Abstract
Twenty-two lycorine-related compounds were investigated for in vitro anti-tumor activity using four cancer cell lines displaying different levels of resistance to pro-apoptotic stimuli and two cancer cell lines sensitive to pro-apoptotic stimuli. Lycorine and six of its congeners exhibited potency in the single-digit micromolar range, while no compound appeared more active than lycorine. Lycorine also displayed the highest potential (in vitro) therapeutic ratio, being at least 15 times more active against cancer than normal cells. Our studies also showed that lycorine exerts its in vitro anti-tumor activity through cytostatic rather than cytotoxic effects. Furthermore, lycorine provided significant therapeutic benefit in mice bearing brain grafts of the B16F10 melanoma model at non-toxic doses. Thus, the results of the current study make lycorine an excellent lead for the generation of compounds able to combat cancers, which are naturally resistant to pro-apoptotic stimuli, such as glioblastoma, melanoma, non-small-cell-lung cancers, metastatic cancers, among others.
Introduction
Lycorine (1) is a pyrrolo[de]phenanthridine ring-type alkaloid (Fig. 1) extracted from different Amaryllidaceae genera, whose structure was firstly determined by Nagakawa et al. in 1956.1 Various biological properties of lycorine, including the inhibition of ascorbic acid (AAa) biosynthesis,2-5 inhibition of growth and cell division in higher plants, algae and yeasts,6 and prevention of cyanide-insensitive respiration,7 have made this substance a valuable tool for studying a number of important physiological processes.8 Useful structure-activity relationship (SAR) data were obtained in a study evaluating the effect of lycorine, its synthetic derivatives, and naturally occurring alkaloids structurally related to 1, on AA biosynthesis in potato tubers.4,5 Specifically, the structural features required for the inhibition of AA biosynthesis are intact A, B and C rings, β-configuration of the D ring when the C/D ring junction changes, and the presence of a “nucleophilic site” at positions C-1 and C-2 of the C-ring.4,5 Furthermore, lycorine,9-11 along with a number of other Amaryllidaceae small molecule constituents, such as pancratistatin12,13 and narciclasine,14-16 have been investigated for their potent anti-tumor effects, both in vitro and in vivo, in various pre-clinical models of human cancers.17-20 Importantly, it has been reported that lycorine,11 pancratistatin12 and narciclasine14,15 display significantly higher anti-proliferative activity in cancer than in normal cells and that these natural products can kill cancer cells by induction of the intrinsic apoptosis pathway.9-16 The inspection of the literature data also reveals that these pro-apoptotic effects are observed at pharmacological doses, i.e. at doses which range between 5- and 20-fold the IC50 in vitro growth inhibitory values determined for each of these compounds.9-16 Our working hypothesis relates to the fact that apoptosis induction by lycorine and its analogues is not the principal mechanism of action by which these compounds exert their anti-tumor effects. The present study therefore aims i) to characterize the anticancer activity of lycorine in terms of cytostatic versus cytotoxic (including potential pro-apoptotic) effects at its IC50 in vitro growth inhibitory concentrations in carcinoma, melanoma and glioma cells, and ii) to investigate the anti-tumor activity of a number of lycorine derivatives and related Amaryllidaceae alkaloids for comparison with lycorine itself (Figure 1). In order to validate our working hypothesis, we made use of six cancer cell lines, four of which are known to be resistant to various pro-apoptotic stimuli, while the remaining two are sensitive. The four cancer cell lines that display resistance to pro-apoptotic stimuli include human U373 glioblastoma (GBM, from astroglial origin21),15,22 human A549 non-small-cell-lung cancer (NSCLC),15,23 human OE21 esophageal cancer24 and human SKMEL-28 melanoma25 models. The two cancer cell lines that are sensitive to pro-apoptotic stimuli include human Hs683 anaplastic oligodendroglioma15,22 and mouse B16F10 melanoma25,26 models. Lycorine derivatives that displayed the most marked anti-tumor activities were also evaluated for their in vitro growth inhibitory effects in normal fibroblasts. We utilized the MTT colorimetric assay14-16,25 in order to determine the IC50 in vitro growth inhibitory activity of each compound under study. Lycorine-induced changes in cell proliferation and cell migration were monitored by means of computer-assisted phase-contrast microscopy,15,16 whereas modifications in actin cytoskeleton organization were observed with computer-assisted fluorescence microscopy.15,16 Apoptosis levels were quantified by means of flow cytometry.15,23 Finally, we evaluated lycorine's in vivo toxicity in healthy mice and its in vivo anti-tumor activity in the B16F10 mouse melanoma model.25,26
Figure 1.
Chemical structures of compounds under study.
Results
Previously, we developed a new purification method to extract lycorine in large amounts from bulbs of Sternbergia lutea Ker Gawl.27 We also reported a procedure for lycorine analysis in crude extracts by HPLC28 and an NMR study aimed at the assignment of all the chemical shifts to all protons and carbons in 1.29 Following these previous efforts, a significant quantity of lycorine was obtained and used for the preparation of its derivatives 2-12, 14-17 and 20 according to the previously reported procedures.4,5,27,30 In addition, lycorine derivative 13 as well as the minor Amaryllidaceae alkaloids pseudolycorine (18), norpluvine (19), ungeremine (21) and amarbellisine (22) were obtained as described in the Experimental Section.
Determination of the IC50 In Vitro Growth Inhibitory Activities of Lycorine and its Analogues: Structure-Activity Relationship Analysis
In this first set of experiments (Table 1), we analyzed the IC50 in vitro growth inhibitory activities of the twenty-two compounds under study at concentrations of up to 10 μM (testing at higher concentrations was not feasible due to insufficient quantity of material available for some of the analogues). The data indicate that lycorine exhibits a marked growth inhibitory activity toward all the cancer cell lines tested regardless of whether the cancer cells are resistant (A549, U373, OE21 and SKMEL-28 models) or sensitive (Hs683 and B16F10 models) to pro-apoptotic stimuli. However, the activity was significantly affected by structural modifications within the phenanthridine skeleton as revealed by the IC50 values of lycorine analogues. Thus, the presence of the unaltered diol functionality in the C-ring in its original configuration, stereochemistry of the C/D ring junction and conformational freedom of the C-ring appear to be critical for anticancer activity. In fact, while activity of the hydrochloride salt of lycorine (15) was unaltered compared with the parent neutral alkaloid, analogues incorporating changes of the hydroxyl groups at C-1 and C-2 of the C-ring, as well as their stereochemistry, lacked anti-tumor activity at concentrations of up to 10 μM. This was observed for 2-epi-lycorine (4), lycorine-2-one (7), lycorene (16) and caranine (17). In contrast, good activity of lycorine chlorohydrin (6) and 1,2-epoxylycorine (14) should likely be attributed to the intracellular conversion of these derivatives to 1 by nucleophilic substitution with water. The lack of activity of 1-O- (2), 2-O- (5) and 1,2-diacetyllycorine (3) is likely due to significant steric bulk introduced into the C-ring and inability of cancer cells to completely hydrolyse the ester groups and convert these derivatives to lycorine within the treatment time period. Further inspection of the SAR data reveals that stereochemistry and conformational freedom of the C-ring are additional important features for the antitumor activity. Thus, α- and β-dihydroderivatives (8 and 10), obtained by catalytic hydrogenation at C(3)-C(3a), proved to be inactive. The similar result was obtained with their 1,2-O,O′-diacetyl derivatives (9 and 11). The same modification may serve as an explanation for the absence of anti-tumor activity of α–dihydrolycorine lactam (12). This analogue additionally incorporates a modification of the B-ring resulting in non-basic character of the nitrogen atom, providing another point of difference from lycorine. Further, quaternization of the nitrogen atom of the B-ring, as found in N-methyllycorine iodide (13), also resulted in a loss of activity. Interestingly, ring-C aromatized lycorine analogues ungeremine (21) and anhydrolycorine (20) are active against some of the cancer cell lines, but not all. It is noteworthy, however, that the responsive cells include human U373 GBM model, which is resistant to pro-apoptotic stimuli.15,22 Pseudolycorine (18) and amarbellisine (22), two alkaloids closely related to lycorine, exhibit growth inhibitory potencies that are very similar to those of lycorine in all the cancer cell lines studied. These results show that the transposition of the double bond from C(3)-C(3a) to C(2)-C(3) and the presence of the methoxy group at C-2, as found in amarbellisine (22), do not constitute structural changes that abolish anti-tumor activity. Similarly, good activity of pseudolycorine (18), which incorporates an open dioxole ring A, suggests that this structural feature is not essential for the anti-tumor activity. Indeed, the lack of anticancer activity of norpluvine (19), which also contains an open A-ring, should be due to the absence of the hydroxyl group at C-2, as is found in caranine (17). Finally, the analysis of the data in Table 1 would be incomplete without an important observation that all active analogues display similar anti-tumor activites in resistant as well as cancer cells sensitive to proapoptotic stimuli.
Table 1.
Lycorine, lycorine analogues, and their in vitro growth inhibitory activity in cancer cell lines.
| Lycorine Analogue | # | Cell Lines : In Vitro IC50 Growth Inhibitory Values (μM)a | |||||
|---|---|---|---|---|---|---|---|
| A549 | OE21 | Hs683 | U373 | SKMEL | B16F10 | ||
| Lycorine | 1 | 4.3 ± 0.3 | 5.1 ± 0.4 | 6.7 ± 0.3 | 7.6 ± 0.2 | 8.5 ± 0.3 | 6.3 ± 0.4 |
| 1-O-Acetyllycorine | 2 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 |
| 1,2-O,O′-Diacetyllycorine | 3 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 |
| 2-epi-Lycorine | 4 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 |
| 2-O-Acetyllycorine | 5 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 |
| Lycorine chlorohydrin | 6 | 3.8 ± 0.2 | 9.6 ± 0.7 | 3.1 ± 0.3 | 2.3 ± 0.1 | > 10 | 6.9 ± 0.5 |
| Lycorine-2-one | 7 | 9.9 ± 0.5 | > 10 | > 10 | > 10 | > 10 | > 10 |
| α–Dihydrolycorine | 8 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 |
| 1,2-Diacetyl-α-dihydrolycorine | 9 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 |
| β–Dihydrolycorine | 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 |
| 1,2-O,O′-Diacetyl-β-dihydrolycorine | 11 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 |
| α–Dihydrolycorine lactam | 12 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 |
| Lycorine N-methyl iodide | 13 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 |
| 1,2-epoxylycorine | 14 | 3.4 ± 0.1 | 8.5 ± 0.5 | 3.3 ± 0.2 | 2.4 ± 0.1 | 9.5 ± 0.4 | 4.6 ± 0.2 |
| Lycorine hydrochloride | 15 | 4.3 ± 0.2 | 4.6 ± 0.1 | 6.5 ± 0.2 | 8.6 ± 0.3 | 8.3 ± 0.3 | 5.5 ± 0.2 |
| Lycorene | 16 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 |
| Caranine | 17 | > 10b | > 10 | > 10 | > 10 | > 10 | > 10 |
| Pseudolycorine | 18 | 7.5 ± 0.4 | 7.7 ± 0.3 | 7.9 ± 0.2 | 7.8 ± 0.3 | > 10 | 7.5 ± 0.3 |
| Norpluvine | 19 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 |
| Anhydrolycorine | 20 | 4.5 ± 0.1 | 8.8 ± 0.2 | 7.1 ± 0.3 | 5.1 ± 0.1 | > 10 | > 10 |
| Ungeremine | 21 | > 10 | > 10 | > 10 | 8.3 ± 0.2 | > 10 | > 10 |
| Amarbellisine | 22 | 7.2 ± 0.3 | 6.7 ± 0.2 | 8.3 ± 0.3 | 7.3 ± 0.2 | 8.3 ± 0.2 | 6.7 ± 0.3 |
U373 (ECACC code 89081403) cell line was cultured in MEM medium supplemented with 5% heat inactivated fetal bovine serum; Hs683 (ATCC code HTB-138), SKMEL-28 (ATCC code HTB-72), A549 (DSMZ code ACC107) and B16F10 (ATCC code CRL-6475) cell lines were cultured in RPMI medium supplemented with 10% heat inactivated fetal bovine serum; MEM and RPMI cell culture media were supplemented with 4mM glutamine, 100μg/mL gentamicin and penicillin-streptomycin (200U/mL and 200μg/mL).
The mean IC50 value could not be determined as one or more of the corresponding data points were higher than the threshold value.
Lycorine Displays Higher In Vitro Anti-Proliferative Effects in Cancer than in Normal Cells
Liu et al.11 demonstrated that lycorine displays higher in vitro growth inhibitory activity in human leukemic cells than in normal white blood cells. Leukemic cells normally grow in suspension, while solid cancer cells are usually adherent. There are, however, significant differences in biological properties of cells growing in suspension compared to adherent ones, especially with respect to their sensitivity to apoptosis. In the second set of experiments (Table 2), we analyzed the IC50 in vitro growth inhibitory activity (using the MTT colorimetric assay) of eight of the most active compounds (as detailed in Table 1) at concentrations of up to 100 μM in six cancer and three normal cell lines. The results illustrated in Table 2 attest to a high potential (in vitro) therapeutic ratio for lycorine because its in vitro anti-growth activity is 15 times higher in cancer than in normal cells. Amarbellisine (22) displays similar features. In contrast, anhydrolycorine (20), which exhibits anti-tumor activity similar to that of lycorine, displays a weaker potential therapeutic ratio than lycorine (Table 2). Thus, the analysis of the combined data from Tables 1 and 2 leads us to conclude that lycorine displays the highest anti-tumor activity and the highest potential (in vitro) therapeutic ratio in comparison to the other investigated compounds. We thus decided to pursue our investigation with lycorine as a potential anti-cancer lead.
Table 2.
Lycorine, lycorine analogues, and their in vitro growth inhibitory activity in cancer versus normal cell lines.
| Lycorine Analogue | # | Therapeutic ratio | IC50In Vitro Growth Inhibitory Values (μM)a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer Cell Lines | Normal Cell Lines | ||||||||||||
| A549 | OE21 | Hs683 | U373 | SKMEL | B16F10 | Mean ± SEM | Wi38 | WS1 | NHDF | Mean ± SEM | |||
| Lycorine | 1 | > 15 | 4.3 ± 0.3 | 5.1 ± 0.4 | 6.7 ± 0.3 | 7.6 ± 0.2 | 8.5 ± 0.3 | 6.3 ± 0.4 | 6.4 ± 0.6 | > 100 | > 100 | > 100 | > 100 |
| 2-O-Acetyllycorine | 5 | > 2 | 47.6 ± 2.3 | 77.4 ± 3.1 | 43.0 ± 2.0 | 63.1 ± 4.1 | 87.8 ± 3.9 | 37.1 ± 2.8 | 59.3 ± 8.2 | 88.5 | > 100 | > 100 | > 96.2 ± 2.7 |
| Lycorine chlorohydrin | 6 | > 11 | 3.8 ± 0.2 | 9.6 ± 0.7 | 3.1 ± 0.3 | 2.3 ± 0.1 | 14.8 ± 1.1 | 6.9 ± 0.5 | 6.8 ± 2.0 | 46.0 | > 100 | 72.1 | > 72.7 ± 11.0 |
| 1,2-epoxy-lycorine | 14 | 5 | 3.4 ± 0.1 | 8.5 ± 0.5 | 3.3 ± 0.2 | 2.4 ± 0.1 | 9.5 ± 0.4 | 4.6 ± 0.2 | 5.3 ± 1.2 | 28.6 | 36.8 | 9.3 | 24.9 ± 8.2 |
| Lycorine hydrochloride | 15 | > 15 | 4.3 ± 0.2 | 4.6 ± 0.1 | 6.5 ± 0.2 | 8.6 ± 0.3 | 8.3 ± 0.3 | 5.5 ± 0.2 | 6.3 ± 0.8 | > 100 | > 100 | > 100 | > 100 |
| Pseudolycorine | 18 | 7 | 7.5 ± 0.4 | 7.7 ± 0.3 | 7.9 ± 0.2 | 7.8 ± 0.3 | 15.5 ± 1.1 | 7.5 ± 0.3 | 9.0 ± 1.3 | 44.0 | 79.1 | 66.9 | 63.3 ± 7.3 |
| Anhydrolycorine | 20 | 2 | 4.5 ± 0.1 | 8.8 ± 0.2 | 7.1 ± 0.3 | 5.1 ± 0.1 | 20.9 ± 1.2 | 23.3 ± 1.2 | 11.6 ± 3.4 | 30.1 | 23.7 | 26.6 | 26.8 ± 1.3 |
| Amarbellisine | 22 | > 13 | 7.2 ± 0.3 | 6.7 ± 0.2 | 8.3 ± 0.3 | 7.3 ± 0.2 | 8.3 ± 0.2 | 6.7 ± 0.3 | 7.4 ± 0.3 | > 100 | 97.6 | > 100 | > 99.2 ± 0.6 |
Cancer cell lines were cultured as specified in Table 1. WI-38 fibroblasts (ATCC code CCL-75; see Fig. 2) were cultured in MEM medium supplemented with 10% heat inactivated fetal bovine serum and 100μM non-essential amino acids; WS-1 (ECACC code 88021104) fibroblasts were cultured in MEM medium supplemented with 10% heat inactivated fetal bovine serum; NADH (PromoCell code c-12300) were cultured in MEM medium supplemented with 10% heat inactivated fetal bovine serum and 100 μM non-essential amino acids.
Lycorine is a Cytostatic Compound in U373 GBM and A549 NSCLC Cancer Cells that Are Resistant to Various Proapoptotic Stimuli
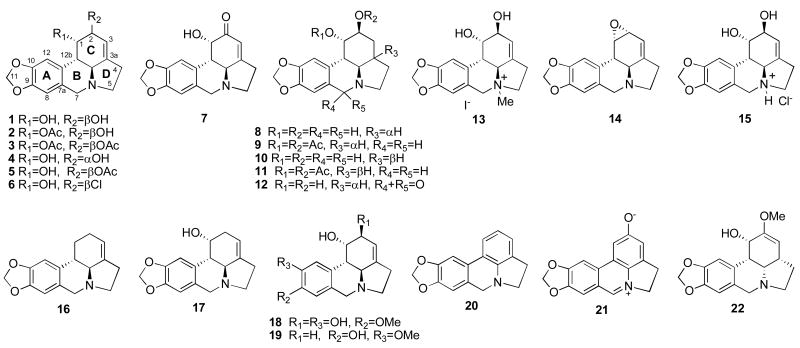
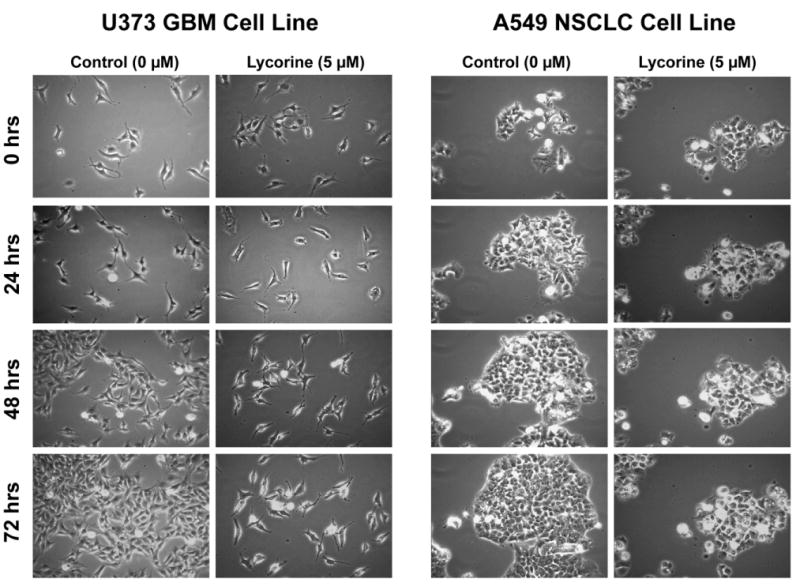
We made use of computer-assisted phase-contrast microscopy (quantitative videomicroscopy) to analyze the principal mechanism of action associated with lycorine's in vitro growth inhibitory effects as first revealed by the MTT colorimetric assay (Tables 1 and 2). Figure 2 shows that lycorine inhibits cancer cell proliferation without inducing cell death when assayed at its IC50 in vitro growth inhibitory value (ca. 5 μM, Table 1) in U373 GBM15,22,31 and A549 NSCLC15,23 cells that are resistant to various proapoptotic stimuli. Based on the phase contrast pictures obtained by means of quantitative videomicroscopy (Fig. 2), for each cell line and each set of experimental conditions we calculated the global growth rate (GGR), which corresponds to the ratio of the mean number of cells present in the last image captured in the experiment (conducted at 72 h) to the number of cells present in the first image (at 0 h). We divided this ratio obtained in the lycorine-treated experiment by the ratio obtained in the control (Fig. 3A). A GGR value of 0.2 in Fig. 3A means that 20% of cells grew in the lycorine-treated set of conditions as compared to the control experiment over a 72-hr observation period. Fig. 3A, based on GGR calculations, thus confirms the MTT colorimetric assay-related data detailed in Tables 1 and 2, e.g. 5 μM lycorine displays marked in vitro growth inhibitory activity in both U373 GBM and A549 NSCLC cells, which display resistance to various proapoptotic stimuli. The quantitative determination of mitosis occurrence (using software that was developed in our laboratory32) made it possible to demonstrate the cytostatic effects associated with lycorine's anti-tumor properties (Fig. 3B). Indeed, lycorine decreased by ≥ 90% the mitosis occurrence in U373 GBM as well as in A549 NSCLC cells at 5 μM for 72 hrs (Fig. 3B). The white arrow in Fig. 3Ca points to an individual A549 NSCLC cell belonging to an A549 NSCLC colony treated with lycorine at 5 μM and analyzed for 72 hrs. The use of computer-assisted phase-contrast microscopy31,33 made it possible to analyze the behaviour of this cell at high magnifications over the 72-hr period of treatment with 5 μM lycorine as illustrated in Fig. 3Cb. The NSCLC cell (Fig. 3Cb; 0 hrs) entered mitosis 7 hrs after 5 μM lycorine was added to the culture medium and this cell almost succeeded in accomplishing its mitosis at the 21st hr post-lycorine addition into the culture medium (Fig. 3Cb). However, 3 hrs later this cell failed in completing its mitosis and the two daughter cells merged at the 24th hr (Fig. 3Cb; 24 hrs). At the 41st hr post-lycorine addition, this cell once more tried to enter mitosis and once more almost succeeded in completing its second mitotic attempt (see Fig. 3Cb; 43 and 48 hrs), but lastly failed to achieve it (Fig. 3Cb; 72 hrs). This feature is typical of what we observed on almost all U373 GBM and A549 NSCLC cells we analyzed and it strongly suggests that the lycorine-induced cytostatic effects on cancer cells mainly occur during cytokinesis. Lycorine-induced impairment of cytokinesis in cancer cells could occur, at least partly, through a lycorine-mediated increase in rigidity of actin cytoskeleton as revealed by fluorescence microscopy analyses (Fig. 3D). Indeed, 5 μM lycorine markedly increased the levels of polymerized actin in U373 GBM cells (green fluorescence in Fig. 3Db) when compared to control (Fig. 3Da). Similar features were observed with respect to A549 NSCLC cells (data not shown). These lycorine-induced cytostatic effects, occurring partly through an increase in rigidity of actin cytoskeleton, closely resemble those of narciclasine, as we recently reported.16
Figure 2.
Morphological illustrations of U373 GBM and A549 NSCLC cell populations left untreated (control) or treated for 72 hrs with 5 μM lycorine. The illustrations reveal cytostatic effects for lycorine, rather than cytotoxic ones. The 5 μM concentration used here approximately corresponds to the IC50 in vitro growth inhibitory value obtained in the MTT colorimetric assay (see Table 1).
Figure 3.
A: In each (control or treated) set of conditions, the cell growth levels after 72 hrs of culture were evaluated by the ratio of the numbers of cells counted in the last and first frames of the image sequence. The global growth ratio (GGR) for a given set of experimental conditions was defined by the ratio of the two growth levels obtained in the control and the treated conditions (control / treated). All the cell counts were performed in triplicate using an interactive computer tool. A GGR value of 0.6 in Fig. 3A means that 60% of cells grew in the treated as compared to the control set of conditions. B: the number of mitoses occurring in a 2 mm2 frame during 72 hrs of observation was quantitatively determined with computer-assisted phase-contrast microscopy (quantitative videomicroscopy) utilizing software packages developed in our laboratory.32 Ca: a A549 NSCLC colony was treated with 5 μM lycorine and then observed by means of quantitative videomicroscopy with the focus on the single cell indicated with the white arrow. Cb: behavior of the single cell indicated with a white arrow in Ca was monitored for 72 hrs. D: immunofluorescence analyses were performed to reveal in green fluorescence the fibrilar (polymerized) actin and in red fluorescence the globular (non polymerized) actin in U373 GBM cells (Da: control; Db: 5 μM lycorine for 6 hrs).
Lycorine Impairs In Vitro U373 GBM and A549 NSCLC Migration
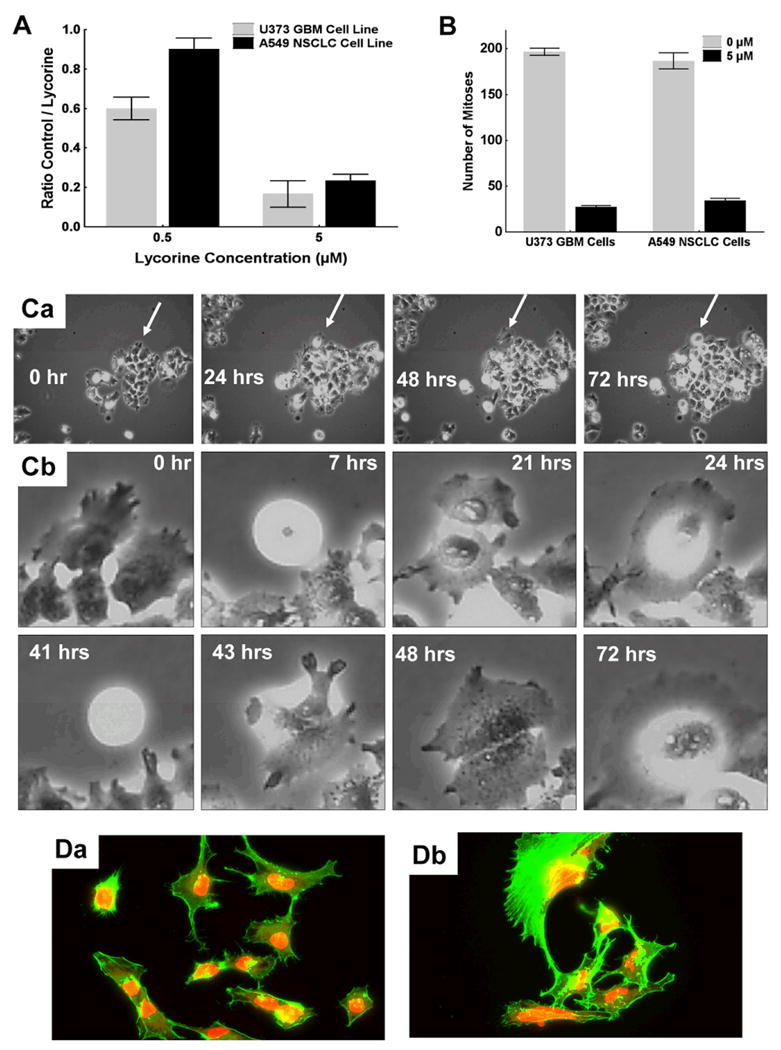
A common property that can impair both cell proliferation and migration, with no evidence of cell death, is associated with disorganization of actin cytoskeleton.15,16,34-36 A lycorine-induced increase in rigidity of actin cytoskeleton through the elevated polymerized actin, prompted us to investigate whether lycorine could also impair cancer cell migration in addition to its cytostatic effects. We utilized computer-assisted phase-contrast microscopy (quantitative videomicroscopy) to calculate the Maximum Relative Distance to the Origin (the MRDO variable in Fig. 4A) in A549 NSCLC (Fig. 4Aa) and U373 GBM (Fig. 4Ab) cells left untreated (control; “0 μM” in Fig. 4A) or treated with 0.5 or 5 μM lycorine. The MRDO variable represents the greatest linear distance travelled by each single cell from the moment it was caught by the quantitative videomicroscopy system through the end of the experiment, as detailed elsewhere.16,21,33 The data illustrated in Fig. 4Aa (A549 NSCLC cells) and 4Ab (U373 GBM cells) show that 5 μM lycorine significantly impaired migration processes in cancer cell populations that display resistance to various proapoptotic stimuli.
Figure 4.
A: Characterization of the effect of 0.5 and 5 μM lycorine (0 μM = control) on migration levels of A549 NSCLC and U373 GBM cells. Migration levels were determined by computing the MRDO variable (by means of quantitative videomicroscopy), which represents the Maximal Relative Distance to the Origin (the largest linear distance) travelled by each single cell during 12 (the gray bars) or 24 (the black bars) hrs of observation. Data are presented as means ± SEMs. Morphological illustrations of MRDO variable computation are provided in reference #50. B: morphological illustrations of A549 NSCLC cell colonies developing on soft agar for 38 days after cell plating procedure without (control; Ba) or with 5 μM lycorine (Bb). The number of colonies was determined in three 0.4-mm2 areas for each set of experimental conditions, which was repeated as hexaplicate. Data are presented as means ± SEMs.
Lycorine Reduces Colony Formation of Undifferentiated A549 NSCLC Cells Growing Under Anchorage-Independent Culture Conditions
Undifferentiated cancer cells, including cancer stem cells, are able to develop as colonies when cultured under anchorage-independent culture conditions (as on soft agar), while differentiated cancer cells necessitate anchorage-dependent culture conditions (as on solid supports when using the MTT colorimetric assay).37 Figs. 4Ba and 4Bb illustrate A549 NSCLC colony formation after 38 days when the culture is under control and 5 μM lycorine-treated set of conditions, respectively. Although undifferentiated cancer cells are biologically more aggressive than differentiated ones and more prone to metastasize (as evidenced in any pathology grading system of human cancers), the data illustrated in Fig. 4Bc reveal that lycorine significantly impairs colony formation in the A549 NSCLC model when assayed between 0.5 (p < 0.05) and 5 (p < 0.01) μM. We obtained similar data with respect to the U373 GBM model (data not shown).
Lycorine Does Not Induce Apoptosis in U373 GBM Cells that Display Resistance to Various Proapoptotic Stimuli
Flow cytometry analyses based on double annexin V (AV) / propidium iodide (PI) staining revealed that narciclasine (positive control (“+Control” in Fig. 5A) used in the present study) induced significant pro-apoptotic effects in the MCF-7 breast cancer cell line (Fig. 5A), as was shown previously.14 Early apoptosis relates to AV+/PI- cells, while late apoptosis relates to AV+/PI+ cells.14,15 Lycorine did not induce pro-apoptotic effects in U373 GBM cell line at 5 μM or even at 50 μM (Fig. 5A). Rather, at 50 μM, lycorine induced non-apoptotic cell death (AV-/PI+ cells) in U373 GBM cells (Fig. 5A). This process is unrelated to necrotic cell death because it is known that when dying cells still stain heavily with PI, necrotic processes are unlikely to occur.23 Quantitative videomicroscopy, which allows the monitoring of morphological changes induced by lycorine, revealed that U373 (Fig. 5B) and A549 (data not shown) cell death occurred when actin cytoskeleton collapsed as illustrated for U373 GBM cells in Fig. 5B.
Figure 5.
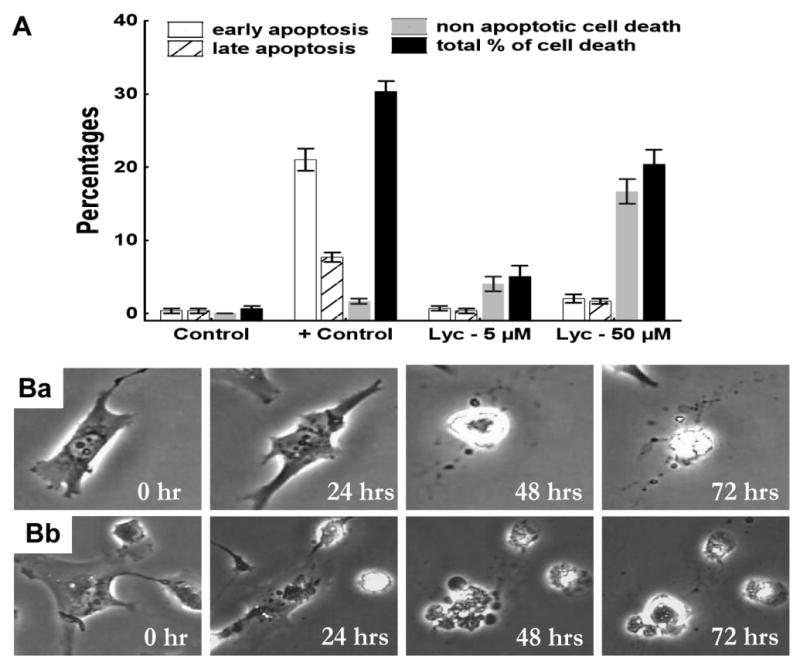
A: determination of apoptotic and non-apoptotic cell death levels in U373 GBM cells after 72 hrs in presence of 5 (Lyc − 5 μM) and 50 (Lyc − 50 μM) μM lycorine as compared to control (absence of lycorine). Human MCF-7 breast cancer cells treated with 1 μM narciclasine for 72 hrs used as positive control (+ Control). U373 cells were stained with propidium iodide (PI) and Annexin-V (AV). Early apoptosis corresponds to AV+/PI- U373 cells, while late apoptosis corresponds to AV+/PI+ U373 cells. Non-apoptotic cell death corresponds to AV-/PI+ cells. Data are presented as means ± SEMs. Ba and Bb: the behavior of two individual U373 GBM cells cultured in presence of 50 μM lycorine was monitored for 72 hrs.
Lycorine-Induced Toxicity in Mice: Determination of the Chronic Maximal Tolerated Dose
Lycorine was administered i.v. at 5, 10, 20, 40 and 80 mg/kg, chronically, i.e. once a day during 5 consecutive days, to healthy mice whose body weights were being recorded over 28 days. The data obtained revealed no gross toxicity displayed by lycorine at these doses (Fig. 6A). The MTD for lycorine under i.v. chronic administrations is therefore higher than 80 mg/kg.
Figure 6.
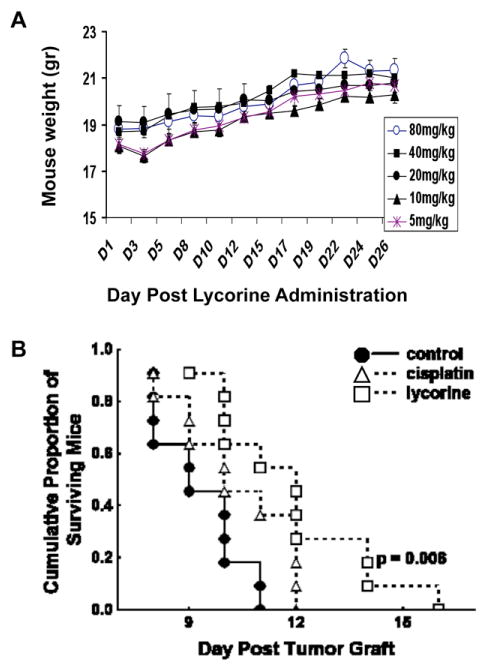
A: body weight measurements of mice having received i.v. administrations (tail vein; three mice per group) of 5, 10, 20, 40 and 80 mg/kg lycorine during 5 consecutive days. Data are presented as means ± SEMs. No single mouse died over a 28-day period of observation and the mice did not loose weights, emphasizing absence of lycorine-induced toxicity. B: Survival analyses of mice orthotopically grafted into their brains with the mouse melanoma B16F10 which have been left untreated (control; the black dots) or treated 3 times a week (Monday, Wednesday, Friday) during 3 consecutive weeks (treatment starting the 5th day post-tumor graft) with 5 mg/kg (the maximal tolerated dose) cisplatin (intraperitoneal administrations) or 40 mg/kg lycorine (i.v. administrations into the tail vein). There were 11 mice per experimental group.
Demonstration of In Vivo Anti-Tumor Activity of Lycorine in the Mouse B16F10 Melanoma Model
We determined the in vivo anti-tumor activity of lycorine in the B16F10 mouse melanoma model,26 orthotopically grafted into the brains of mice in order to mimic melanoma brain metastases, a feature that occurs in a large proportion of melanoma patients38 and contributes to an exceedingly poor prognosis.39 The data we obtained when assaying lycorine i.v. at 40 mg/kg three times a week (Monday, Wednesday, Friday) during three consecutive weeks (with treatment starting at the 5th day post-tumor grafting) revealed that lycorine significantly (p = 0.006) increased the survival of B16F10 melanoma-bearing mice, while cisplatin (chosen as a reference compound for melanoma treatment40,41) failed provide significant therapeutic benefit (Fig. 6B). The active dose for lycorine in terms of in vivo anti-tumor activity identified in the current experiment, i.e. 40 mg/kg, is thus significantly lower than its maximal tolerated dose (> 80 mg/kg; Fig. 6A). It must be additionally emphasized that lycorine succeeded in passing the blood brain barrier because while administered i.v. it significantly increased the survival of mice bearing melanoma brain-related metastases (Fig. 6B).
Discussion
The increase in incidence of various types of cancers associated with dismal prognoses, such as gliomas, melanomas, oesophageal cancers, NSCLCs, among others, has not been paralleled by improved therapeutic options over the years. More than 90% of cancer patients die from their metastases, which are naturally resistant to apoptosis. Nonetheless, a large majority of therapeutic agents used by oncologists to treat cancer patients are pro-apoptotic.42,43 Glioma,42 melanoma,43 oesophageal cancer,44 non-small-cell lung cancers,45 and a number of others, are also resistant to apoptosis. New types of drugs are, therefore, urgently needed to combat cancers that are resistant to proapoptotic stimuli and that are therefore associated with dismal prognoses. Lycorine could be a potential candidate to combat cancer cells that display resistance to proapoptotic stimuli as demonstrated in the current study. While lycorine has been suggested to exert its anti-tumor activity in leukemia cells through pro-apoptotic effects,9-11 our current working hypothesis indeed relates to the fact that apoptosis induction by lycorine is not the principal mechanism of action by which this compound exerts its anti-tumor effects in solid cancers. In addition, we observed significant similarities between the anticancer properties of lycorine and narciclasine. We previously demonstrated that narciclasine is unable to induce apoptosis at a pharmacological concentration of 1 μM in glioma cells, which display resistance to proapoptotic stimuli,15,16 while the IC50 in vitro growth inhibitory values (i.e. the “physiological” values) for narciclasine are around 40 nM in carcinoma cells that are sensitive to proapoptotic stimuli as well as in glioma cells that display resistance to proapoptotic stimuli.15,16 The same observations were made in the current study with respect to lycorine (Fig. 5). Furthermore, we showed that narciclasine impairs both cell proliferation and cell migration in a large panel of cancer cell lines15 by disorganizing actin cytoskeleton.16 We report here that lycorine also impairs actin cytoskeleton organization (Fig. 3D), a feature which in turn impairs both cell migration and cell proliferation in differentiated as well as in undifferentiated cancer cell populations (Figs. 2-4). The current study reveals that lycorine increases actin cytoskeleton rigidity, as we recently demonstrated with respect to narciclasine.16 Indeed, we observed that narciclasine modulates the Rho/Rho kinase/LIM kinase/cofilin signaling pathway, greatly increasing GTPase RhoA activity as well as inducing actin stress fiber formation in a RhoA-dependent manner.16 The possibility remains that lycorine also induces higher rigidity in actin cytoskeleton of cancer cells by modulating the Rho/Rho kinase/LIM kinase/cofilin signaling pathway.
Liu et al.9 also observed a cytostatic effect of lycorine in leukemic cells when the alkaloid was assayed at its IC50 in vitro growth inhibitory concentration (i.e. at physiological concentration) and it resulted in the increased population of cells in G2/M phase. Only after the concentration of lycorine was increased by 5-10 times above the IC50 in vitro growth inhibitory values in leukemic cells the actual apoptotic features were observed.9-11
The current study demonstrates that lycorine is able to cross the blood brain barrier (Fig. 6), as we showed earlier with respect to narciclasine.15,16 However, the in vivo therapeutic ratio of narciclasine appears inferior15 to that of lycorine (Fig. 6). Thus, lycorine seems to be less toxic than narciclasine and, therefore, it should be more facile to manage lycorine clinically. Additionally, any modifications we attempted to bring on narciclasine led to a loss of its anti-tumor activity.15 A final point giving advantage to lycorine over narciclasine as a potential candidate for clinical development in oncology relates to the fact that lycorine induces no CYP3A4 inhibitory activity, while narciclasine does.46 Altogether, the current investigation provides significant impetus for the development of lycorine analogues possessing enhanced anti-tumor effects without concomitant elevation of toxicity levels.
The current study provides important preliminary SAR data that could be used for the design of novel lycorine derivatives to be used in oncology. Thus, 1-O-acetyllycorine (2), 1,2-O,O′-diacetyllycorine (3), 2-epi-lycorine (4), 2-O-acetyllycorine (5), lycorine chlorohydrin (6), lycorin-2-one (7), 1,2-epoxylycorine (14), lycorene (16) and caranine (17), incorporate modifications of one or both hydroxyl groups located at C-1 and C-2 of the C-ring. It is noteworthy that 6 and 14 are likely converted in vivo into lycorine by nucleophilic substitution with water and, therefore, their contribution to the SAR data should be treated with caution. Furthermore, 4 possesses altered stereochemistry of the C-ring, while 14 incorporates a fused three-membered ring bringing about significant conformational constraints. The conformational freedom of the C-ring is raised in α- and β-dihydrolycorines (8 and 10), which also contain modified stereochemistry of the C/D ring junction. The same stereochemistry is present in the corresponding diacetyl derivatives (9 and 11) that additionally incorporate the altered diol system at C-1 and C-2. α-Dihydrolycorine lactam (12) contains a modified B-ring and non-basic nitrogen atom. Both the diol system and the double bond of C-ring are modified in amarbellisine (24). The basic nature of nitrogen and stereochemistry of the B/D ring junction are altered in N-methyllycorine iodide (13), which is a salt containing the quaternized nitrogen atom. A similar modification is present in lycorine hydrochloride (15), a salt reversibly converted to 1 in vivo. Anhydrolycorine and ungeremine (20 and 21) contain the aromatized C-ring. In addition, the latter is a betaine compound incorporating the quaternized nitrogen atom. Finally, pseudolycorine and norpluvine (18 and 19) possess an open dioxole ring with the inverted methoxy and hydroxyl groups at C-9 and C-10, respectively. The latter alkaloid also lacks the hydroxyl group at C-2 of the C-ring.
Conclusions
In conclusion, the current study reveals that lycorine modifies the organization of actin cytoskeleton in cancer cells and, in so doing, markedly impairs both cancer cell proliferation and migration. Lycorine is at least 15 times more active as a cytostatic compound in cancer than in normal cells. This alkaloid displays anti-tumor activity against cancer cells regardless of whether the cells display resistance or sensitivity to apoptosis and it is also active against undifferentiated cancer cells growing under anchorage-independent conditions, thus potentially it will be active against cancer stem cells. It is able to provide significant therapeutic benefit in aggressive brain melanoma models with i.v. administrations and at doses that are non-toxic. Altogether, these data should encourage synthetic organic chemists to further explore chemistry associated with lycorine and related phenanthridine alkaloids. Such efforts are expected to identify compounds capable of combating cancers associated with dismal prognoses irrespective of whether they are resistant to proapoptotic stimuli or include significant proportions of cancer stem cells.
Experimental Section
In Vitro Pharmacology
Cell Lines
Human cancer cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, USA), the European Collection of Cell Culture (ECACC, Salisbury, UK) and the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ, Braunschweig, Germany). The code numbers and histological types of all the cell lines used in the current study are detailed in Table 1.
Biology and Biochemistry Related Experiments
The overall growth level of human cancer cell lines was determined using the colorimetric MTT (3-[4,5-dimethylthiazol-2yl]-diphenyl tetrazolium bromide, Sigma, Belgium) assay.14-16,25 Briefly, the cell lines were incubated for 24 h in 96-microwell plates (at a concentration of 10,000 to 40,000 cells/mL culture medium depending on the cell type) to ensure adequate plating prior to cell growth determination. The assessment of cell population growth by means of the MTT colorimetric assay is based on the capability of living cells to reduce the yellow product MTT (3-(4,5)-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) to a blue product, formazan, by a reduction reaction occurring in the mitochondria. The number of living cells after 72 h of culture in the presence (or absence: control) of the various compounds is directly proportional to the intensity of the blue, which is quantitatively measured by spectrophotometry – in our case using a Biorad Model 680XR (Biorad, Nazareth, Belgium) at a 570 nm wavelength (with a reference of 630 nm). Each experiment was carried out in sextuplicate.
Cell proliferation and cell migration in differentiated cancer cell populations were determined by means of computer-assisted phase contrast microscopy.15,16,32,33 Cell proliferation in undifferentiated cancer cell populations was determined by means of the soft-agar assay.37 The actin cytoskeleton organization was determined by means of computer-assisted fluorescence microscopy.15,16 Phallacidin conjugated with the green-fluorescent Alexa Fluor488 (Molecular Probes Inc., Eugene, Oregon) was used to label the fibrillar actin and Alexa Fluor594 conjugated DNAse-I (Molecular Probes Inc.) to label the globular actin.15,16 Apoptotic features were determined by means of flow cytometry using double iodide propidium and Annexin-V staining, as detailed elsewhere.15,23
In Vivo Testing
All the in vivo experiments described in the present study were performed on the basis of authorization No. LA1230568 of the Animal Ethics Committee of the Belgian Federal Department of Health, Nutritional Safety and the Environment.
In Vivo Toxicology Study of Lycorine in Mice
The chronic treatment (one i.v. (tail vein) administration (50 μL in saline) per day during 5 consecutive days (Monday-Friday)) of healthy mice with lycorine (doses ranging from 5 to 80 mg/kg) enabled the maximal tolerated dose (the MTD index) to be determined as detailed elsewhere.15,60 The MTD index is defined as the dose just below the lowest dose level that kills at least one mouse in a treatment group of 3 mice after a maximum of 28 days.15,60
In Vivo Activity of Lycorine in the Mouse B16F10 Metastatic Melanoma
In vivo orthotopic grafts of mouse B16F10 melanoma cells into the brains of B6D2F1 mice (female mice; 17-19 gr; Iffa Credo, Charles Rivers, Arbresle, France) were obtained as described previously by our group for other tumor types.15,16 Briefly, all mice in a given experimental group had B16F10 melanoma cells stereotactically implanted into their brains on the same day. Mice in the control group received an i.v. (tail vein) injection of 50 μL saline. Cisplatin (5 mg/Kg; intraperitoneal administrations in 50 μL volume; 3 times (Monday, Wednesday, Friday) a week during three consecutive weeks) was used as a reference compound for melanoma treatment. Lycorine has been administered i.v. at 40 mg/Kg in 50 μL according to an experimental schedule identical to the one described above for cisplatin. All treatments began on the 5th day post-tumor graft. There were 11 mice per experimental group.
Statistical Analyses
Statistical comparison of control and treated groups was initially undertaken with the Kruskal-Wallis test (a non-parametric one-way analysis of variance). Where this revealed significant differences, the Dunn multiple comparison procedure (2-sided test) was applied. However, this was adapted to the special case of comparing treatment and control groups in which only (k-1) comparisons were undertaken among the k groups tested by the Kruskal-Wallis test (instead of the possible k(k-1)/2 comparisons considered in the general procedure). The levels of statistical significance associated with survival indices were determined by using Gehan's generalized Wilcoxon test. All these statistical analyses were carried out using Statistica (Statsoft, Tulsa, Oklahoma).
Plant Material
Bulbs of Sternbergia lutea Ker Gawl were collected near Bari, Italy and identified by Prof. O. Arrigoni, Dipartimento di Botanica e Patologia Vegetale, Universita di Bari, Italy, where a voucher sample was deposited. Bulbs and whole plants of Amaryllis belladonna L. and Pancratium maritimum L. were collected from flowering plants cultivated in Alexandria, Egypt and sandy hills on the northern coast of Egypt (Baltim) and were identified by Prof. Alam El-Din Negm, University of Alexandria, and Prof. N. El Hadidy, University of Cairo, Egypt, respectively. The voucher samples of the two plants were deposited in the Collection of Department of Pharmacognosy, Faculty of Pharmacy, University of Alexandria, Egypt.
Alkaloids and their synthetic derivatives
Lycorine (1) was isolated from dried bulbs of S. lutea using a published procedure.27 Ungeremine (21) and amarbellisine (22) were isolated from P. maritimun L. and A. belladonna L., respectively, as previously reported.47,48 1-O-acetyllycorine (2), 1,2-O,O-diacetyllycorine (3), 2-epi-lycorine(4), 2-O-acetyllycorine (5), lycorine chlorohydrin (6), lycorine-2-one (7), α-dihydrolycorine (8), 1,2-O,O-diacetyl-α-dihydrolycorine (9), β-dihydrolycorine (10), 1,2-O,O′-diacetyl-β-dihydrolycorine (11), α-dihydrolycorine lactam (12), 1,2-α-epoxylycorine (14), lycorine hydrochloride (15), lycorene (16), caranine (17), and anhydrolycorine (20) were prepared from lycorine according to the previously reported procedures.4,5,27,30 N-methyllycorine iodide (13), pseudolycorine (18), and norpluvine (19) were generously supplied by Prof. H.M. Fales, Department of Health, Education and Welfare, Bethesda, MD, USA, and Prof. C. Fuganti, Istituto di Chimica, Politecnico di Milano, Italy, respectively
General Methods
General laboratory chemicals and solvents were purchased from commercial sources and used without purification. Reactions were performed in a reaction vessel open to the atmosphere and monitored by thin layer chromatography (TLC). Melting points were determined with Axioskop Zeiss microscope and optical rotations were measured on a Jasco P-1010 digital polarimeter. IR spectra were recorded on a Perkin-Elmer Spectrum One FT-IR Spectrometer and UV spectra were taken on a Perkin-Elmer Lambda 25 UV-Vis spectrophotometer. 1H and 13C NMR spectra were recorded at 600, 400 and 75 MHz, on Bruker spectrometers. EI MS were taken at 70 ev on QP 5050 Shimadzu spectrometer. ESI MS spectra were recorded on Waters Micromass Q-TOF Micro and Agilent 1100 coupled to a JOEL AccuTOF (JMS-T100LC) spectrometers. Analytical and preparative TLCs were performed on silica gel (Merck, Kieselgel 60 F254, 0.25 and 0.50 mm, respectively) plates; the spots were visualized by exposure to UV light and/or I2 vapour. The > 95% purity of all compounds was ascertained by Elemental Analyses, performed on EA 1108 Elemental Analyzer, Fisons.
Lycorine or (1S,2S,12bS,12cS)-1,2-Diol,2,4,5,7,12b,12c-hexahydro-1H-[1,3]dioxolo[4,5-j]pyrrolo[3,2,1-de]phenanthridine (1)
1,27,29 1H NMR (400 MHz, CDCl3-CD3COOD, 3:1 v/v): δ 6.98 (s, H-12), 6.80 (s, H-8), 5.95 (s, H2-11), 5.77 (br s, H-3), 4.58 (br s, H-1), 4.48 and 4.19 (1H each, d, J = 14.0 Hz, H2-7), 4.26 (m, H-2), 3.95 (d, J = 11.8 Hz, H-12c), 3.75 and 3.49 (1H each, m, H2-5), 2.99 (d, J = 11.8 Hz, H-12b), 2.88, (m, H2-4). 13C NMR (75 MHz, CDCl3-CD3COOD, 3:1 v/v): δ 149.6 (C-9), 148.1 (C-10), 137.9 (C-3a), 130.7 (C-7a), 125.7 (C-12a), 122.9 (C-3), 108.8 (C-8), 106.4 (C-12), 102.8 (C-11), 71.9 (C-2), 70.1 (C-1), 61.8 (C-12c), 55.1 (C-5), 54.2 (C-7), 38.2 (C-12b), 30.3 (C-4). Mp: 276-277 °C (lit.1 mp 277 °C). [α]25D +1.40 (c 3, 1% H2SO4); UV (1% H2SO4) nm (log ε): 288 (3.55), 238 (3.45); IR (nujol) 3320 cm-1; EI MS, m/z: 287 [M]+, 286, 268, 252, 250, 227, 226. Elemental analysis C, 66.94; H, 5.95, N, 4.84; O, 22.23 (Calcd. for C16H17NO4, C, 66.89; H, 5.90; N, 4.88; O, 22.27).
1-O-Acetyllycorine or (1S,2S,12bS,12cS)-1,2-Diol-2,4,5,7,12b,12c-hexahydro-1H-[1,3]dioxolo[4,5-j]pyrrolo[3,2,1-de]phenanthridine 1-acetate (2)
4,49,50 1H NMR (400 MHz, CDCl3): δ 6.70 (s, H-8), 6.44 (H-12), 5.89 (s, H2-11), 5.56 (m, H-3), 5.50 (m, H-1), 4.14 and 3.48 (1H each, d, J = 14.0 Hz, H2-7), 4.13 (m, H-2), 3.32 and 2.35 (br dd, J = 8.5 and 8.5 Hz, and br q, J = 8.5 and 5.2 Hz, H2-5), 2.84 (d, J = 10.3 Hz, H-12c), 2.84 (dd, J = 10.3 and 2.2 Hz, H-11b) and 2.59 (1H each, m, H2-4). 13C NMR (75 MHz, CDCl3): δ 171.8 (MeCO), 146.5 (C-9), 143.7 (C-10), 136.1 (C-3a), 129.3 (C-7a), 127.2 (C-12a), 117.4 (C-3), 107.3 (C-8), 104.9 (C-12), 100.9 (C-11), 72.7 (C-1), 69.4 (C-2), 61.5 (C-12c), 56.8 (C-5), 53.6 (C-7), 39.2 (C-12b), 28.5 C-4), 20.9 (MeCO). Mp: 215-216 °C (lit.49 mp 215-216 °C). [α]25D -64.8 (c 0.9, CHCl3); UV (CHCl3) nm (log ε): 290 (3.70), 240 (3.60); IR (CHCl3) 3610, 1733 cm-1; EI MS, m/z: 329 [M]+, 328, 269 252, 250, 227, 226. Elemental analysis C, 65.66; H, 5.86, N, 4.28; O, 24.25 (Calcd. for C18H19NO5, C, 65.64; H, 5.81; N, 4.25; O, 24.29).
1,2-O,O′-Diacetyllycorine or (1S,2S,12bS,12cS)-1,2-Diol-2,4,5,7,12b,12c-hexahydro-1H-[1,3]dioxolo[4,5-j]pyrrolo[3,2,1-de]phenanthridine-1,2-diacetate (3)
4,49 1H NMR (600 MHz, CDCl3): δ 6.76 (s, H-8), 6.59 (s, H-12), 5.94 (s, H2-11), 5.75 (s, H-1), 5.45 (s, H-3), 5.27 (s, H-2), 4.18 and 3.55 (1H each, d, J = 13.9 Hz, H2-7), 2.89 (d, J = 10.3 Hz, H-12c), 3.38 and 2.42 (1H each, m, H2-5), 2.79 (d, J = 10.3 Hz, H-12b), 2.67 (m, H2-4), 2.09 and 1.97 (3H, each, 2×MeCO). 13C NMR (75 MHz, CDCl3): δ 171.8 and 172.3 (2× MeCO), 146.5 (C-3a), 146.4 (C-9), 146.2 (C-10), 129.5 (C-7a), 126.6 (C-12a), 113.9 (C-3), 107.4 (C-12), 105.1 (C-8), 101.0 (C-11), 71.0 (C-2), 69.3 (C-1), 61.3 (C-12c), 58.9 (C-7), 53.9 (C-5), 40.6 (C-12b), 28.7 (C-4) 21.3 and 20.9 (2×MeCO). Mp: 216-217 °C (lit.49 mp 215-216 °C). [α]25D - 31.1 (c 1.1, CHCl3); UV (CHCl3) nm (log ε): 290 (3.70), 240 (3.59); IR (CHCl3) 1740 cm-1; EI MS, m/z: 371 [M]+, 370, 328, 311, 252, 250, 227, 226. Elemental analysis C, 64.72; H, 5.67, N, 3.80; O, 25.81 (Calcd. for C20H21NO6, C, 64.68; H, 5.70; N, 3.77; O, 25.85).
2-epi-Lycorine or (1S,2R,12bS,12cS)-1,2-Diol-2,4,5,7,12b,12c-hexahydro-1H-[1,3]dioxolo[4,5-j]pyrrolo[3,2,1-de]phenanthridine (4)
4,49 Mp: 167- 168 °C (lit.50 mp 167-168 °C). [α]25D -217 (c 1.1, CHCl3); UV (MeOH) nm (log ε): 290 (3.67); IR (nujol) 3472, 3413 cm-1. Elemental analysis C, 66.85; H, 5.93, N, 4.82; O, 22.24 (Calcd. for C16H17NO4, C, 66.89; H, 5.90; N, 4.88; O, 22.27).
2-O-Acetyllycorine or (1S,2S,12bS,12cS)-1,2-Diol-2,4,5,7,12b,12c-hexahydro-1H-[1,3]dioxolo[4,5-j]pyrrolo[3,2,1-de]phenanthridine-2-acetate (5)
1,4 1H NMR (400 MHz, CDCl3-CD3COOD, 3:1 v/v): δ 6.94 (s, H-12), 6.78 (s, H-8), 5.96 (s, H2-11), 5.69 (s, H-3), 5.36 (s, H-2), 4.67 (s, H-1), 4.60 and 3.98 (1H each, d, J = 13.6 Hz, H2-7), 4.13 (d, J = 12.1 Hz, H-12c), 3.87 and 3.32 (1H each, m, H2-5), 2.90 (J = 12.1 Hz, H-12b), 2.80 (m, H2-4), 2.04 (MeCO). Mp: 231-232 °C (lit.1 mp 230-232 °C). [α]25D +22.4 (c 0.2, CHCl3); UV (CHCl3) nm (log ε): 291 (3.67), 240 (3.60); IR (CHCl3) 3580, 1718 cm-1; EI MS, m/z: 329 [M]+, 328, 269, 252, 250, 227, 226. Elemental analysis C, 65.67; H, 5.84, N, 4.21; O, 24,25 (Calcd. for C18H19NO5, C, 65.64, H 5.81; N, 4.25; O, 24.29).
Lycorine chlorohydrin or (1S,2S,12bS,12cS)-1-ol-2-Chloro-2,4,5,7,12b,12c-hexahydro-1H-[1,3]Dioxolo[4,5-j]pyrrolo[3,2,1-de]phenanthridine (6)
5,51 1H NMR (400 MHz, CDCl3): δ 6.79 (s, H-12), 6.59 (s, H-8), 5.93 (d, J = 1.5 Hz, H-11A) 5.91 (d, J = 1.5 Hz, H-11b), 5.56 (m, H-3), 4.78 (dd, J =2.2 and 2.0 Hz, H-1), 4.65 (m, H-2), 4.13 and 3.51 (1H each, d, J = 14.0 Hz, H2-7), 3.35 and 2.37 (1H each, m, H2-5) 2.99 (d, J = 10.7 Hz, H-12c), 2.85 (dd, J = 10.7 and 2.0 Hz, H-12b), 2.63 (m, H2-4). UV (EtOH) nm (log ε): 292 (3.68), 280 (3.60); IR (CHCl3) 3520, 1625, 1505, 1485 cm-1; EI MS, m/z: 307 [M+2]+, 305 [M]+, 270, 250, 227, 226. Elemental analysis C, 62.88; H, 5.23, N, 4.61; (Calcd. for C16H16NO3, C 62.85; H, 5.27; N, 4.58).
Lycorin-2-one or (1α)-3,12-Didehydro-1-hydroxy-9,10-[methylenebis(oxy)]-galanthan-2-one (7)
4 1H NMR (400 MHz, CDCl3-CD3COOD, 3:1 v/v): δ 6.82 (s, H-12), 6.65 (s, H-8), 6.05 (s, H-3) 5.92 (s, H2-11), 4.73 (d, J =2.6 Hz, H-1), 4.36 (d, J = 14.7 Hz, H-7A), 4.29 (1H, d, J = 10.7 Hz, H-12c; 1H, d, J = 14.7 Hz, H-7B; by NMDR), 3.74 and 3.56 (1H each m, H2-5), 3.49 (dd, J = 10.7 and 2.6 Hz, H-12b), 3.17 (m, H2-4). Mp: 143-145 °C (lit.4 mp 143-145 °C). [α]25D -99.7 (c 2.5, 1% H2SO4); UV (CHCl3) nm (log ε): 292 (3.47), 245 (3.50); IR (nujol) 3150, 1655 cm-1; EI MS, m/z: 285 [M]+, 284, 267, 226. Elemental analysis C, 67.40; H, 5.26, N, 4.95; O, 22.48 (Calcd. for C16H15NO4, C, 67.36; H, 5.30; N, 4.91; O, 22.43).
α-Dihydrolycorine or (1S,2S,3aR,12bS,12cR)-1,2-Diol-2,3,3a,4,5,7,12b,12c-octahydro-1H-[1,3]dioxolo[4,5-j]pyrrolo[3,2,1-de]phenanthridine (8)
4,29,49 1H NMR (400 MHz, CD3OD-CD3COOD, 3:1 v/v): δ 7.08 (s, H-12), 6.85 (s, H-8), 5.98 (s, H2-11), 4.60 and 4.0 (1H each, d, J = 14.0, Hz H2-7), 4.53 (dd, J = 2.0 and 2.0 Hz, H-1), 4.07 (m, H-2), 3.65 (d, J = 11.7 Hz, 12c), 3.24 (dd, J = 11.7 and 2.0 Hz, 12b), 3.63 and 3.40 (1H each, m, H2-5), 2.59 (m, H-3a), 2.59 and 2.10 (1H each, m, H2-4), 2.59 and 2.10 (1H each, m, H2-3). 13C NMR (75 MHz, CD3OD-CD3COOD, 3:1 v/v): δ 149.7 (C-9), 147.7 (C-10), 133.0 (C-7a), 124.5 (C-12a), 108.6 (C-8), 106.9 (C-12), 102.6 (C-11), 70.5 (C-2), 69.6 (C-1), 64.3 (C-12c), 56.5 (C-5), 53.8 (C-7), 36.6 (C-3a), 33.2 (C-12b), 30.5 (C-4), 27.8 (C-3). Mp: 247 °C. [α]25D +99.5 (c 3, 1% H2SO4); UV (1% H2SO4) nm (log ε): 287 (3.66), 238 (3.58); IR (nujol) 3410 cm-1; EI MS, m/z: 289 [M]+, 288, 270, 254. Elemental analysis C, 66.46; H, 6.65, N, 4.80; O, 22.16 (Calcd. for C16H19NO4, C, 66.42; H, 6.62; N, 4.84; O, 22.12).
1,2-O,O′-Diacetyl-α-dihydrolycorine or (1S,2S,3aR,12bS,12cR)-1,2-Diol-2,3,3a,4,5,7,12b,12c-octahydro-1H-[1,3]dioxolo[4,5-j]pyrrolo[3,2,1-de]phenanthridine diacetate (9)
4,52 Compound was prepared as described in the literature4 from α-dihydrolycorine (8). The physical and spectral properties were identical to those previously reported.52 More specifically the 1H NMR spectrum (400 MHz, CD3OD-CD3COOD, 3:1 v/v) differed from that of 8 by the downfield shift of H-1 and H-2 (Δδ 1.42 and 1.34) and the two singlets due to the acetyl groups (δ 2.04 and 1.97). Mp 178-180 °C (lit.52 179-180 °C). Elemental analysis C, 64.30; H, 6.25, N, 3.79; O, 25.84 (Calcd. for C20H23NO6, C, 64.33; H, 6.21; N, 3.75; O, 25.71).
β-Dihydrolycorine or (1S,2S,3aS,12bS,12cR)-1,2-Diol-2,3,3a,4,5,7,12b,12c-octahydro-1H-[1,3]dioxolo[4,5-j]pyrrolo[3,2,1-de]phenanthridine (10)
4 1H NMR (400 MHz, CDCl3-CD3COOD, 3:1 v/v): δ 6.91 (s, H-12), 6.70 (s, H-8), 5.96 (s, H2-11), 4.52 (1H, d, J = 14.0 Hz H-7A; 1H, t, J = 1.9 Hz, H-1; by NMDR), 4.27 (d, J = 14.0 Hz, H-7b), 4.09 (m, H-2), 3.82 and 3.45 (1H each, m, H2-5), 3.31 (dd, J = 12.1 and 1.9 Hz, H-12B), 3.14 (t, J = 12.1 Hz, H-12c), 2.36 (m, H-3a), 2.23 (m, H-4A), 2.11 (dt, J = 13.7 and 3.8 Hz, H-3A), 1.81 (1H, dd, J = 13.7 and 2.3 Hz, H-3b; 1H, m, H-4B; by NMDR). Mp: 120-123 °C (lit.4 mp 120-123 °C). [α]25D -85.0 (c 0.5, 1% H2SO4); UV (1% H2SO4) nm (log ε): 289 (3.37), 236 (3.29); IR (nujol) 3400cm-1; EI MS, m/z: 289 [M]+, 288, 270. Elemental analysis C, 66.45; H, 6.59, N, 4.87; O, 22.16 (Calcd. for C16H19NO4, C, 64.42; H, 6.62; N, 4.84; O, 22.12).
1,2-O,O′-Diacetyl-β-dihydrolycorine or (1α,2β,12β)-1,2-Diol-9,10-[methylenebis(oxy)]-galanthan-diacetate (11)
4 Compound was prepared from lycorine as white prisms and as described in the literature4. 1H NMR spectrum (400 MHz, CDCl3-CD3COOD, 3:1 v/v) differed from that of the β-dihydrolycorine by the downfield shift of H-1 and H-2 (Δδ 1.03 and 1.24) and the two singlets due to the acetyl groups (δ 2.02 and 1.98). Elemental analysis C, 64.38; H, 6.25, N, 3.71; O, 25.74 (Calcd. for C20H23NO6, C, 64.33; H, 6.21; N, 3.75; O, 25.71).
α-Dihydrolycorine-lactam or (1α,2β)-1,2-Dihydroxy-9,10-[methylenebis(oxy)]-galanthan-7-one (12)
4,29,52 Compound was prepared from lycorine as white prisms and as described in the literature.4 1H NMR spectrum (400 MHz, CDCl3-CD3COOD, 3:1 v/v) differed from that of the α-dihydrolycorine by the absence of the AB system of H2-7. 13C NMR (75 MHz, CD3OD-CD3COOD, 3:1 v/v): δ 165.1 (C-7), 152.3 (C-10), 147.8 (C-9), 137.3 (C-12a), 126.0 (C-7a), 108.9 (C-8), 106.4 (C-12), 103.0 (C-11), 73.9 (C-2), 70.4 (C-1), 57.3 (C-12c), 46.8 (C-5), 39.4 (C-3a), 36.1 (C-12b), 32.9 (C-4), 30.8 (C-3). Mp 268-270 °C (lit.52 mp 270 °C). Elemental analysis C, 63.32; H, 5.68, N, 4.66; O, 26.33 (Calcd. for C16H17NO5, C, 63.36; H, 5.65; N, 4.62; O, 26.37).
N-methyl lycorine iodide or (1α,2β)-3,12-didehydro-1,2-dihydroxy-6-methyl-9,10-[methylenebis(oxy)]-galanthanium iodide (13)
53 The compound was generously supplied by Prof. H. M. Fales, Department of Health Education and Welfare, Bethesda, MD, USA. The 1H NMR spectrum (400 MHz, CDCl3-CD3COOD, 3:1 v/v) differed from that of the lycorine for the presence of the singlet at δ 7.90 due to the N-methyl. Elemental analysis C, 47.60; H, 4.64, N, 3.29; O, 26.33 (Calcd. for C17H20NO4, C 47.57, H, 4.70; N, 3.26).
1,2-α-Epoxy-lycorine: or (1α,2α)-3,12-didehydro-1,2-epoxy-9,10-[methylenebis(oxy)]-galanthan (14)
5,53 1H NMR (400 MHz, CDCl3): δ 6.79 (s, H-12), 6.56 (s, H-8), 5.93 (d, J = 1.5 Hz, H-11A) 5.90 (d, J = 1.5 Hz, H-11b), 5.45 (m, H-3), 5.01 (m, H-2), 4.64 (dd, J =2.2 and 2.0 Hz, H-1), 4.12 and 3.52 (1H each, d, J = 14.0 Hz, H2-7), 3.36 and 2.40 (1H each, m, H2-5), 3.11 (dd, J = 10.7, 2.0 Hz, H-12b), 2.82 (d, J = 10.7, H-12c), 2.63 (m, H2-4). [α]25D -194.4 (c 0.1, CHCl3); UV (EtOH) nm (log ε): 290 (3.74), 235 (sh); IR (CHCl3) 1620, 1505, 1485 cm-1; EI MS, m/z: 269 [M]+, 265, 250, 227, 226. Elemental analysis C, 71.32; H, 5.64, N, 5.23; O, 17.79 (Calcd. for C16H15NO3, C, 71.36; H, 5.61; N, 5.20; O, 17.82).
Lycorine hydrochloride or (1S,2S,12bS,12cS)-1,2-diol-2,4,5,7,12b,12c-hexahydro-1H-[1,3]dioxolo[4,5-j]pyrrolo[3,2,1-de]phenanthridine hydrochloride (15)
27 Compound was prepared as described in the literature27 from lycorine. The spectroscopic data (1H and 13C NMR) were identical to those of lycorine. Elemental analysis C, 59.32; H, 5.64, N, 4.37 (Calcd. for C16H18NO4, C, 59.35; H, 5.60; N, 4.33).
Lycorene or (12bS,12cS)-2,4,5,7,12b,12c-hexahydro-1H-[1,3]-dioxolo[4,5-j]pyrrolo[3,2,1-de]phenanthridine (16)
5,55 1H NMR (400 MHz, CDCl3): δ 6.77 (s, H-12), 6.58 (s, H-8), 5.92 (d, J = 1.5 Hz, H-11A) 5.90 (d, J = 1.5 Hz, H-11b), 5.44 (m, H-3), 4.11 (d, J = 14.0 Hz, H-7A), 3.54 (dd J = 14.0 and 2.2 Hz, H-7B), 3.23 and 2.32 (m, H2-5), 2.54 and 2.37 (1H each, m, H2-1), 2.54 and 2.30 (1H each, m, H2-2), 2.45 (ddd, J = 10.7, 2.2 and 2.0 Hz, H-12b), 2.43 (dd, J = 10.7 and 2.0 Hz, H-12c), 2.35 (m, H2-4). Mp 112-116 °C (lit.56 mp 120-121); UV (EtOH) nm (log ε): 290 (3.67), 237 (sh); IR (CHCl3) 1605, 1505, 1485 cm-1; EI MS, m/z: 255 [M]+, 254, 252, 227, 226. Elemental analysis C, 75.30; H, 6.75, N, 5.44; O, 12.58 (Calcd. for C16H17NO2, C, 75.27; H, 6.71; N, 5.49; O, 12.53).
Caranine or (1R,12bS,12cS)-1-ol-2,4,5,7,12b,12c-hexahydro-1H-[1,3]Dioxolo[4,5-j]pyrrolo[3,2,1-de]phenanthridine (17)
5,55 1H NMR (400 MHz, CDCl3): δ 6.82 (s, H-12), 6.58 (s, H-8), 5.92 (d, J = 1.5 Hz, H-11A) 5.90 (d, J = 1.5 Hz, H-11b), 5.41 (m, H-3), 4.70 (m, H-1), 4.13 (d, J = 14.0 Hz, H-7A), 3.52 (dd J = 14.0 and 2.2 Hz, H-7B), 3.32 and 2.33 (m, H2-5), 2.59 (m, H-2), 2.78 (dd, J = 10.7 and 2.6 Hz, H-12c), 2.41 (ddd, J = 10.7, 2.2 and 2.0 Hz, H-12b), 2.59 (m, H2-4). Mp 176-180 °C (lit.55 mp 178-181 °C); UV (EtOH) nm (log ε): 291 (3.44), 280 (3.49), 237 (3.49); IR (CHCl3) 3680, 1605, 1505, 1485 cm-1; EI MS, m/z: 271 [M]+, 270, 252, 227, 226. Elemental analysis C, 70.86; H, 6.36, N, 5.13; O, 17.70 (Calcd. for C16H17NO3, C, 70.83; H, 6.32; N, 5.16; O, 17.69).
Pseudolycorine or (1S,2S,11bS,11cS)-1,2,10-triol-2,4,5,7,11b,11c-hexahydro-9-methoxy-1H-pyrrolo[3,2,1-de]phenanthridine (18)
56 The compound was generously supplied by Prof. H. M. Fales, Department of Health Education and Welfare, Bethesda, MD, USA. 1H NMR (400 MHz, CDCl3-DMSOd6): δ 6.78 (s, H-11), 6.62 (br s H-8), 5.41 (m, H-3), 4.5-2.0 (10H). Mp: 247-249 °C (lit.56 mp 247-249 °C); [α]25D -57.8 (c 0.3 EtOH); EI MS, m/z: 289 [M]+, 274, 270, 258, 229, 228. Elemental analysis C, 66.46; H, 6.67, N, 4.81; O, 22.16 (Calcd. for C16H19NO4, C 66.42; H, 6.62; N, 4.84; O, 22.12).
Norpluvine or (1R,11bS,11cS)-1,9-diol-10-methoxy-2,4,5,7,11b,11c-hexahydro-1H-Pyrrolo[3,2,1-de]phenanthridine (19)
57 The compound was generously supplied by Prof. C. Fuganti, Istituto di Chimica, Politecnico di Milano, Italy. 1H NMR (400 MHz, CDCl3): δ 6.74 (s, H-11), 6.64 (s H-8), 5.97 (dd, J = 5.9, 1.0, Hz, H-1); 5.39 (d, J = 2.2 Hz, H-3), 4.26 (dd, J = 5.9, 1.0, Hz, H-1); 4.13 and 3.50 (1H each, d, J = 14.5 Hz, H2-7), 3.34 and 2.37 (1H, each, m, H2-5), 2.76 (d, J = 9.9 Hz, H-11c 2.66 (d, J = 9.9 Hz, H-11b), 2.62 and 2.33 m, H2-2), 2.59 (m, H2-4) 114.5-2.0 (10H). Mp: 274-275 °C (lit.57 274-275°C); [α]25D -160 (c 0.15 MeOH); EI MS, m/z: 272 [M]+, 273, 252, 253, 244, 229, 228. Elemental analysis C, 70.28; H, 7.06, N, 5.17; O, 17.53 (Calcd. for C16H19NO3, C, 70.31; H, 7.01; N, 5.12; O, 17.56).
Anhydrolycorine or 4,5-dihydro-7H-[1,3]Dioxolo[4,5-j]pyrrolo[3,2,1-de]phenanthridine (20)
30,58 1H NMR (400 MHz, CDCl3): δ 7.28 (d, J =8.1 Hz, H-1), 7.16 (s, H-11), 7.00 (dt, J = 8.1 and 1.5 Hz, H-3), 6.75 (dd, J = 8.1 and 8.1 Hz, H-2), 6.63 (s, H-8), 5.97 (s, H2-11) 4.06 (m, H2-7), 3. 32 (t, J = 7.4, H2-5), 3.02 (td, J = 7.4 and 1.5 Hz, H2-4). Mp 115-120 °C (lit.58 mp 122-123 °C); UV (EtOH) nm (log ε): 342 (4.02), 287 (3.82), 280 (3.85), 249 (4.32); IR (CHCl3) 1630 cm-1; EI MS, m/z: 251 [M]+, 250. Elemental analysis C, 76.44; H, 5.25, N, 5.54; O, 12.70 (Calcd. for C16H13NO2, C, 76.48; H, 5.21; N, 5.57; O, 12.73).
Ungeremine or 2-hydroxy-4,5-dihydro-[1,3]Dioxolo[4,5-j]pyrrolo[3,2,1-de]phenanthridinium (21)
30,47,59 Compound was isolated from whole plant of Pancratium maritimum L. as previously reported in the literature.47 1H NMR (400 MHz, CD3OD-CD3COOD 3:1 v/v): δ 9.32 (s, H-7), 7.96 (s, H-11), 7.66 (s, H-8), 7.33 (dt, J = 1.8 and 1.8 Hz, H-3) 6.36 (s, H2-11), 5.22 (t, J = 7.0 Hz, H2-5), 3.73 (td, J = 7.0, 1.8 Hz, H2-4). 13C NMR (CD3OD-CD3COOD, 3:1 v/v): δ 162. 6 (C-2), 157.3 (C-9), 151.8 (C-10), 141.9 (C7), 139.8 (C-3a), 132.9 (C-12c), 132.0 (C-12b), 126.2 (C-7a), 124.0 (C-12a), 118.1 (C-3), 108.2 (C-8), 105.4 (C-11), 104.1 (C-1), 102.0 (C-12), 57.0 (C-5), 28.3 (C-4). Mp 268-270 °C (lit.59 mp 260-270 decomp.); UV (0.1 N NaOH) nm (log ε): 405 (3.90), 272 (4.57); IR (nujol) 1615 cm-1; EI MS, m/z: 265 [M]+, 264. Elemental analysis C, 72.43; H, 4.14, N, 5.31; O, 18.12 (Calcd. for C16H11NO3, C, 72.45; H, 4.18; N, 5.28; O, 18.09).
Amarbellisine or (1S,3aS,12bS,12cS)-1-ol-2-methoxy-3a,4,5,7,12b,12c-hexahydro-1H-[1,3]Dioxolo[4,5-j]pyrrolo[3,2,1-de]phenanthridine (22)
48 Compound was isolated from bulbs of Amaryllis belladonna L. as previously reported in the literature.48 1H NMR (400 MHz, CDCl3): δ 6.54 (s, H-12), 6.45 (s, H-8), 5.88 and 5.86 (1H each, d, J = 1.1 Hz, H2-11), 5.56 (br s, H-3), 4.33 and 3.79 (1H each, d, J = 16.7 Hz, H2-7), 4.08 (br s, H-12c), 3.48 (br s, H-1), 3.43 (s, OMe), 3.41 (br ddd, J = 11.8, 5.4 and 2.3 Hz, H-3a), 3.28 (br s, H-12b), 3.07 and 3.02 (dd, J= 11.2 and = 2.2 Hz, and d, J = 11.2 H2-5), 2.14 (ddd, J = 12.9, 5.4 and 3.4 Hz, H2-4). 13C NMR (75 MHz, CDCl3): δ 154.2 (C-2), 146.7 (C-10), 146.0 (C-9), 132.5 (C-7a), 124.6 (C-12a), 112.9 (C-3), 107.3 (C-8), 106.8 (C-12), 100.7 (C-11), 79.8 (C-1), 69.1 (C-12c), 60.9 (C-7), 58.6 (C-3a), 57.6 (OMe), 55.4 (C-5), 45.6 (C-12b), 32.7 (C-4). Mp: < 300 °C (lit.48 mp < 300 °C); [α]25D (c 0.7 CHCl3) -39.2; UV (MeOH) nm (log ε): 293 (2.90), 244 (2.90); IR (KBr) 3439, 1645 cm-1; ESI MS, m/z: 340 [M+K]+, 324 [M+Na]+, 302 [M+H]+. Elemental analysis C, 67.80; H, 6.33, N, 4.69; O, 21.22 (Calcd. for C17H19NO4, C, 67.76; H, 6.36; N, 4.65; O, 21.24).
Acknowledgments
The authors thank the Italian Ministry University and Research (MIUR, contribution DISSPAPA N. 197), the Fonds National de la Recherche Scientifique (FNRS, Belgium), the Fonds Yvonne Boël (Belgium) and the National Institutes of Health (USA, CA135579) for financial support of this work. R. Kiss is a director of research and Véronique Mathieu a senior research assistant with the FNRS. The staff members of Istituto di Chimica Biomolecolare del CNR, Pozzuoli, Italy are gratefully acknowledged for providing mass and NMR spectra.
Footnotes
Abbreviations: AA, ascorbic acid; AV, Annexin V; DMEM, Dulbecco's modified Eagle's medium; DMSO, dimethyl sulfoxide; FBS, fetal bovine serum; GGR, global growth rate; FTIR, Fourier transform infrared spectroscopy; HRMS, high resolution mass spectrometry; HPLC, high performance liquid chromatography; MEM, modified eagle medium; MRDO, Maximum Relative Distance to the Origin; MTD, maximal tolerated dose; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NMR, nuclear magnetic resonance; PI, propidium iodide; SAR, structure-activity relationship; SEM, standard error of the mean; TLC, thin layer chromatography.
References
- 1.Nakagawa Y, Uyeo S, Yayima H. The double bond in lycorine. Chem Ind. 1956:1238–1239. [Google Scholar]
- 2.Arrigoni O, Arrigoni Liso R, Calabrese G. Lycorine as an inhibitor of ascorbic acid biosynthesis. Nature. 1975;256:513–514. [Google Scholar]
- 3.Arrigoni O, De Gara L, Paciolla C, Evidente A, De Pinto MC, Liso R. Lycorine: a powerful inhibitor of L-galactono-γ-lactone dehydrogenase activity. J Plant Physiol. 1997;150:362–364. [Google Scholar]
- 4.Evidente A, Cicala MR, Randazzo G, Riccio R, Calabrese G, Liso R, Arrigoni O. Lycorine structure-activity relationships. Phytochemistry. 1983;22:2193–2196. and references cited therein. [Google Scholar]
- 5.Evidente A, Arrigoni O, Liso R, Calabrese G, Randazzo G. Further experiments on structure-activity relationships among the lycorine alkaloids. Phytochemistry. 1986;25:2739–2743. and references cited therein. [Google Scholar]
- 6.De Leo P, Dalessandro G, De Santis A, Arrigoni O. Inhibitory effect of lycorine on cell division and cell elongation. Plant Cell Physiol. 1973;14:487–496. [Google Scholar]
- 7.Arrigoni O, Arrigoni Liso R, Calabrese G. Ascorbic acid as a factor controlling the development of cyanide-insensitive respiration. Science. 1976;194:332–333. doi: 10.1126/science.194.4262.332. [DOI] [PubMed] [Google Scholar]
- 8.Arrigoni O. Ascorbate system in plant development. J Bioen Biomem. 1994;26:407–419. doi: 10.1007/BF00762782. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Hu WX, He LF, Ye M, Li Y. Effects of lycorine on HL-60 cells via arresting cell cycle and inducing apoptosis. FEBS Letters. 2004;578:245–250. doi: 10.1016/j.febslet.2004.10.095. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Li Y, Tang LJ, Zhang GP, Hu WX. Treatment of lycorine on SCID mice model with human APL cells. BioMed Pharmacother. 2007;61:229–234. doi: 10.1016/j.biopha.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu XS, Jiang J, Jiao XY, Wu YE, Lin JH, Cai YM. Lycorine induces apoptosis and down-regulation of MCL-1 in human leukemia cells. Cancer Letters. 2009;274:16–24. doi: 10.1016/j.canlet.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 12.McLachlan A, Kekre N, McNulty J, Pandey S. Pancratistatin: a natural anti-cancer compound that targets mitochondria specifically in cancer cells to induce apoptosis. Apoptosis. 2005;10:619–630. doi: 10.1007/s10495-005-1896-x. [DOI] [PubMed] [Google Scholar]
- 13.Griffin C, Sharda N, Sood D, Nair J, McNulty J, Pandey S. Selective cytotoxicity of pancratistatin-related natural Amaryllidaceae alkaloids: evaluation of the activity of two new compounds. Cancer Cell Int. 2007;7:10. doi: 10.1186/1475-2867-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumont P, Ingrassia L, Rouzeau S, Ribaucour F, Thomas S, Roland I, Darro F, Lefranc F, Kiss R. The Amaryllidaceae isocarbostyril narciclasine induces apoptosis by activation of the death receptor and/or mitochondrial pathways in cancer cells but not in normal fibroblasts. Neoplasia. 2007;9:766–776. doi: 10.1593/neo.07535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ingrassia L, Lefranc F, Dewelle J, Pottier L, Mathieu V, Spiegl-Kreinecker S, Sauvage S, El Yazidi M, Dehoux M, Berger W, Van Quaquebeke E, Kiss R. Structure-activity relationship analysis of novel derivatives of narciclasine (an Amaryllidaceae isocarbostyril derivative) as potential anticancer agents. J Med Chem. 2009;52:1100–1114. doi: 10.1021/jm8013585. [DOI] [PubMed] [Google Scholar]
- 16.Lefranc F, Sauvage S, Van Goietsenoven G, Mégalizzi V, Lamoral-Theys D, Debeir O, Spiegl-Kreinecker S, Berger W, Mathieu V, Decaestecker C, Kiss R. Narciclasine, a plant growth modulator, activates Rho and stress fibers in glioblastoma cells. Mol Cancer Ther. 2009;8:OF1–12. doi: 10.1158/1535-7163.MCT-08-0932. [DOI] [PubMed] [Google Scholar]
- 17.Kornienko A, Evidente A. Chemistry, biology, and medicinal potential of narciclasine and its congeners. Chem Rev. 2008;108:1982–2014. doi: 10.1021/cr078198u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ingrassia L, Lefranc F, Mathieu V, Darro F, Kiss R. Amaryllidaceae isocarbostyril alkaloids and their derivatives as promising antitumor agents. Trans Oncol. 2008;1:1–13. doi: 10.1593/tlo.08100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evidente A, Kireev AS, Jenkis AR, Romero AE, Steelant WFA, Van Slambrouck S, Kornienko A. Biological evaluation of structurally diverse Amaryllidaceae alkaloids and their synthetic derivatives: discovery of novel leads for anticancer drug design. Planta Med. 2009;75:501–507. doi: 10.1055/s-0029-1185340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evidente A, Kornienko A. Anticancer evaluation of structurally diverse Amaryllidaceae alkaloids and their synthetic derivatives. Phytochem Rev. 2009;8:449–459. doi: 10.1055/s-0029-1185340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belot N, Rorive S, Doyen I, Lefranc F, Bruyneel E, Dedecker R, Micik S, Brotchi J, Decaestecker C, Salmon I, Kiss R, Camby I. Molecular characterization of cell substratum attachments in human glial tumors relates to prognostic features. Glia. 2001;36:375–390. doi: 10.1002/glia.1124. [DOI] [PubMed] [Google Scholar]
- 22.Branle F, Lefranc F, Camby I, Jeuken J, Geurts-Moespot A, Sprenger S, Sweep F, Kiss R, Salmon I. Evaluation of the efficiency of chemotherapy in in vivo orthotopic models of human glioma cells with and without 1p19q deletions and in C6 rat orthotopic allografts serving for the evaluation of surgery combined with chemotherapy. Cancer. 2002;95:641–655. doi: 10.1002/cncr.10710. [DOI] [PubMed] [Google Scholar]
- 23.Mijatovic T, Mathieu V, Gaussin JF, De Neve N, Ribaucour F, Van Quaquebeke E, Dumont P, Darro F, Kiss R. Cardenolide-induced lysosomal membrane permeabilization demonstrates therapeutic benefits in experimental human non-small cell lung cancers. Neoplasia. 2006;8:402–412. doi: 10.1593/neo.05850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruyere C, Mijatovic T, De Neve N, Gaussin JF, Gras T, Nindfa P, Dehoux M, Saussez S, Kiss R. Combining pro-apoptotic or pro-autophagic chemotherapies with chemokine inhibition in experimental esophageal cancers [abstract]. Proceedings of the 100th Annual Meeting of the American Association for Cancer Research; 2009 Apr 18-22; Denver, CO. Philadelphia (PA). 2009. Abstract nr 4135. [Google Scholar]
- 25.Mathieu V, Pirker C, Martin de Lasalle E, Vernier M, Mijatovic T, De Neve N, Gaussin JF, Dehoux M, Lefranc F, Berger W, Kiss R. The sodium pump alpha-1 subunit: A disease progression-related target for metastatic melanoma treatment. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2009.00708.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathieu V, Le Mercier M, De Neve N, Sauvage S, Gras T, Roland I, Lefranc F, Kiss R. Galectin-1 knockdown increases sensitivity to temozolomide in a B16F10 mouse metastatic melanoma model. J Invest Dermatol. 2007;127:2399–2410. doi: 10.1038/sj.jid.5700869. [DOI] [PubMed] [Google Scholar]
- 27.Evidente A, Iasiello I, Randazzo G. An improved method for the large-scale preparation of lycorine. Chem Ind. 1984:348–349. [Google Scholar]
- 28.Evidente A, Iasiello I, Randazzo G. Rapid quantitative analysis of lycorine by reversed-phase high-performance liquid chromatography. J Cromatogr. 1983a;281:362–366. [Google Scholar]
- 29.Evidente A, Cicala MR, Giudicianni I, Randazzo G, Riccio R. 1H and 13C NMR analysis of lycorine and α-dihydrolycorine. Phytochemistry. 1983b;22:581–584. [Google Scholar]
- 30.Evidente A, Randazzo G, Surico G, Lavermicocca P, Arrigoni O. Degradation of lycorine by Pseudomonas species strain ITEM 311. J Nat Prod. 1985;48:564–570. [Google Scholar]
- 31.Lefranc F, Mijatovic T, Kondo Y, Sauvage S, Roland I, Krstic D, Vasic V, Gailly P, Kondo S, Blanco G, Kiss R. Targeting the alpha-1 subunit of the sodium pump (the Na+/K+-ATPase) to combat glioblastoma cells. Neurosurgery. 2008;62:211–222. doi: 10.1227/01.NEU.0000311080.43024.0E. [DOI] [PubMed] [Google Scholar]
- 32.Debeir O, Mégalizzi V, Warzée N, Kiss R, Decaestecker C. Videomicroscopic extractions of specific information on cell proliferation and migration in vitro. Exp Cell Res. 2008;314:2985–2998. doi: 10.1016/j.yexcr.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Debeir O, Van Ham P, Kiss R, Decaestecker C. Tracking of migrating cells under phase-contrast videomicroscopy with combined mean-shift processes. IEEE Trans Med Imaging. 2005;24:697–711. doi: 10.1109/TMI.2005.846851. [DOI] [PubMed] [Google Scholar]
- 34.Giganti A, Friederich E. The actin cytoskeleton as a therapeutic target: state of the art and future directions. Prog Cell Cycle Res. 2003;5:511–525. [PubMed] [Google Scholar]
- 35.Buda A, Pignatelli M. Cytoskeletal network in colon cancer: from genes to clinical application. Int J biochem Cell Biol. 2004;36:759–765. doi: 10.1016/j.biocel.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Lindberg U, Karlsson R, Lassing I, Schutt CE, Höglund AS. The microfilament system and malignancy. Semin Cancer Biol. 2008;18:2–11. doi: 10.1016/j.semcancer.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Darro F, Cahen P, Vianna A, Decaestecker C, Nogaret JM, Leblond B, Chaboteaux C, Ramos C, Petein M, Budel V, Schoofs A, Pourrias B, Kiss R. Growth inhibition of human in vitro and mouse in vitro and in vivo mammary tumor models by retinoids in comparison with tamoxifen and RU-486 anti-progestagen. Breast Cancer Res Treat. 1998;51:39–55. doi: 10.1023/a:1006098124087. [DOI] [PubMed] [Google Scholar]
- 38.Aragon-Ching JB, Zujewski JA. CNS metastasis: an old problem in a new guise. Clin Cancer Res. 2007;13:1644–1647. doi: 10.1158/1078-0432.CCR-07-0096. [DOI] [PubMed] [Google Scholar]
- 39.McWilliams RR, Rao RD, Brown PD, Link MJ, Buckner JC. Treatment options for brain metastases from melanoma. Expert Rev Anticancer Ther. 2005;5:809–820. doi: 10.1586/14737140.5.5.809. [DOI] [PubMed] [Google Scholar]
- 40.Atallah E, Flaherty L. Treatment of metastatic melanoma. Curr Treat Options Oncol. 2005;6:185–193. doi: 10.1007/s11864-005-0002-5. [DOI] [PubMed] [Google Scholar]
- 41.Kersey P. Apoptosis and melanoma: how new insights are effecting the development of new therapies for melanoma. Curr Opin Oncol. 2006;18:189–196. doi: 10.1097/01.cco.0000208794.24228.9f. [DOI] [PubMed] [Google Scholar]
- 42.Lefranc F, Brotchi J, Kiss R. Possible future issues in the treatment of glioblastomas: Special emphasis on cell migration and the resistance of migrating glioblastoma cells to apoptosis. J Clin Oncol. 2005;23:2411–2422. doi: 10.1200/JCO.2005.03.089. [DOI] [PubMed] [Google Scholar]
- 43.La Porta CA. Drug resistance in melanoma: new perspectives. Curr Med Chem. 2007;14:387–391. doi: 10.2174/092986707779941078. [DOI] [PubMed] [Google Scholar]
- 44.D'Amico TA, Harpole DH., Jr Molecular biology of esophageal cancer. Chest Surg Clin N Am. 2000;10:451–469. [PubMed] [Google Scholar]
- 45.Fenell DA. Caspase regulation in non-small cell lung cancer and its potential for therapeutic exploitation. Clin Cancer Res. 2005;11:2097–2105. doi: 10.1158/1078-0432.CCR-04-1482. [DOI] [PubMed] [Google Scholar]
- 46.McNulty J, Nair JJ, Singh M, Cranksaw DJ, Holloway AC, Bastida J. Selective cytochrome P450 3°4 inhibitory activity of Amaryllidaceae alkaloids. Bioorg Med Chem Lett. 2009;19:3233–3237. doi: 10.1016/j.bmcl.2009.04.086. [DOI] [PubMed] [Google Scholar]
- 47.Evidente A, Andolfi A, Abou-Donia AH, Touema SM, Hammoda HM, Shawsky E, Motta A. Two betaine-type alakaloids from Egyptian Pancratium maritimum. Phytochemistry. 1992;31:2139–2141. [Google Scholar]
- 48.Abou-Donia AH, Abib AA, El Din AS, Evidente A, Gaber M, Scopa A. (-)-Amarbellisine, a lycorine-type alkaloid from Amaryllis belladonna L. growing in Egypt. Phytochemistry. 2004;65:2113–2118. doi: 10.1016/j.phytochem.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 49.Nakagawa Y, Uyeo S. Stereochemistry of reduction production of 1-acetyl- lycorin-2-one. J Chem Soc. 1959:3736–3741. [Google Scholar]
- 50.Evidente A. Isolation and structural characterization of lutessine, a new alkaloid from bulbs of Sternbergia lutea. J Nat Prod. 1986;49:90–94. [Google Scholar]
- 51.Takeda K, Kotera K, Mizukami S. A partial synthesis of caranine. J Am Chem Soc. 1958;80:2562–2567. [Google Scholar]
- 52.Yoshisuke Y, Takchiro S, Jun'ichi T, Kimiaki I, Jun T, Shuzo T, Masae Y, Mayumi M, Hiroshi I, Hirokazu T. Total synthesis of the Amaryllidaceae alkaloids, lycorine and zephyranthine. J Chem Soc Perkin I. 1979:1358–1363. [Google Scholar]
- 53.Kotera K, Hamada Y, Mitsui R. Absolute configuration of diastereomeric methiodides in the lycorine-type alkaloid. Tetrahedron. 1968;24:2463–2478. doi: 10.1016/s0040-4020(01)82518-7. [DOI] [PubMed] [Google Scholar]
- 54.Wildman WC, Heimer NE. Alkaloid biosynthesis and interconversions. The conversion of caranine to lycorine. J Am Chem Soc. 1967;89:5265–5269. doi: 10.1021/ja00996a032. [DOI] [PubMed] [Google Scholar]
- 55.Fales HM, Wildman WC. Interconversions of Amaryllidaceae alkaloids by sodium and amyl alcohol. J Am Chem Soc. 1958;80:4395–4404. [Google Scholar]
- 56.Ghosal S, Kumar Y, Signh S. Glucosyloxy alkaloids from pancratium biflorum. Phytochemistry. 1984;23:1167–1171. [Google Scholar]
- 57.Uyeo S, Yanaihara N. Phenolic alkaloids occurring in Lycoris radiata. J Chem Soc. 1959:172–177. [Google Scholar]
- 58.Cook JW, Loudon JD, McCloskey P. Dehydration of lycorine. J Chem Soc. 1954:4176–4181. [Google Scholar]
- 59.Fales HM, Warnhoff EW, Wildman WC. Alkaloids of the Amaryllidaceae. IV. The action of oxidizing agents on lycorine and caranine. J Am Chem Soc. 1955;77:5885–5890. [Google Scholar]
- 60.Van Quaquebeke E, Mahieu T, Dumont P, Dewelle J, Ribaucour F, Simon G, Sauvage S, Gaussin JF, Tuti J, El Yazidi M, Van Vynckt F, Mijatovic T, Lefranc F, Darro F, Kiss R. 2,2,2-Trichloro-N-({2-[2-(dimethylamino)ethyl]-1,3-dihydro-1H-benzo[de]iso-quinolin-5yl}carbamoyl)acetamide (UNBS3157), a novel non-hematotoxic naphthalimide derivative with potent anti-tumor activity. J Med Chem. 2007;50:4122–4134. doi: 10.1021/jm070315q. [DOI] [PubMed] [Google Scholar]