Abstract
Acute gastroenteritis caused by Salmonella infection is a significant public health problem. Using a mouse model of this condition, the authors demonstrated previously that the cytokine gamma interferon (IFN-γ) is required for a normal intestinal inflammatory response to the pathogen. In the present study, these experiments are extended to show that natural killer (NK) cells constitute an early source of intestinal IFN-γ during Salmonella infection, and that these cells have a significant impact on intestinal inflammation. It was found that infection of mice with Salmonella increased both intestinal IFN-γ production and the numbers of NK cells in the intestine and mesenteric lymph nodes. NK cells, along with other types of lymphocytes, produced IFN-γ in response to the bacteria in vitro, while antibody-mediated depletion of NK cells in vivo resulted in a significant reduction in Salmonella-induced intestinal IFN-γ expression. In a mouse strain lacking NK cells and T and B lymphocytes, intestinal production of IFN-γ and Salmonella-induced intestinal inflammation were both significantly decreased compared with a strain deficient only in T and B cells. The authors’ observations point to an important function for NK cells and NK-derived IFN-γ in regulating the intestinal inflammatory response to Salmonella.
Keywords: Salmonella, NK cells, intestinal inflammation
Introduction
Salmonella enterica serovar Typhimurium infection in humans, which is typically acquired by ingestion of contaminated food or water, leads to a diarrheal illness that is responsible for c. 40 000 cases of acute gastroenteritis annually in the United States (Rabsch et al., 2001). The hallmark of this condition is an acute, usually self-limited, intestinal inflammatory response that helps to eliminate the infection, but that also gives rise to the characteristic manifestations of diarrhea, vomiting and abdominal pain. The generation of this response involves a complex interplay between microbial virulence factors and the host immune system (Srikanth & Cherayil, 2007).
In the initial stages of the infection, Salmonella-induced alterations of the cytoskeleton of intestinal epithelial cells lead to uptake and ultimate transcytosis of the bacteria across the epithelium into the lamina propria (Patel & Galan, 2005). During this process, activation of host pattern recognition receptors that are expressed on both epithelial and subepithelial cells results in the secretion of chemoattractant molecules that recruit neutrophils, monocytes, dendritic cells and lymphocytes from the circulation to the site of infection. These molecules include cytokines and chemokines such as tumor necrosis factor (TNF)-α, IL-8, CXCL-2 and monocyte chemoattractant protein 1 (MCP-1), and also various lipid mediators produced from arachidonic acid and its metabolites (McCormick & Mrsny, 2006; Srikanth & Cherayil, 2007). The recruited phagocytes engulf and destroy the invading bacteria, and initiate the process of healing and tissue restitution, while the dendritic cells and lymphocytes contribute to the development of Th1-type adaptive immunity, a key aspect of defense against Salmonella in mice and humans (Mastroeni, 2002; van de Vosse et al., 2004). Detailed understanding of how this complex response is orchestrated is necessary in order to advance the development of new approaches to preventing and treating the clinical abnormalities associated with salmonellosis.
The cellular and molecular analyses of Salmonella-induced intestinal inflammation have been greatly facilitated by the recent development of a mouse model that allows dissection of the bacterial and host factors involved in pathogenesis (Barthel et al., 2003). In this model, adult mice are given a single oral dose of streptomycin, followed by oral infection with the streptomycin-resistant SL1344 strain of S. Typhimurium. An acute colitis develops and reaches its peak at about 48 h after infection. It manifests most prominently in the cecum and has many of the pathologic features of Salmonella enterocolitis in humans. This experimental system has confirmed the importance of the Salmonella type III secretion systems and flagellin-based motility in inducing the inflammatory response, and has revealed an important role for MyD88-dependent signals activated by host Toll-like receptors (TLRs) (Stecher et al., 2004; Hapfelmeier et al., 2005). Using this model, it was recently shown that IFN-γ plays an important role in the development of Salmonella enterocolitis. In mice lacking this cytokine, Salmonella-induced intestinal expression of mediators such as TNF-α is diminished, neutrophil recruitment is impaired and the intestinal inflammation is significantly attenuated (Rhee et al., 2005). In this work, these experiments are extended to identify the cellular source of IFN-γ in the gut during Salmonella infection.
Materials and methods
Animals
Wild-type C57BL/6 mice, IFN-γ knock-out mice on the C57BL/6 background, as well as the T and B cell-deficient NOD.CB17-Prkdcscid/J and the T, B and natural killer (NK) cell-deficient NOD.Cg-PrkdcscidIL2rgtm1Wjl/SzJ congenic strains (referred to hereafter as SCID and SCID/γc −/− mice, respectively) were purchased from the Jackson Laboratory (Bar Harbor, ME) and housed in a specific pathogen-free facility at the Massachusetts General Hospital. They were given food and water ad libitum and used at 6–8 weeks of age.
Mouse model of Salmonella -induced intestinal inflammation
The protocol described by Barthel et al. (2003) was followed. In brief, groups of three to five animals were given 20 mg of streptomycin in water by gavage with a 21-gauge feeding needle. Twenty-four hours later, they were infected orally with 108 CFU of the naturally streptomycin-resistant SL1344 strain of S. Typhimurium, and then sacrificed 48 h after infection. Intestinal inflammation was assessed at necropsy based on gross appearance and length of the cecum, histopathology and quantitative reverse transcriptase PCR (RT-PCR)-based measurement of TNF-α, CXCL-2, CXCL-9 and CXCL-10 mRNA levels, all as described in detail earlier (Rhee et al., 2005).
Cecal organ culture
To measure intestinal cytokine production, ceca from control and infected animals were removed and flushed gently with sterile phosphate-buffered saline (PBS). Triplicate 2 mm ×2 mm pieces were excised and each piece placed in 0.5 mL of complete Dulbecco’s modified Eagle medium (DMEM) tissue culture medium with 10% fetal calf serum. After overnight incubation at 37 °C, 5% CO2, the supernatants were collected, centrifuged briefly to remove insoluble material and then analyzed by enzyme-linked immunosorbent assay (ELISA) to determine IFN-γ and CXCL-10 concentrations, using the relevant antibody pairs from R&D Systems (Minneapolis, MN). Standard curves for the ELISAs were generated with known concentrations of recombinant mouse IFN-γ and CXCL-10.
Flow cytometry analysis
Single-cell suspensions of mesenteric lymph node (MLN) cells from control and infected animals were prepared by crushing the tissue through a 70-μm nylon mesh. Cecal lamina propria cells were prepared following a standard protocol (Scheiffele & Fuss, 2003). Briefly, ceca pooled from several control or infected animals were rinsed free of contents, and cut into small pieces. Epithelial cells were removed by shaking for 30 min at 37 °C in Hank’s buffered saline solution (HBSS) containing 5 mM EDTA, 10% fetal calf serum and 0.014% dithiothreitol. The tissue fragments were collected by sedimentation, washed several times with HBSS and digested with a mixture of collagenase and DNase for 1 h at 37 °C. The digested fragments were passed through a 70 μm nylon mesh, the cells collected by centrifugation and washed several times before trypan blue staining and counting. The cells were surface stained with fluorescein-conjugated anti-NK1.1 and cychrome-conjugated anti-CD3 antibodies before being subjected to flow cytometry analysis with a Becton–Dickinson FACScan using CELLQUEST software. Forward and side scatter were used to gate on lymphoid cells.
In vitro stimulation experiments
Spleens from uninfected C57BL/6 mice were excised and crushed through a 70-μm nylon mesh. After lysing red blood cells, the leukocytes were washed, stained with trypan blue to confirm viability (> 95% routinely), and cultured in triplicate at a density of 3 × 106 cells per 0.5 mL of complete Roswell Park Memorial Institute (RPMI) tissue culture medium with 10% fetal calf serum. Some cultures received 108 heat-killed SL1344 suspended in 25 μL of PBS. After overnight incubation at 37 °C, 5% CO2, the cultures were collected, spun at 4 °C at 13 000 g for 5 min and the supernatants used to estimate IFN-γ concentrations by ELISA. For intracellular staining of IFN-γ expression, the cells were treated for 2 h with 10 μg mL−1 of brefeldin A to block secretion, starting 4 h after exposure to the heat-killed Salmonella. The cells were then chilled on ice, surface stained with fluorescein-conjugated anti-NK1.1 and cychrome-conjugated anti-CD3 antibodies, fixed in 3.7% formaldehyde, permeabilized with 0.1% saponin and then stained with phycoerythrin-conjugated anti-IFN-γ. Three-color flow cytometry analysis was carried out as described above to determine the proportions of IFN-γ+ cells that expressed NK1.1 and CD3.
NK cell depletion
Groups of C57BL/6 mice were injected intraperitoneally with two doses of rabbit anti-asialoGM1 antiserum (Wako Pure Chemicals, Richmond, VA), 25 μL dose−1 diluted in 300 μL endotoxin-free, sterile PBS given 2 days apart, or an equivalent amount of nonimmune rabbit serum. For assessment of depletion, the animals were euthanized 2 days after the second injection and the splenocytes stained with a mixture of fluorescein-conjugated anti-NK1.1 and cychrome-conjugated anti-CD3 for flow cytometry analysis. To examine the effect of NK depletion on the response to Salmonella infection, the animals were treated with oral streptomycin on the same day as the second injection of anti-asialoGM1 or control antiserum, infected orally 24 h later and then euthanized 48 h after infection.
Statistical analysis
Student’s t test was used to compare results, except for the experiments on tissue bacterial burden, where a nonparametric (Mann–Whitney) analysis was carried out. A P value of <0.05 was considered significant.
Results
Up-regulation of IFN-γ and IFN-γ-induced chemokines in the intestine during Salmonella infection
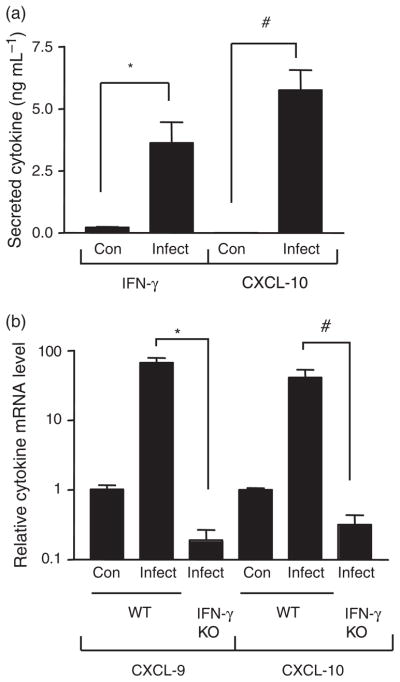
Earlier experiments had demonstrated an important role for IFN-γ in the regulation of Salmonella enterocolitis, but did not indicate whether intestinal expression of this cytokine was increased during the infection (Rhee et al., 2005). To address this issue, the use of the mouse model of Salmonella-induced intestinal inflammation was extended to examine IFN-γ production in cecal organ cultures as described in ‘Materials and methods’. As shown in Fig. 1a, the amount of IFN-γ released from the ceca of the infected mice was significantly higher than that from the uninfected controls, showing that Salmonella increased the intestinal expression of the cytokine. As a further indicator of the effects of Salmonella-induced IFN-γ, the expression of several proinflammatory chemokines that are known to be up-regulated by IFN-γ was examined (Schroder et al., 2004). Figure 1a demonstrates that the cecal production of one such chemokine, CXCL-10, was significantly increased following Salmonella infection. The secretion of three other IFN-γ-regulated chemokines, CCL5, CXCL-9 and CXCL-11, was also increased (data not shown). The chemokines CXCL-9, CXCL-10 and CXCL-11 are involved in the recruitment of Th1-type helper T cells (Luster et al., 2005). These molecules, along with CCL-5, have also been implicated in attracting NK cells to the site of bacterial infection (Morris & Ley, 2004; Widney et al., 2005). Thus, the IFN-γ-regulated chemokines induced by Salmonella in the gut are likely to play important roles in the inflammatory response to the infection. These observations were extended using real-time quantitative RT-PCR to demonstrate that cecal mRNA levels of both CXCL-9 and CXCL-10 were increased following infection (Fig. 1b). Furthermore, it was confirmed that the Salmonella-induced increases in cecal CXCL-9 and CXCL-10 required IFN-γ by showing that they did not occur in IFN-γ-deficient mice (Fig. 1b).
Fig. 1.
Effect of Salmonella infection on cecal cytokine expression. (a) Fragments of control and infected ceca were cultured and the supernatants analyzed for IFN-γ and CXCL-10 by ELISA. The means and SEs are depicted and are representative of three independent experiments. *P = 0.003, #P <0.0001 (n = 6 control and nine infected fragments). (b) Total cecal RNA from control and infected wild-type or IFN-γ knock-out mice was subjected to quantitative RT-PCR analysis with CXCL-9- or CXCL-10-specific primers. The means and SEs of relative mRNA levels (normalized to GAPDH) are depicted and are from two independent experiments. *P = 0.03, #P = 0.03 (n = 2 control wild-type, five infected wild-type and three infected knock-out mice).
Thus, these experiments revealed that Salmonella infection leads to the increased intestinal expression of IFN-γ and several IFN-γ-regulated proinflammatory chemokines. Together with earlier findings on the attenuation of Salmonella-induced enterocolitis and TNF-α expression in IFN-γ knock-out animals (Rhee et al., 2005), these observations indicate that IFN-γ is a key regulator of multiple aspects of the intestinal inflammatory response in this infection.
Recruitment of NK cells to the intestine during Salmonella infection
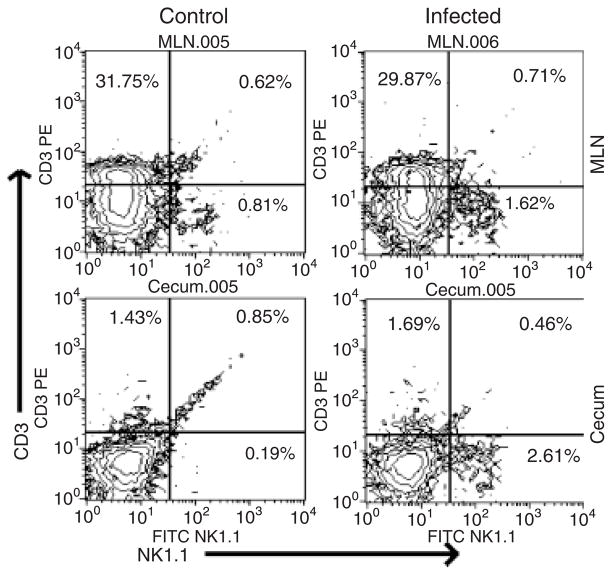
These findings prompted the authors to try to identify the cellular source of IFN-γ in the gut during Salmonella infection. Although the cytokine can be produced by several different cell types (Schroder et al., 2004), attention was focused on NK cells, components of the innate immune system that have been shown to respond rapidly with IFN-γ secretion during infection (Lodoen & Lanier, 2006). The authors started by using flow cytometry to examine NK cell numbers in the intestine and gut-associated lymphoid tissue (GALT) during Salmonella infection. This analysis demonstrated that there was a clear increase in the proportion of cells that were NK1.1+CD3−, a phenotype characteristic of NK cells (Lanier, 2005), in both the cecum and MLN following infection (Fig. 2). These results are consistent with the idea that Salmonella infection leads to the recruitment of NK cells to the intestine and GALT, and suggest that these cells might contribute to IFN-γ production in the gut.
Fig. 2.
Flow cytometry analysis of cecal and MLN cells from control and infected mice. Single-cell suspensions were prepared from MLN and ceca, stained with anti-NK1.1 and anti-CD3 antibodies and subjected to FACS analysis. The percentages of NK cells (NK1.1+CD3−) as well as T cells (NK1.1−CD3+) and NK T cells (NK1.1+CD3+) are indicated in the relevant quadrants.
Salmonella stimulates NK cells to produce IFN-γ in vitro
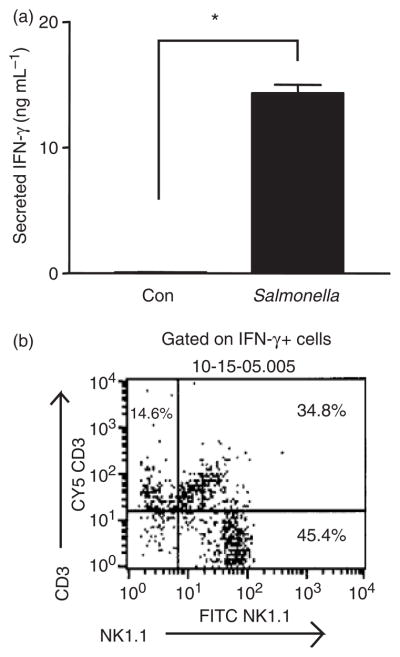
In vitro experiments were then carried out to determine whether NK cells can respond to Salmonella with IFN-γ production. Splenocytes prepared from uninfected C57BL/6 mice were incubated overnight with heat-killed Salmonella and cell supernatants examined by ELISA for the presence of the cytokine. The results shown in Fig. 3a indicate that exposure to the bacteria led to a marked increase in IFN-γ secretion. In order to identify the source of the cytokine, a parallel experiment was carried out in which the cells were subjected to three-color fluorescence-activated cell sorting (FACS) analysis with anti-NK1.1, anti-CD3 and anti-IFN-γ antibodies. The results indicated that most of the IFN-γ-expressing cells in the Salmonella-exposed cultures were NK1.1+CD3 − cells (NK cells), although NK T cells (NK1.1+CD3+) and T cells (NK1.1 −CD3+) also contributed significantly to production of the cytokine (Fig. 3b). Thus, at least three types of lymphocytes are able to produce IFN-γ rapidly in response to Salmonella, with NK cells making up a major component of this response.
Fig. 3.
In vitro induction of IFN-γ expression in splenocytes by Salmonella. (a) Control splenocytes or cells exposed to heat-killed Salmonella were cultured overnight and supernatants assayed for IFN-γ by ELISA. The means and SEs from three independent experiments, each carried out in triplicate, are represented. *P <0.0001 (n = 9 cultures in each group). (b) Splenocyte cultures exposed to heat-killed Salmonella were stained for surface NK1.1 and CD3 and intracellular IFN-γ. Results show NK1.1 and CD3 expression on the IFN-γ-expressing cell population and are representative of two independent experiments.
NK cell depletion leads to a reduction in Salmonella -induced expression of intestinal IFN-γ in vivo
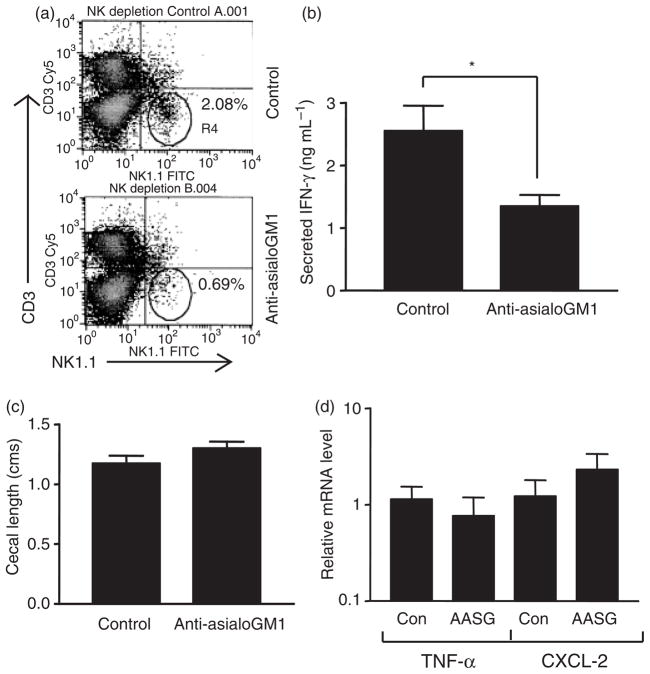
In order to assess the in vivo contribution of NK cells to the anti-Salmonella response, depletion experiments were carried out with anti-asialoGM1 antibody. AsialoGM1 is a membrane glycolipid that is expressed at high levels on murine NK cells, and injection of a polyclonal rabbit antiserum to this marker is widely used to achieve the specific depletion of this cell population in vivo (Kasai et al., 1980). Groups of C57BL/6 mice were injected intraperitoneally with two doses of either anti-asialoGM1 antiserum or an equivalent amount of non-immune serum following the protocol described in ‘Materials and methods’. Forty-eight hours after the second dose, the animals were sacrificed and the splenocytes subjected to FACS analysis with anti-NK1.1 and anti-CD3 antibodies. The results shown in Fig. 4a indicate that the anti-asialoGM1 treatment produced an c. 70% reduction in the number of NK1.1+CD3 − NK cells compared with animals injected with the control antibody. To examine the effect of NK depletion on the response to Salmonella infection, groups of mice were given oral streptomycin at the same time as the second dose of anti-asialoGM1 or control antiserum, followed a day later by infection with S. Typhimurium SL1344. The animals were euthanized 48 h after the infection. Analysis of cecal IFN-γ secretion revealed a modest, but nevertheless statistically significant, reduction resulting from the treatment with anti-asialoGM1 (Fig. 4b), indicating that NK cells are at least partly responsible for production of this cytokine in the gut in response to Salmonella infection in vivo. However, no difference in Salmonella-induced intestinal inflammation was noted between the control and anti-asialoGM1-treated groups, as indicated by gross appearance and length of the cecum (Fig. 4c and data not shown), and the cecal expression of two mediators, TNF-α and CXCL-2, that are involved in the acute inflammatory recruitment of neutrophils (Fig. 4d) (Yang et al., 2002; Kobayashi, 2006; Tukel et al., 2006). Salmonella-induced cecal histopathology was also not appreciably altered by NK depletion (data not shown).
Fig. 4.
Effect of NK cell depletion on intestinal IFN-γ secretion and inflammation. (a) Splenocytes from mice injected with either control or anti-asialoGM1 antiserum were stained with anti-NK1.1 and anti-CD3 antibodies and subjected to FACS analysis. Results shown are representative of two independent experiments. (b) Cecal fragments from infected, control or anti-asialoGM1-treated mice were cultured and the supernatants analyzed for IFN-γ by ELISA. The means and SEs from three independent experiments, each involving triplicate fragments from two mice in each group, are shown. *P = 0.01 (n = 18 fragments in each group). (c) Ceca were excised from infected, control or anti-asialoGM1-treated mice and the maximum length of the organ recorded. The means and SEs of four or five mice in each group from two independent experiments are depicted. (d) Total cecal RNA from control and anti-asia-loGM1 (AASG)-treated mice were subjected to quantitative RT-PCR analysis with primers specific for TNF-α or CXCL-2. The means and SEs of relative mRNA levels (normalized to GAPDH) from one experiment involving three mice in each group are shown.
NK cell-deficient mice have significantly reduced intestinal IFN-γ expression and attenuated enterocolitis during Salmonella infection
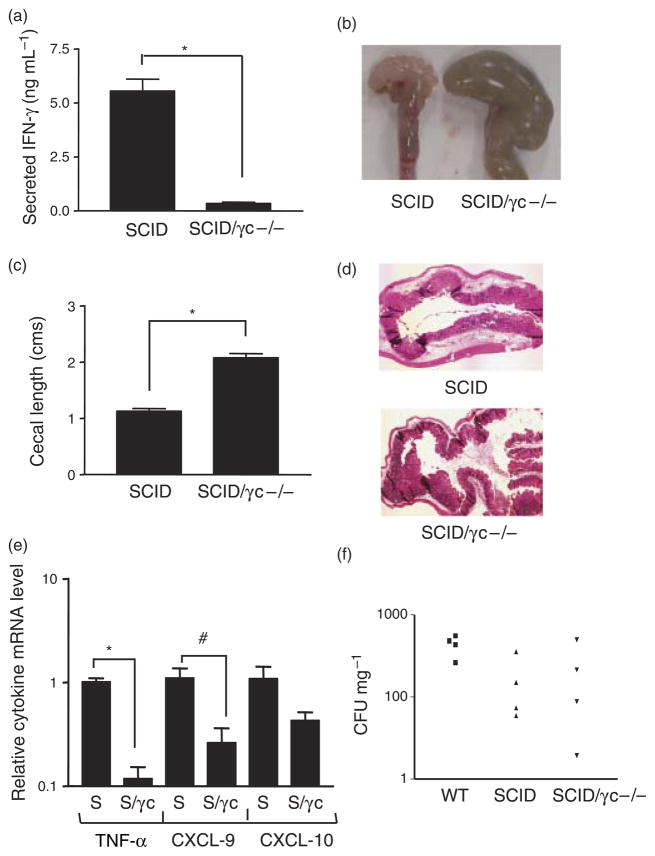
The results of Fig. 4b indicate that although NK cells do contribute to the intestinal IFN-γ response to Salmonella in vivo, other cell types are also likely to produce the cytokine, a conclusion that is consistent with in vitro observations of this study (Fig. 3b). The amount of IFN-γ produced by these other cells (NK T cells and T cells, as suggested by the data of Fig. 3b) is sufficient to allow a normal intestinal inflammatory response to Salmonella, indicating a degree of cellular redundancy with respect to control of this function. To circumvent this problem of redundancy, our investigation of the in vivo role of NK cells was continued by making use of a pair of congenic mouse strains, one of which, referred to here as the SCID strain, lacks all T and B lymphocytes because of mutation of the Prkdc gene (Bosma & Carroll, 1991). The other strain, referred to here as the SCID/γc −/− strain, lacks NK cells as well as all T and B lymphocytes because of the presence of the SCID mutation and a knock-out of the cytokine receptor common γ chain (γc) (Leonard et al., 1995). Thus, analysis of the responses in these congenic strains provides information on the role of NK cells in the absence of any contributions from Tand B lymphocytes. Groups of SCID and SCID/γc −/− mice were infected orally with S. Typhimurium following oral streptomycin pretreatment and then euthanized 48 h after infection. Cecal IFN-γ secretion was markedly and significantly reduced in the SCID/γc −/− animals compared with the SCID mice (Fig. 5a). Furthermore, Salmonella-induced intestinal inflammation was clearly attenuated in the SCID/γc −/− mice as indicated by several parameters. Firstly, the ceca of the infected SCID animals were pale, thick-walled, shrunken and devoid of stool, in keeping with the development of a robust intestinal inflammatory response. In contrast, the organs in the SCID/γc −/− mice were large, stool-filled and thin-walled (Fig. 5b). This difference in macroscopic appearance was quantitated by measurement of cecal length, which indicated that the SCID/γc −/− organs were significantly larger (Fig. 5c). On microscopic examination, ceca from the SCID mice displayed appreciable submucosal edema and a prominent inflammatory infiltrate, whereas both these features were reduced in the SCID/γc −/− animals (Fig. 5d). Consistent with the reduction in IFN-γ, cecal expression of the inflammatory mediators TNF-α and CXCL-9 were significantly decreased in the SCID/γc −/− mice and expression of CXCL-10 showed a trend in the same direction (Fig. 5e). The bacterial burden in the MLN (Fig. 5f), as well as spleen and liver (data not shown), was similar in the SCID and SCID/γc −/− mice and was not significantly different from wild-type animals. These results suggest that in the absence of T and B lymphocytes, NK cells are the major, if not sole, source of IFN-γ in the gut during Salmonella infection, and that these cells are required for the development of a normal intestinal inflammatory response to this pathogen but not for control of bacterial multiplication.
Fig. 5.
Outcome of Salmonella infection in SCID and SCID/γc −/− mice. (a) Fragments of ceca from infected SCID and SCID/γc −/− mice were cultured and the supernatants analyzed for IFN-γ by ELISA. The means and SEs of three or four mice in each group from two independent experiments are depicted. *P <0.0001 (n = 9 SCID and 12 SCID/γc −/− fragments). (b) Ceca from infected SCID and SCID/γc −/− mice were excised and photographed. The images are representative of two independent experiments with a total of four mice in each group. (c) Ceca from infected SCID and SCID/γc −/− mice were excised and the maximum length of the organ recorded. The means and SEs of four mice in each group from two independent experiments are shown. *P = 0.001 (n = 4 mice in each group). (d) Ceca from infected SCID and SCID/γc −/− mice were excised and sections stained with hematoxylin and eosin. Original magnification: ×40. The images are representative of sections from two or three mice of each type. (e) Total cecal RNA from infected SCID (S) and SCID/γc −/− (S/γc) mice were subjected to quantitative RT-PCR analysis with primers specific for TNF-α, CXCL-9 and CXCL-10. The means and SEs of relative mRNA levels (normalized to GAPDH) from three independent experiments with a total of four or five mice in each group are shown. *P = 0.001, #P = 0.045 (n = 4 SCID and five SCID/γc −/− mice). (f) Serial dilutions of homogenates of the MLN of infected wild-type (WT), SCID and SCID/γc −/− mice were cultured to determine the tissue bacterial burden. Each symbol represents an individual animal.
Discussion
Observations of this study demonstrate that enteric Salmonella infection is associated with an increase in the intestinal expression of NK cell recruiting chemokines and a corresponding increase in NK cell numbers in the gut. They show further that depletion of NK cells in wild-type mice results in a modest but significant reduction of Salmonella-induced IFN-γ expression in the intestine, with no effect on the inflammatory response. However, the absence of NK cells in a T and B cell-deficient background results in a dramatic decrease in intestinal IFN-γ production following infection, and a clear attenuation of intestinal inflammation. Taken together, these findings are consistent with the idea that NK cells play an important role in the intestinal immune response to Salmonella infection by rapidly up-regulating expression of IFN-γ, and that this function is probably carried out redundantly with other cell types (Kirby et al., 2002; Berntman et al., 2005; Rydstrom & Wick, 2007).
Observations of this study are consistent with previous reports documenting early IFN-γ production by NK cells, as well as NK T cells, T cells, macrophages and neutrophils, during Salmonella infection (Kirby et al., 2002; Berntman et al., 2005; Rydstrom & Wick, 2007), and by NK cells and CD8+ memory T cells in protection against Listeria monocytogenes infection (Dunn & North, 1991; Andersson et al., 1998; Berg et al., 2003). A more recent study in a Shigella lung inflammation model also demonstrated an essential role for IFN-γ produced by NK cells and T cells in the generation of the inflammatory response and control of bacterial replication (Le-Barillec et al., 2005). In many of these experiments, the IFN-γ was shown to be required for inhibiting bacterial growth, presumably as a result of macrophage activation. Tissue bacterial burdens were also analyzed in this study of Salmonella infection, but no differences were found that could be attributed to the presence or absence of NK cells (Fig. 5f). Thus, in this model, the major effect of NK cells is on the intestinal inflammatory response, whereas the control of bacterial multiplication appears to involve other innate immune mechanisms. The effect on intestinal inflammation is likely to be the consequence of NK cell-derived IFN-γ. IFN-γ directly or indirectly increases the expression of a number of genes involved in the recruitment of inflammatory cells, including TNF-α, CXCL-9 and CXCL-10 (Schroder et al., 2004). In its absence, expression of these mediators is decreased, leading to attenuation of inflammation, as seen in the authors’ earlier work (Rhee et al., 2005) and in the present study.
The cytokine receptor γc chain carries out important functions in signal transduction by the receptors for IL-2, IL-4, IL-7, IL-9 and IL-15 (Di Santo et al., 1995; Leonard et al., 1995). Lack of responsiveness to these cytokines gives rise to the major phenotypic characteristic of mice that lack γc, namely, defects in development of lymphoid cells, including NK cells. These mice have not been reported to display deficiencies of innate immunity or inflammatory responses other than those related to the absence of NK cells. In fact, older γc −/− animals develop a spontaneous colitis, indicating that their ability to mount an intestinal inflammatory response is not inherently impaired (Kai et al., 2005). However, given the wide-ranging functions of many cytokines, it is formally possible that the reduction in Salmonella-induced intestinal IFN-γ production and inflammation in the SCID/γc −/− mice is caused by γc-dependent effects other than the lack of NK cells. Despite repeated attempts, Salmonella-induced intestinal IFN-γ expression and inflammation could not be restored to the levels of SCID mice by transfer of SCID splenic NK cells to the SCID/γc −/− mice (data not shown). Therefore, the possibility that the decrease in IFN-γ expression and inflammation in these animals is related to NK cell-independent consequences of the γc deficiency cannot be definitively excluded, but it seems unlikely in view of the authors’ other data indicating the involvement of NK cells in the response to Salmonella.
The molecular mechanism by which Salmonella infection leads to NK cell activation in vivo requires further investigation. Data from previously published in vitro work indicate a role for macrophage-derived TNF-α in this process (Ramarathinam et al., 1993). TNF-α has also been implicated, along with IL-12, in Listeria-induced activation of NK cells (Tripp et al., 1993). The authors and others have shown that macrophage TNF-α production in response to Salmonella depends on lipopolysaccharide–TLR4 interactions (Li & Cherayil, 2003; Royle et al., 2003; Weiss et al., 2004), while other pattern-recognition receptors are involved in the induction of IL-12 (Merlin et al., 2001). Putting these findings together, a likely in vivo scenario is that TNF-α and IL-12 secreted by Salmonella-activated intestinal macrophages (either resident or recruited) act on NK cells to induce production of IFN-γ.
NK cells are clearly not the only source of IFN-γ during Salmonella infection. The authors’ in vitro experiments indicate that in addition to NK cells, Salmonella activates both T cells and NK T cells to produce the cytokine (Fig. 3b). Furthermore, NK depletion in wild-type mice resulted in only a partial reduction of intestinal IFN-γ production in vivo (Fig. 4b). A more complete attenuation was seen only in the SCID/γc −/− mice, which lack T and B lymphocytes in addition to NK cells (Fig. 5), suggesting the redundant involvement of multiple cell types in the production of IFN-γ in the gut. This redundancy is consistent with the previously documented role of NK cells, memory T cells and other cell types in the early production of IFN-γ in response to Salmonella, Listeria and Shigella (Dunn & North, 1991; Andersson et al., 1998; Kirby et al., 2002; Berg et al., 2003; Berntman et al., 2005; Le-Barillec et al., 2005), and helps to explain why Salmonella-induced enterocolitis occurs normally in mouse strains that lack T and B cells, such as Rag-deficient and SCID mice (Barthel et al., 2003; the present study). The participation of NK T cells and memory T cells in the IFN-γ response to Salmonella is in keeping with the fact that cytokines such as IL-12 can activate these cells in addition to NK cells (Berg et al., 2003; Brigl et al., 2003). Dendritic cells and macrophages can also be sources of IFN-γ (Wick, 2004; Chan et al., 2006; Rydstrom & Wick, 2007), but the almost complete absence of the cytokine in the infected ceca of the SCID/γc−/− mice (Fig. 5a) makes it unlikely that these cells contribute significantly to the intestinal IFN-γ response during Salmonella infection.
It is intriguing that the expression of CXCL-9 and CXCL-10, chemokines that have been implicated in NK cell recruitment to the site of bacterial infection (Widney et al., 2005), appears to be dependent on IFN-γ and NK cells (Figs 1b and 5e). This finding raises the possibility that cells other than NK cells may produce a low, baseline level of IFN-γ in the gut that is required for subsequent responses, including the recruitment of the NK cells that provide more of the cytokine following activation. An alternate explanation, one that does not exclude the first, is that the IFN-γ produced by NK cells serves to amplify other inflammatory responses, including the expression of CXCL-9 and CXCL-10.
NK cells may carry out functions other than producing IFN-γ. They can secrete a variety of additional cytokines and chemokines that assist in the recruitment of inflammatory cells (Lodoen & Lanier, 2006). Furthermore, they have been shown to lyse phagocytes infected with intracellular bacteria such as Mycobacterium tuberculosis (Vankayalapati et al., 2005). Whether these other NK functions contribute to the response to Salmonella infection remains to be seen. A more detailed investigation of the involvement of NK cells in antimicrobial protection and inflammation during salmonellosis is indicated.
Acknowledgments
This work was supported by Public Health Service grants AI-48815 and AI-065619 from the National Institute of Allergy and Infectious Diseases.
References
- Andersson A, Dai WJ, Di Santo JP, Brombacher F. Early IFN-γ production and innate immunity during Listeria monocytogenes infection in the absence of NK cells. J Immunol. 1998;161:5600–5606. [PubMed] [Google Scholar]
- Barthel M, Hapfelmeier S, Quintanilla-Martinez L, Kremer M, Rohde M, Hogardt M, Pfeffer K, Russmann H, Hardt WD. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun. 2003;71:2839–2858. doi: 10.1128/IAI.71.5.2839-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg RE, Crossley E, Murray S, Forman J. Memory CD8+T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J Exp Med. 2003;198:1583–1593. doi: 10.1084/jem.20031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntman E, Rolf J, Johansson C, Anderson P, Cardell SL. The role of CD1d-restricted NK T lymphocytes in the immune response to oral infection with Salmonella typhimurium. Eur J Immunol. 2005;35:2100–2109. doi: 10.1002/eji.200425846. [DOI] [PubMed] [Google Scholar]
- Bosma MJ, Carroll AM. The SCID mouse mutant: definition, characterization, and potential uses. Annu Rev Immunol. 1991;9:323–350. doi: 10.1146/annurev.iy.09.040191.001543. [DOI] [PubMed] [Google Scholar]
- Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- Chan CW, Crafton E, Fan HN, et al. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat Med. 2006;12:207–213. doi: 10.1038/nm1352. [DOI] [PubMed] [Google Scholar]
- Di Santo JP, Kuhn R, Muller W. Common cytokine receptor gamma chain (γc)-dependent cytokines: understanding in vivo functions by gene targeting. Immunol Rev. 1995;148:19–34. doi: 10.1111/j.1600-065x.1995.tb00091.x. [DOI] [PubMed] [Google Scholar]
- Dunn PL, North RJ. Early γ interferon production by natural killer cells is important in defense against murine listeriosis. Infect Immun. 1991;59:2892–2900. doi: 10.1128/iai.59.9.2892-2900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapfelmeier S, Stecher B, Barthel M, et al. The Salmonella pathogenicity island (SPI)-2 and SPI-1 type III secretion systems allow Salmonella serovar Typhimurium to trigger colitis via MyD88-dependent and MyD88-independent mechanisms. J Immunol. 2005;174:1675–1685. doi: 10.4049/jimmunol.174.3.1675. [DOI] [PubMed] [Google Scholar]
- Kai Y, Takahashi I, Ishikawa H, et al. Colitis in mice lacking the common cytokine receptor gamma chain is mediated by IL-6-producing CD4+ T cells. Gastroenterology. 2005;128:922–934. doi: 10.1053/j.gastro.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Kasai M, Iwamori M, Nagai Y, Okumura K, Tada T. A glycolipid on the surface of mouse natural killer cells. Eur J Immunol. 1980;10:175–180. doi: 10.1002/eji.1830100304. [DOI] [PubMed] [Google Scholar]
- Kirby AC, Yrlid U, Wick MJ. The innate immune response differs in primary and secondary Salmonella infection. J Immunol. 2002;169:4450–4459. doi: 10.4049/jimmunol.169.8.4450. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y. Neutrophil infiltration and chemokines. Crit Rev Immunol. 2006;26:307–316. doi: 10.1615/critrevimmunol.v26.i4.20. [DOI] [PubMed] [Google Scholar]
- Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- Le-Barillec K, Magalhaes JG, Corcuff E, Thuizat A, Sansonetti PJ, Phalipon A, Di Santo JP. Roles for T and NK cells in the innate immune response to Shigella flexneri. J Immunol. 2005;175:1735–1740. doi: 10.4049/jimmunol.175.3.1735. [DOI] [PubMed] [Google Scholar]
- Leonard WJ, Shores EW, Love PE. Role of the common cytokine receptor gamma chain in cytokine signaling and lymphoid development. Immunol Rev. 1995;148:97–114. doi: 10.1111/j.1600-065x.1995.tb00095.x. [DOI] [PubMed] [Google Scholar]
- Li Q, Cherayil BJ. Role of Toll-like receptor 4 in macrophage activation and tolerance during Salmonella enterica serovar Typhimurium infection. Infect Immun. 2003;71:4873–4882. doi: 10.1128/IAI.71.9.4873-4882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodoen MB, Lanier LL. Natural killer cells as an initial defense against pathogens. Curr Opin Immunol. 2006;18:391–398. doi: 10.1016/j.coi.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- Mastroeni P. Immunity to systemic Salmonella infections. Curr Mol Med. 2002;2:393–406. doi: 10.2174/1566524023362492. [DOI] [PubMed] [Google Scholar]
- McCormick BA, Mrsny RJ. NF-κB-independent responses activated by bacterial–epithelial interactions: role of arachidonic acid metabolites. In: McCormick BA, editor. Bacterial–Epithelial Cell Cross-Talk. Molecular Mechanisms in Pathogenesis. Cambridge University Press; Cambridge, UK: 2006. pp. 269–298. [Google Scholar]
- Merlin T, Sing A, Nielsen PJ, Galanos C, Freudenberg MA. Inherited IL-12 unresponsiveness contributes to the high LPS resistance of the Lpsd C57BL/10ScCr mouse. J Immunol. 2001;166:566–573. doi: 10.4049/jimmunol.166.1.566. [DOI] [PubMed] [Google Scholar]
- Morris MA, Ley K. Trafficking of natural killer cells. Curr Mol Med. 2004;4:431–438. doi: 10.2174/1566524043360609. [DOI] [PubMed] [Google Scholar]
- Patel JC, Galan JE. Manipulation of the host actin cytoskeleton by Salmonella – all in the name of entry. Curr Opin Microbiol. 2005;8:10–15. doi: 10.1016/j.mib.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Rabsch W, Tschape H, Baumler A. Non-typhoidal salmonellosis: emerging problems. Microbes Infect. 2001;3:237–247. doi: 10.1016/s1286-4579(01)01375-2. [DOI] [PubMed] [Google Scholar]
- Ramarathinam L, Niesel DW, Klimpel GR. Salmonella typhimurium induces IFN-γ production in murine splenocytes. Role of natural killer cells and macrophages. J Immunol. 1993;150:3973–3981. [PubMed] [Google Scholar]
- Rhee SJ, Walker WA, Cherayil BJ. Developmentally-regulated intestinal expression of IFN-γ and its target genes and the age-specific response to enteric Salmonella infection. J Immunol. 2005;175:1127–1136. doi: 10.4049/jimmunol.175.2.1127. [DOI] [PubMed] [Google Scholar]
- Royle MC, Totemeyer S, Alldridge LC, Maskell DJ, Bryant CE. Stimulation of Toll-like receptor 4 by lipopolysaccharide during cellular invasion by live Salmonella typhimurium is a critical but not exclusive event leading to macrophage responses. J Immunol. 2003;170:5445–5454. doi: 10.4049/jimmunol.170.11.5445. [DOI] [PubMed] [Google Scholar]
- Rydstrom A, Wick MJ. Monocyte recruitment, activation and function in the gut-associated lymphoid tissue during oral Salmonella infection. J Immunol. 2007;178:5789–5801. doi: 10.4049/jimmunol.178.9.5789. [DOI] [PubMed] [Google Scholar]
- Scheiffele F, Fuss IJ. Induction of TNBS colitis in mice. In: Coligan JE, Bierer B, Margulies DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. John Wiley & Sons; Hoboken, NJ: 2003. pp. 15.19.6–15.19.7. [DOI] [PubMed] [Google Scholar]
- Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon γ: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- Srikanth CV, Cherayil BJ. Intestinal innate immunity and the pathogenesis of Salmonella enteritis. Immunol Res. 2007;37:61–77. doi: 10.1007/BF02686090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecher B, Hapfelmeier S, Muller C, Kremer M, Stallmach T, Hardt WD. Flagella and chemotaxis are required for efficient induction of Salmonella enterica serovar Typhimurium colitis in streptomycin-pretreated mice. Infect Immun. 2004;72:4138–4150. doi: 10.1128/IAI.72.7.4138-4150.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp CS, Wolf SF, Unanue ER. Interleukin 12 and tumor necrosis factor α are costimulators of interferon γ production by natural killer cells in severe combined immunodeficiency mice with listeriosis and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci USA. 1993;90:3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukel C, Raffatellu M, Chessa D, Wilson RP, Akcelik M, Baumler AJ. Neutrophil influx during non-typhoidal salmonellosis: who is in the driver’s seat? FEMS Immunol Med Microbiol. 2006;46:320–329. doi: 10.1111/j.1574-695X.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- Vankayalapati R, Garg A, Porgador A, Griffin DE, Klucar P, Safi H, Girard WM, Cosman D, Spies T, Barnes PF. Role of NK cell activating receptors and their ligands in the lysis of mononuclear phagocytes infected with an intracellular bacterium. J Immunol. 2005;175:4611–4617. doi: 10.4049/jimmunol.175.7.4611. [DOI] [PubMed] [Google Scholar]
- van de Vosse E, Hoeve MA, Ottenhof TH. Human genetics of intracellular infectious diseases: molecular and cellular immunity against mycobacteria and salmonellae. Lancet Infect Dis. 2004;4:739–749. doi: 10.1016/S1473-3099(04)01203-4. [DOI] [PubMed] [Google Scholar]
- Weiss DS, Raupach B, Takeda K, Akira S, Zychlinsky A. Toll-like receptors are temporally involved in host defense. J Immunol. 2004;172:4463–4469. doi: 10.4049/jimmunol.172.7.4463. [DOI] [PubMed] [Google Scholar]
- Wick MJ. Living in the danger zone: innate immunity to Salmonella. Curr Opin Microbiol. 2004;7:51–57. doi: 10.1016/j.mib.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Widney DP, Hu Y, Foreman-Wykert AK, Bui KC, Nguyen TT, Lu B, Gerard C, Miller JF, Smith JB. CXCR3 and its ligands participate in the host response to Bordetella bronchiseptica infection of the mouse respiratory tract but are not required for clearance of bacteria from the lung. Infect Immun. 2005;73:485–493. doi: 10.1128/IAI.73.1.485-493.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang KK, Dorner BG, Merkel U, Ryffel B, Schutt C, Golenbock D, Freeman MW, Jack RS. Neutrophil influx in response to peritoneal infection with Salmonella is delayed in LPS-binding protein or CD14-deficient mice. J Immunol. 2002;169:4475–4480. doi: 10.4049/jimmunol.169.8.4475. [DOI] [PubMed] [Google Scholar]