Abstract
Background
Prior studies show that lactate is a useful prognostic marker in sepsis.
Objective
To study the feasibility and accuracy of a point-of-care (POC) analyzer capable of performing bedside serum lactate measurements; and to determine if other measurements (pH, base excess) are predictive of mortality.
Methods:
Design: prospective cohort study of adult (age 18 years or older) Emergency Department (ED) patients with suspected infection during the study period of May 2006 through March 2007.
Setting
A 55,000-annual-visit urban tertiary care ED.
Intervention
A point-of-care device (i-STAT, Abbott Point of Care Inc., Princeton, NJ) was deployed using a standardized training and quality assurance process. Using POC testing, we measured serum lactate, pH, and base excess, as well as concomitant lactate measurement via a central laboratory.
Statistics
Area under the curve (AUC) for receiver operator characteristic curve, Bland-Altman statistics along with a correlation coefficient, and relative risk with 95% confidence intervals reported.
Results
There were 699 patients enrolled, of whom 34 (4.9%) died. The AUCs for mortality prediction were: POC lactate 0.72, laboratory lactate 0.70, pH measurement 0.60, and base excess 0.60. Bland-Altman showed that POC lactate was, on average, 0.32 (95% confidence interval −0.35– 0.98) lower than laboratory lactate, with agreement kappa = 0.97.
Conclusions
A point-of-care testing device provides a reliable and feasible way to measure serum lactate at the bedside. The pH and base excess were less helpful.
Keywords: sepsis, lactic acid, infection, mortality, risk assessment
INTRODUCTION
There are an estimated 571,000 cases of severe sepsis that present to United States emergency departments (EDs) each year, with an unacceptably high mortality rate between 20% and 50% (1–3). Early identification of the “at-risk” patient represents a challenge to the ED physician, as the presentation of sepsis is often subtle and difficult to assess. Although there is no universally accepted gold-standard screening test, the measurement of serum lactate level is useful for the identification of ED patients at increased risk of mortality from sepsis (3–5). The Surviving Sepsis Campaign, an international, multidisciplinary consensus effort, endorses obtaining a serum lactate as one of its core sepsis bundles (6). Additionally, the use of similar markers of hypoperfusion, namely low pH and base excess, have been proposed but not previously well studied. The identification process of patients at increased risk of adverse outcome is important, as septic patients benefit from early and aggressive resuscitation protocols (1).
In order for a blood lactate level to provide utility for clinical decision-making, an accurate result must be readily available in a timely fashion. A major problem in obtaining accurate blood lactate levels relates to sample handling before analysis. Once the blood sample is drawn, lactate levels continue to rise in the sample as the result of red blood cell metabolism. If the sample can be analyzed immediately, the effect is minimal. But, if the sample needs to be transported to a central laboratory or requires centrifugation before analysis, the delay results in falsely elevated lactate levels. From a practical standpoint, one may divide the currently available lactate methods into three groups: 1) standard enzymatic spectrophotometric methods, performed with blood collected in special preservative tubes, requiring centrifugation; 2) electrode-based amperometric methods, performed on anticoagulated whole blood but which may require transport to a laboratory (see Methods); 3) electrode-based amperometric methods, performed on whole blood at the bedside (see Methods) For the first method, delays are unavoidable, thus the recommendation that samples be collected in tubes that minimize red cell metabolism (e.g., so-called “gray-top” tubes containing NaF). Due to the need for transportation, centrifugation, and typical instrument analysis times, turnaround times are often 2–3 h (or longer). For the electrode-based methods, centrifugation is not required and analysis time is minimal (<5 min). For the second method, though, actual turn-around time may be prolonged significantly by transportation delay; during those delays, lactate levels will rise, potentially significantly. As a result, there are criticisms of this methodology, but it was, in fact, the method used in many of the studies establishing blood lactate as a valuable ED risk stratification tool for patients with infection (3–5). With a turnaround time of typically 30 min or less, this is within a time frame that is useful for clinical decision-making. The third method, point-of-care lactate, offers the advantage of rapidly available results at the bedside with reduced time-to-assay that would potentially reduce time for in vitro metabolism; however, its feasibility and reliability is relatively unproven in this setting.
Because conventional measurement in a central laboratory is not available in all institutions, or may be associated with significant delays, a rapid and accurate point-of-care (POC) test could contribute to improved and timely care of the septic patient. Accordingly, we undertook this study to determine if a POC device would be reliable in the ED for identification of patients at risk for adverse outcomes in sepsis. The objective of this investigation was to study the feasibility and accuracy of a POC analyzer capable of performing bedside serum lactate measurements in patients with suspected infection, and to determine if other POC acid-base measurements (pH, base excess) hold similar predictive ability.
MATERIALS AND METHODS
Study Design and Selection of Participants
This was a prospective cohort study of a convenience sample of adult (age 18 years or older) ED patients with suspected infection during the study period of May 1, 2006 –March 15, 2007 who had a POC lactate measurement obtained with a mandatory confirmatory lactate measurement performed by the hospital’s clinical laboratory. The central laboratory determinations of lactate were done using whole blood on a Siemens/Bayer RapidLab 1265 Analyzer (Siemens Healthcare Diagnostics, Deerfield, IL), which was run according to the manufacturer’s recommendations. The measurement is based on amperometry and an immobilized lactate oxidase electrode. The suspicion of infection in the ED was initially determined by the clinician ordering the test based on a combination of factors from history (e.g., fever, productive cough, dysuria), vital signs and physical examination (e.g., temperature, crackles on lung examination), laboratory (e.g., elevated white blood cell count or bandemia), or diagnostic testing (e.g., pneumonia on chest X-ray study). The presence of infection was confirmed by an independent researcher reviewing the medical decision-making portion of the ED chart or by the decision to administer antibiotics. Exclusion criterion was absence of suspected infection. The setting was a 55,000-visit-per-year urban tertiary care ED. Laboratory blood lactate measurements were performed on a Siemens 1265 Blood Gas Analyzer. This study was approved by our institutional ethics committee with a waiver of consent.
Intervention
It is a clinical guideline at our institution to obtain a lactate level on all patients with a suspected infection. For this investigation, after a standardized training program, a POC device (i-STAT; Abbott Point-of-Care, Inc., Princeton, NJ) was made available for use by the clinical team, along with ICG4 blood gas cartridge, which measures a blood lactate, base excess, and pH. The ED technicians who routinely perform phlebotomy were individually trained and certified for competency before performing the test. The training occurred through one-on-one training that took approximately 5 min to teach proper use of the device. The protocol called for each i-STAT lactate measurement to be confirmed by a laboratory measurement. Venous blood was collected in a heparinized tube, an aliquot was withdrawn using a needleless system and assayed by the POC device; the remaining blood was sent to the hospital laboratory. We performed quality assurance checks on the device according to manufacturer specifications and routinely checked for accuracy as compared to laboratory measurements. To comply with CLIA (Clinical Laboratory Improvement Amendments of 1988) standards and regulations, we created a compliant quality assurance protocol (Figure 1). This study was approved by our institution’s investigational review board and informed consent was waived given the use of a Food and Drug Administration-approved device, the lack of a need for an additional blood draw, and practice of obtaining a confirmatory measurement by the hospital laboratory.
Figure 1.
Quality assurance process for ED I-STAT initiative.
Data Collection and Processing
We collected routine demographic and clinical characteristics to describe our patient population along with pertinent laboratory values of POC lactate results and matched them to corresponding laboratory lactate levels that were run in parallel. We also recorded the results from the POC base excess and pH measurements. The primary clinical outcome was in-hospital mortality.
Statistics
Descriptive statistics are reported along with each measurement, and 95% confidence intervals are displayed around mean values. The area under the curve for receiver operator characteristic curve was used as a summary measure of each measurement’s predictive ability. Bland-Altman statistics of mean difference and limits of agreement were reported along with a correlation coefficient for POC vs. laboratory lactate. Logistic regression models were used to determine if a single measurement or combination of measurements offers incremental advantage. For clinically meaningful outcomes assessment, patients were divided on the basis of their test results into low, medium, and high lactate levels based on previously reported thresholds, adjusted for bias in lactate measurement, and rounded off to the nearest 0.5 level for ease of use.
RESULTS
Characteristics of Study Subjects
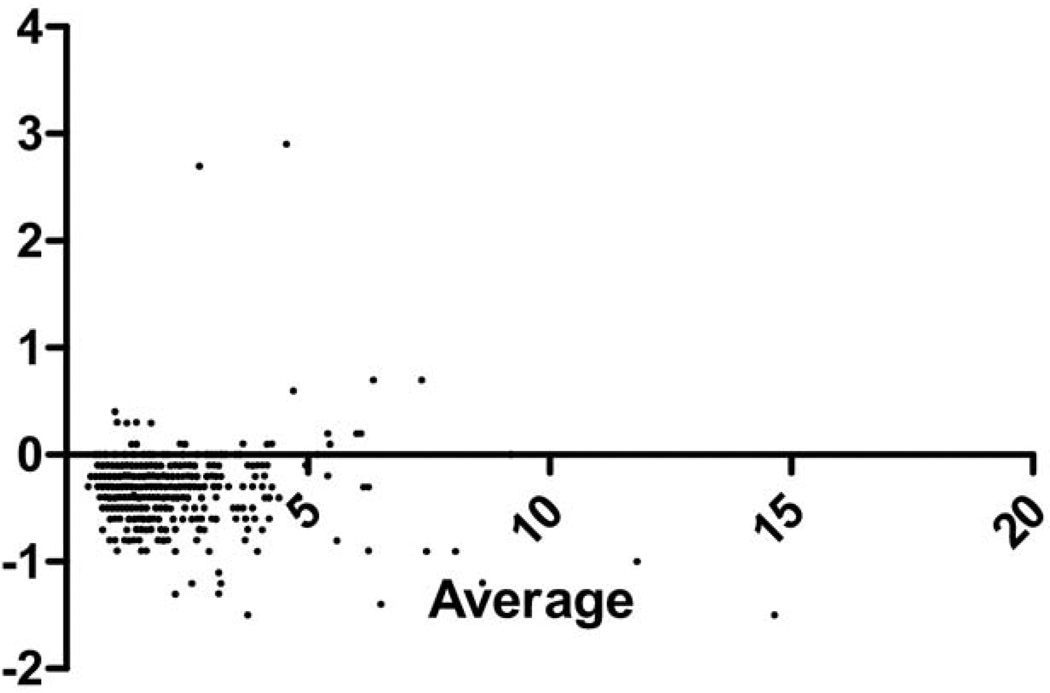
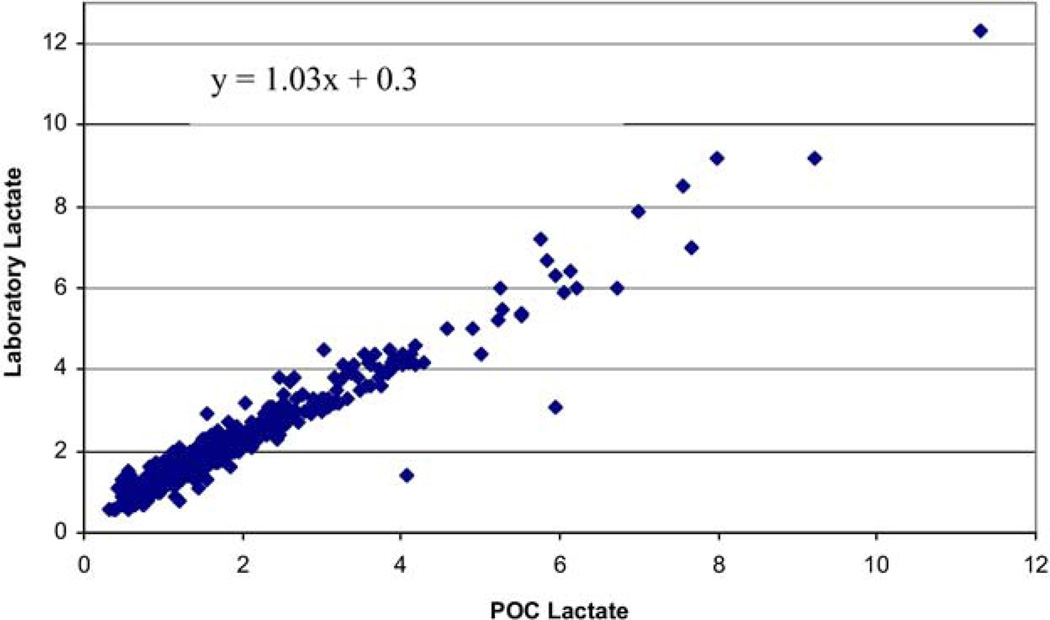
There were 699 patients enrolled, of whom 34 (4.9%) died. The population had a mean age of 60.4 years (95% confidence interval [CI] 58.9–61.2) (Table 1). Bland-Altman statistics showed that POC lactate was accurate for clinical decision-making compared to the laboratory lactate. There was an average bias for POC lactate of 0.32 (SD 0.45) lower than laboratory lactate, with the limits of agreement ranging from –1.1 to 0.50 (the range over which 95% of the differences between the POC and laboratory lactate will be contained) (Figure 2). The POC lactate was highly correlated with the laboratory lactate, r = 0.97 (Figure 3).
Table 1.
Demographics of the Study Population
| All subjects (n = 699) |
|
|---|---|
| Age, years [mean (SD)] | 60.4 (± 20.0) |
| Gender, % female | 408 (58.3%) |
| Comorbidities [n (%)] | |
| Hypertension | 290 (41.5%) |
| Cardiovascular disease | 107 (15.3%) |
| Diabetes | 154 (22.1%) |
| End-stage renal disease/hemodialysis | 29 (4.2%) |
| History of stroke | 46 (6.6%) |
| History of congestive heart failure | 70 (10.0%) |
| Most common infection sources [n (%)] | |
| Lower respiratory/pneumonia | 132 (18.9%) |
| Skin/soft tissue/wound | 129 (18.5%) |
| Urogenital | 91 (13.0%) |
| Intra-abdominal | 73 (10.4%) |
| Temperature, °C [mean (SD)] | 37.8 (1.2) |
| Heart rate, beats/min [mean (SD)] | 97.0 (20.8) |
| Respiratory rate, per minute [mean (SD)] | 19.7 (5.6) |
| Systolic blood pressure, mm Hg [mean (SD)] | 129.5 (28.8) |
Figure 2.
Bland-Altman of data 1: difference vs. average.
Figure 3.
Comparison of point-of-care with laboratory lactate level.
Next, we assessed the prognostic ability of the POC lactate along with the other POC tests of pH and base excess. The mean POC lactate of 3.2 mmol/L (95% CI 2.05– 4.37) in those who died was higher than 1.65 (1.56 –1.74) in those who lived. Mean laboratory lactate levels also differed between the dead and survivors: 3.83 (2.20 –5.47) vs. 1.95 (1.86 –2.04), respectively, as did pH: 7.42 (7.42–7.43) vs. 7.37 (7.33–7.42), respectively. Base excess did not show a statistically significance difference: 1.71 (1.32–2.10) vs. 0.62 (−4.09 –2.85), respectively. The area under the curve (AUC) for the receiver operator characteristic (ROC) curve for the different laboratory predictors tested for mortality prediction were: POC lactate 0.72, laboratory lactate 0.70, pH measurement 0.60, and base excess 0.60. Each of the parameters tested was significantly associated with death on a univariate basis; however, in a logistic regression, there was no advantage to using more than one parameter. The AUC for the POC lactate level (and laboratory lactate level) was higher than the other parameters, which performed poorly based on ROC analysis.
DISCUSSION
The findings from this study support the use of POC lactate as one clinically useful methodology to measure a blood lactate level to risk-stratify patients with suspected sepsis. One may surmise that POC is feasible by virtue of conducting 699 tests in the ED, and accurate based on the Bland-Altman results, yielding a mean difference of 0.32 with reasonable limits of agreement, which may be interpreted as clinically acceptable. Although we did not assess the impact of POC testing on time to result in the current study, nor did we study if POC testing alters outcomes, we did establish that POC testing is feasible, accurate, and reasonably prognostic in the ED setting. It is a particularly attractive option if lactate levels are not available from a central laboratory within useful time frame for clinical decision-making. Furthermore, despite theoretical promise for pH and base excess to serve as useful prognostic marker, we did not find them to be predictive enough alone or in combination for clinical decision-making in this setting.
We did find a bias of a mean difference whereby the POC lactate is, on average, 0.32 mmol/L lower than laboratory lactate. The first explanation is that POC lactate measurements are systematically lower than laboratory measured values due to the assay techniques. A second explanation is that the laboratory lactate is falsely elevated (slightly) as it is processed after about 15–30 min of transport time and run in the laboratory. Because our lactate testing was performed on a lithium heparin tube, without the preservatives known to freeze metabolism, this type of inflated result is possible.
A number of prior initiatives have established the measurement of blood lactate levels as helpful to the clinician assessing patients with suspected sepsis (3–5). Furthermore, in a smaller study, Goyal et al. showed a significant reduction in time to result through the use of POC testing of fingerstick blood samples from triage (7). Finally, the Surviving Sepsis Campaign has recommended obtaining a serum lactate within 3 h from ED presentation as a target quality assurance measure (8). The cumulative evidence is certainly in support of routine lactate screening.
Limitations
There are a number of limitations to this study. First, there is potential for selection bias, as not all patients received POC testing; instead, patients were included only if the clinicians elected to use the device. Thus, convenience sampling may have biased our results. We left the decision to obtain a lactate to the clinician, based on a suspicion of infection; we did not rigorously define the criteria for suspected infection. We did not have synchronized clocks between the i-STAT and the actual laboratory result, nor did we follow the samples in real time, so we did not compare the time to results, which would have been an informative comparison. We have a sepsis protocol that calls for the implementation of early goal-directed therapy treatment strategy for patients with lactate levels over 4.0 mmol/L; thus, these patients received therapies that may have altered the natural course of outcomes (1,9).
CONCLUSION
A POC testing device provides a practical, feasible, and reliable way to measure blood lactate at the bedside that is predictive of death in sepsis. The venous pH and base excess were less helpful. Future experimental studies that directly test whether the use of POC lactate levels improve outcomes in patients with sepsis compared to routine testing would be informative.
ARTICLE SUMMARY.
-
Why is this topic important?
Serum lactate is demonstrated to risk-stratify patients with sepsis, and has been used as an indicator for therapies such as early goal-directed therapy in sepsis. However, this measurement is not always readily available for clinical decision-making.
-
What does this study attempt to show?
This study attempts to show that serum lactate measurement using a point-of-care (POC) device is feasible and useful for risk prognostication. We also tested the prognostic ability of pH and base excess.
-
What are the key findings?
The areas under the curve for mortality prediction were: POC lactate 0.72, laboratory lactate 0.70, pH measurement 0.60, and base excess 0.60. Bland-Altman showed that POC lactate was, on average, 0.32 (95% confidence interval −0.35− 0.98) lower than laboratory lactate, with agreement kappa = 0.97.
-
How is patient care impacted?
This study shows that POC lactate is a useful alternative if a laboratory lactate is not readily available.
Acknowledgments
This study was funded by Abbott Point of Care Inc. Nathan I. Shapiro, MD, MPH, is funded in part by grants from the National Institutes of Health/National Institute of Heart, Lung, and Blood (1R01HL091757-01A1) and National Institute of General Medical Sciences (1P50GM076659-01). Dr. Trzeciak is supported by a grant from the National Institutes of Health/National Institute of General Medical Sciences (K23GM083211).
Stephen Trzeciak, MD, MPH, receives research support from Novo Nordisk, Eli Lilly, and Inverness. Nathan I. Shapiro, MD, MPH, receives research support from Eli Lilly, Abbot Point of Care, Inverness, and Hutchinson technologies. Michael Donnino, MD, is funded in part by a grant from the American Heart Association (0735533T).
REFERENCES
- 1.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro N, Howell MD, Bates DW, Angus DC, Ngo L, Talmor D. The association of sepsis syndrome and organ dysfunction with mortality in emergency department patients with suspected infection. Ann Emerg Med. 2006;48:583–590. doi: 10.1016/j.annemergmed.2006.07.007. 590.e.1. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro NI, Howell MD, Talmor D, et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45:524–528. doi: 10.1016/j.annemergmed.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Trzeciak S, Dellinger RP, Chansky ME, et al. Serum lactate as a predictor of mortality in patients with infection. Intensive Care Med. 2007;33:970–977. doi: 10.1007/s00134-007-0563-9. [DOI] [PubMed] [Google Scholar]
- 5.Howell MD, Donnino M, Clardy P, Talmor D, Shapiro NI. Occult hypoperfusion and mortality in patients with suspected infection. Intensive Care Med. 2007;33:1892–1899. doi: 10.1007/s00134-007-0680-5. [DOI] [PubMed] [Google Scholar]
- 6.Dellinger P, Carlet J, Masur H, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858–873. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]
- 7.Goyal M, Pines JM, Drumheller BC, Gaieski DF. Point-of-care testing at triage decreases time to lactate level in septic patients. J Emerg Med. 2008 Jul 8; doi: 10.1016/j.jemermed.2007.11.099. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro NI, Howell MD, Talmor D, et al. Implementation and outcomes of the Multiple Urgent Sepsis Therapies (MUST) protocol. Crit Care Med. 2006;34:1025–1032. doi: 10.1097/01.CCM.0000206104.18647.A8. [DOI] [PubMed] [Google Scholar]