Abstract
The present study tested the hypothesis that prenatal nicotine exposure (PNE) induces sex specific alternations in indices of cardiorespiratory coupling during early development. Rat pups exposed to either nicotine (6mg/kg/day) or saline (control) in utero were chronically instrumented with ECG electrodes for measurement of heart rate (HR) and respiratory frequency (RF) was monitored by whole body plethysmography on postnatal days (P)13, P16 and P26. PNE had no identifiable effect on resting respiratory frequency (RF) in either sex. There was however a strong trend (p=0.057) for resting HR to be elevated by PNE in male offspring only. Alternatively, the HR response to hypoxia (10% O2), was significantly blunted at P13 but significantly elevated at P26 s in the absence of any significant change in RF in PNE males only. Indicators of respiratory sinus arrhythmia (RSA) were also significantly reduced in P26 PNE males. No significant effects of PNE on HR, RF or RSA were identified in female offspring at any age. Our results demonstrate that PNE induces very specific changes in cardiorespiratory integration at select postnatal ages and these changes are more prominent in males. Additionally, alternations in cardiorespiratory integration appear to persist into later development in males only, potentially increasing the risk for cardiovascular diseases such as hypertension later in life.
Keywords: heart rate, respiratory sinus arrhythmia, prenatal nicotine, sex differences, heart rate variability, development
INTRODUCTION
A good indicator of cardiorespiratory integration is the presence of respiratory sinus arrhythmia (RSA) (Yasuma and Hayano, 2004). RSA is characterized by the acceleration of heart rate (HR) during inspiration and a subsequent slowing during expiration. In adults, respiratory-related fluctuations in HR are primarily associated with moment-to-moment changes in vagal drive (McAllen and Spyer, 1978). The strength of cardiorespiratory coupling can be evaluated by spectral analysis of the beat to beat interval of HR and quantified by the amplitude or the power in the high frequency range (HF) (Pagani et al., 1986). The coupling between respiratory and cardiovascular control systems can also be identified by the relationship between respiratory frequency and the mean spectral frequency within the HF band following HR spectral analysis (Bernardi et al., 1989, Hirsch and Bishop, 1981). In the rodent RSA is not fully established until late postnatal development (postnatal day 21–30) (Kelly and Richards, 1997, Adolph, 1967, Adolph, 1971, Tucker and Johnson, 1984, Seidler and Slotkin, 1979), yet data from the neonatal rat in vitro suggest the neural circuitry involved in coordinating RSA-related modulation of parasympathetic drive is present as early as postnatal day 5 (P5) and is mediated by increases in inhibitory neurotransmission to cardiac vagal motoneurons during fictive inspiration (Neff et al., 2003). Moreover, this in vitro work suggests that this respiratory-related inhibitory input is selectively modulated by nicotinic receptors (Neff et al., 2003).
In the neonatal rodent, the inspiratory-related increase in GABAergic input to vagal motoneurons in vitro has been shown to be augmented by prenatal nicotine exposure (PNE). This presumably would trigger elevated resting HRs and enhanced cardiorespiratory coupling. Alternatively, PNE has been shown to evoke an accelerated decrease in inspiratory-related inhibitory neurotransmission and recruitment of excitatory drive to cardiac vagal motoneurons after hypoxia/hypercapnia in vitro (Neff et al., 2004). These observations suggest that in addition to altering RSA, another main impact of PNE on early postnatal development may be to increase the risk of fatal bradycardias during exposure to hypoxia. Since approximately 11% of pregnant women continue to smoke during pregnancy (Martin et al., 2003), and PNE has been linked to an increased incidence of sudden infant death syndrome (SIDS), these observations suggest that one impact of PNE may be to significantly alter cardiorespiratory integration. Unfortunately, to date few animal studies have examined the impact of PNE on cardiorespiratory integration beyond the first week of life in vivo, a factor which may be critical since there is some evidence to suggest that the first week of a rodent’s life may actually correspond to the last trimester of human pregnancy (Quinn, 2005). Furthermore, the end of the second week of rodent postnatal life, particularly P13, also appears to be an important period for developmental changes in several cardiorespiratory brain regions (Liu et al., 2006, Liu and Wong-Riley, 2006, Dutschmann et al., 2009). In addition, in rodents, the contribution of the parasympathetic system to resting HR does not appear to be fully integrated until P30 (Nagaoka et al., 2003, Kelly and Richards, 1997), supporting the need for additional studies in PNE animals beyond the first few days of life to investigate whether indicators of altered cardiorespiratory integration can be identified in vivo.
To further our understanding of the impact of PNE on cardiorespiratory integration during early postnatal development the current study was undertaken. Indicators of cardiorespiratory integration at rest and during hypoxia were evaluated in offspring at three postnatal time points. It was hypothesized that PNE would induce developmental differences in both resting RSA and cardiorespiratory responses to hypoxia by the end of the second week of life (P13 and P16), a critical developmental time point in rodent brain development (Liu et al., 2006, Liu and Wong-Riley, 2006, Dutschmann et al., 2009). Furthermore, pups were separated by sex and it was hypothesized that PNE-induced changes would be most dramatic in male offspring but would be resolved by adolescence or P26.
MATERIALS AND METHODS
All experiments were performed on Sprague Dawley rats (Harlan Industries, Minneapolis, IN) housed in the University of Florida (UF) animal care facility. All procedures were approved by the UF Institutional Animal Care and Use Committee (IACUC) prior to use. Rats were maintained under standard environmental conditions (12:12h light:dark cycle) with free access to food and water.
Prenatal Nicotine Exposure
Dams (231±5g) were implanted with a 28 day osmotic mini-pump (2ML4: Durect Corporation Alzet Osmotic Pumps, Cupertino, CA) subcutaneously on day 6 of gestation (of a normal 22 day gestation period). Osmotic mini-pumps were used to prevent the systematic vasoconstriction and decreased birth weights often associated with bolus injections of nicotine (Slotkin et al., 1987). The osmotic pumps contained either nicotine tartrate (PNE, n=16: 6mg/kg/day of nicotine) or vehicle (CON, n=10: 2mL of saline).The nicotine dose was based on previous studies by Slotkin and colleagues (Navarro et al., 1989). The day of parturition was termed P0. On P3 pup sex was determined, and litters were culled to 8 (4 females: 4 males whenever possible) to ensure proper nutrition. Pups were weaned on P21 and housed in same sex pairs. All pups remained with the litter or littermates until the day they were selected for the study; P13, P16 or P26. To minimize litter effects, whenever possible, only one pup of either sex per litter was used for a single developmental time point.
General Instrumentation
On P12, 14, or 24, pups were retrieved from their home cages, anesthetized with isoflurane to a surgical plane, and placed on a warming blanket (37° C). A small incision was made along the dorsal mid-line surface of the neck. Two thin wire electrodes with barred tips (Teflon coated; 0.005 mm diameter, A-M Systems Inc., Carlsborg, WA) were placed subcutaneously, directed toward the forelimbs for electrocardiogram recording (ECG: Lead I). Another thin wire electrode was placed subcutaneously along the dorsal surface of the back to serve as a ground. For P12 and P14 pups, all three electrodes were connected to a back mounted pedestal (E363-BM - PlasticsOne Inc., Roanoke, VA) secured with sutures just between the shoulder blades. At P24, ECG and ground wires were secured into a head-mounted pedestal (MS363 - PlasticsOne Inc) cemented to the skull. Following instrumentation, the incision was closed and pups recovered on a warming blanket and received subcutaneous fluids, analgesics (Buprenorphine, 0.01mg/kg), and topical antibiotic cream. P12 and P14 pups were also fed every half hour with a milk substitute (30% fat milk-based cream) until they began feeding on their own and the mother returned to grooming them once they were returned to the litter.
Data Collection
One (P13) or two days (P16 & P26) following surgery, the pup was removed from its home cage and the back- or head-mounted pedestal was tethered to a single brush 6 channel commutator (SL6C/SB - PlasticsOne Inc.). The pup was then placed in a sealed plethysmograph chamber (Buxco Electronic Inc, Wilmington, NC) ventilated with room air (21% O2) at a rate of 2mL/min. A thin wire thermister was inserted into one of the airflow holes in the plethysmograph to monitor chamber temperature. Normal ambient temperature was maintained in the plethymograph with heat lamps to the temperature appropriate for each age (P13: 33–34°C, P16: 29–30°C, and P25: 22°C) (Frank and Heller, 1997). The plethysmograph was fitted with a pressure transducer (TRD5700 - Buxco Electronic Inc) to allow non-invasive monitoring respiratory frequency (RF). The signal from the ECG leads was directed to a Grass pre-amplifier (P511, Grass Instruments, West Warwick, RI), amplified (1–5,000×) and filtered (0.3–3 KHz). The ECG signal and analog output from the plethysmograph pressure transducer were recorded on-line (500 Hz) via a data acquisition interface (Power1404, Cambridge Electronics Design (CED), Cambridge, UK) connected to a PC computer equipped with a complimentary data analysis software system (Spike2, CED, Cambridge, UK).
All experiments occurred between 1230 –1700 h. Each pup was acclimated to the plethysmograph for at least 20 min. After acclimation, baseline measurements were recorded for 20 min followed immediately by a 5 (P13) or 10 min (P16 and P26) period of hypoxia (air inflow was switched from room air to 10% O2). Oxygen levels were monitored by an oximeter inserted into a chamber air port (AX-300, Teledyne, CA). In the smaller chambers (P13 and P16), it took ~2 min for the exiting O2 to reach 10%. In the larger chambers (P26), it took ~4 min for the exiting O2 to reach 10%. Following completion of the experiment, animals were euthanized with an overdose of pentobarbital (200 mg/kg, i.p.).
Data Analysis
All data were analyzed off-line. HR was calculated from the interval between successive R-wave peaks. RF was calculated from the time interval between plethysmograph pressure troughs, or time of peak inspiration (see Fig.1). Baseline measurements of HR and RF were taken from two separate 2 min segments when the animals were quiet.
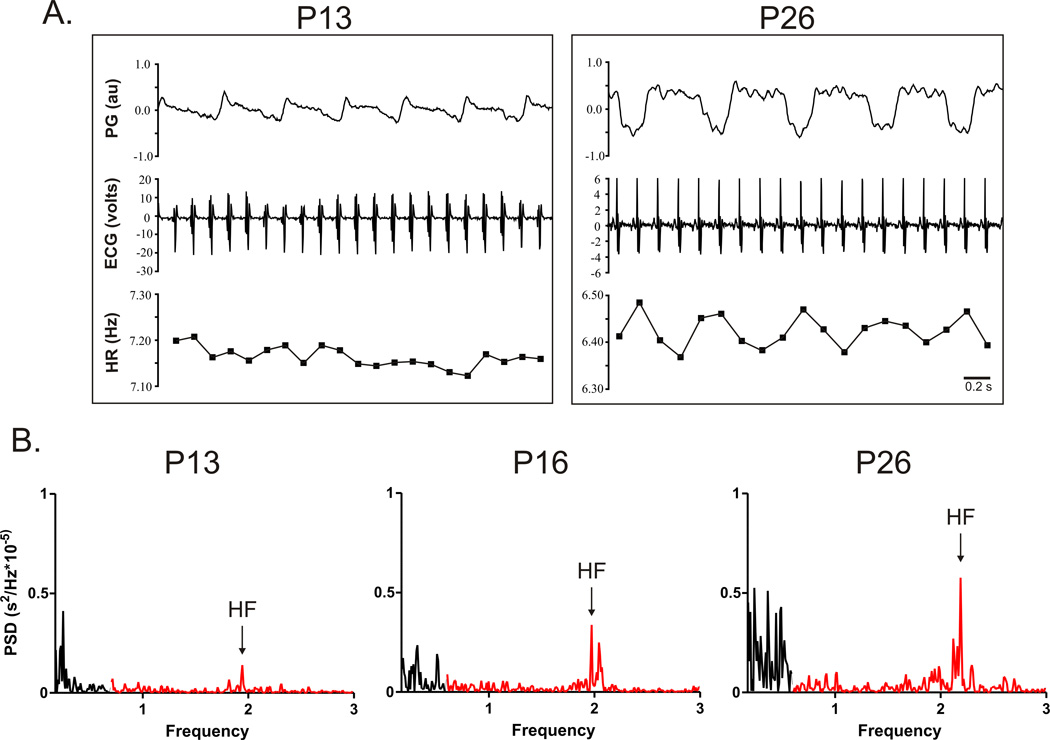
Figure 1. Representative tracings from chronically instrumented rat pups illustrating developmental change in indicators of respiratory sinus arrhythmia (RSA).
A. The tracing on the upper left versus right show the maturation of a noticeable fluctuation in heart rate (HR) with each breath (downward pressure change in the plethysmograph (PG) between P13 and P26. B. Typical changes in individual HRV analysis profiles across development. Power in the high frequency range (HF; 0.6–3 Hz) in shown in red and power in the low frequency range (LF, 0.06–0.6 Hz) is shown in black. Tracings in A reflect data from CON males; tracings in B reflect data from CON females.
Heart rate variability (HRV) analysis was performed on each 2 min baseline period per animal. Tachograms (successive R to R intervals) were generated from each segment, interpolated at 10 Hz, detrended via the smoothness priors formulation (alpha=1000) (Niskanen et al., 2004, Tarvainen et al., 2002) and analyzed using fast Fourier transfer as previously described (Porter et al., 2009). The absolute power within the low frequency (LF; 0.06–0.6 Hz) and high frequency (HF; 0.6–2.4 Hz) range (Akselrod et al., 1981, Malliani et al., 1991) for each of the two baseline segments were then averaged (Dahlstrom et al., 2008). The power in the HF range was used as an indicator of parasympathetic drive and the ratio of LF to HF power (LF/HF) was used as an index of sympatho-vagal balance (Akselrod et al., 1981, Malliani et al., 1991). To evaluate developmental changes in RSA, the ratio of the location of the HF peak frequency (Hz) identified through HRV analysis and the average RF during the same 2 min period was calculated (Pagani et al., 1986).
During hypoxia, two separate 1 min segments were selected for analysis, including a transition time period (Trans) defined as the period immediately after chamber O2 levels began to drop (18%→13%) and then again during the first min of sustained 10% O2 (10%). Averages of HR and RF were measured within each segment and hypoxia-induced changes in HR and RF were then calculated from the difference between values obtained during hypoxia and those values averaged during baseline.
To identify specific effects of PNE on HR, RF, HRV, and RSA during both normoxia and hypoxia either a one-way or two-way (treatment*postnatal day) ANOVA was used within each sex. (Bernardi et al., 1989, Hirsch and Bishop, 1981). When appropriate, a Scheffe’s post-hoc test was used to assess differences between treatment groups. P-value of 0.05 was accepted for significance. All data are presented as means±SEM.
RESULTS
Impact of PNE and sex on resting HR and RF
As shown in Table 1, in both males and females, weight increased significantly with age (p <0.0001) but no significant effect of PNE on weight identified. In males there was no significant effect of age or treatment on resting RF, but there was a strong trend for resting HR to be elevated at rest in PNE versus control pups (p=0.057). Alternatively, in females, independent of treatment, RF was significantly lower at P26 compared to both P13 and P16 (p<0.001). No parallel effect of age or PNE on HR was identified in the females.
TABLE 1.
Baseline Physiological Parameters at Three Developmental Time Points
| MALES | FEMALES | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | Age | N | Weight | HR | RF | N | Weight | HR | RF |
| CON | P13 | 5 | 29±1 | 393±15 | 149±21 | 4 | 29±2 | 431±20 | 146±5 |
| P16 | 5 | 33±2* | 420±8 | 121±8 | 4 | 32±1* | 425±17 | 142±5 | |
| P26 | 5 | 70±3^ | 421±11 | 135±28 | 5 | 65±4^ | 439±20 | 114±9^ | |
| PNE | P13 | 5 | 28±2 | 431±16 | 136±15 | 4 | 25±1 | 403±12 | 128±12 |
| P16 | 5 | 36±2* | 450±14 | 166±22 | 6 | 33±2* | 423±15 | 130±8 | |
| P26 | 5 | 70±3^ | 409±6 | 110±8 | 6 | 61±1^ | 407±14 | 107±8^ | |
All data represented as Mean±SEM.
represents significant difference from P13 and P26 regardless of treatment,
represents significant increase from P13 and P16 regardless of treatment. N= number of individuals, HR = heart rate (beats/min), RF=respiratory frequency (breaths/min).
Developmental changes in HRV and RSA
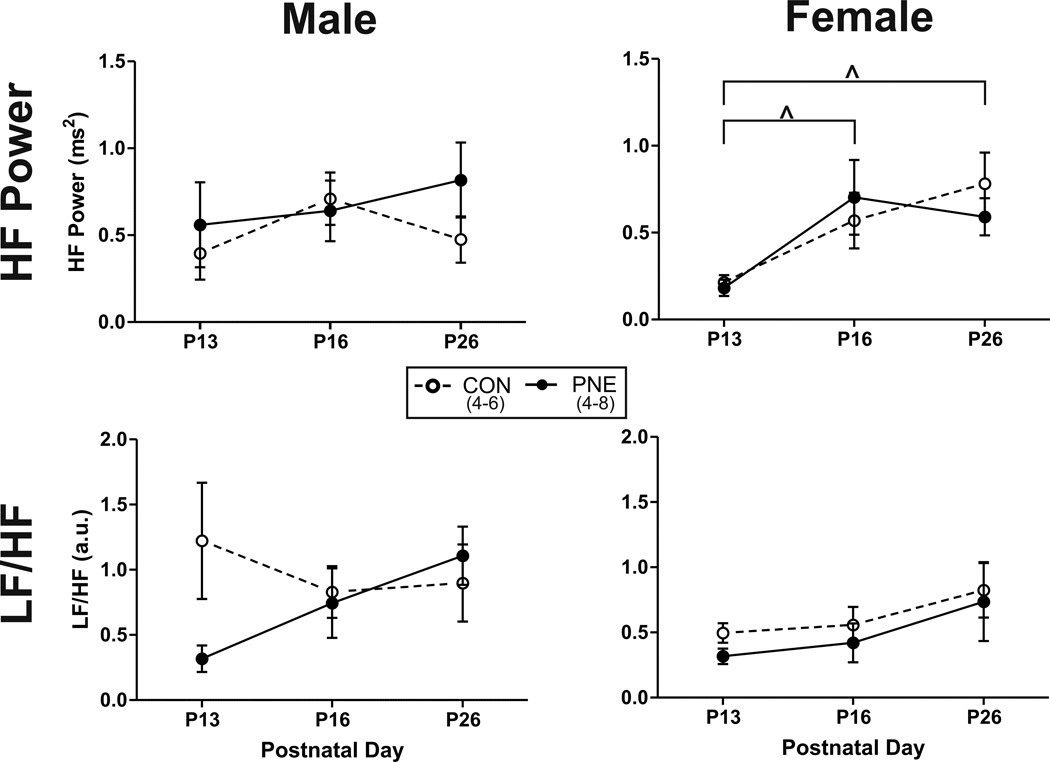
Figure 1A illustrates the typical fluctuations in HR relative to respiration observed in controls at each developmental age. At P13 some fluctuation in HR relative to respiration was observed and by P26 respiratory-related fluctuations in HR were more obvious, reflecting maturation of cardiorespiratory coupling or RSA. The development of observable RSA with increasing age was paralleled by an increase in the peak power in the HF range following HRV. Figure 1B illustrates the typical pattern of HRV at each developmental age and the developmental increase in power in the HF range and the shift in the balance of power in the LF and HF range (LF/HF) from a value ≤1 at P13 to a ratio ≥1 at P26. In both males and females no significant effect of PNE was identified for either HF power or LF/HF ratio (Figure 2). In females however, independent of treatment, there was a significant effect of age on HF power from P13 compared to P16 (P=0.008) and P26 (P=0.004). No age dependent effect on HRV was identified in males.
Figure 2. Impact of PNE on developmental changes in heart rate variability (HRV) during normoxia in both males and females.
Males are represented in the left columns and females are on the right. Open circles and dashed lines illustrate mean values from controls (CON) and closed circles and solid lines represent PNE data. ^ represents a significant difference from P13.
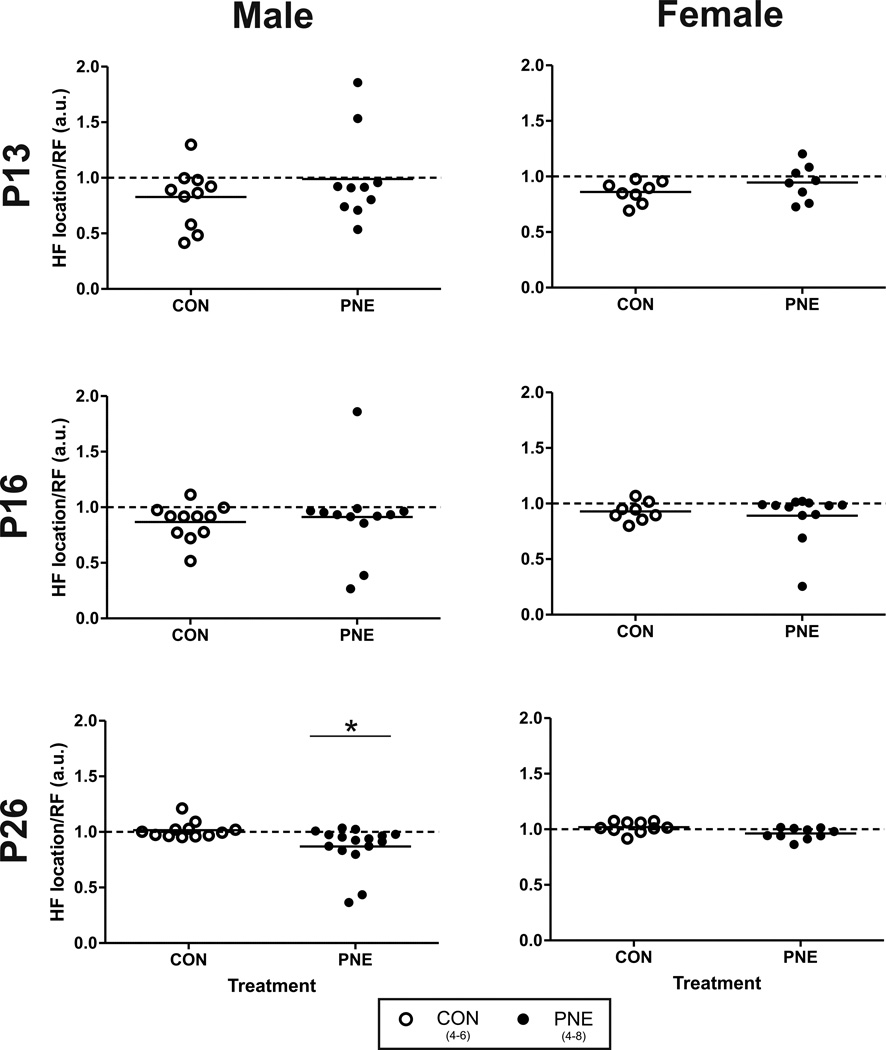
The presence of RSA was further evaluated by plotting the ratio of the location of the HF peak (depicted by arrows in Figure 1B: left panel) and the average RF from the same data segment; ratios approaching a value of 1 suggest a high correlation between the periodicity of HR and RF. As shown in Figure 3, at P13 and P16 the average HF/RF ratio was slightly below 1 and there was considerable variability but no significant effect of PNE on these ratios in male or female offspring. At P26 however the mean ratio was significantly lower in PNE males compared to CON (P=0.03). In contrast, at P26, no significant effect of PNE on the HF/RF ratio was found in female animals.
Figure 3. The effect of PNE on the relationship between the location of the high frequency (HF) component of HRV and respiratory frequency (RF) during normoxia.
Left columns represent data from male offspring across development and female offspring are represented in the right column. Open circles show data from control (CON) animals. Closed circles are data from PNE offspring. *represents a significant difference from CON animals.
Cardiorespiratory responses to hypoxia
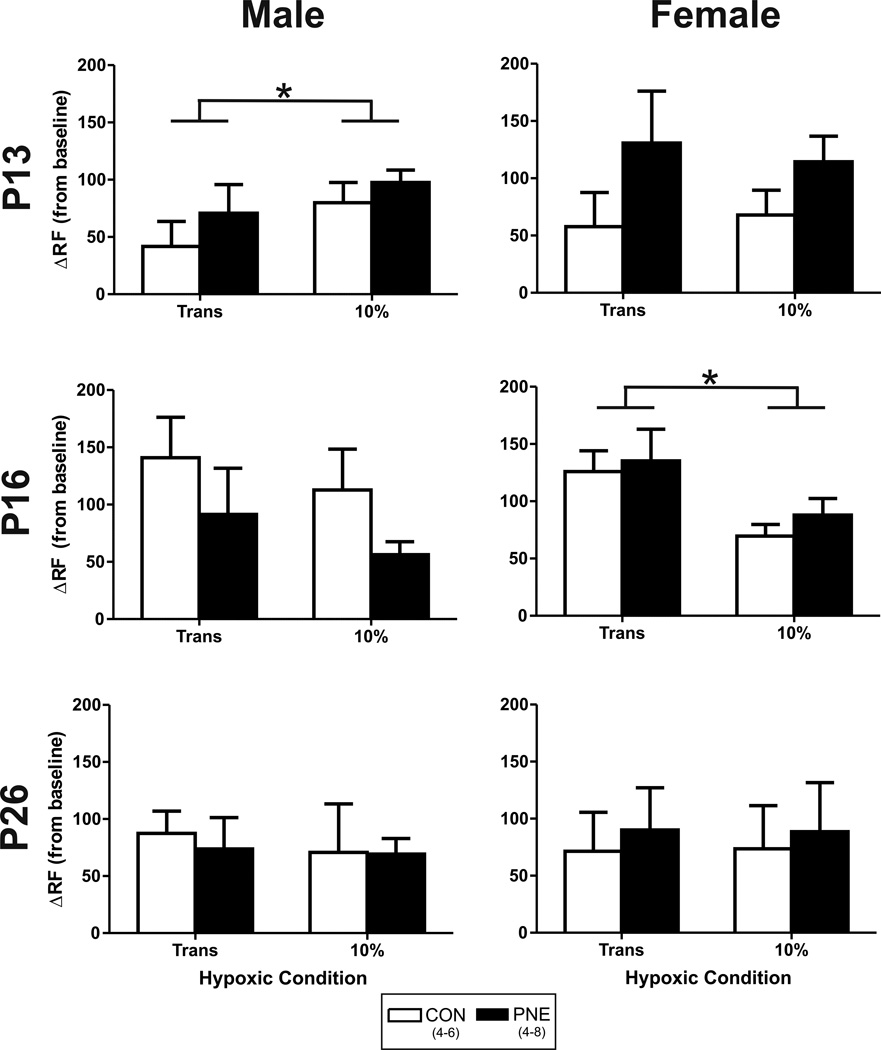
The typical response to hypoxia at P13 in CON versus PNE males is shown in Figure 4. Both animals responded to hypoxia with an increase in RF and HR. In the CON pup however HR appeared to rise at a much faster rate during the initial phase of hypoxia compared to the PNE pup. Conversely, no obvious differences were seen in the mean rise in RF during the onset of hypoxia. The average change in HR during hypoxia as a function of resting HR is shown in Figure 5. At P13 (Figure 5, top left side) a significant effect of both time in hypoxia (Trans vs 10%; P=0.01; Figure 5: top left panel) and treatment on HR in male pups was identified (P=0.05), but the interaction of the two factors was not significant (P=0.88). Further analysis identified that HR increased significantly as the level of hypoxia increased and the overall response of the PNE male pups was significantly blunted compared to the CON males. At P16 the significant effect of time in hypoxia was still present (HR increased significantly from Trans to 10%; P=0.0004), but the effect of PNE on HR in the males was no longer significant (P>0.05). Alternatively, at P26, there was a significant interaction between time and treatment (P=0.01) and PNE males demonstrated a significant increase in HR over time (P=0.04) and the mean increase in HR from baseline was significantly greater at 10% hypoxia in the PNE pups compared to CON animals (P=0.04). There was no significant effect of time on HR in the CON animals at P26.
Figure 4. Representative tracings from one CON (A) and one PNE (B) male pup at postnatal day 13 during an acute hypoxic exposure.
Tracings include heart rate (HR), respiratory frequency (RF) and pressure fluctuations associated with respiratory effort in the plethysmograph (PG).
Figure 5. Effect of PNE on the heart rate (HR) response to hypoxia.
Data derived from the change from baseline. Open bars show average data from control (CON) animals. Closed bars represent PNE animal data. * represents a significant effect of O2 level regardless of treatment (P<0.05). ^ indicates a significant treatment effect regardless of O2 level (P=0.05). # indicates a treatment effect during 10% hypoxia (P=0.02). † indicates a significant effect of O2 level only in PNE animals (P=0.04).
No significant effect of PNE on the HR response to hypoxia was identified in the female offspring at any of the postnatal days examined. Independent of PNE however, at P13 (Figure 5: top right panel), there was a strong trend for HR to increase with time (P=0.06) and at P16, all female pups significantly increased HR over time (from Trans to 10%, P=0.0006). At P26 however, the effect of time in hypoxia on HR was no longer significant (P>0.05).
Unlike HR, no significant effect of PNE on RF in response to hypoxia was identified in either sex (see Figure 6), but at P13 and P16 significant effects of time on RF were identified. Specifically, at P13, all male pups (regardless of treatment) significantly increased their RF from the Trans period to 10% hypoxia (P=0.03). By P16 and P26, however no further effect of time was identified. Conversely, females demonstrated no significant differences in RF from Trans to 10% oxygen at P13 or P26. At P16, the effect of time on RF however was significant (P=0.012), reflecting a decrease in RF from the Trans period to 10%.
Figure 6. Effect of PNE on respiratory frequency (RF) in response to hypoxia.
Data reflects change from baseline. Open bars show average data from control (CON) animals. Closed bars represent average data from PNE animals. * represents a significant effect of O2 level regardless of treatment (P<0.05).
DISCUSSION
The major finding of the present study is that the effect of PNE on cardiorespiratory integration in vivo during early postnatal development is sex-dependent and primarily effects male offspring only. Sex-specific PNE effects included a trend toward an overall increase in resting HR across development, a reduction the HF/RF ratio at P26, and a significant blunting at P13 versus a significant elevation at P26 of the HR response to acute hypoxia. These results support previous observations that the effects of PNE on cardiorespiratory function are identifiable during the second week of life (P13), a critical developmental time point in rodent brain development (Liu et al., 2006, Liu and Wong-Riley, 2006, Dutschmann et al., 2009). These results also corroborate finding from in vitro studies that GABAergic drive to cardiac vagal motoneurons may be elevated at baseline in P13 PNE offspring but during hypoxia inhibitory inputs are reduced (Neff et al., 2004). Somewhat unexpected however was the observation that the autonomic effects of PNE lingered into the third week of life, at which point both a significant reduction in indicators of RSA and an elevation in the HR response to hypoxia in males only was identified. This observation provides the first indication that markers of sustained cardiovascular dysfunction previously reported to be present in adult PNE offspring (Pausova et al., 2003, Xiao et al., 2008) may actually be identifiable as early as in adolescence and may be limited to males.
PNE and developmental changes in resting HR, RF and RSA
Previous developmental studies investigating postnatal changes in autonomic function have identified that HR increases most notably from birth through P10–13 (Kelly and Richards, 1997). Beyond P13, resting HR slowly declines as the parasympathetic system begins to integrate with the sympathetic system (Kelly and Richards, 1997, Kelly and Richards, 1998). Similarly, a developmental peak in resting RF was reported to occur sometime during the second postnatal week in rodents (Liu et al., 2006, Huang et al., 2004), suggesting that the P10–P14 age may be a critical time period for cardiorespriatory integration. In the present study no significant age-dependent change in resting HR was identified between P13–26 in either CON males or females, an observation that supports previous reports from similarly aged rodents (Kelly and Richards, 1998, Kelly and Richards, 1997, Longin et al., 2006). However, when HRV analysis was used to evaluate changes in autonomic balance with age, subtle sex-based differences were identified. For example, only females demonstrated a significant increase in HF power from P13 to P16 and P26. This result might be expected, as stated above, since tonic parasympathetic input has been reported to begin to integrate into autonomic control around P15 and increase out to ~P21 in rodents (Kelly and Richards, 1997, Adolph, 1971). This was paralleled by a significant developmental drop in resting RF between P16 and P26 in females only. A similar pattern however was not observed in the males, suggesting that development of cardiorespiratory integration may follow a different development time course in males versus females.
Interestingly, evaluation of resting cardiorespiratory function in PNE offspring at P13 and P16 did not identify any significant developmental changes in either sex. Alternatively, PNE did significantly reduce the ratio of the mean HF power/ RF, an index of RSA in males only at P26. These data agree with previous studies demonstrating that other prenatal insults can change the development of RSA at specific time points (Kelly and Richards, 1998). Since low RSA is an indicator of poor cardiovascular health, this present study provides further evidence that PNE may trigger a phenotype with a predisposition to for developing cardiovascular disease, specifically in males (Pausova et al., 2003, Xiao et al., 2008), and suggests that HRV analysis, specifically the determination of RSA indexes, during adolescence may predict abnormalities in autonomic function after PNE.
PNE and developmental changes in the response to acute hypoxia
As stated above, all indications are that the parasympathetic system does not appear to play a major role in resting HR during early postnatal development (< P16) in rodents (Nagaoka et al., 2003, Kelly and Richards, 1997). Parasympathetic drive can however, be recruited in response to hypoxia. In a recent study by Fewell and colleagues, for example, cervical vagotomy was shown to significantly elevate the HR response to 5 min of hypoxia in both P1–P2 and P10–P11 rodent pups, in the absence of changes in baseline HR (Fewell et al., 2007). In the present study, both males and females at all ages demonstrated increased HR and RF throughout hypoxia compared to resting conditions. However, following PNE at P13 there was a significant blunting of the HR response to hypoxia. This PNE-related blunting of the HR response to hypoxia has been previously reported in P1–P5 rodents (Slotkin et al., 1997, Hafstrom et al., 2002). However, our results document for the first time that this PNE-induced attenuation of HR is sex-specific (only occurs in males) and persists beyond the first few days of life (at least until P13). The mechanism(s) underlying this sex-based difference is currently unknown but similar disturbances have been reported following other perinatal insults such as early postnatal hypoxia, (Bavis et al., 2004). Thus, it appears that females may be better suited to adapt to perinatal insults compared to their male counterparts. It should be noted however, that because the observations from the present study occurred prior to the onset of puberty, it is unlikely that the potential for greater plasticity in females is exclusively estrogen based.
Finally, the results of the present study identified that PNE-induced a sustained increase in the HR during hypoxia in males at P26. In CON animals the HR response to hypoxia at P26 was characterized by an initial increase in HR, followed by a decline in HR as hypoxia persisted, Conversely, PNE males expressed a progressive rise in HR as oxygen levels fell. This sustained effect of PNE in males was not originally predicted; however in retrospect these results may not be all that surprising. For example, epidemiological data suggests that PNE can have long-term effects, including adolescent hyperactivity and attention deficient disorders (Ernst et al., 2001, DiFranza et al., 2004), reduced memory and spatial ability (Cutler et al., 1996), and increased incidence of cigarette use (Oncken et al., 2004). Moreover, some of these longer lasting effects are sex specific (Oncken et al., 2004, Eppolito and Smith, 2006). Morphological evidence from rodents suggests that these behavioral effects of PNE may be caused by first by significant increase in cellular death within specific brain regions during neuralation (Roy et al., 1998); and second by slight changes in neural maturation during adolescence (P21), including a reduction in neuronal cell size and increased numbers of glia (Roy et al., 2002). Moreover, it has been suggested that PNE alters the tone of cholinergic neurons through a programmed disruption of cholinergic activity that develops during early adolescence (Abreu-Villaca et al., 2004a), but is not evident during early postnatal development (Navarro et al., 1989). Similar markers of synaptic abnormalities appear in the serotonergic system and both cholinergic and serotonergic deficiencies appear to be more prominent in males than females (Slotkin et al., 2007, Abreu-Villaca et al., 2004b, Slotkin et al., 2006). Additionally, both basal and neurotransmitter-stimulated activity of adenylyl cyclase has been reported to decline after PNE, but only during adolescence, suggesting a dysfunction in overall neuronal signaling and not just a deficiency in cholinergic or serotonergic activity (Slotkin et al., 2006).
It is possible that similar mechanisms underlie the present results, however, to date only a few studies have examined the impact of PNE on cardiovascular health. Indeed, one study demonstrated that a PNE-associated reduction in cardiac adrenergic sensitivity persists even into adulthood (Britos et al., 1992) and another identified an angiotensin-II dependent hyperresponsiveness that occurs only in male PNE offspring (Xiao et al., 2008). Thus, the elevated HR response documented here in PNE males during hypoxia may be mediated by chronic changes in central sympathetic drive elicited either as a compensatory response to reduced cardiac sensitivity or abnormalities in the brain-angiotensin system. Of particular importance, in regards to the results of the present study, is the potential for PNE to predispose male offspring to an elevated incidence of cardiovascular disease in later life (Barker, 2000).
Methodical Considerations
A main factor to take into consideration when evaluating the results from the present study is the time allowed for recovery after surgery. The pups were chronically instrumented for a more realistic evaluation of HR regulation in the conscious unrestrained animal. However, because of difficulties in pup weight gain when instrumentation took place prior to P12, animals recorded at P13 were only allowed one day of recovery. Although both treatment groups received surgery at the same developmental time point, there is some evidence to suggest that prenatal stress affects the responsiveness of the hypothalamic-pituitary adrenal axis (Xiao et al., 2008). Thus, it is possible that some of the alterations seen as a result of PNE were due to different responses to the stress of surgery. However, we tried to minimize all stress associated with instrumentation by making sure that the pups were returned to their home cage and received maternal care following their return. Moreover, any differences in stress responses to surgery may have been minimal since our results are similar to those in other animals models (Hafstrom et al., 2002) and uninstrumented pups (Fuller et al., 2009). Additionally, surgery recovery time has not been shown to effect developmental changes in autonomic function (Egbert and Katona, 1980).
CONCLUSIONS
The results of the present study demonstrate for the first time that development of the parasympathetic control of HR at rest and in response to hypoxia is subtly different in young males and females and as a consequence the sex of an individual should always be factored into the analysis of future cardiovascular developmental studies. The results of the present study also extend previous reports of alterations in the hypoxic response after PNE to later developmental time points, including the persistence of a reduced tachycardic response to hypoxia in the second postnatal week and a novel reversal of this response to an exaggerated tachycardic response to hypoxia in early adolescence. These results are in agreement with previous theories (Guntheroth and Spiers, 2002) that early postnatal dysregulation induced by certain risk factors, such as PNE, may predispose infants of a certain age to severe bradycardia during hypoxic challenges. This dysregulation may be related to sex specific alterations in the timing of developmental integration of cardiorespiratory networks. Finally, the effects of PNE on autonomic function were identified to persist into adolescence only in male offspring, an observation that may provide new insights into an increased incidence of cardiovascular disease in certain adult populations, mainly those exposed to prenatal nicotine.
Highlights.
PNE did not affect resting cardiorespiratory variables at postnatal day 13 or 16.
PNE blunted the heart response to hypoxia in males only at postnatal day 13.
PNE reduced respiratory sinus arrhythmia in males during early adolescence.
PNE augments the heart rate responses to hypoxia during early adolescence.
ACKNOWLEDGEMENTS
Authors thank Mr. Jessie Stanley for his technical help on the project and acknowledge grant support from the University of Florida Alumni Scholarship, Florida Department of Health James and Esther King Biomedical Research Program and NIH (HL-76518).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abreu-Villaca Y, Seidler FJ, Slotkin TA. Does prenatal nicotine exposure sensitize the brain to nicotine-induced neurotoxicity in adolescence? Neuropsychopharmacology. 2004a;29:1440–1450. doi: 10.1038/sj.npp.1300443. [DOI] [PubMed] [Google Scholar]
- Abreu-Villaca Y, Seidler FJ, Tate CA, Cousins MM, Slotkin TA. Prenatal nicotine exposure alters the response to nicotine administration in adolescence: effects on cholinergic systems during exposure and withdrawal. Neuropsychopharmacology. 2004b;29:879–890. doi: 10.1038/sj.npp.1300401. [DOI] [PubMed] [Google Scholar]
- Adolph EF. Ranges of heart rates and their regulations at various ages (rat) Am J Physiol. 1967;212:595–602. doi: 10.1152/ajplegacy.1967.212.3.595. [DOI] [PubMed] [Google Scholar]
- Adolph EF. Ontogeny of heart-rate controls in hamster, rat, and guinea pig. Am J Physiol. 1971;220:1896–1902. doi: 10.1152/ajplegacy.1971.220.6.1896. [DOI] [PubMed] [Google Scholar]
- Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- Barker DJ. In utero programming of cardiovascular disease. Theriogenology. 2000;53:555–574. doi: 10.1016/s0093-691x(99)00258-7. [DOI] [PubMed] [Google Scholar]
- Bavis RW, Olson EB, Jr, Vidruk EH, Fuller DD, Mitchell GS. Developmental plasticity of the hypoxic ventilatory response in rats induced by neonatal hypoxia. J Physiol. 2004;557:645–660. doi: 10.1113/jphysiol.2004.061408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi L, Keller F, Sanders M, Reddy PS, Griffith B, Meno F, Pinsky MR. Respiratory sinus arrhythmia in the denervated human heart. J Appl Physiol. 1989;67:1447–1455. doi: 10.1152/jappl.1989.67.4.1447. [DOI] [PubMed] [Google Scholar]
- Britos SA, Keller EA, Orsingher OA. Prenatal nicotine exposure induces cardiac adrenergic subsensitivity in adult rats. Acta Physiol Pharmacol Ther Latinoam. 1992;42:217–224. [PubMed] [Google Scholar]
- Cutler AR, Wilkerson AE, Gingras JL, Levin ED. Prenatal cocaine and/or nicotine exposure in rats: preliminary findings on long-term cognitive outcome and genital development at birth. Neurotoxicol Teratol. 1996;18:635–643. doi: 10.1016/s0892-0362(96)00125-0. [DOI] [PubMed] [Google Scholar]
- Dahlstrom A, Ebersjo C, lundell B. Nicotine in breast milk influences heart rate variability in the infant. Acta Paediatr. 2008;97:1075–1079. doi: 10.1111/j.1651-2227.2008.00785.x. [DOI] [PubMed] [Google Scholar]
- Difranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children's health. Pediatrics. 2004;113:1007–1015. [PubMed] [Google Scholar]
- Dutschmann M, Morschel M, Rybak IA, Dick TE. Learning to breathe: control of the inspiratory-expiratory phase transition shifts from sensory- to central-dominated during postnatal development in rats. J Physiol. 2009;587:4931–4948. doi: 10.1113/jphysiol.2009.174599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egbert JR, Katona PG. Development of autonomic heart rate control in the kitten during sleep. Am J Physiol. 1980;238:H829–H835. doi: 10.1152/ajpheart.1980.238.6.H829. [DOI] [PubMed] [Google Scholar]
- Eppolito AK, Smith RF. Long-term behavioral and developmental consequences of pre- and perinatal nicotine. Pharmacol Biochem Behav. 2006;85:835–841. doi: 10.1016/j.pbb.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adolesc Psychiatry. 2001;40:630–641. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Fewell JE, Zhang C, Gillis AM. Influence of adenosine A(1)-receptor blockade and vagotomy on the gasping and heart rate response to hypoxia in rats during early postnatal maturation. J Appl Physiol. 2007;103:1234–1241. doi: 10.1152/japplphysiol.01421.2006. [DOI] [PubMed] [Google Scholar]
- Frank MG, Heller HC. Development of diurnal organization of EEG slow-wave activity and slow-wave sleep in the rat. Am J Physiol. 1997;273:R472–R478. doi: 10.1152/ajpregu.1997.273.2.R472. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Dougherty BJ, Sandhu MS, Doperalski NJ, Reynolds CR, Hayward LF. Prenatal nicotine exposure alters respiratory long-term facilitation in neonatal rats. Respir Physiol Neurobiol. 2009;169:333–337. doi: 10.1016/j.resp.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntheroth WG, Spiers PS. The triple risk hypotheses in sudden infant death syndrome. Pediatrics. 2002;110:e64. doi: 10.1542/peds.110.5.e64. [DOI] [PubMed] [Google Scholar]
- Hafstrom O, Milerad J, Sundell HW. Prenatal nicotine exposure blunts the cardiorespiratory response to hypoxia in lambs. Am J Respir Crit Care Med. 2002;166:1544–1549. doi: 10.1164/rccm.200204-289OC. [DOI] [PubMed] [Google Scholar]
- Hirsch JA, Bishop B. Respiratory sinus arrhythmia in humans: how breathing pattern modulates heart rate. Am J Physiol. 1981;241:H620–H629. doi: 10.1152/ajpheart.1981.241.4.H620. [DOI] [PubMed] [Google Scholar]
- Huang YH, Brown AR, Costy-Bennett S, Luo Z, Fregosi RF. Influence of prenatal nicotine exposure on postnatal development of breathing pattern. Respir Physiol Neurobiol. 2004;143:1–8. doi: 10.1016/j.resp.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Richards JE. Development of heart inter-beat interval variability in preweanling rats: effects of exposure to alcohol and hypoxia. Physiol Behav. 1997;61:231–241. doi: 10.1016/s0031-9384(96)00365-4. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Richards JE. Heart rate orienting and respiratory sinus arrhythmia development in rats exposed to alcohol or hypoxia. Neurotoxicol Teratol. 1998;20:193–202. doi: 10.1016/s0892-0362(97)00090-1. [DOI] [PubMed] [Google Scholar]
- Liu Q, Lowry TF, Wong-Riley MT. Postnatal changes in ventilation during normoxia and acute hypoxia in the rat: implication for a sensitive period. J Physiol. 2006;577:957–970. doi: 10.1113/jphysiol.2006.121970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Developmental changes in the expression of GABAA receptor subunits alpha1, alpha2, and alpha3 in brain stem nuclei of rats. Brain Res. 2006;1098:129–138. doi: 10.1016/j.brainres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Longin E, Gerstner T, Schaible T, Lenz T, Konig S. Maturation of the autonomic nervous system: differences in heart rate variability in premature vs. term infants. J Perinat Med. 2006;34:303–308. doi: 10.1515/JPM.2006.058. [DOI] [PubMed] [Google Scholar]
- Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84:482–492. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Munson ML. Births: final data for 2002. Natl Vital Stat Rep. 2003;52:1–113. [PubMed] [Google Scholar]
- Mcallen RM, Spyer KM. Two types of vagal preganglionic motoneurones projecting to the heart and lungs. J Physiol. 1978;282:353–364. doi: 10.1113/jphysiol.1978.sp012468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka S, Bito Y, Sakuma R, Nomura H, Hata T, Nishiyama J, Hirata Y. Comparative physiology of postnatal developments of cardiopulmonary reflex. Biol Sci Space. 2003;17:265–266. [PubMed] [Google Scholar]
- Navarro HA, Seidler FJ, Schwartz RD, Baker FE, Dobbins SS, Slotkin TA. Prenatal exposure to nicotine impairs nervous system development at a dose which does not affect viability or growth. Brain Res Bull. 1989;23:187–192. doi: 10.1016/0361-9230(89)90146-9. [DOI] [PubMed] [Google Scholar]
- Neff RA, Simmens SJ, Evans C, Mendelowitz D. Prenatal nicotine exposure alters central cardiorespiratory responses to hypoxia in rats: implications for sudden infant death syndrome. J Neurosci. 2004;24:9261–9268. doi: 10.1523/JNEUROSCI.1918-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff RA, Wang J, Baxi S, Evans C, Mendelowitz D. Respiratory sinus arrhythmia: endogenous activation of nicotinic receptors mediates respiratory modulation of brainstem cardioinhibitory parasympathetic neurons. Circ Res. 2003;93:565–572. doi: 10.1161/01.RES.0000090361.45027.5B. [DOI] [PubMed] [Google Scholar]
- Niskanen JP, Tarvainen MP, Ranta-Aho PO, Karjalainen PA. Software for advanced HRV analysis. Comput Methods Programs Biomed. 2004;76:73–81. doi: 10.1016/j.cmpb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Oncken C, Mckee S, Krishnan-Sarin S, O'malley S, Mazure C. Gender effects of reported in utero tobacco exposure on smoking initiation, progression and nicotine dependence in adult offspring. Nicotine Tob Res. 2004;6:829–833. doi: 10.1080/1462220042000282555. [DOI] [PubMed] [Google Scholar]
- Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell'orto S, Piccaluga E, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res. 1986;59:178–193. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- Pausova Z, Paus T, Sedova L, Berube J. Prenatal exposure to nicotine modifies kidney weight and blood pressure in genetically susceptible rats: a case of gene-environment interaction. Kidney Int. 2003;64:829–835. doi: 10.1046/j.1523-1755.2003.00172.x. [DOI] [PubMed] [Google Scholar]
- Porter K, Ahlgren J, Stanley J, Hayward LF. Modulation of heart rate variability during severe hemorrhage at different rates in conscious rats. Auton Neurosci. 2009;150:53–61. doi: 10.1016/j.autneu.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn R. Comparing rat's to human's age: how old is my rat in people years? Nutrition. 2005;21:775–777. doi: 10.1016/j.nut.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Roy TS, Andrews JE, Seidler FJ, Slotkin TA. Nicotine evokes cell death in embryonic rat brain during neurulation. J Pharmacol Exp Ther. 1998;287:1136–1144. [PubMed] [Google Scholar]
- Roy TS, Seidler FJ, Slotkin TA. Prenatal nicotine exposure evokes alterations of cell structure in hippocampus and somatosensory cortex. J Pharmacol Exp Ther. 2002;300:124–133. doi: 10.1124/jpet.300.1.124. [DOI] [PubMed] [Google Scholar]
- Seidler FJ, Slotkin TA. Presynaptic and postsynaptic contributions to ontogeny of sympathetic control of heart rate in the pre-weanling rat. Br J Pharmacol. 1979;65:431–434. doi: 10.1111/j.1476-5381.1979.tb07847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Mackillop EA, Rudder CL, Ryde IT, Tate CA, Seidler FJ. Permanent, sex-selective effects of prenatal or adolescent nicotine exposure, separately or sequentially, in rat brain regions: indices of cholinergic and serotonergic synaptic function, cell signaling, and neural cell number and size at 6 months of age. Neuropsychopharmacology. 2007;32:1082–1097. doi: 10.1038/sj.npp.1301231. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Orband-Miller L, Queen KL, Whitmore WL, Seidler FJ. Effects of prenatal nicotine exposure on biochemical development of rat brain regions: maternal drug infusions via osmotic minipumps. J Pharmacol Exp Ther. 1987;240:602–611. [PubMed] [Google Scholar]
- Slotkin TA, Saleh JL, Mccook EC, Seidler FJ. Impaired cardiac function during postnatal hypoxia in rats exposed to nicotine prenatally: implications for perinatal morbidity and mortality, and for sudden infant death syndrome. Teratology. 1997;55:177–184. doi: 10.1002/(SICI)1096-9926(199703)55:3<177::AID-TERA2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Tate CA, Cousins MM, Seidler FJ. Prenatal nicotine exposure alters the responses to subsequent nicotine administration and withdrawal in adolescence: Serotonin receptors and cell signaling. Neuropsychopharmacology. 2006;31:2462–2475. doi: 10.1038/sj.npp.1300988. [DOI] [PubMed] [Google Scholar]
- Tarvainen MP, Ranta-Aho PO, Karjalainen PA. An advanced detrending method with application to HRV analysis. IEEE Trans Biomed Eng. 2002;49:172–175. doi: 10.1109/10.979357. [DOI] [PubMed] [Google Scholar]
- Tucker DC, Johnson AK. Development of autonomic control of heart rate in genetically hypertensive and normotensive rats. Am J Physiol. 1984;246:R570–R577. doi: 10.1152/ajpregu.1984.246.4.R570. [DOI] [PubMed] [Google Scholar]
- Xiao D, Xu Z, Huang X, Longo LD, Yang S, Zhang L. Prenatal gender-related nicotine exposure increases blood pressure response to angiotensin II in adult offspring. Hypertension. 2008;51:1239–1247. doi: 10.1161/HYPERTENSIONAHA.107.106203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuma F, Hayano J. Respiratory sinus arrhythmia: why does the heartbeat synchronize with respiratory rhythm? Chest. 2004;125:683–690. doi: 10.1378/chest.125.2.683. [DOI] [PubMed] [Google Scholar]