Abstract
Neuromuscular compartments are subvolumes of muscle that have unique biomechanical actions and can be activated singly or in groups to perform the necessary task. Beside unique biomechanical actions, other evidence that supports the neuromuscular compartmentalization of muscles includes segmental reflexes that preferentially excite motoneurons from the same compartment, proportions of motor unit types that differ among compartments and a central partitioning of motoneurons that innervate each compartment. The current knowledge regarding neuromuscular compartments in representative muscles involved in locomotion, respiration and mastication is presented to compare and contrast these different motor systems. Developmental features of neuromuscular compartment formation in these three motor systems are reviewed to identify when these compartments are formed, their innervation patterns and the process of refinement to achieve the adult phenotype. Finally, the role of androgen modulation of neuromuscular compartment maturation in representative muscles of these motor systems is reviewed and the impact of testosterone on specific myosin heavy chain fiber types is discussed based on recent data. In summary, neuromuscular compartments are pre-patterned output elements in muscle that undergo refinement of compartment boundaries and muscle fiber phenotype during maturation. Further studies are needed to understand how these output elements are selectively controlled during locomotion, respiration and mastication.
English et al (1993) proposed the partitioning hypothesis to describe the relationship between compartmentalization of muscles and the spatial organization of their motor nuclei. This hypothesis was based on mounting evidence that many classically defined muscles including: lateral gastrocnemius (English and Letbetter 1982); medial gastrocnemius (Letbetter 1974); biceps femoris and semitendinosus (English and Weeks 1987); trapezius (Keane and Richmond 1981); biventer cervicis (Armstrong et al. 1988); extensor digitorum longus (Balice-Gordon and Thompson 1988); gluteus maximus (English 1990), biceps brachii (Segal 1992); and masseter (Herring et al. 1979; Widmer et al. 1997), have a complex anatomical organization composed of multiple, functionally distinct, neuromuscular compartments. Thus these compartments represent output elements that may be independently activated to generate unique biomechanical actions. It has also been shown that neuromuscular compartments are differentially recruited in specific reflex pathways (Ia afferents, flexion reflex), further supporting the idea that these compartments have functionally distinct roles as unique output elements. Centrally, the motoneurons that innervate these distinct muscle regions have been found to have a spatial organization within the muscle motoneuron pool. Recently, to further characterize these compartments, we, as well as others, have begun to investigate the factors responsible for the developmental patterning of compartments, the spatial organization of motoneuron subpopulations in the motoneuron pool responsible for the innervation of specific neuromuscular compartments, and hormonal influences that might affect both compartment motoneurons centrally and the phenotype of compartment muscle fibers peripherally. Knowledge of the properties, organization and activation of these output elements as they function as synergists or antagonists in the context of motor control in locomotion, respiration and mastication is required for refinement of models for these three motor systems. The focus of this paper is to provide an overview of neuromuscular compartmentalization and to review developmental aspects and hormonal influences that affect compartment formation and functional characteristics in the lateral gastrocnemius, diaphragm and masseter muscles, representing muscles of locomotion, respiration and mastication.
Neuromuscular Compartmentalization of Muscle
Neuromuscular compartments are discrete subvolumes of muscle that are innervated by a unique collection of motoneurons (English and Letbetter 1982). The original work by Letbetter (1974) on cat medial gastrocnemius laid the foundation for examination of other limb, neck, respiratory and masticatory muscles that have been shown to be compartmentalized into functional subvolumes. The number of neuromuscular compartments that are contained within any specific muscle varies widely and may be related to the complexity of the biomechanical actions that are performed by the muscle/neuromuscular compartments. Different neuromuscular compartments may also contain muscle fibers with different proportions of particular myosin heavy chain (MyHC) isoforms and, thus, the speed of contraction and fatigue characteristics will vary between neuromuscular compartments.
Lateral Gastrocnemius
The lateral gastrocnemius (LG) is composed of four neuromuscular compartments. Four regions of the lateral gastrocnemius muscle in the cat and rat: LG1, LG2, LG3 and LGm (English 1984; Donahue and English 1987) are innervated by four primary nerve branches of the lateral gastrocnemius muscle nerve, each of which contain a unique collection of motor axons. English and Letbetter (1982) used the method of glycogen depletion to determine the innervation territories of the four primary nerve branches and found four distinct and non-overlapping regions of muscle fibers. The compartmentalization of the gastrocnemius, defined by the primary motor nerve branches, was further analyzed using evoked electromyographic (EMG) mapping of stimulated isolated single motor axons (English and Weeks 1989) in conjunction with glycogen depletion (English and Weeks 1984). It was found that the motor unit territories were confined to a single compartment. Thus, individual compartments in the lateral gastrocnemius are independently innervated and have the potential to be activated either singly or as groups of compartments to perform specific tasks. For example, slow walking involves preferential activation of compartment LG3 over compartments LG1 and LG2, with the LGm compartment remaining almost totally inactive (English 1984).
Further evidence for the differential activation of lateral gastrocnemius neuromuscular compartments has been provided through the study of reflex partitioning. Nichols et al (1993) evaluated the activation of LG compartments by various reflexes (flexion-withdrawal reflex, caudal cutaneous sural nerve reflex, crossed-extension reflex) in the cat. Statistically significant differences in LG compartment recruitment were observed only during the flexion-withdrawal reflex with the LGm compartment having a higher response than the LG1, LG2 or LG3 compartments. This outcome is as one might predict given the dorsal location of this compartment provides a mechanical advantage for LGm in this flexion-withdrawal reflex (Vanden Noven et al. 1986). No differences in activation were found among compartments after the caudal cutaneous sural nerve or crossed-extension reflexes were elicited. Ia afferent sensory partitioning has also been observed in lateral gastrocnemius after electrical stimulation of LG primary nerve branches. Significantly greater amplitude monosynaptic EPSPs were recorded from homonomous LG motoneurons innervating the same compartment compared to EPSPs recorded from heteronomous motoneurons innervating the three other compartments after normalization for different motoneuron types in each compartment (Vanden Noven et al. 1986). In addition, statistically greater amplitude heteronomous EPSPs were found in the LG2 branch after stimulation of the soleus nerve compared to the other LG nerve branches. Similar compartment Ia afferent partitioning has been reported for the medial gastrocnemius (Lucas and Binder 1984). Detailed reviews of monosynaptic Ia EPSP afferent excitation of motoneurons of neuromuscular compartments in various muscles has been published (Stuart et al. 1988; Windhorst et al. 1989).
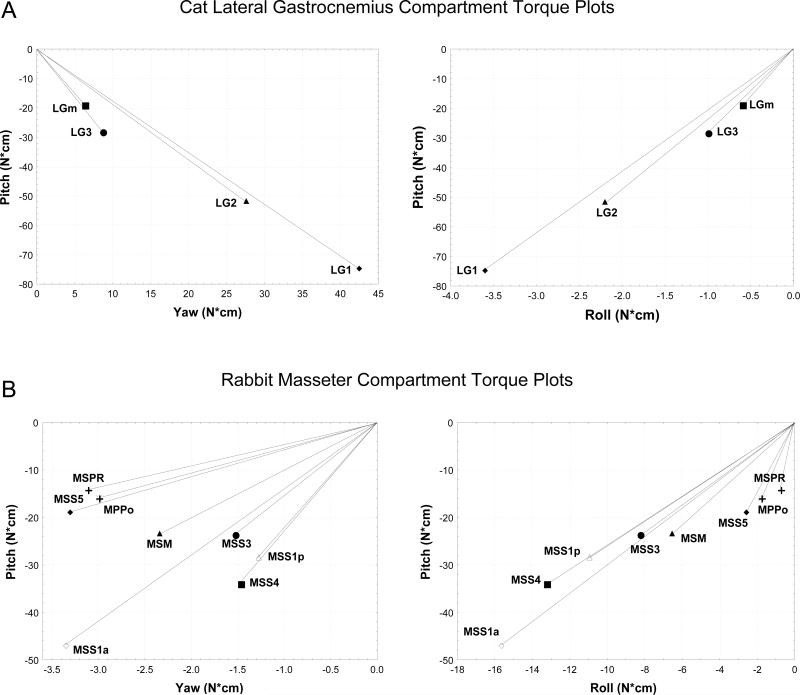
Further evidence for the autonomy of neuromuscular compartments is also provided by the distinctly different biomechanical actions that are elicited from each compartment. Average torque trajectories and magnitudes differ significantly among lateral gastrocnemius neuromuscular compartments (Fig. 1a) (Carrasco et al. 1999). The LG1 compartment has a statistically greater plantarflexion torque than the other compartments while LGm has the smallest. Compartments located more laterally (LG1 and LG2) produce higher off-sagittal torques (abduction-adduction, inversion-eversion) about the ankle than those located near the mid-sagittal plane (LG3, LGm). These recordings were conducted with a fixed ankle joint angle (100°) relative to the tibia and it is possible that further differences in torques produced by the individual LG compartments may be observed as the foot is positioned in functional 3D space.
Figure 1.
Vector plots of mean lateral gastrocnemius compartment torques about the ankle in the cat (n=6) (A) (Carrasco et al. 1999) and typical masseter compartment torques about the temporomandibular joint in rabbit (B). Each plot represents pitch vs yaw or pitch vs roll for the two muscles. For the LG compartments, negative pitch is plantarflexion, negative roll is eversion, and negative roll is adduction. For the masseter compartments, negative pitch is jaw closing, negative yaw is working side rotation and negative roll is lingual tipping. All compartments were statistically different (ANOVA, Bonferroni or LSD test, p<0.05) in at least one torque component (pitch, yaw, roll or magnitude) for both the LG (n=6) and masseter (n=5) muscles.
Diaphragm
The mammalian diaphragm is a major inspiratory muscle that is composed of at least two architecturally distinct regions: the sternocostal region and the crus region surrounding the esophagus (Pickering and Jones 2002). These two regions have functionally distinct actions. During respiration, both the sternocostal region and the crus are activated. However, during emesis, regurgitation, swallowing or eructation, there is activation of only the sternocostal region with inhibition of the crus to allow the bolus to move in the lower esophagus (Monges et al. 1978). The innervation of the sternocostal diaphragm and crus consists of four branches of the phrenic nerve (SC1, SC2, SC3 and Cr) (Hammond et al. 1989) with potentially additional innervation of costal regions of the sternocostal diaphragm by cervical regions (C4, C5) and innervation of the crus by branches from the vagus nerve and cervical regions (C5, C6) (Young et al. 2009). Unilateral electrical stimulation of the crural branch has been shown to only activate the ipsilateral crus diaphragm while stimulation of the three branches innervating the sternocostal diaphragm elicits responses in distinct but overlapping regions (Duron et al. 1979b; Hammond et al. 1989). These findings were verified using glycogen depletion studies that mapped the depleted muscle fiber territories. Differential recruitment of the sternal but not the mid-costal regions during post-inspiratory activity has been shown using electromyographic recordings in sleeping or anesthetized lambs and is consistent with the observed innervation of these regions by different nerve branches (Henderson-Smart et al. 1982). Thus some investigators describe the diaphragm as having three distinct neuromuscular compartments: sternal, costal, and crus.
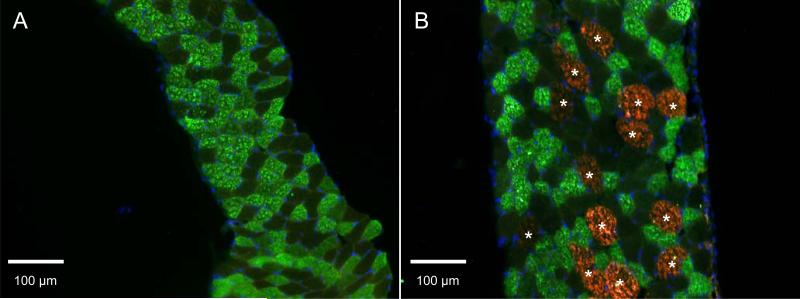
Mechanical actions of the sternocostal and crus regions of the diaphragm have also been reported to be different, further establishing their differential roles. Activation of the sternocostal diaphragm elicits an outward movement of the lower rib cage while activation of the crus had no effect on the rib cage (De Troyer et al. 1982). There has as yet been no reported assessment of the biomechanical actions of different regions of the sternocostal diaphragm during the stimulation of different nerve branches. Therefore, it is unclear whether there are any mechanical advantages to compartmentalization of the sternocostal portion of the diaphragm. Several studies have reported finding no differences in the proportion of muscle fiber types comprising the different diaphragm compartments. However, we have recently detected significant differences between the expression of type IIb MyHC isoform within the sternocostal and crural diaphragm regions in the mouse (Fig. 2). Type IIb MyHC is not expressed in the lateral costal regions of the sternocostal diaphragm but is readily detected in the sternal regions and in the crus. This differential expression of MyHC mirrors the observed different mechanical actions of these diaphragm regions and provides additional support for the uniqueness of these compartments.
Figure 2.
Parasagittal sections of mouse diaphragm representing mid-costal (A) and sternocostal (B) regions of an adult male mouse. Fibers containing MyHC type IIa can be observed in both regions of the diaphragm. However, the sternocostal region (B) and the crural region (not shown) have fibers containing type IIb MyHC (asterisks). The discrete localization of MyHC type IIb fibers supports the presence of individual compartments in the mouse diaphragm.
Masseter
The masseter muscle is one of four muscles of mastication and has the primary role of closing the jaw in conjunction with two other jaw closing muscles, the temporalis and medial pterygoid muscles. The fourth masticatory muscle, the lateral pterygoid, causes jaw protrusion and jaw opening when activated. It has been known for many years that the masseter is composed of superficial, intermediate and deep layers that have different functions (Schumacher 1961; Hannam et al. 1977; Herring et al. 1979; Weijs and Dantuma 1981; Widmer et al. 1997). However, a more thorough evaluation of the rabbit masseter muscle architecture revealed multiple anatomical partitions (Weijs and Dantuma 1981; Widmer et al. 1997) that could be further subdivided into neuromuscular compartments (English et al. 1999a; Widmer et al. 2003). Unlike the lateral gastrocnemius where each of four neuromuscular compartments is innervated by a primary muscle nerve branch, the rabbit masseter has been shown to be composed of least 23 separate compartments that are innervated by unique motor unit axons detected in secondary and tertiary branches of the masseteric motor nerve (Widmer et al. 2003). A similar complexity of the anatomical organization and neural innervation has been reported in the human masseter (Widmer et al. 1996). The complexity of the organization of the masseter muscle output elements makes this muscle an excellent model to investigate compartment characteristics and their recruitment during complex movement patterns such as those associated with mastication.
Reflex partitioning to specific masseter compartment motoneurons has not yet been examined. However, the biomechanical actions of the individual masseteric compartments have been shown to produce unique torques about the temporomandibular joint (English et al. 1999a) (Fig. 1b). The biomechanical actions involve large jaw closing torques as the predominant action, but also produce significant off-sagittal torques to rotate the mandibular condyle about a dorsal-ventral axis to promote medial-lateral movement of the mandible and to rotate the condyle in the rostral-caudal axis to tip the teeth slightly during the power stroke. Comparison of masseter and LG compartment torques reveals larger plantarflexion (pitch) torques and off-sagittal (yaw) torques about the ankle than masseter compartment jaw closing (pitch) torques and off-sagittal torques (yaw) (Fig. 1). However, these differences are more than compensated by the larger number of compartments in the masseter muscle that represent a greater diversity of potential torque production about the temporomandibular joint.
Central Partitioning of Motoneurons Innervating Neuromuscular Compartments
The central organization of motoneurons innervating neuromuscular compartments has been examined for a number of compartmentalized muscles. Several studies suggest a distinct somatotopy of compartment motoneurons in the motor nucleus while some studies of diaphragm, neck, sartorius and medial gastrocnemius muscles have reported considerable overlap of compartment motoneurons. The differences reported in the observed organization of compartmental motoneurons between different studies may be due in part to differences in the methodology, i.e., labeling nerve branches or labeling discrete regions of the muscle to determine the organization of subpopulations of motoneurons. The functional significance of a somatotopic organization of the compartment motoneurons may be related to an increased success of establishing appropriate synaptic contacts from segmental or descending connections during development.
Lateral Gastrocnemius
For each of the four neuromuscular compartments of the lateral gastrocnemius in the cat, a spatially organized group of motoneurons, or compartment nuclei, have been identified (Weeks and English 1985). A distinct topography of compartment nuclei was reported using HRP labeling of primary nerve branches, with more proximal compartments (innervated by LGm, LG2 nerve branches) generally located more rostrally in the lateral gastrocnemius motoneuron pool and the more distal compartments (innervated by LG1, LG3 nerve branches) located in the caudal region of the LG motor nucleus. Although an extensive overlap was observed in the distribution of the various compartment nuclei, the central tendencies of each compartment nucleus were significantly different (Weeks and English 1985). A motoneuron size difference was also identified with a higher proportion of large cells located in the proximal compartment nuclei. This finding is consistent with the prevalent muscle fiber phenotype found in these compartments. The proximal compartments consist of predominantly faster contracting muscle fibers, fast-twitch glycolytic (type FG), that are associated with larger fast-twitch fatigable (FF) motor units. The more distal compartment nuclei have an approximately equal numbers of large and small motoneurons, have a higher number of muscle spindles and a higher proportion of slow oxidative (SO) fibers and slow-twitch (type S) motor units (Weeks and English 1985). The smaller sized motoneurons (<800 μm2) in these compartments were assumed to be gamma motoneurons.
Diaphragm
The spatial distribution of diaphragm compartment nuclei have been investigated using different combinations of fluorescent tracers or HRP retrograde labeling of phrenic nerve primary branches or the injection of tracers into different regions of the diaphragm (Duron et al. 1979a; Rikard-Bell and Bystrzycka 1980; Tan and Miller 1986; Laskowski and Sanes 1987; Gordon and Richmond 1990). The results of these studies were generally similar, with the sternal portion of the sternocostal diaphragm innervated by motoneurons located more rostrally in the phrenic motor nucleus while motoneurons innervating the lateral costal portion of the diaphragm were found more caudally. The crural motoneurons were found to have a broad spatial distribution in the middle and caudal regions of the phrenic motoneuron pool when muscle nerve branches were retrogradely labeled (Duron et al. 1979a; Rikard-Bell and Bystrzycka 1980). However, when labeling was performed by HRP injections into the diaphragm, crural motoneurons were observed to be more extensively distributed throughout the whole phrenic motoneuron pool (Tan and Miller 1986). Differences in the spatial distribution of motoneurons observed between studies using muscle injection and muscle nerve labeling may be the result of diffusion of the tracer in the muscle (Gordon and Richmond 1990). Another possible interpretation may be that primary nerve branches innervating the diaphragm contain axons innervating multiple compartments and retrograde labeling of any one primary nerve branch would label multiple compartment nuclei. Since motor unit territories are estimated to be 10-15% the size of the whole diaphragm, it is conceivable that each primary nerve branch could innervate multiple compartments. When discrete regions (potentially single compartments) of the diaphragm were labeled using Nuclear Yellow and Fast Blue, the motoneuron density for each fluorescent label was much more clustered (Laskowski and Sanes 1987). However, overlap of each labeled set of motoneurons in the middle of the motor nucleus was still observed. Thus, the spatial distribution of compartment nuclei of the diaphragm (determined by the primary nerve branches of the phrenic nerve) has less distinct somatotopy compared to compartment nuclei of the lateral gastrocnemius.
Masseter
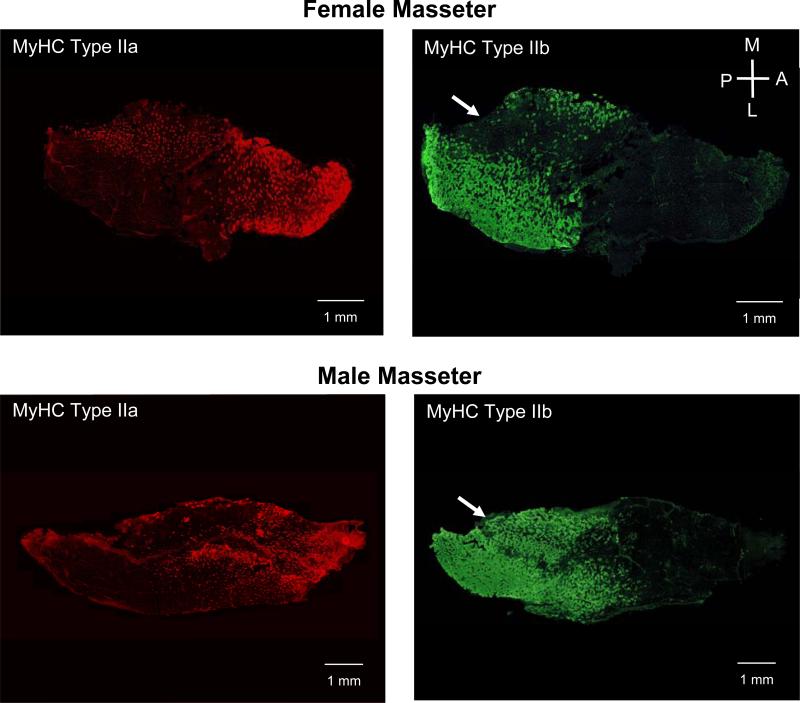
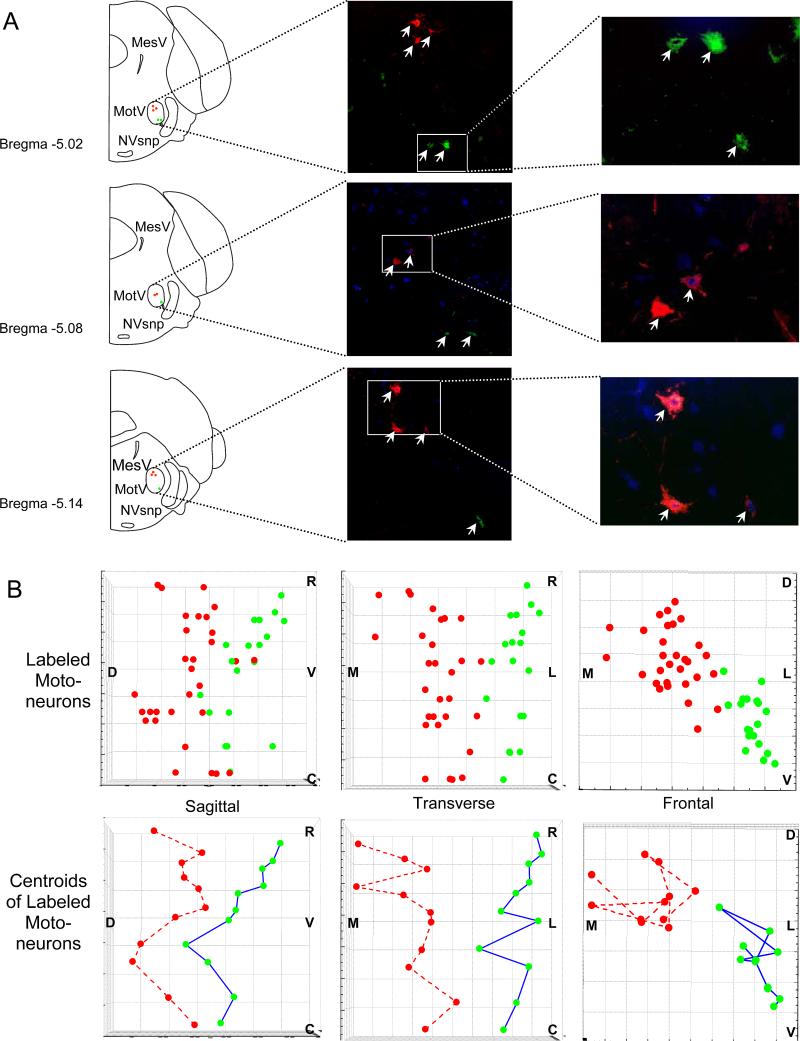
Retrograde labeling of pairs of primary branches innervating different (anterior/posterior) regions of the masseter were investigated using fluorogold and fast blue tracers and no simple spatial patterning of subpopulations of motoneurons could be detected in the rabbit masseteric motoneuron pool (Saad et al. 1997). However, a study using HRP retrograde labeling of motoneurons innervating defined regions of the rabbit masseter muscle found statistically different distributions within the masseteric motor nucleus (Weijs 1996). The superficial regions of the masseter muscle were innervated by neurons occupying the dorsolateral aspect of the masseteric motoneuron pool while the deep regions were innervated by motoneurons occupying the dorsomedial and central ventromedial aspects. Extensive spatial overlap of motoneurons innervating all masseter regions was found in the rostrocaudal dimension of the masseteric motor nucleus. However, this study was limited by the spread of HRP resulting in the labeling of multiple compartments and the inability to compare retrogradely labeled regions simultaneously using multiple tracers. We have recently conducted retrograde labeling of two discrete regions (anterior and posterior regions of the intermediate layer) of the mouse masseter muscle using injections of cholera toxin B conjugated to two different Alexa fluorochromes. The intermediate layer of the mouse masseter muscle has three distinct regions (at least three compartments) that are each composed of muscle fibers with different proportions of MyHC fiber types (Fig. 3). The anterior third has a high proportion of MyHC IIa fibers; the middle third of the muscle has a high proportion of fibers containing MyHC IIx; while the posterior third is almost exclusively occupied by fibers containing MyHC IIb. Retrograde labeling of the anterior and posterior regions of the intermediate layer allowed the identification of compartment motoneurons to assess their spatial organization within the masseteric motoneuron pool. A discrete somatotopy was observed in the masseteric motor nucleus. The anterior region of the masseter was innervated by motoneurons that occupied the dorsomedial aspect of the masseteric motor nucleus while motoneurons innervating the posterior masseter were observed in the ventrolateral masseteric motoneuron pool (Fig. 4a). The labeled motoneurons representing each compartment were highly clustered and had no overlap (Fig. 4b). Thus, accurate assessment of the central partitioning of motoneurons innervating compartments in complex muscles such as the masseter muscle appears to depend on the ability to label motor units of individual compartments and these compartments may not be represented by the first order branching of the muscle nerve.
Figure 3.
Representative transverse cryosections of male and female mouse adult mouse masseter muscles immunolabeled for MyHC type IIa (red) or IIb (green). MyHC fiber types are non-uniformly distributed within the masseter in both male and female mice. A higher density of IIa-containing fibers is observed within the anterior region while IIb-containing fibers occupy the posterior region of the muscle. A sexual dimorphic distribution of MyHC IIb is apparent in the posterior region of the masseter. Arrows denote regions in which females are devoid of IIb-containing fibers. In contrast males have a relatively uniform distribution of MyHC IIb in the posterior masseter.
Figure 4.
Spatial organization of motoneurons innervating the anterior and posterior regions of the intermediate masseter layer identified by retrograde labeling using cholera toxin B conjugated to Alexa 555 (anterior region-red) or Alexa 488 (posterior region-green). Tracer (1-3 μl) was injected into the anterior or posterior intermediate masseter of anesthetized mice and allowed to transport for four-five days prior to harvesting of the brainstem. Cryosections were mounted on slides and immunostained for NeuN to identify motoneurons. Illustrated are labeled motoneurons (inset magnified) detected within the masseteric motor nucleus (motV) at the brainstem level shown in the linked left panel (A). A three-dimensional map of the labeled motoneurons for each tracer was constructed and centroids for each group of motoneurons in each section were calculated and plotted (B). An obvious central partitioning of motoneurons innervating these two masseter intermediate layer compartments of the muscle was observed.
Development of Neuromuscular Compartments
Recent evidence suggests that cues that may regulate muscle nerve innervation of appropriate muscle targets involve the developmentally-regulated expression of homeodomain transcription factors within motoneuron pools (Marco Garcia and Jessell 2008; Dalla Torre di Sanguinetto SA et al. 2008). It remains to be determined whether transcription factors that regulate the central organization of motoneurons and the patterning of innervation for whole muscles also regulate the patterning of innervation for neuromuscular compartments. Most of the research examining developmental aspects of neuromuscular compartmentalization has concentrated on the timing and role of nerve-muscle interactions in specifying compartments and on factors involved in the refinement of innervation.
Two different mechanisms have been considered for the development of neuromuscular compartments: (1) the muscle is innervated by arbitrary motoneurons in the muscle motoneuron pool, with muscle fibers having polyneuronal innervation, and subsequent refinement of connections are made after birth to form adult neuromuscular compartments by synapse elimination; or (2) compartments are patterned prior to birth with their appropriate motoneuron innervation and the small amount of cross-compartment innervation is refined by selective synapse elimination. Examination of prenatal events in the development of limb and masticatory muscle has provided evidence that nerve-muscle interactions do not play a role in the initial partitioning of muscle groups (Condon et al. 1990; Morris-Wiman and Widmer 2001; Morris-Wiman and Widmer 2003). Additionally, studies examining the neonatal development of lateral gastrocnemius (Donahue and English 1987; Donahue and English 1989), extensor digitorum longus (Balice-Gordon and Thompson 1988), gluteus maximus (English 1990), tibialis anterior (Iliya and Dum 1984) and diaphragm (Laskowski and High 1989) support the conclusion that partitioned innervation to neuromuscular compartments is established at birth.
Lateral Gastrocnemius
In studies using EMG recordings to assess responses to electrical stimulation of primary nerve branches in neonatal rat pups, evoked responses were mapped from as many as 60 sites in the four LG neuromuscular compartments (Donahue and English 1987). Cross-compartmental potentials were rarely observed indicating specificity of the axons in each primary nerve branch. Axon specificity was further tested by electrically stimulating the proximal stump of each severed primary nerve branch and broadly recording from the LG muscle. No EMG potentials were detected after stimulation of each proximal end of the severed primary branch indicating that motor axons did not split prior to the nerve branches and that early postnatally each primary nerve branch contained unique motoneuron axons. To determine if increased synapse elimination in cross-compartment innervation compared to general synapse elimination played a role in the refinement of compartment innervation, unilateral tenotomy of the tendocalcaneus was performed in a group of P0 rat pups to delay synapse elimination and the percentage of polyneuronal innervation in LG was compared on the tenotomy and unoperated sides (Donahue and English 1989). Cross-compartmental innervation was observed to be eliminated at an earlier time compared to polyneuronal innervation of the compartment. Interestingly, cross-compartmental innervation to LGm was found more frequently in the distal part of the compartment where no physical boundaries are present to separate the compartments (Bennett and Ho 1988; Donahue and English 1989). No cross-compartmental innervation was found after postnatal day 8. These findings support the prenatal patterning of compartments in the lateral gastrocnemius.
Diaphragm
The existence in early postnatal rat pups (P0-P3) of a rostral-caudal organization (cervical roots C3-C6) of the innervation of the rostral (sternal and upper costal region) and caudal (mid and lower costal region) diaphragm was demonstrated by recording synaptic potentials or muscle contraction in response to electrical stimulation of cervical ventral roots (Laskowski and High 1989). Interestingly, the cervical root C3 was found to innervate rostral regions of the diaphragm in the neonate but not in adults. Also, early postnatally, 65% of the diaphragm muscle fibers were innervated by at least two cervical roots and this multi-root innervation was not observed in adults. This postnatal refinement of the C3-C6 rostral-caudal segmental innervation of diaphragm could involve two potential mechanisms (Laskowski and High 1989): (1) synapse elimination which has been shown to continue through postnatal day 14 (Redfern 1970); and (2) generation of new muscle fibers during secondary myogenesis which has been observed to continue through postnatal day 21 (Prakash et al. 1993). Although these studies did not investigate the specificity of compartment innervation by primary nerve branches, nevertheless, the data support a patterned segmental organization of the innervation of the diaphragm at birth that is subsequently refined early postnatally.
Masseter
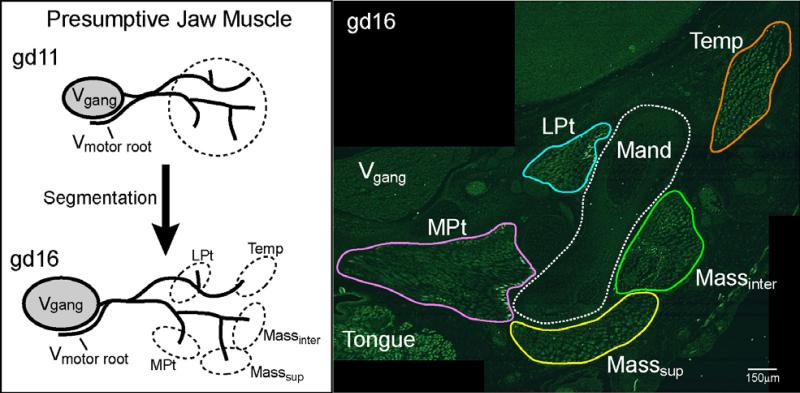
The three layers of the mouse masseter muscle: superficial, intermediate and deep, form prenatally from distinct muscle masses (Fig. 5). Each of these anatomical layers contains multiple neuromuscular compartments in the adult mouse; however, no studies to date have examined the boundaries of these compartments or their development. Our lab has investigated the early postnatal development and the influence of the muscle nerve on the patterning of the masseter layers and the phenotype of muscle fibers in the respective layers. Early postnatally, a regionalization of fiber types based on MyHC phenotype can be observed in the mouse that parallels what is observed in the adult masseter with MyHC type IIa distributed in the rostral (anterior) region, type IIb fibers distributed in the caudal (posterior) region and presumed type IIx fibers located centrally (Widmer et al. 2002). Unilateral injection of β-bungarotoxin into the amnionic sac on gestational day (gd) 12 to eliminate sensory and motor innervation in mouse embryos did not affect the patterning of the masseter layers (Morris-Wiman and Widmer 2001) and produced no change in the distribution of muscle fiber MyHC content (Morris-Wiman and Widmer 2003). However, secondary myogenesis appeared to be affected; the volumes of individual masticatory muscle masses as well as the masseter muscle layers were significantly smaller. These data support the premise that neuromuscular compartments, as well as the phenotype of the muscle fibers within each compartment, are pre-patterned in the masseter by birth.
Figure 5.
The developmental process of presumptive jaw muscle mass segmentation begins concomitant with motor root nerve branching at gd11. This mass eventually segments into specific jaw muscles and, in the mouse masseter, into anatomical layers. The right image is a frontal section through the jaw muscles (Temp, temporalis; Masssup, superficial masseter; Massinter, intermediate masseter; LPt, lateral pterygoid; MPt, medial pterygoid) at gd16 that was immunolabeled for desmin to identify the muscle masses. Other structures labeled for orientation include tongue and trigeminal ganglion (V-gang).
Hormonal Influences on Compartment Muscle Phenotype
Several studies over the past decades have examined the postnatal effects of androgen on muscle at the cellular and molecular levels. Most studies have focused on the effects of supraphysiological levels of testosterone exposure on sexually dimorphic muscles such as the pelvic floor muscles (levator ani) and these studies have provided valuable insight into the target sites of androgen action, the muscle fiber and the motoneuron. More recent studies have examined anabolic effects of androgens on other muscles and have demonstrated that these effects vary among muscles, muscle fiber types, and experimental conditions. For example: in limb muscle, anabolic effects of androgens on muscle mass are augmented when combined with exercise; the anabolic effects of androgens on diaphragm involve alterations in motoneuron membrane properties, as well as muscle mass; and, in masticatory muscle, the effects of androgen exposure at puberty include alterations in muscle fiber phenotype.
Lateral Gastrocnemius
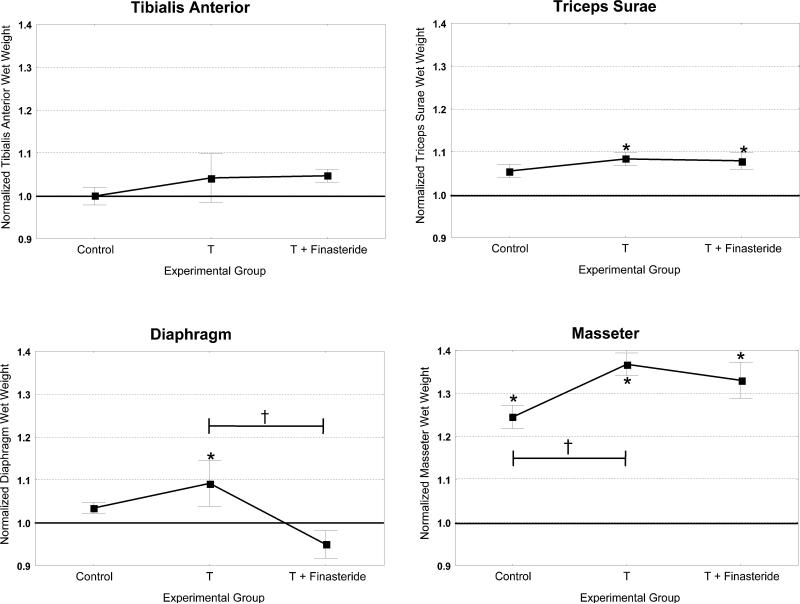
Limb muscles such as the lateral gastrocnemius show very modest responses to androgens either after castration or after supplementation. Castration of male rats or mice has been shown to cause a 10% reduction in gastrocnemius muscle mass after 9 or 11 weeks (Jiao et al. 2009) (Fig. 6). Supplementation of normal physiological levels of testosterone for three weeks after prolonged castration caused a modest increase in the muscle wet weight of triceps surae (medial and lateral gastrocnemius, soleus and plantaris muscles) but had no effect on tibialis anterior in mice (Fig. 6). It has been suggested that testosterone supplementation must be accompanied by exercise in order to have an anabolic effect on limb muscle. However, endurance exercise combined with testosterone supplementation does not produce significant increases in muscle mass (Borst and Mulligan 2007; Brown 2008). Resistance exercise involving eccentric contractions has been shown to produce a modest increase in muscle mass in some studies (Latham et al. 2004; Petrella et al. 2006).
Figure 6.
Androgen responsiveness of adult limb, diaphragm and masseter muscles. CD-1 male mice at 11 weeks post-castration were supplemented for three weeks with testosterone (T) or testosterone and finasteride. Controls had no supplementation. Graphs represent wet weights of muscle from the supplementation/control groups normalized to corresponding wet weights from a parallel group of castrated animals. Significant differences in wet weights between experimental groups and castrated condition are indicated by an asterisk (*). The masseter muscle was the only one of the four muscles examined that had a significant reduction in wet weight after castration. Three muscles were found to significantly increase their wet weight after testosterone supplementation (triceps surae, diaphragm and masseter). Only diaphragm had a significant reduction with testosterone and finasteride. Significant differences between experimental groups are indicated by a † symbol (p < 0.05).
Diaphragm
In previous studies it was observed that after castration diaphragm wet weight of male rats did not change and fiber type proportions were not altered (Prezant et al. 1997). However, supraphysiological levels of testosterone have been shown to produce increases in cross-sectional area of specific fiber types, type IIx and IIb, in the diaphragm (Prezant et al. 1997; Bisschop et al. 1997; Lewis et al. 2002). Additionally, testosterone supplementation has been shown to improve neuromuscular transmission in male rats (Blanco et al. 2001). We have evaluated the effects of 11 weeks of castration and the subsequent addition of physiological levels of testosterone for three weeks on the diaphragm in male mice. Diaphragm wet weight increased 10% in response to testosterone after prolonged castration (Fig. 6). However, fiber type proportions within each compartment (sternocostal and crural) were not changed (unpublished data). These data would indicate that testosterone at normal physiological levels does not preferentially affect muscle fibers of a particular phenotype as has been suggested by studies in other muscles. Exposure to finasteride, an inhibitor of the enzyme 5α-reductase responsible for converting testosterone to the more potent DHT, caused a significant decrease in the testosterone-mediated increase in diaphragm wet weight (Fig. 6). Whereas muscle has little 5α-reductase, many motoneurons are enriched in this enzyme. Studies examining androgens effects on the sexual dimorphic muscle levator ani have demonstrated these effects are at least partially mediated through 5α-reductase conversion of testosterone within motoneurons. Our results with finasteride suggest that in diaphragm, the motoneuron, as well as the muscle fiber, may be a target of testosterone action.
Masseter
Sexual dimorphism has been reported for both muscle fiber phenotype and motoneuron properties in adult rodent, rabbit and macaque masseter muscles. In all species examined, a higher proportion of faster MyHC fiber types has been identified in the adult male than in adult female masseter (Maxwell et al. 1979; English et al. 1999b; Eason et al. 2000b; Widmer et al. 2002). In the adult mouse, the male has a higher proportion of MyHC IIb fibers while the female mouse has a higher proportion of IIa fibers (Fig. 3) (Eason et al. 2000b; Widmer et al. 2002). Likewise, in the adult male rabbit masseter, 80% of the muscle fibers contain MyHC type IIa while this same phenotype is found in only 50% of the adult female masseter fibers. Interestingly, the proportion of MyHC type I and IIa fibers and their distribution in the female rabbit masseter are similar to that observed in young adult male rabbits (Eason et al. 2000a). The effect on the rabbit masseter muscle of endogenous androgen during puberty in males or androgen supplementation in castrated young males is to cause a change from a slower to faster MyHC phenotype (Reader et al. 2001). However, castration of adult older male rabbits did not alter the patterning of MyHC phenotype in the masseter, suggesting that this patterning once established is not affected by androgen levels. In male mice, the masseter muscle was observed to decrease by approximately 15% in wet weight 11 weeks after castration when compared to unmanipulated controls (Fig. 6). Testosterone supplementation at physiological levels for three weeks resulted in an average increase of 38% in masseter wet weight when compared to masseter from castrated animals. These data indicate that the male masseter response is much more robust than that observed for the triceps surae muscles or the diaphragm and suggest an enhanced androgen sensitivity of the masseter muscle. The increase in masseter muscle weight was not accompanied by an alteration in the normal patterning of MyHC fiber phenotype, in agreement with what has been observed in adult male rabbit masseter. Median firing rates have been shown to be faster in adult male rabbit masseter motoneurons than in female and the duration of motor unit activity shorter in males (English and Widmer 2003). These results suggest that androgens may mediate changes in masseter muscle fiber phenotype directly by action on the muscle fibers, indirectly by action on motoneurons, or both. The functional significance of sexual dimorphism in the masseter muscle may be related to the requirements of the jaw system for rapid, high force production such as defense or food gathering.
Summary
Neuromuscular compartmentalization can be found in limb, respiratory and masticatory muscles and these compartments serve to subdivide the muscle into functional output elements that can be activated singly or in combination with other compartments/muscles depending on the functional needs. While in lateral gastrocnemius primary nerve branches contain unique motor axons innervating each compartment, the diaphragm and masseter muscle have a more complex arrangement of compartments that are not represented by the primary nerve branches. Ia afferent reflex excitation of motoneurons innervating homonomous compartments over other compartments suggests that motor control mechanisms are specific at the compartment level in limb muscles, but more information is needed to evaluate if respiratory and masticatory muscle compartments have similar Ia afferent controls. Although the spatial organization of compartment motoneurons has substantial overlap with other compartment nuclei and is not as distinct as the muscle motoneuron pool, there is a consistent general somatotopy found for limb, diaphragm and masseter muscle compartments and this organization may have a role in the targeting of segmental and descending inputs. The formation of neuromuscular compartments in muscles associated with locomotion, respiration and mastication appears to be based on a patterned developmental program that occurs prior to birth with refinement of innervation to compartments achieved by selective synapse elimination and potentially secondary myogenesis. The specific mechanisms by which appropriate innervation of compartments by their motoneurons is accomplished still remains to be determined, but may be similar to those active in whole muscle development. During puberty, androgens appear to influence the proportion of fibers with a fast MyHC phenotype in some muscles such as masseter, while causing hypertrophy of fast MyHC fiber types in other muscles such as the diaphragm. The pre-programmed formation of neuromuscular compartments involved in locomotion, respiration and mastication, combined with hormonally-induced maturational changes, establish a repertoire of functional output elements to generate the biomechanical actions necessary to perform the varied tasks of each of these three motor systems. Due to the more complex organization of muscle into neuromuscular compartments, it would seem advantageous to record activity at the compartment level rather than a single muscle site (when appropriate) so that investigations of motor control mechanisms in these three motor systems might have a common anatomical substrate to better understand the selective recruitment of these output elements for specific tasks.
Acknowledgements
Supported by the National Institutes of Health Grants DE12207, HD055286 and FL. Dept. of Health 07BB-11.
Abbreviations
- Cr
crural nerve branch of the phrenic nerve
- EMG
electromyography
- LG
lateral gastrocnemius
- LG1
LG compartment 1
- LG2
LG compartment 2
- LG3
LG compartment 3
- LGm
medial LG compartment
- MyHC
myosin heavy chain
- SC1
sternocostal nerve branch 1 of the phrenic nerve
- SC2
sternocostal nerve branch 2 of the phrenic nerve
- SC3
sternocostal nerve branch 3 of the phrenic nerve
- SO
slow oxidative
References
- Armstrong JB, Rose PK, Vanner S, Bakker GJ, Richmond FJ. Compartmentalization of motor units in the cat neck muscle, biventer cervicis. J Neurophysiol. 1988;60:30–45. doi: 10.1152/jn.1988.60.1.30. [DOI] [PubMed] [Google Scholar]
- Balice-Gordon RJ, Thompson WJ. The organization and development of compartmentalized innervation in rat extensor digitorum longus muscle. J Physiol. 1988;398:211–231. doi: 10.1113/jphysiol.1988.sp017039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MR, Ho S. The formation of topographical maps in developing rat gastrocnemius muscle during synapse elimination. J Physiol. 1988;396:471–496. doi: 10.1113/jphysiol.1988.sp016973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisschop A, Gayan-Ramirez G, Rollier H, Dekhuijzen PN, Dom R, de B,V, Decramer M. Effects of nandrolone decanoate on respiratory and peripheral muscles in male and female rats. J Appl Physiol. 1997;82:1112–1118. doi: 10.1152/jappl.1997.82.4.1112. [DOI] [PubMed] [Google Scholar]
- Blanco CE, Zhan WZ, Fang YH, Sieck GC. Exogenous testosterone treatment decreases diaphragm neuromuscular transmission failure in male rats. J Appl Physiol. 2001;90:850–856. doi: 10.1152/jappl.2001.90.3.850. [DOI] [PubMed] [Google Scholar]
- Borst SE, Mulligan T. Testosterone replacement therapy for older men. Clin Interv Aging. 2007;2:561–566. doi: 10.2147/cia.s1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. Skeletal muscle and bone: effect of sex steroids and aging. Adv Physiol Educ. 2008;32:120–126. doi: 10.1152/advan.90111.2008. [DOI] [PubMed] [Google Scholar]
- Carrasco DI, Lawrence J, III, English AW. Neuromuscular compartments of cat lateral gastrocnemius produce different torques about the ankle joint. Motor Control. 1999;3:436–446. doi: 10.1123/mcj.3.4.436. [DOI] [PubMed] [Google Scholar]
- Condon K, Silberstein L, Blau HM, Thompson WJ. Differentiation of fiber types in aneural musculature of the prenatal rat hindlimb. Developmental Biology. 1990;138:275–295. doi: 10.1016/0012-1606(90)90197-q. [DOI] [PubMed] [Google Scholar]
- Dalla Torre di Sanguinetto SA, Dasen JS, Arber S. Transcriptional mechanisms controlling motor neuron diversity and connectivity. Curr Opin Neurobiol. 2008;18:36–43. doi: 10.1016/j.conb.2008.04.002. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Sampson M, Sigrist S, Macklem PT. Action of costal and crural parts of the diaphragm on the rib cage in dog. J Appl Physiol. 1982;53:30–39. doi: 10.1152/jappl.1982.53.1.30. [DOI] [PubMed] [Google Scholar]
- Donahue SP, English AW. The role of synapse elimination in the establishment of neuromuscular compartments. Dev Biol. 1987;124:481–489. doi: 10.1016/0012-1606(87)90501-x. [DOI] [PubMed] [Google Scholar]
- Donahue SP, English AW. Selective elimination of cross-compartmental innervation in the rat lateral gastrocnemius muscle. J Neurosci. 1989;9:1621–1627. doi: 10.1523/JNEUROSCI.09-05-01621.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duron B, Marlot D, Larnicol N, Jung-Caillol MC, Macron JM. Somatotopy in the phrenic motor nucleus of the cat as revealed by retrograde transport of horseradish peroxidase. Neurosci Lett. 1979a;14:159–163. doi: 10.1016/0304-3940(79)96141-x. [DOI] [PubMed] [Google Scholar]
- Duron B, Marlot D, Macron JM. Segmental motor innervation of the cat diaphragm. Neurosci Lett. 1979b;15:93–96. doi: 10.1016/0304-3940(79)96095-6. [DOI] [PubMed] [Google Scholar]
- Eason JM, Schwartz G, Shirley KA, English AW. Investigation of sexual dimorphism in the rabbit masseter muscle showing different effects of androgen deprivation in adult and young adult animals. Arch Oral Biol. 2000a;45:683–690. doi: 10.1016/s0003-9969(00)00030-3. [DOI] [PubMed] [Google Scholar]
- Eason JM, Schwartz GA, Pavlath GK, English AW. Sexually dimorphic expression of myosin heavy chains in the adult mouse masseter. J Appl Physiol. 2000b;89:251–258. doi: 10.1152/jappl.2000.89.1.251. [DOI] [PubMed] [Google Scholar]
- English AW. An electromyographic analysis of compartments in cat lateral gastrocnemius muscle during unrestrained locomotion. J Neurophysiol. 1984;52:114–125. doi: 10.1152/jn.1984.52.1.114. [DOI] [PubMed] [Google Scholar]
- English AW. Development of compartmentilized innervation of the rat gluteus maximus muscle. J Comp Neurol. 1990;301:104–113. doi: 10.1002/cne.903010110. [DOI] [PubMed] [Google Scholar]
- English AW, Carrasco DI, Widmer CG. Torques produced by different compartments of the rabbit masseter muscle. J Appl Biomech. 1999a;15:348–360. [Google Scholar]
- English AW, Eason J, Schwartz G, Shirley A, Carrasco DI. Sexual dimorphism in the rabbit masseter muscle: myosin heavy chain composition of neuromuscular compartments. Cells Tissues Organs. 1999b;164:179–191. doi: 10.1159/000016658. [DOI] [PubMed] [Google Scholar]
- English AW, Letbetter WD. Anatomy and innervation patterns of cat lateral gastrocnemius and plantaris muscles. Am J Anat. 1982;164:67–77. doi: 10.1002/aja.1001640107. [DOI] [PubMed] [Google Scholar]
- English AW, Weeks OI. Compartmentalization of single muscle units in cat lateral gastrocnemius. Exp Brain Res. 1984;56:361–368. doi: 10.1007/BF00236292. [DOI] [PubMed] [Google Scholar]
- English AW, Weeks OI. An anatomical and functional analysis of cat biceps femoris and semitendinosus muscles. J Morphol. 1987;191:161–175. doi: 10.1002/jmor.1051910207. [DOI] [PubMed] [Google Scholar]
- English AW, Weeks OI. Electromyographic cross-talk within a compartmentalized muscle of the cat. J Physiol (Lond ) 1989;416:327–336. doi: 10.1113/jphysiol.1989.sp017763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW, Widmer CG. Sex differences in rabbit masseter motoneuron firing behavior. J Neurobiol. 2003;55:331–340. doi: 10.1002/neu.10217. [DOI] [PubMed] [Google Scholar]
- English AW, Wolf SL, Segal RL. Compartmentalization of muscles and their motor nuclei: the partitioning hypothesis. Phys Ther. 1993;73:857–867. doi: 10.1093/ptj/73.12.857. [DOI] [PubMed] [Google Scholar]
- Gordon DC, Richmond FJR. Topography in the phrenic motoneuron nucleus demonstrated by retrograde multiple-labelling techniques. J Comp Neurol. 1990;292:424–434. doi: 10.1002/cne.902920308. [DOI] [PubMed] [Google Scholar]
- Hammond CG, Gordon DC, Fisher JT, Richmond FJ. Motor unit territories supplied by primary branches of the phrenic nerve. J Appl Physiol. 1989;66:61–71. doi: 10.1152/jappl.1989.66.1.61. [DOI] [PubMed] [Google Scholar]
- Hannam AG, De Cou RE, Scott JD, Wood WW. The relationship between dental occlusion, muscle activity and associated jaw movement in man. Arch Oral Biol. 1977;22:25–32. doi: 10.1016/0003-9969(77)90135-2. [DOI] [PubMed] [Google Scholar]
- Henderson-Smart DJ, Johnson P, McClelland ME. Asynchronous respiratory activity of the diaphragm during spontaneous breathing in the lamb. J Physiol. 1982;327:377–391. doi: 10.1113/jphysiol.1982.sp014237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring SW, Grimm AF, Grimm BR. Functional heterogeneity in a multipinnate muscle. Am J Anat. 1979;154:563–576. doi: 10.1002/aja.1001540410. [DOI] [PubMed] [Google Scholar]
- Iliya AR, Dum RP. Somatotopic relations between the motor nucleus and its innervated muscle fibers in the cat tibialis anterior. Exp Neurol. 1984;86:272–292. doi: 10.1016/0014-4886(84)90186-9. [DOI] [PubMed] [Google Scholar]
- Jiao Q, Pruznak AM, Huber D, Vary TC, Lang CH. Castration Differentially Alters Basal and Leucine-Stimulated Tissue Protein Synthesis in Skeletal Muscle and Adipose Tissue. Am J Physiol Endocrinol Metab. 2009 doi: 10.1152/ajpendo.00473.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane JM, Richmond FJ. Compartmentalized organization of fibre types and receptors in the trapezius muscle in the cat. Soc. Neurosci. Abstr. 1981;7:687. [Google Scholar]
- Laskowski MB, High JA. Expression of nerve-muscle topography during development. J Neurosci. 1989;9:175–182. doi: 10.1523/JNEUROSCI.09-01-00175.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski MB, Sanes JR. Topographic mapping of motor pools onto skeletal muscles. J Neurosci. 1987;7:252–260. doi: 10.1523/JNEUROSCI.07-01-00252.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham NK, Bennett DA, Stretton CM, Anderson CS. Systematic review of progressive resistance strength training in older adults. J Gerontol A Biol Sci Med Sci. 2004;59:48–61. doi: 10.1093/gerona/59.1.m48. [DOI] [PubMed] [Google Scholar]
- Letbetter WD. Influence of intramuscular nerve branching on motor unit organization in medial gastrocnemius muscle. Anat Rec. 1974;178:402. [Google Scholar]
- Lewis MI, Horvitz GD, Clemmons DR, Fournier M. Role of IGF-I and IGF-binding proteins within diaphragm muscle in modulating the effects of nandrolone. Am J Physiol Endocrinol Metab. 2002;282:E483–E490. doi: 10.1152/ajpendo.00191.2001. [DOI] [PubMed] [Google Scholar]
- Lucas SM, Binder MD. Topographic factors in distribution of homonymous group Ia-afferent input to cat medial gastrocnemius motoneurons. J Neurophysiol. 1984;51:50–63. doi: 10.1152/jn.1984.51.1.50. [DOI] [PubMed] [Google Scholar]
- Marco Garcia NV, Jessell TM. Early motor neuron pool identity and muscle nerve trajectory defined by postmitotic restrictions in Nkx6.1 activity. Neuron. 2008;57:217–231. doi: 10.1016/j.neuron.2007.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell LC, Carlson DS, McNamara JA, Jr., Faulkner JA. Histochemical characteristics of the masseter and temporalis muscles of the rhesus monkey (Macaca mulatta). Anat Rec. 1979;193:389–402. doi: 10.1002/ar.1091930306. [DOI] [PubMed] [Google Scholar]
- Monges H, Salducci J, Naudy B. Dissociation between the electrical activity of the diaphragmatic dome and crura muscular fibers during esophageal distension, vomiting and eructation. An electromyographic study in the dog. J Physiol (Paris) 1978;74:541–554. [PubMed] [Google Scholar]
- Morris-Wiman J, Widmer CG. Myosin heavy chain (MyHC) isoform expression in early mouse masseter development. J Dent Res. 2003;82:1770. doi: 10.1177/002203450208100108. [Spec Iss A] [DOI] [PubMed] [Google Scholar]
- Morris-Wiman JA, Widmer CG. Early nerve-muscle interactions influence jaw muscle architecture. J Dent Res. 2001;80:130. [Spec Iss A] [Google Scholar]
- Nichols TR, Lawrence JH, III, Bonasera SJ. Control of torque direction by spinal pathways at the cat ankle joint. Exp Brain Res. 1993;97:366–371. doi: 10.1007/BF00228708. [DOI] [PubMed] [Google Scholar]
- Petrella JK, Kim JS, Cross JM, Kosek DJ, Bamman MM. Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Physiol Endocrinol Metab. 2006;291:E937–E946. doi: 10.1152/ajpendo.00190.2006. [DOI] [PubMed] [Google Scholar]
- Pickering M, Jones JF. The diaphragm: two physiological muscles in one. J Anat. 2002;201:305–312. doi: 10.1046/j.1469-7580.2002.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash YS, Fournier M, Sieck GC. Effects of prenatal undernutrition on developing rat diaphragm. J Appl Physiol. 1993;75:1044–1052. doi: 10.1152/jappl.1993.75.3.1044. [DOI] [PubMed] [Google Scholar]
- Prezant DJ, Karwa ML, Kim HH, Maggiore D, Chung V, Valentine DE. Short- and long-term effects of testosterone on diaphragm in castrated and normal male rats. J Appl Physiol. 1997;82:134–143. doi: 10.1152/jappl.1997.82.1.134. [DOI] [PubMed] [Google Scholar]
- Reader M, Schwartz G, English AW. Brief exposure to testosterone is sufficient to induce sex differences in the rabbit masseter muscle. Cells Tissues Organs. 2001;169:210–217. doi: 10.1159/000047884. [DOI] [PubMed] [Google Scholar]
- Redfern PA. Neuromuscular transmission in new-born rats. J Physiol. 1970;209:701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikard-Bell GC, Bystrzycka EK. Localization of phrenic motor nucleus in the cat and rabbit studied with horseradish peroxidase. Brain Res. 1980;194:479–483. doi: 10.1016/0006-8993(80)91227-5. [DOI] [PubMed] [Google Scholar]
- Saad M, Dubuc R, Widmer CG, Westberg KG, Lund JP. Anatomical organization of efferent neurons innervating various regions of the rabbit masseter muscle. J Comp Neurol. 1997;383:428–438. [PubMed] [Google Scholar]
- Schumacher GH. Funktionelle morphologie der kaumuskulatur. VEB Gustav Fischer; Jena: 1961. [Google Scholar]
- Segal RL. Neuromuscular compartments in the human biceps brachii muscle. Neurosci Lett. 1992;140:98–102. doi: 10.1016/0304-3940(92)90691-y. [DOI] [PubMed] [Google Scholar]
- Stuart DG, Hamm TM, Vanden Noven S. Partitioning of monosynaptic Ia EPSP connections with motoneurons according to neuromuscular topography: generality and functional implications. Prog Neurobiol. 1988;30:437–447. doi: 10.1016/0301-0082(88)90010-x. [DOI] [PubMed] [Google Scholar]
- Tan LK, Miller AD. Innervation of periesophageal region of cat's diaphragm: implication for studies of control of vomiting. Neurosci Lett. 1986;68:339–344. doi: 10.1016/0304-3940(86)90513-6. [DOI] [PubMed] [Google Scholar]
- Vanden Noven S, Hamm TM, Stuart DG. Partitioning of monosynaptic Ia excitatory postsynaptic potentials in the motor nucleus of the cat lateral gastrocnemius muscle. J Neurophysiol. 1986;55:569–586. doi: 10.1152/jn.1986.55.3.569. [DOI] [PubMed] [Google Scholar]
- Weeks OI, English AW. Compartmentalization of the cat lateral gastrocnemius motor nucleus. J Comp Neurol. 1985;235:255–267. doi: 10.1002/cne.902350208. [DOI] [PubMed] [Google Scholar]
- Weijs WA. Functional somatotopic organisation of motoneurons supplying the rabbit masseter muscle. J Comp Neurol. 1996;364:279–289. doi: 10.1002/(SICI)1096-9861(19960108)364:2<279::AID-CNE7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Weijs WA, Dantuma R. Functional anatomy of the masticatory apparatus in the rabbit. Neth J Zool. 1981;31:99–147. [Google Scholar]
- Widmer CG, Bui AT, Clark WJ. Human masseteric nerve branching and innervation patterns. J Dent Res. 1996;75:242. [Spec Iss A] [Google Scholar]
- Widmer CG, Carrasco DI, English AW. Differential activation of neuromuscular compartments in the rabbit masseter muscle during different oral behaviors. Exp Brain Res. 2003;150:297–307. doi: 10.1007/s00221-003-1464-y. [DOI] [PubMed] [Google Scholar]
- Widmer CG, Klugman D, English AW. Anatomical partitioning and nerve branching patterns in the adult rabbit masseter. Acta Anat (Basel) 1997;159:222–232. doi: 10.1159/000147988. [DOI] [PubMed] [Google Scholar]
- Widmer CG, Morris-Wiman JA, Nekula C. Spatial distribution of myosin heavy-chain isoforms in mouse masseter. J Dent Res. 2002;81:33–38. doi: 10.1177/002203450208100108. [DOI] [PubMed] [Google Scholar]
- Windhorst U, Hamm TM, Stuart DG. On the function of muscle and reflex partitioning. Behavioral and Brain Sciences. 1989;12:629–681. [Google Scholar]
- Young RL, Page AJ, Cooper NJ, Frisby CL, Blackshaw LA. Sensory and motor innervation of the crural diaphragm by the vagus nerves. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.08.053. [DOI] [PubMed] [Google Scholar]