponding to fraction no. 42 to 52 (Fig. 6A). The active fractions were designated as Q and were used for further purification by HPLC. A tentative elution profile of 30% ACN fractions from permeates under 3 kD of β-CN digests by reverse phase HPLC yielded about 10 major peaks (Fig. 6B). When the Q fraction from anion-exchange chromatography was fractionated, 3 peptide peaks were detected and were designated as Q1, Q2 and Q3, which had identical retention time as the peaks appeared in the profiles of the 30% ACN fractions (Fig. 6B). The ratio of peptide quantity in Q1, Q2 and Q3 was estimated as 91%, 4% and 4%, respectively. All peaks were collected individually through repeated chromatography. The purity of the peptides was verified by further chromatography as a single component. The Q2 and Q3 fractions could not be tested for chemotactic activity because of their low yield, while Q1 induced potent macrophage chemotaxis at a level comparable to the original β-CN digests (Fig. 6C).
Fig. 6.
Fractionation of chemotactic peptides from enzymatic digests of β-CN. (A) One milligram of 30% ACN fraction was dissolved in 1 mL of 20mM phosphate buffer and potential chemotactic peptides were fractionated by anion exchange chromatography on a HiTrap™ Q HP (Amersham Pharmacia Biotech AB, Uppsala, Sweden) in the Bio Logic chromatography system (Bio-Rad, CA, USA). Fractions elutated from a linear gradient of 0 to 1 M NaCl in 20mM Tris-HCl buffer (pH 8.2) were monitored at 214 nm (a) and 0.25 ml for each fraction was collected and tested for mouse macrophage chemotaxis (b). Purification of macrophage chemotactic peptides (Q1, Q2 and Q3) collected from 30% ACN fractions by HPLC. (B) The high-performance liquid chromatogram of 30% ACN and Q fractions. (C) Q1 peptide was tested for mouse macrophage chemotaxis. N.T.; not tested. (D) Ca2+ mobilization in mouse macrophages induced by β-casochemotide-1. Calcium mobilization in J774-1 macrophage cells induced by β-casochemotide-1 was detected by a confocal laser microscope (a) (fluorescence was observed as right photo). The Ca2+ flux induced by β-casochemotide-1 was detected in gated cells and compared with that by fMLF (b).
To further examine the biological activity of Q1, we measured its capacity to induce Ca2+ mobilization in mouse macrophages. Figure 6D shows that Q1 at a concentration of 10−3 M elicited a significant increase in Ca2+ flux in mouse macrophages. In contrast, the bacterial chemotactic peptide fMLF only induced Ca2+ flux in human monocytes but not in mouse macrophages (Fig. 3B, Fig. 6D), consistent with our observations with β-CN digests that the chemotactic activity was mediated by receptor(s) distinct from those used by fMLF.
3.5. Structural analysis of chemotactic peptides
The amino acid sequences of the three peptides Q1, Q2 and Q3 purified by HPLC were analyzed by a protein sequencer with automatic Edman degradation. The sequence of peptide Q1 was shown to be YPVE… with a molecular weight of 604 (m/z) as determined by fast atom bombardment-mass spectrometry. Based on the sequence of β-CN, Q1 was estimated as YPVEP (f114-118 of β-CN). Q2 and Q3 were determined as a tetrapeptide EMPF (f108-111 of β-CN) and a hexapeptide YPVEPF (f114-119 of β-CN), respectively. Q1 was thus designated as “β-casochemotide-1” based on its origin and monocyte/macrophage chemotactic property.
4. Discussion
There is a growing interest in identifying biologically active components in natural products such as cow milk. Milk proteins constitute a rich source of peptides which are potential biological response modifiers defined in Food Specified Health Uses (FOSHU) authorized by the Ministry of Health, Labor and Welfare (MHLW) in Japan [16]. In fact, CPP [17] and antihypertension peptides [18] have been identified as enzymatic digestion products of milk casein. The objective of this study was to identify and isolate β-CN components that may regulate the function of the mammal immune system. We found that actinase E digests exert potent chemotactic activity for monocytes and macrophages but not other cell types of the hematopoietic origin. Our effort yielded a novel macrophage chemotactic peptide with the amino acid sequence of YPVEP, designated β-casochemotide-1, derived from milk β-CN in its low molecular fraction of actinase E digests. β-CN is highly conserved in mammals with low degree of variation in amino acid composition among different species. For instance, in human the same segment is composed of YPFVEP in comparison with the bovine sequence YPVEP. However at this stage it is not clear whether the human β-CN segment with an additional amino acid F between P and V would possess the same biological activity as the bovine counterpart. We identified two more peptides in addition to β-casochemotide-1, Q2 (EMPF) of β-CN f108-111 and Q3 (YPVEPF) of β-CN f114-119. These three peptides were apparently adsorbed on anion-exchange chromatography with the pI point of 3 to 4. As early as in 1972, when the primary amino acid sequence of β-CN was reported, the f117 was Gln [19]. However, a subsequent study identified β-CN f117 as Glu [20]. The inconsistency may be caused by genetic polymorphism. The β-CN used in our study has been confirmed to contain Glu as its f117.
Phagocytic leukocytes respond to a large number of chemoattractants with directional cell movement, activation of integrins, generation of superoxide anions, and release of granule contents. These functions constitute the first line of host defense against invading microorganisms or to repair tissue injury. Over the past three decades, numerous chemoattractants have been identified, which include the “classical” chemoattractants such as the bacterial chemotactic peptide fMLF, C5a, LTB4 and platelet-activating factor (PAF) [21–23], as well as a superfamily of chemokines [24–26]. Both classical chemoattractants and chemokines activate GPCRs expressed not only on cells of hematopoietic origin, but also on other cell types. In addition to their role in infection and inflammation, there is growing evidence for the involvement of one or more chemoattractants in lymphocyte trafficking [27, 28], coagulation [29], hematopoiesis [30], wound healing [31], allergy [32], atherogenesis [33], angiogenesis [31, 34], and malignancy [35]. The scope of chemoattractant receptor research has greatly expanded due to the discovery that several G protein-coupled chemokine receptors, CCR5 and CXCR4 in particular, also serve as co-receptors for human immunodeficiency virus type 1 (HIV-1) [26]. As one of the first identified leukocyte chemoattractants, the bacterial fMLF containing only three amino acids is a highly potent chemoattractant and activator of phagocytic leukocytes. The binding of GPCRs by chemoattractants results in a pertussis toxin (PT)-sensitive signaling cascade that effect a variety of cell functions [36]. In an effort to characterize the putative receptor(s) used by β-CN components, we found that the chemotactic activity of the β-CN digests was completely inhibited by PTX but not CTX, indicating the involvement of Gαi proteins. However, the Ca2+ flux and chemotaxis induced by the digests in human monocytes was not desensitized by fMLF and by a variety of other chemoattractants of both “classical” and “chemokine” families tested. β-CN digests also did not chemoattract fMLF receptor-transfected cell lines (data not shown). Thus, despite the early report that β-CN might share with fMLF for receptor [37], our study clearly indicates that β-CN or its enzymatic digests did not use fMLF receptors to chemoattract monocyte/macrophage. Further research is underway to characterize the chemotactic activity of synthetic β-CN peptides to facilitate the identification of their cellular receptor(s).
Our identification of small peptide fragments of β-CN as selective monocyte/macrophage chemoattractants may have important biological and pharmacological significance. In addition to its monocyte/macrophage chemotactic components, an octapeptides that contain β-casochemotide-1 from β-CN exhibited anti-hypertension activity in rat (unpublish data). These results suggest that peptide fragments in milk proteins may have multiple biological functions, which are beneficial to human and other mammals. It is interesting to note that the sequence of β-casochemotide-1 was not listed as an allergenic peptide in the milk [38], indicating that β-casochemotide-1 can be considered as a GRAS (Generally Recognized as Safe) components. Moreover, monocyte/macrophage chemotaxis and activation are essential events in host innate immune responses [11]. Therefore, β-CN digests containing β-casochemotide-1 and other GRAS components of milk may have important role in establishing and maintaining a competent immune system in the digestive tract despite the notion that the existence and identity of enzymes capable of cleaving β-casein in the mammal guts and blood remain to be determined. We have also identified fermented milk products and their components as activators of the immune responses [39–46]. Thus, identification of bioactive agents in the milk products will facilitate the development of novel regulators of immune system assisting the host defense against inflammation and infection.
Acknowledgments
We thank Dr. Joost J. Oppenheim for reviewing the manuscript, Drs. K. Chen, J. Huang and Z. Howard, and N. Dunlop and W. Gong for their technical assistance. Dr. H. Kitazawa was supported by Program to Enhance International Standing of Higher Education in Japan 2006 (MEXT: Ministry of Education, Culture, Sports, Science and Technology). M. Tohno is supported by JSPS research fellow (Research Fellowships for Young Scientists Program, No. 18005121). This project has been funded in part with federal funds from the National Cancer Institutes, National Institutes of Health, under Contract No. NO1-CO-12400. The research was also supported [in part] by the Intramural Research Program of the NIH, NCI. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huth PJ, Layman DK, Brown PH. The Emerging Role of Dairy Proteins and Bioactive Peptides in Nutrition and Health. J Nutr. 2004;134:961s. [Google Scholar]
- 2.Brantl V, Teschemacher H, Henschen A, Lottspeich F. Novel opioid peptides derived from casein (beta-casomorphins). I. Isolation from bovine casein peptone. Hoppe Seylers Z Physiol Chem. 1979;360:1211–1216. doi: 10.1515/bchm2.1979.360.2.1211. [DOI] [PubMed] [Google Scholar]
- 3.Abubakar A, Saito T, Kitazawa H, Kawai Y, Itoh T. Structural analysis of new antihypertensive peptides derived from cheese whey protein by proteinase K digestion. J Dairy Sci. 1998;81:3131–3138. doi: 10.3168/jds.S0022-0302(98)75878-3. [DOI] [PubMed] [Google Scholar]
- 4.Kawahara T, Otani H. Stimulatory effect of a casein phosphopeptide preparation on cytokine mRNA expression by human intestinal epithelial-like Caco-2 cells. Biosci Biotech Biochem. 2004;68:1779–1781. doi: 10.1271/bbb.68.1779. [DOI] [PubMed] [Google Scholar]
- 5.Kawahara T, Katayama D, Otani H. Effect of β-casein (1–28) on proliferative responses and secretory functions of human immunocompetent cell lines. Biosci Biotech Biochem. 2004;68:2091–2095. doi: 10.1271/bbb.68.2091. [DOI] [PubMed] [Google Scholar]
- 6.Coste M, Rochet V, Leonil J, Molle D, Bouhallab S, Tome D. Identification of C-terminal peptides of bovine beta-casein that enhance proliferation of rat lymphocytes. Immunol Lett. 1992;33:41–46. doi: 10.1016/0165-2478(92)90091-2. [DOI] [PubMed] [Google Scholar]
- 7.Matin DM, Otani H. Cytotoxic and antimicrobial activities of chemically synthesized k-casecidin and its partial peptide fragments. J Dairy Res. 2002;69:329–334. doi: 10.1017/s0022029902005435. [DOI] [PubMed] [Google Scholar]
- 8.Hata I, Higashiyama S, Otani H. Identification of a phosphopeptide in bovine alpha s1-casein digest as a factor influencing proliferation and immunoglobulin production in lymphocyte cultures. J Dairy Res. 1998;65:569–578. doi: 10.1017/s0022029998003136. [DOI] [PubMed] [Google Scholar]
- 9.Migliore-Samour D, Floc'h F, Jolles P. Biologically active casein peptides implicated in immunomodulation. J Dairy Res. 1989;56:357–362. doi: 10.1017/s0022029900028806. [DOI] [PubMed] [Google Scholar]
- 10.Otani H, Wakatsuki S. Reduction of allergic symptoms in NC/Jic Jcl mice given a diet containing a commercially avairable casein phosphopeptide preparation, CPP-III. Animal Sci J. 2004;75:147–153. [Google Scholar]
- 11.Jung HC, Eckmann L, Yang SK, Panja A, Fierer J, Wroblewska ME, et al. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Y, Gong W, Li B, Dunlop NM, Shen W, Su SB, et al. Utilization of Two seven-transmembrane, G protein-coupled receptors, formyl peptide receptor-like 1 and formyl peptide receptor, by the synthetic hexapeptide WKYMVm for human phagocyte activation. J Immunol. 1999;163:6777–6784. [PubMed] [Google Scholar]
- 13.Yang D, Chen Q, Le Y, Wang JM, Oppenheim JJ. Differential regulation of formyl peptide receptor-like 1 expression during the differentiation of monocytes to dendritic cells and macrophages. J Immunol. 2001;166:4092–4098. doi: 10.4049/jimmunol.166.6.4092. [DOI] [PubMed] [Google Scholar]
- 14.Lowry OH, Resebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 15.Kitazawa H, Ino T, Kawai Y, Itoh T, Saito T. A novel immunostimulating aspect of Lactobacillus gasseri: induction of “Gasserokine” as chemoattractants for macrophages. Int J Food Microbiol. 2002;77:29–38. doi: 10.1016/s0168-1605(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 16.Ohama H, Ikeda H, Moriyama H. Health foods and foods with health claims in Japan. Toxicol. 2006;221:95–111. doi: 10.1016/j.tox.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Holt C, Wahlgren NM, Drakenberg T. Ability of a beta-casein phosphopeptide to modulate the precipitation of calcium phosphate by forming amorphous dicalcium phosphate nanoclusters. Biochem J. 1996;314:1035–1039. doi: 10.1042/bj3141035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto N, Akino A, Takano T. Antihypertensive effect of the peptides derived from casein by an extracellular proteinase from Lactobacillus helveticus CP790. J Dairy Sci. 1994;77:917–922. doi: 10.3168/jds.S0022-0302(94)77026-0. [DOI] [PubMed] [Google Scholar]
- 19.Brignon G, Dumas BR, Grosclaude F, Mercier JC. Primary structure of bovine casein. Partial sequence. Eur J Biochem. 1971;22:179–185. doi: 10.1111/j.1432-1033.1971.tb01530.x. [DOI] [PubMed] [Google Scholar]
- 20.Yan SB, Wold F. Neoglycoproteins: in vitro introduction of glycosyl units at glutamines in beta-casein using transglutaminase. Biochemistry. 1984;23:3759–3765. doi: 10.1021/bi00311a030. [DOI] [PubMed] [Google Scholar]
- 21.Hwang SB. Specific receptors of platelet-activation factor, receptor heterogeneity, and signal transduction mechanisms. J Lipid Med. 1990;2:123–158. [PubMed] [Google Scholar]
- 22.Goldstein IM. Complement: biologically active products. In: Gallin JI, Goldstein IM, Snyderman R, editors. Inflammation: basic principles and clinical correlates, 2nd. New York: Raven Press; 1992. pp. 55–74. [Google Scholar]
- 23.Snyderman R, Uhing RJ. Chemoattractant stimulus-response coupling. In: Gallin JI, Goldstein IM, Snyderman R, editors. Inflamation: basic principles and clinical correlates, 2nd. New York: Raven Press; 1992. pp. 421–439. [Google Scholar]
- 24.Oppenheim JJ, Murphy WJ, Chertov O, Schirrmacher V, Wang JM. Prospects for cytokine and chemokine biotherapy. Clin Cancer Res. 1997;3:2682–2686. [PubMed] [Google Scholar]
- 25.Howard OM, Oppenheim JJ, Wang JM. Chemokines as molecular targets for therapeutic intervention. J Clin Immunol. 1999;19:280–292. doi: 10.1023/A:1020587407535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su SB, Ueda H, Howard OM, Grimm MC, Gong W, Ruscetti FW, et al. Inhibition of the expression and function of chemokine receptors on human CD4+ leukocytes by HIV-1 envelope protein gp120. Chem Immunol. 1999;72:141–160. doi: 10.1159/000058731. [DOI] [PubMed] [Google Scholar]
- 27.Schall TJ, Bacon K, Camp RD, Kaspari JW, Goeddel DV. Human macrophage inflammatory protein (MIP-1) and MIP-1 chemokines attract distinct populations of lymphocytes. J Exp Med. 1993;177:1821–1826. doi: 10.1084/jem.177.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taub DD, Conlon K, Lloyd AR, Oppenheim JJ, Kelvin DJ. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1 and MIP-1. Science. 1993;260:355–358. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- 29.Zucker MB, Katz IR. Platelet factor 4: production, structure, and physiologic and immunologic action. Proc Soc Exp Biol Med. 1991;198:693–702. doi: 10.3181/00379727-198-43309. [DOI] [PubMed] [Google Scholar]
- 30.Dunlop DJ, Wright EG, Lorimore S, Graham GJ, Holyoake T, Kerr DJ, et al. Demonstration of stem cell inhibition and myeloprotective effects of SCI/rhMIP1 in vivo. Blood. 1992;79:2221–2225. [PubMed] [Google Scholar]
- 31.Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 32.Rot A, Krieger M, Brunner T, Bischoff SC, Schall TJ, Dahinden CA. RANTES and macrophage inflammmatory protein 1 induce the migration and activation of normal human eosinophil granulocytes. J Exp Med. 1992;176:1489–1495. doi: 10.1084/jem.176.6.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang JM, Su S, Gong W, Oppenheim JJ. Chemokines, receptors, and their role in cardiovascular pathology. Int J Clin Lab Res. 1998;28:83–90. doi: 10.1007/s005990050024. [DOI] [PubMed] [Google Scholar]
- 34.Maione TE, Gray GS, Petro J, Hunt AJ, Donner AL, Bauer SI, et al. Inhibition of angiogenesis by recombinant platelet factor 4 and related peptides. Science. 1990;247:77–79. doi: 10.1126/science.1688470. [DOI] [PubMed] [Google Scholar]
- 35.Wang JM, Deng X, Gong W, Su S. Chemokines and their role in tumor growth and metastasis. J Immunol Methods. 1998;220:1–17. doi: 10.1016/s0022-1759(98)00128-8. [DOI] [PubMed] [Google Scholar]
- 36.Le Y, Murphy PM, Wang JM. Formyl-peptide receptors revisited. Trends Immunol. 2002;23:541–548. doi: 10.1016/s1471-4906(02)02316-5. [DOI] [PubMed] [Google Scholar]
- 37.Katagiri T, Adachi I, Terao T, Osawa T. Alpha-casein-binding proteins of guinea pig macrophage membranes and their possible roles in chemotaxis. J Biochem. 1980;87:1421–1430. doi: 10.1093/oxfordjournals.jbchem.a132883. [DOI] [PubMed] [Google Scholar]
- 38.Bernard H, Negroni L, Chatel JM, Clement G, Adel-Patient K, Peltre G, et al. Molecular basis of IgE cross-reactivity between human beta-casein and bovine beta-casein, a major allergen of milk. Mol Immunol. 2000;37:161–167. doi: 10.1016/s0161-5890(00)00029-8. [DOI] [PubMed] [Google Scholar]
- 39.Shimosato T, Kitazawa H, Katoh S, Tohno M, Iliev ID, Nagasawa C, et al. Augmentation of T(H)-1 type response by immunoactive AT oligonucleotide from lactic acid bacteria via Toll-like receptor 9 signaling. Biochem Biophys Res Commun. 2005;326:782–787. doi: 10.1016/j.bbrc.2004.11.119. [DOI] [PubMed] [Google Scholar]
- 40.Iliev ID, Kitazawa H, Shimosato T, Katoh S, Morita H, He F, et al. Strong immunostimulation in murine immune cells by Lactobacillus rhamnosus GG DNA containing novel oligodeoxynucleotide pattern. Cell Microbiol. 2005;7:403–414. doi: 10.1111/j.1462-5822.2004.00470.x. [DOI] [PubMed] [Google Scholar]
- 41.Tohno M, Kitazawa H, Shimosato T, Matsumoto M, Katoh S, Kawai Y, et al. A swine toll-like receptor 2-expressing transfectant as a potential primary screening system for immunobiotic microorganisms. FEMS Immunol Med Microbiol. 2005;44:283–288. doi: 10.1016/j.femsim.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 42.Tohno M, Shimazu T, Ueda W, Anzawa D, Aso H, Nishimura J, et al. Molecular cloning of porcine RP105/MD-1 involved in recognition of extracellular phosphopolysaccharides from Lactococcus lactis ssp. cremoris. Mol Immunol. 2007;44:2566–2577. doi: 10.1016/j.molimm.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 43.Tohno M, Shimosato T, Kawai Y, Aso H, Ikegami S, Taketomo N, et al. Advanced molecular immunoassay system for immunobiotic lactic acid bacteria using a transfectant of Toll-like receptor 2. Anim Sci J. 2007;78:195–205. [Google Scholar]
- 44.Kitazawa H, Shimosato T, Tohno M, Saito T. Immunostimulatory activities of Lactic acid bacteria via Toll-like receptors. Jpn J Lactic acid bacteria. 2005;16:11–20. [Google Scholar]
- 45.Shimosato T, Kimura T, Tohno M, Iliev ID, Katoh S, Ito Y, et al. Strong immunostimulatory activity of AT-oligodeoxynucleotide requires a six-base loop with a self-stabilized 5 -C…G-3 stem structure. Cell Microbiol. 2006;8:485–495. doi: 10.1111/j.1462-5822.2005.00640.x. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi N, Kitazawa H, Iwabuchi N, Xiao JZ, Miyaji K, Iwatsuki K, et al. Immunostimulatory oligodeoxynucleotide from Bifidobacterium longum suppresses Th2 immune responses in a murine model. Clin Exp Immunol. 2006;145:130–138. doi: 10.1111/j.1365-2249.2006.03111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]