Abstract
The enantioselective total synthesis of the rearranged spongian diterpene aplyviolene has been completed in 14 steps from the known hydroazulenone 8. The key junction of the hydrocarbon and oxygenated fragments to form the critical C8 quaternary carbon stereocenter and set the stage for elaborating the delicate bicyclic lactone functionality was accomplished in high yield and exquisite stereoselectivity by Michael addition of an enantioenriched hydroazulenone enolate to an enantiopure α-bromocyclopentenone.
Keywords: chemical synthesis, natural product
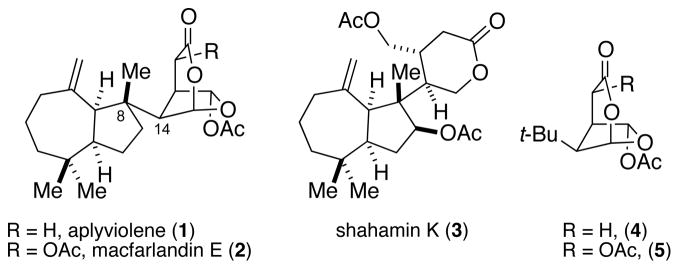
Rearranged spongian diterpenes are bioactive natural products isolated exclusively from sponges and nudibranchs.1 The most structurally complex contain adjoined polycyclic hydrocarbon and highly oxidized lactone fragments, of which the most intricate is the 6-acetoxy-2,7-dioxabicyclo[3.2.1]octan-3-one ring system found in aplyviolene (1),2 macfarlandin E (2),2a,3 and 13 related natural products (Figure 1).1 In 2001 we disclosed the first total synthesis of a rearranged spongian diterpene, shahamin K (3), which possesses a cis-hydroazulene and a relatively simple monocyclic lactone subunit.4 We recently demonstrated the first construction of the sensitive bicyclic lactone fragment of diterpenes 1 and 2 by the synthesis of simplified congeners 4 and 5.5 We report herein the enantioselective total synthesis of aplyviolene (1); the first total synthesis of a natural product containing a 6-acetoxy-2,7-dioxabicyclo[3.2.1]octan-3-one ring system.
Figure 1.
Rearranged Spongian Diterpenes and Substructures
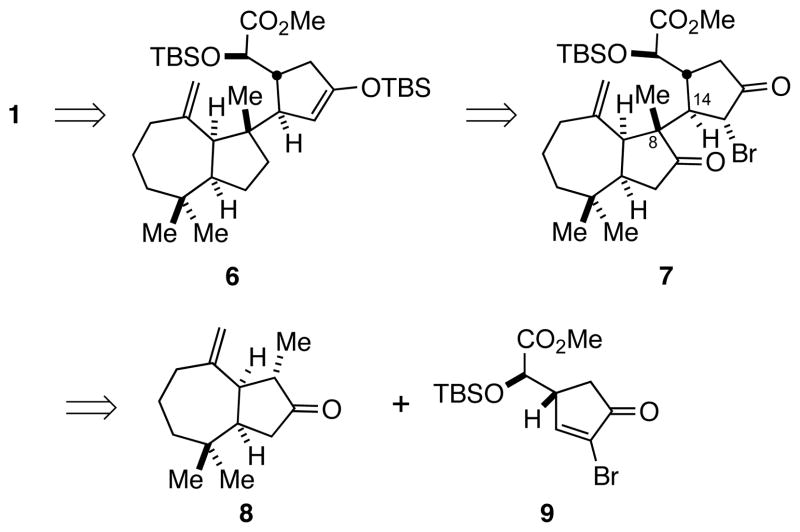
The substantial challenges associated with a total synthesis of aplyviolene (1) center on constructing the C8–C14 σ-bond that joins the quaternary carbon stereocenter of the cis-hydroazulene fragment with the one-carbon bridge of the 6-acetoxy-2,7-dioxabicyclo[3.2.1]octan-3-one moiety, and the inherent lability of the diacyloxy acetal functionality of the latter unit. We envisaged assembling 1 from tricyclic precursor 6 along the lines developed in our construction of analog 4 (Scheme 1). α-Bromoketone 7 was seen as a potential precursor of enoxysilane 6. Compound 7 is recognizably the Michael-addition product of the thermodynamic enolate of hydroazulenone 84 and α-bromocyclopentenone 9, with the proper relative configuration of the C8–C14 σ-bond expected to arise from the facial bias of the two enantioenriched coupling partners. Unknown at the onset was the compatibility of the exo-methylene functionality of the hydroazulene fragment with the oxidative and acidic steps that would be required to elaborate the bicyclic lactone subunit from precursor 6. We hoped that the sterically hindered environment of this exocyclic double bond would allow its incorporation early in the synthetic sequence.
Scheme 1.
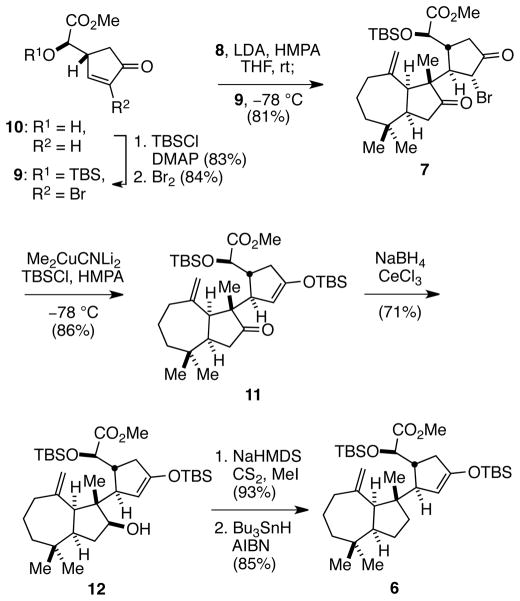
The sequence developed to prepare intermediate 6 is summarized in Scheme 2. Enantiomerically pure cyclopentenone 105 was first converted in two routine steps to α-bromocyclopentenone 9. In the critical fragment coupling event, the thermodynamic enolate of hydroazulenone 8 (95% ee) was generated with LDA in 2:1 THF/HMPA at room temperature over 4 h4 and coupled with cyclopentenone 9 at −78 °C to give a single crystalline product, 7, in 81% yield.6 Reductive silylation was best achieved by slow addition of 7 to a solution containing dilithium dimethyl(cyano)cuprate and TBSCl in 10:1 THF/HMPA at −78 °C, affording enoxysilane 11 in 86% yield.7,8 Reduction of ketone 11 with NaBH4 and CeCl3 at 0 °C in EtOH supplied secondary alcohol 12, which was converted to its xanthate ester in high yield. Exposure of this intermediate to Bu3SnH, AIBN in toluene for 5 min at 100 °C cleanly afforded the deoxygenated product 6 in 56% overall yield from ketone 11.9
Scheme 2.
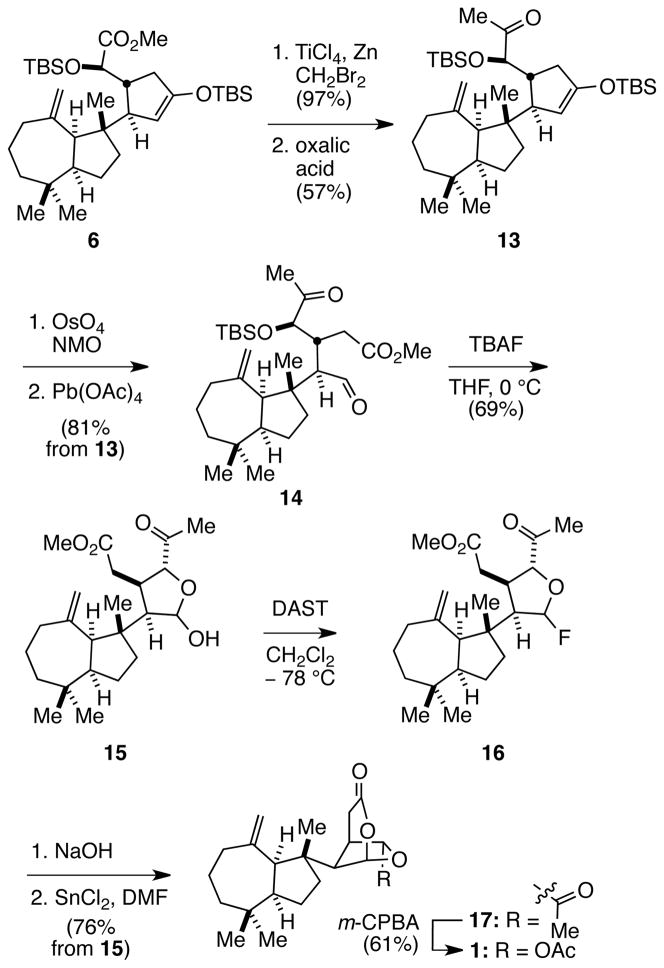
With tricyclic intermediate 6 in hand, its elaboration to aplyviolene (1) was initiated by transforming the ester of the α-siloxyacetic acid side chain to a methyl ketone (Scheme 3). This transformation was accomplished by the two-step sequence we had developed in our earlier model studies,5 delivering intermediate 13 in 55% yield. The enoxysilane double bond of 13 was selectively cleaved by oxidation at room temperature with catalytic OsO4 and NMO, followed by cleavage of the resulting crude α-hydroxycyclopentanone with Pb(OAc)4 to give tricarbonyl intermediate 14 in 81% yield. Cleavage of the silyl ether with TBAF in THF at 0 °C provided hemiacetal 15, which was transformed to anomeric fluoride 16 upon reaction with diethylaminosulfur trifluoride (DAST) at −78 °C in CH2Cl2. Saponification of the methyl ester with NaOH, followed by lactonization of the crude carboxylic acid product by exposure to 1.5 equiv of SnCl2 in DMF at room temperature gave rise to the dioxabicyclo[3.2.1]octan-3-one product 17 in good overall yield from intermediate 16.10 Initiating the lactonization from anomeric fluoride 16 permitted the use of non-acidic conditions, which were tolerant of the exo-methylene unit. The delicate α-acetoxy acetal functionality was then introduced by Baeyer–Villiger oxidation of 17 with m-CPBA at 0 °C in CH2Cl2 to afford aplyviolene (1) in 61% yield. The optical rotation of synthetic 1, −26.2 (c 0.1, CH2Cl2), compared well with the values reported for the natural sample, −29.2 (c 1.0, CH2Cl2)2a and −26.1,2b as did all spectroscopic data.
Scheme 3.
In summary, the total synthesis of the diterpene aplyviolene, 1, was completed in 14 linear steps and 5.6% overall yield from hydroazulenone 8, which is available in 11 steps from 3-methyl-2-cyclohexenone.4. This synthesis required orchestrating the stereochemical complexity of 7 contiguous stereocenters and the reactivity of the diacyloxy acetal and exo-methylene functionality, and sets the stage for future efforts in this area directed at more complex diterpenes containing the 6-acetoxy-2,7-dioxabicyclo[3.2.1]octan-3-one ring system.5
Supplementary Material
Acknowledgments
This research was supported by the NIH Neurological Disorders & Stroke Institute (Grant NS-12389), NIH National Institutes of General Medical Sciences (Grant GM-098601), and NIH postdoctoral fellowship support for M.J.S. (CA-138084). Professor Christopher M. Beaudry and Nicholas L. Untiedt are acknowledged for contributions to the early stages of the synthesis. We thank Professor Gerwick, Scripps Institute of Oceanography (SIO), and Dr. Hyukjae Choi, SIO, for purification and access to authentic aplyviolene. NMR and mass spectra were determined at UC Irvine using instruments purchased with the assistance of NSF and NIH shared instrumentation grants. We thank Dr. Joseph Ziller and Dr. John Greaves, Department of Chemistry, UC Irvine, for their assistance with X-ray and mass spectrometric analyses.
Footnotes
Supporting Information. Experimental details, characterization data and copies of 1H and 13C NMR spectra of new compounds, and CIF file for 7. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Keyzers RA, Northcote PT, Davies-Coleman MT. Nat Prod Rep. 2006;23:321. doi: 10.1039/b503531g. [DOI] [PubMed] [Google Scholar]; (b) Gonzalez M. Cur Bioact Comp. 2007;3:1. [Google Scholar]
- 2.(a) Hambley TW, Poiner A, Taylor WC. Tetrahedron Lett. 1986;27:3281. [Google Scholar]; (b) Buckleton JS, Bergquist PR, Cambie RC, Clark GR, Karuso P, Rickard CEF. Acta Cryst. 1986;C42:1846. [Google Scholar]
- 3.Molinski TF, Faulkner DJ, He CH, Van Duyne GD, Clardy J. J Org Chem. 1986;51:4564. [Google Scholar]
- 4.Lebsack AD, Overman LE, Valentekovich RJ. J Am Chem Soc. 2001;123:4851. doi: 10.1021/ja015802o. [DOI] [PubMed] [Google Scholar]
- 5.(a) Schnermann MJ, Beaudry CM, Egovora AV, Polishchuk RS, Sütterlin C, Overman LE. Proc Nat Acad Sci USA. 2010;107:6158. doi: 10.1073/pnas.1001421107. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Schnermann MJ, Beaudry CM, Genung NE, Canham SM, Untiedt NL, Karanikolas BDW, Sütterlin C, Overman LE. J Am Chem Soc. 2011;133 doi: 10.1021/ja207727h. accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The relative configuration of 7 was secured by X-ray crystallographic analysis: CCDC 840473.
- 7.The use of dialkylcuprates to transform a α-bromoenone to an enoxy silane has not been reported. For reductive enolsilylation of an α-acetoxy enone, see: Paquette LA, Ross RJ, Shi Y. J Org Chem. 1990;55:1589.For the related formation of an enol triflate, see: Tius MA, Kannangara GSK. Tetrahedron. 1992;48:9173.
- 8.The use of HMPA was required, as the reductive loss of bromide was the predominant process observed in its absence.
- 9.Barton DHR, McCombie SW. J Chem Soc, Perkin Trans. 1975;1:1574. [Google Scholar]
- 10.Mukaiyama T, Murai Y, Shoda S. Chemistry Letters. 1981;10:431. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.