Abstract
Objective
To determine the effects of malnutrition on hemodynamic status of adolescents hospitalized for anorexia nervosa.
Design
Longitudinal observational study.
Setting
Tertiary care pediatric hospital.
Patients
Thirty-eight adolescents with anorexia nervosa, aged 13 to 21 years, with a mean (SD) body mass index (calculated as weight in kilograms divided by height in meters squared) of 15.9 (1.8).
Intervention
Subjects received standard care, including bed rest and graded nutritional therapy. A subsample of subjects (n = 19) returned 11 to 57 weeks following hospitalization for a second cardiac evaluation.
Main Outcome Measures
Results from a 15-lead electrocardiogram, echocardiogram, treadmill stress test, and spinal bone mineral density measurement.
Results
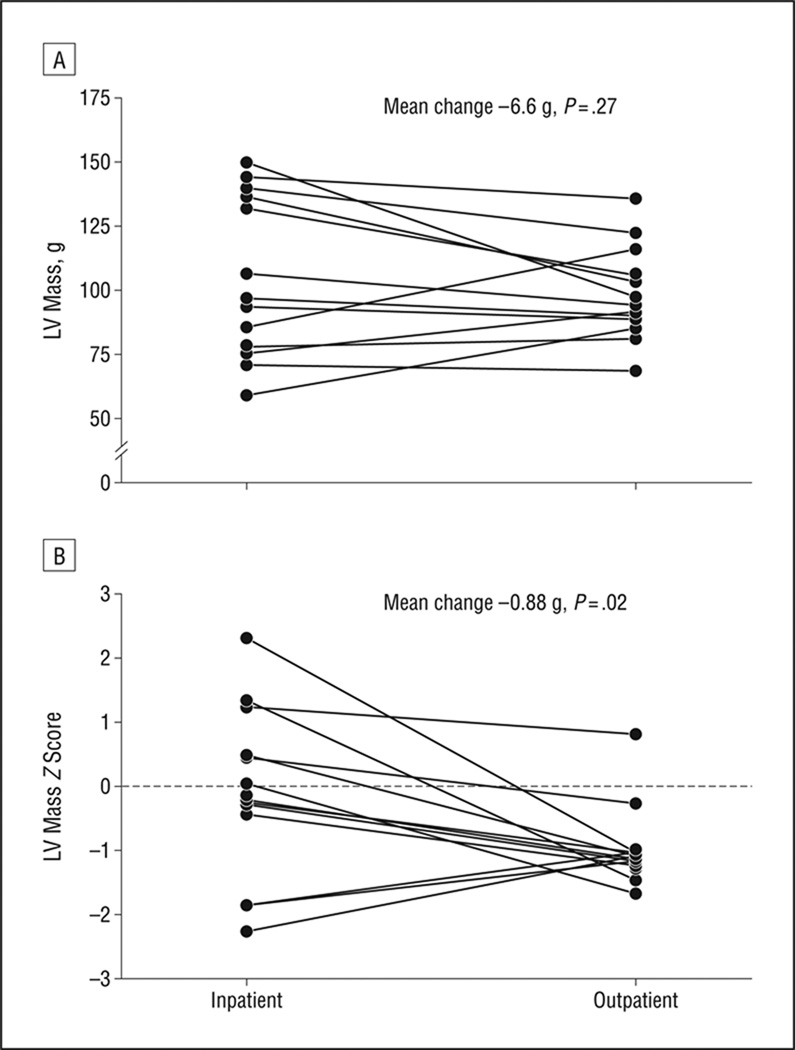
On admission, 26 subjects (68%) had sinus bradycardia. Bradycardia was less common in participants with a longer duration of illness (P = .04). Left ventricle mass was lower than predicted for age (Z score <−1.0) in 11 subjects (31%). Exercise tolerance was normal by all measures. Both heart rate and QT interval were predictors of spinal bone mineral density. In those who returned for follow-up, absolute measures of left ventricle mass did not change (P = .27). However, the corresponding Z scores declined over time (mean [SD] change, −0.9 [1.3]; P = .02).
Conclusions
In acutely malnourished adolescents with anorexia nervosa, few truly pathologic cardiac findings were identified. Sinus bradycardia was observed in most cases. Mild reductions in left ventricle mass and left ventricle function were seen both at baseline and at follow-up, suggesting early sparing of cardiac muscle in the face of moderate malnutrition as well as a relative delay of cardiac muscle restoration. The association of hemodynamic status with altered spinal bone mineral density emphasizes the range of systems affected by malnutrition in anorexia nervosa.
Cardiac events are major concerns of clinicians caring for adolescents with eating disorders, particularly those so severely malnourished that medical hospitalization is required. Consensus, rather than evidence, has held that cardiac dysfunction is responsible for much of the morbidity and mortality of patients with anorexia nervosa (AN).1,2 Hospitalization for AN at many institutions follows a structured protocol of refeeding and relative immobilization.3,4 These activity limitations are based on concerns for cardiovascular safety and a desire to maximize weight gain. However, the degree of concern for a cardiac event may overshadow true risk and lead to other potential complications associated with bed rest.5
The current literature regarding cardiovascular health for patients with AN has focused on adult populations or extremely ill cohorts. The existing data are in conflict. Reduced cardiac dimensions have been shown, with concurrent preservation of left ventricle (LV) function and cardiac output.6–8 In contrast, LV dysfunction and/or decreased exercise capacity have also been demonstrated.9–11 These investigations have been limited by small sample size, retrospective designs, and a focus on adults with chronic malnutrition. Even less is known about the hemodynamic status of adolescents with AN. Mont et al12 demonstrated decreased LV mass and diminished cardiac wall thickness in teens with AN, which improved after refeeding.
Our study objective was to prospectively determine the effects of malnutrition on the cardiovascular health of adolescents and young women hospitalized for AN. We hypothesized that ill adolescents with AN would show few abnormalities on cardiac evaluation.
METHODS
SUBJECTS
All female adolescents and young women, aged 13 to 21 years, admitted to Children’s Hospital Boston, Boston, Massachusetts, between April 29, 2005, and February 5, 2008, for medical complications of AN were screened for study participation. To be eligible, patients (1) met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria for AN (as determined by psychiatric consultation); (2) were at least 2 years post menarche; (3) had no preexisting conditions or medication use affecting cardiac health; (4) were admitted at a time permitting scheduling of the cardiac evaluation; and (5) were willing to return for a follow-up visit. Thirty-eight adolescents were enrolled. Study procedures were reviewed and approved by the local institutional review board. Informed consent was obtained from all subjects or their parents. Minor subjects also provided assent for participation.
STUDY DESIGN AND TREATMENT
Per our standardized eating disorder inpatient protocol, all subjects were placed on bed rest and graded nutritional therapy during the admission.3 Subjects consumed a baseline meal plan between 1250 and 1750 kcal/d and advanced by 250 kcal/d toward their individualized nutritional goal as determined by the hospital nutritionist. Subjects were confined to their beds throughout the day, including meal times, as per our institutional standard of care.
DATA COLLECTION
Height (in centimeters) was measured using a standardized procedure with a wall-mounted stadiometer. Weight (in kilograms) was measured each morning, following voiding and in the fasting state, with subjects wearing a hospital gown. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. For each subject aged 20 years or younger, percentage of median body weight (MBW) was determined using the 2000 Centers for Disease Control and Prevention growth charts and the following formula: percentage of MBW = 100 × [patient BMI/median BMI for age].13 For each subject older than 20 years, percentage of MBW was determined by the following formula: percentage of MBW = 100 × [patient BMI/median BMI for adults], where the median BMI for adults is 21.7.14 Semistructured interviews were used to obtain demographic information, health history, and exercise history (hours per week of physical activity prior to admission, type of exercise [eg, walking or running, swimming, strength training]).
BIOCHEMICAL ASSESSMENTS
Fasting venous blood samples were drawn each morning. Serum samples were analyzed in the Children’s Hospital Boston Core Laboratory for concentrations of calcium, phosphorus, and magnesium. Any electrolyte imbalance was corrected prior to echocardiography or stress test.
As a pilot study to determine whether subclinical myocardial injury was present, serum levels of aldolase, creatine kinase MB, and troponin I were measured 30 minutes before and 30 minutes following the cardiopulmonary stress test in a convenience subset of subjects (n = 35).
CARDIAC EVALUATION
Orthostatic vital signs were measured each morning in supine and standing positions. On admission, a standard 15-lead electrocardiogram (ECG) was obtained. All ECGs were interpreted by a single pediatric cardiologist (M.E.A.). The QT interval was corrected for heart rate by the Bazett formula: QTc = QT/√RR. The QT dispersion was defined as the difference between the maximum QT and minimum QT intervals measured in all leads. An echocardiographic assessment of systolic and diastolic function (complete 2-dimensional echocardiogram and Doppler evaluation including stress-velocity analysis) was conducted within the first 1 or 2 days of hospital stay. Standard short- and long-axis views of the LV were used to assess regional wall motion. End-diastolic and end-systolic volumes, mass, and ejection fraction were obtained from 2-dimensional images using a biplane modified Simpson rule. Ventricular sphericity was calculated as the ratio of short-axis dimension to long-axis dimension. Calculations for stress-velocity analysis were performed to obtain ventricular end-diastolic and end-systolic dimensions and wall thicknesses, fractional shortening, velocity of fiber shortening, end-systolic stress, preload, and contractility. Contractility is quantified as the relationship between end-systolic stress and velocity of circumferential fractional contraction, a previously validated stress-velocity index that incorporates afterload and is independent of preload.15 The Z scores were calculated to allow comparison between study measurements and established age- and sex-specific normative data. Physicians at the Children’s Hospital Boston Echocardiography Laboratory interpreted the echocardiograms using standard clinical procedures.
Additionally, we evaluated functional capacity and exercise performance via weight-bearing, upright cardiopulmonary exercise testing. The exercise stress test was performed on the day of hospital discharge using a standard Bruce protocol, directly supervised by an exercise physiologist. Each subject walked on a treadmill and breathed through a mouthpiece that permitted measurement and analysis of expired gases. The subject’s heart rhythm, heart rate, and blood pressure were monitored throughout the test. The test was stopped if the full 21-minute protocol was completed, if the subject felt she had reached her maximum exertion level, or if any cardiac symptoms developed.
The entire cardiac evaluation was repeated at least 11 weeks following discharge. At the follow-up visit, weight and vital signs were also measured. Nineteen subjects did not return for a wide variety of reasons, including loss to follow-up, transfer of care to another facility, inpatient psychiatric admission, or refusal of a second examination. There was only 1 difference in baseline characteristics between those who did and did not return for follow-up. Participants who returned were more likely to report regular exercise prior to hospitalization (P = .03); however, there was no difference in mean hours of exercise per week between the 2 groups (P = .15).
BONE DENSITY
Anteroposterior lumbar spine (L1–L4) areal bone mineral density (aBMD; in grams per centimeters squared) was measured by dual-energy x-ray absorptiometry in 28 subjects using the QDR-4500 Delphi scanner (Hologic, Inc, Waltham, Massachusetts). Measurements were compared with those of age- and sex-matched reference data.16,17 For participants younger than 20 years, pediatric normative data were used.18
STATISTICAL ANALYSIS
Repeated-measures analysis was used to determine the linear trends for weight, cardiac parameters (pulse, blood pressure), and electrolytes over the first 5 days of hospitalization.
Simple associations were assessed by Pearson correlation coefficient. Bivariate associations were measured using linear regression. Paired t tests were used to compare continuous variables measured at the baseline assessment and follow-up. Mean Z score measurements of echocardiogram parameters were compared with established normal data using 1-sample t tests. All computations were performed with SAS version 9.1 statistical software (SAS Institute, Inc, Cary, North Carolina). Results are presented as mean (standard deviation) unless otherwise indicated. Results with P < .05 were considered statistically significant.
RESULTS
DEMOGRAPHIC DATA
We studied 38 adolescents and young women with AN, ranging in age from 13 to 21 years (Table 1). All subjects used restrictive eating behaviors; 8 subjects (21%) also reported regular purging. One subject was African American (self-reported); all others were white. The duration of amenorrhea ranged from 1.0 to 42.0 months, with a median of 6.0 months. Subjects with amenorrhea for less than 3 months had menses only with oral contraceptives; all hormonal medications had been stopped at least 1 month prior to study participation. The subjects were moderately malnourished; the mean (SD) BMI was 15.9 (1.8), corresponding to a mean (SD) percentage of MBW of 78% (9%). Prior to hospitalization, 27 subjects (71%) reported participating in regular physical exercise, for a median duration of 6.0 h/wk. Of those who exercised, most were involved in weight-bearing pursuits such as running, dancing, and strength training.
Table 1.
Baseline Characteristics of 38 Young Women Hospitalized for Anorexia Nervosa
| Characteristic | Mean (SD)a | Median (Range) |
|---|---|---|
| Age, y | 16.5 (2.2) | 16.0 (13.2–21.9) |
| Height, cm | 162.7 (6.4) | 162.9 (147.1–175.0) |
| Weight, kg | 42.1 (5.6) | 43.1 (31.6–54.2) |
| BMI | 15.9 (1.8) | 15.8 (12.1–19.9) |
| Weight relative to median, % | 78 (9) | 78 (57–96) |
| Duration of amenorrhea, mob | 8.3 (8.2) | 6.0 (1.0–42.0) |
| Regular exercise, No. (%) | 27 (71) | NA |
| Regular exercise, h/wk | 6.8 (6.4) | 6.0 (0.0–20.0) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NA, not applicable.
Values are expressed as mean (SD) unless indicated otherwise.
Subjects with amenorrhea for less than 3 months had menses only with the use of oral contraceptives; all hormonal medications had been stopped at least 1 month prior to study participation.
ALTERATIONS DURING REFEEDING
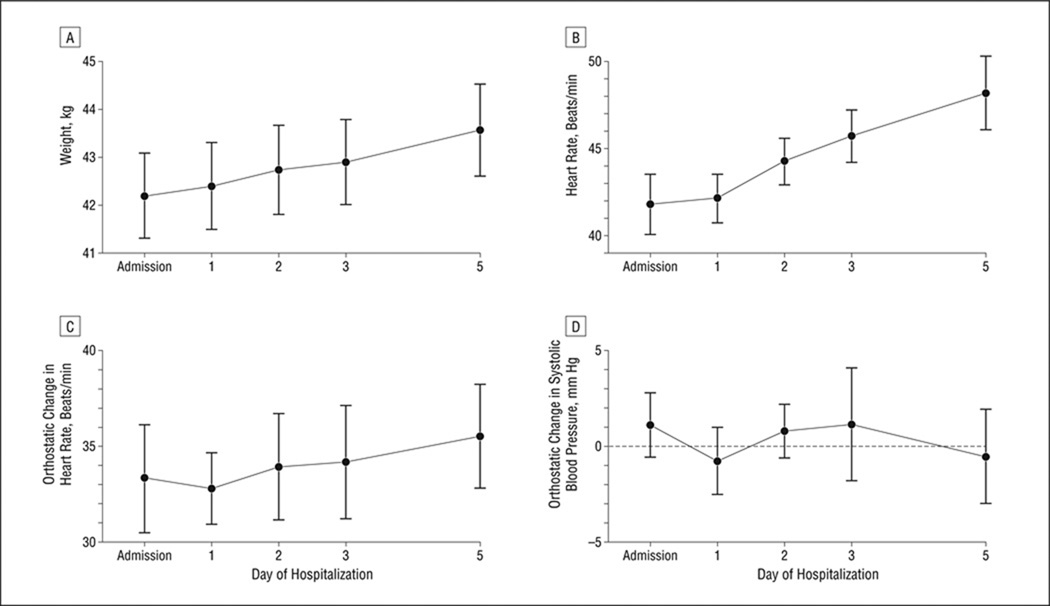
The regression trend (SE) of the weight, adjusted for age, baseline percentage of MBW, and regular exercise, increased by 0.3 (0.0) kg/d, leading to clinically significant weight gain by discharge (P < .001) (Table 2 and Figure 1). Morning supine pulse increased with each subsequent day of refeeding (linear trend [SE] additionally adjusted for weight, 1.4 [0.2] beats/min per day; P < .001). The degree of orthostasis by blood pressure or pulse did not change (Figure 1). All subjects remained on the adolescent medicine service for their entire medical admission.
Table 2.
Trends in Weight and Cardiac Parameters Over 5 Days in 38 Young Women Hospitalized for Anorexia Nervosa
| Adjusted for Covariatesa | Adjusted for Covariates and Weighta | |||
|---|---|---|---|---|
| Parameter | Linear Trend/d (SE) | P Valueb | Linear Trend/d (SE) | P Valueb |
| Weight, kg | 0.3 (0.0) | <.001 | NA | NA |
| Pulse, beats/min | 1.3 (0.2) | <.001 | 1.4 (0.2) | <.001 |
| Minimum SBP, mm Hg | 0.3 (0.3) | .32 | 0.1 (0.3) | .67 |
| Orthostatic pulse change, beats/min | 0.4 (0.5) | .42 | 0.2 (0.5) | .75 |
| Orthostatic SBP change, mm Hg | −0.2 (0.5) | .72 | −0.5 (0.4) | .19 |
| Phosphorus, mg/dL | −0.1 (0.0) | <.001 | −0.1 (0.0) | <.001 |
Abbreviations: NA, not applicable; SBP, systolic blood pressure.
SI conversion factor: To convert phosphorus to millimoles per liter, multiply by 0.323.
Covariates are age, baseline percentage of median body weight, and hours of regular exercise per week.
From repeated-measures analysis; P tests for linear trend are significantly different from 0.
Figure 1.
Trends in weight (A), morning resting heart rate (B), and morning orthostatic change in heart rate (C) and systolic blood pressure (D) (change when moving from supine to standing position) in 38 young women hospitalized for anorexia nervosa. Data are presented as estimated means; error bars indicate standard error. See Table 2 for statistical assessment of trends.
All subjects maintained calcium levels within the normal range (range, 9.0–10.7 mg/dL; to convert to millimoles per liter, multiply by 0.25).19 One subject was hypokalemic on admission (potassium level of 2.83 mEq/L; to convert to millimoles per liter, multiply by 1.0); by the next morning, the potassium level had normalized (4.26 mEq/L). Although oral phosphorus supplementation (250 mg orally twice daily to 500 mg orally thrice daily) kept values within normal limits (median, 3.8 mg/dL; range, 2.9–4.5 mg/dL; to convert to millimoles per liter, multiply by 0.323), serum phosphorus levels declined by a mean (SE) of −0.1 (0.0) mg/dL per day (P < .001) (Table 2).
ECG ANALYSES
On admission, 26 subjects (68%) had sinus bradycardia (heart rate <50 beats/min) (Table 3). Eleven subjects (29%) had heart rates less than 40 beats/min. Participants with a longer duration of illness had higher heart rates (Table 4). Admission BMI was predictive of a lower heart rate (mean [SE], −3.27 [1.36] beats/min per kg/m2; P = .02). Right-axis deviation (QRS axis 90°–180°) was identified in 3 subjects (8%) (mean [SD] QRS axis, 72° [18°]) (Table 3). While the baseline QT interval was prolonged (mean [SD], 0.45 [0.05] seconds), the heart rate–corrected QTc interval was within the normal range (mean [SD], 0.40 [0.03] seconds). The QTc interval was longer in subjects with longer duration of amenorrhea (P = .05) (Table 4). Reported duration of illness, reported exercise habits, and BMI did not affect the QTc interval. Additional ECG measurements, including amplitudes in all leads, were normal (Table 3). Benign arrhythmias were observed. Physiological sinus arrhythmia was identified in 4 subjects (11%), while an additional 2 participants (5%) demonstrated isolated atrial premature beats. No sustained tachyarrhythmias were observed.
Table 3.
Admission Electrocardiogram and Echocardiogram Findings of 38 Young Women Hospitalized for Anorexia Nervosa
| Finding | Mean (SD)a | Median (Range) |
|---|---|---|
| ECG data | ||
| Heart rate, beats/min | 48.6 (15.6) | 45.0 (30.0 to 100.0) |
| Bradycardia, beats/min, No. (%) | ||
| <50 | 26 (68) | NA |
| <40 | 11 (29) | NA |
| QRS axis, degrees | 72 (18) | 76 (32 to 112) |
| QRS right-axis deviation, No. (%) | 3 (8) | NA |
| Interval, s | ||
| PR | 0.14 (0.03) | 0.15 (0.08 to 0.19) |
| QT | 0.45 (0.05) | 0.45 (0.33 to 0.58) |
| QTc | 0.40 (0.03) | 0.40 (0.34 to 0.45) |
| QT dispersion | 0.04 (0.02) | 0.04 (0.01 to 0.12) |
| JT | 0.37 (0.05) | 0.37 (0.26 to 0.49) |
| JTc | 0.32 (0.03) | 0.32 (0.27 to 0.38) |
| Echocardiogram data | ||
| LV mass, g | 100.1 (23.8) | 97.2 (58.6 to 151.5) |
| LV mass Z score | −0.5 (1.1) | −0.4 (−2.3 to 2.3) |
| Z score <−1, No. (%) | 11 (31) | NA |
| Z score >+1, No. (%) | 3 (9) | NA |
| Septal thickness, cm | 0.8 (0.1) | 0.7 (0.6 to 0.9) |
| Septal thickness Z score | −0.7 (0.7) | −0.6 (−1.9 to 0.7) |
| Z score <−1, No. (%) | 12 (34) | NA |
| Z score >+1, No. (%) | 0 | NA |
| Posterior wall thickness, cm | 0.7 (0.1) | 0.7 (0.5 to 0.9) |
| Posterior wall thickness Z score | −0.6 (0.8) | −0.6 (−2.6 to 1.0) |
| Z score <−1, No. (%) | 9 (26) | NA |
| Z score >+1, No. (%) | 1 (3) | NA |
| LV ESS, g/cm2 | 37.1 (7.6) | 37.0 (14.9 to 51.2) |
| LV ESS Z score | −1.5 (1.0) | −1.5 (−4.3 to 0.3) |
| Z score <−1, No. (%) | 23 (70) | NA |
| Z score >+1, No. (%) | 0 | NA |
| VCFc, count/s | 1.2 (0.2) | 1.2 (0.9 to 1.7) |
| VCFc Z score | 1.8 (2.0) | 1.8 (−2.9 to 6.5) |
| Z score <−1, No. (%) | 3 (9) | NA |
| Z score >+1, No. (%) | 25 (71) | NA |
| Fractional shortening, % | 37.7 (5.1) | 37.2 (27.6 to 55.3) |
| Fractional shortening Z score | 0.6 (1.4) | 0.6 (−2.4 to 4.6) |
| Z score <−1, No. (%) | 4 (11) | NA |
| Z score >+1, No. (%) | 13 (37) | NA |
| Ejection fraction | 0.66 (0.05) | 0.67 (0.56 to 0.77) |
| Ejection fraction Z score | 0.8 (1.2) | 0.9 (−1.5 to 3.2) |
| Z score <−1, No. (%) | 3 (9) | NA |
| Z score >+1, No. (%) | 16 (47) | NA |
Abbreviations: ECG, electrocardiogram; ESS, end-systolic stress; LV, left ventricle; NA, not applicable; VCFc, velocity of circumferential fractional contraction.
Values are expressed as mean (SD) unless indicated otherwise.
Table 4.
Bivariate Associations Between Cardiac Parameters and Demographic Variables at Baseline
| Regression Coefficient (SE) | ||||
|---|---|---|---|---|
| Outcome | Duration of Illness, mo | Duration of Amenorrhea, mo | BMI | Exercise, h/wk |
| Heart rate, beats/min | 0.24 (0.11)a | 0.38 (0.27) | −3.27 (1.36)a | −0.51 (0.40) |
| QTc interval, ms | 0.10 (0.20) | 1.20 (0.60)a | −2.50 (2.70) | −0.30 (0.80) |
| LV mass, g | −0.07 (0.18) | −0.63 (0.50) | 8.34 (1.77)b | 1.11 (0.60) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); LV, left ventricle.
P < .05.
P < .001.
ECHOCARDIOGRAPHIC DATA
Echocardiographic evaluation was carried out for 35 hospitalized subjects. Body surface area–corrected LV mass was mildly diminished in 11 subjects (31%) (Table 3) (mean [SD] LV mass Z score, −0.5 [1.1]). Subjects with higher BMI at admission had greater LV mass (Pearson r = 0.63; P < .001). Both posterior wall thickness and septal thickness were lower than predicted (Z score <−1) in 9 subjects (26%) and 12 subjects (34%), respectively. Systemic resistance (afterload) was less than average for age in the majority of subjects (23 subjects [70%]) as measured by LV end-systolic stress (mean [SD], 37.1 [7.6] g/cm2; mean [SD] Z score, −1.5 [1.0]; t test, P < .001). Velocity of circumferential fractional contraction was better than predicted (mean [SD] Z score, 1.8 [2.0]; t test, P < .001). Fractional shortening and ejection fraction were also normal (Table 3).
Three asymptomatic pericardial effusions were detected. These small to moderate effusions were present in young women with BMIs ranging from 12.6 to 17.3 at admission.
TREADMILL EXERCISE STRESS TEST DATA
Serum measurements of creatine kinase MB, troponin I, and aldolase did not differ from normal ranges either before or after the stress test. Neither the degree nor amount of preadmission exercise predicted stress test performance. Exercise tolerance was normal by all measures.
aBMD MEASUREMENTS
Mean (SD) lumbar spine aBMD was 0.886 (0.114) g/cm2, corresponding to a mean (SD) lumbar spine aBMD Z score of −0.889 (1.105). Baseline percentage of MBW was correlated with spinal aBMD (Pearson r = 0.59; P = .001). Higher ECG heart rate was predictive of lower spinal aBMD (regression coefficient [SE], −0.003 [0.001]; R2 = 0.15; P = .04) (Figure 2). While longer QT interval was associated with a higher spinal aBMD (regression coefficient [SE], 1.12 [0.33]; P = .002), this relationship was no longer significant for the QTc interval. Echocardiogram measures such as LV mass were also not predictive of spinal aBMD.
Figure 2.
Changes in echocardiogram measurements of left ventricle (LV) mass (A) and LV mass Z scores (B) between inpatient hospitalization and outpatient follow-up for 14 young women with anorexia nervosa. Mean changes and associated P values are taken from paired t tests (Table 5).
FOLLOW-UP DATA
Nineteen subjects returned for a second cardiovascular evaluation as an outpatient 87 to 411 days (median, 121 days) following admission. Weight restoration occurred between assessments (mean [SD] BMI change, 2.2 [1.6]; P < .001), leading to a significant increase in percentage of MBW and BMI at follow-up (mean [SD] BMI, 18.1 [1.7], corresponding to mean [SD] percentage of MBW of 88% [8%]; P < .001) (Table 5). While heart rate on ECG had improved, 4 subjects (22%) remained bradycardic (<50 beats/min). Right-axis deviation was more frequent in this sample (8 subjects [44%] at follow-up). The QT interval and JT interval were shorter than in previous measurements (P < .001). No arrhythmias, benign or otherwise, were noted on follow-up ECG.
Table 5.
Alterations in Hemodynamic Status of a Subsample of 19 Adolescents With Anorexia Nervosa Returning for Follow-up After Weight Restoration
| Finding | Mean (SD) | Sample for Pairwise Comparison, No. |
95% CI | ||
|---|---|---|---|---|---|
| Inpatient | Outpatient | Change | |||
| Demographic characteristic | |||||
| BMI | 15.9 (1.9) | 18.1 (1.7) | 2.2 (1.6) | 19 | 1.4 to 3.0 |
| Percentage of MBW, % | 78 (9) | 88 (8) | 10 (8) | 19 | 6 to 14 |
| ECG data | |||||
| Heart rate, beats/min | 47.5 (12.7) | 60.3 (13.0) | 12.6 (12.8) | 18 | 6.2 to 19.0 |
| QRS axis, degrees | 76.5 (18.3) | 83.3 (10.9) | 7.4 (19.7) | 18 | −2.4 to 17.2 |
| Interval, sa | |||||
| QT | 0.45 (0.05) | 0.40 (0.03) | −0.06 (0.04) | 18 | −0.08 to −0.04 |
| QTc | 0.39 (0.03) | 0.39 (0.02) | −0.01 (0.03) | 18 | −0.02 to 0.01 |
| JT | 0.37 (0.04) | 0.31 (0.03) | −0.06 (0.04) | 18 | −0.08 to −0.04 |
| JTc | 0.32 (0.03) | 0.31 (0.02) | −0.02 (0.03) | 18 | −0.03 to 0.00b |
| Echocardiogram data | |||||
| LV mass, g | 105.5 (28.0) | 104.4 (17.6) | −6.6 (21.4) | 14 | −18.9 to 5.8 |
| LV mass Z score | −0.2 (1.2) | −0.9 (0.6) | −0.9 (1.3) | 14 | −1.6 to −0.1 |
| Septal thickness, cm | 0.8 (0.1) | 0.7 (0.1) | −0.1 (0.1) | 13 | −0.2 to 0.0c |
| Septal thickness Z score | −0.4 (0.7) | −1.2 (0.6) | −1.0 (0.9) | 13 | −1.6 to −0.5 |
| VCFc | 1.3 (0.2) | 1.1 (0.1) | −0.2 (0.2) | 14 | −0.3 to 0.0d |
| VCFc Z score | 2.0 (2.1) | 0.5 (1.5) | −1.7 (2.7) | 14 | −3.2 to −0.1 |
| Treadmill exercise test | |||||
| Test duration, min | 14.7 (4.3) | 15.3 (3.9) | 0.9 (1.7) | 17 | 0.0 to 1.7e |
| Peak tidal volume, L | 1.6 (0.2) | 1.7 (0.3) | 0.1 (0.2) | 17 | 0.0 to 0.2f |
| Peak ventilatory efficiency | 24.1 (3.2) | 25.9 (3.5) | 2.0 (3.3) | 17 | 0.3 to 3.7 |
| Forced vital capacity, L | 3.3 (0.4) | 3.6 (0.4) | 0.3 (0.4) | 17 | 0.1 to 0.5 |
| Forced expiratory volume in 1 s, L | 3.0 (0.4) | 3.2 (0.4) | 0.3 (0.4) | 17 | 0.1 to 0.4 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CI, confidence interval; ECG, electrocardiogram; LV, left ventricle; MBW, median body weight; VCFc, velocity of circumferential fractional contraction.
The following formulas were used: QTc interval = QT interval/√RR interval; JTc interval = JT interval/√RR interval.
Exact 95% CI is −0.02900 to −0.00089 and does not contain 0. The 95% CI in the table appears to contain 0 due to rounding.
Exact 95% CI is −0.155 to −0.033 and does not contain 0.
Exact 95% CI is −0.285 to −0.021 and does not contain 0.
Exact 95% CI is 0.0050 to 1.7289 and does not contain 0. The 95% CI in the table appears to contain 0 due to rounding.
Exact 95% CI is 0.0431 to 0.2040 and does not contain 0.
Echocardiogram findings changed in unexpected ways given the weight restoration that occurred between visits (Table 5). Seventeen subjects had echocardiograms at both times, permitting paired comparisons. Septal thickness decreased (P = .01), and there was a clinically unimportant decline in velocity of circumferential fractional contraction (P=.03). Absolute measures of LV mass showed no change, while the corresponding Z scores declined over time (mean [SD] LV mass Z score, −0.2 [1.2] for inpatients vs −0.9 [0.6] for outpatients; P = .02). Subjects with lower BMIs no longer had lower measurements of LV mass (ρ = 0.36; P = .16). One pericardial effusion persisted, in the most malnourished patient (BMI of 15.0, improved from 12.1).
On repeated treadmill testing, participants were able to tolerate longer exertion (mean [SD] change, 0.9 [1.7] minute; P = .05) (Table 5). Several pulmonary measures, including peak tidal volume, peak ventilatory efficiency, forced vital capacity, and forced expiratory volume in 1 second, also significantly improved (all P ≤ .02) (Table 5).
COMMENT
Our data demonstrate that despite the high level of concern for sudden death and cardiac morbidity in patients with AN, few cardiac abnormalities exist among the population of malnourished adolescents admitted for nutritional stabilization. Additionally, observed abnormalities resolve on refeeding and restoration of appropriate body weight.
Most of our sample had sinus bradycardia, consistent with other reports of malnutrition.10,20,21 Reduction in heart rate is likely due to increased vagal tone and the body’s attempt to conserve energy in response to starvation.22 Interestingly, subjects with a longer duration of illness were less likely to be bradycardic. Acute reductions in nutritional intake and rapid weight loss have a more profound effect on metabolic regulation of heart rate than chronic malnourishment. In addition, subjects with a higher BMI at admission were more likely to have a lower heart rate. This finding is confounded by the fact that all subjects in this study met medical criteria for hospitalization; thus, patients with lower heart rates were selected for participation. Despite significant increases in weight between assessments, 4 subjects (22%) returning for a second evaluation remained bradycardic.
Repolarization abnormalities are a common concern of clinicians caring for patients with AN. Prolongation of the QT interval is the ECG abnormality most commonly associated with AN and is the most feared given the association between delayed repolarization and sudden death. Prevalence reports of QTc prolongation in subjects with AN range from 0% to 45%.12,23–26 In our small sample, the distribution of QTc intervals was within the normal range. Subjects who had a longer duration of amenorrhea had a longer QT interval. Measurements of weight alone (BMI, percentage of MBW) were not associated with length of the QT interval, suggesting that changes in the hormonal milieu may have an impact on repolarization apart from the effect of malnutrition alone. Refeeding appears to return any QT interval prolongation to normal over time. The QT dispersion has been shown to increase in subjects with AN compared with control subjects and is associated with longer duration of disease and advancing subject age.24,26,27 In the young women in our study, QT dispersion was within normal limits at both times. In our sample, only 1 subject had a metabolic derangement at the time of ECG; abnormal QTc intervals in the acute setting should be interpreted in the context of serum chemistries and clinical history.
Low aBMD is another well-known complication of AN.28–30 Patients with AN possess numerous risk factors for skeletal deficits, including low body weight, poor nutritional intake, and hormonal abnormalities. In this sample, spinal aBMD was within the normal range for age and sex and was strongly correlated with measures of malnutrition. Interestingly and unexpectedly, higher heart rate at admission predicted lower lumbar spine aBMD. Given the other correlations we found, it is likely that this relationship is mediated through duration of illness; subjects with higher heart rates at admission had longer durations of illness, and thus we would expect a more significant effect on spinal aBMD. We also found that a longer QT interval predicted increased spinal aBMD. This cross-system association emphasizes that the combination of malnutrition and behavior associated with AN results in a multisystem disease.
Myocardial mass decreases as a result of self-induced starvation, similar to reductions in skeletal muscle that are visible evidence of malnutrition.2,10 Reduced LV volumes, LV mass, and cardiac output have all been associated with AN.10,31 We found clinically unimportant decreases in LV mass at presentation. In subjects returning for follow-up, the recovery of LV mass was less than predicted by the increase in body surface area, and LV mass Z scores decreased between admission and follow-up assessments. The decline in LV mass Z scores despite weight improvements suggests early sparing of cardiac muscle in the face of moderate malnutrition as well as a relative delay of cardiac muscle restoration. Continued controversy exists as to the etiology of these findings. Possible hypotheses include a direct effect of malnutrition or a secondary effect of preload reduction leading to ventricular atrophy. Despite these decreased measurements, little to no LV dysfunction has been reported,31,32 nor was any noted here. The only truly pathologic observation, a pericardial effusion, was identified in 3 subjects; admission weight was less than 75% of MBW in 2 of the 3 subjects with an effusion. With no intervention other than nutritional rehabilitation, the effusion resolved in 2 of the cases. The outlier in this study who had persistent pericardial effusion and among the lowest LV mass indices had profound malnutrition with a BMI of 12.1 at presentation.
In addition to studying a sample of younger subjects with a shorter duration of illness, we also challenged subjects to perform a treadmill exercise test. This evaluation closely mimics the upright-posture stresses on the cardiovascular system that are associated with typical daily activities, unlike supine exercise tests used previously to assess this population. No significant abnormalities were noted either during acute medical stabilization or at follow-up. No evidence of occult myocardial injury was demonstrated using measurement of cardiac enzymes.
Study limitations deserve acknowledgment and consideration. Our sample is small and thus limits our ability to detect rather rare cardiac abnormalities. Only a subsample underwent cardiac enzyme measurements, which limits our power to detect subtle differences. However, not a single subject had cardiac enzyme results outside the normal range. In this pilot study, we were unable to assess potential hormonal mechanisms that may mediate the effects of malnutrition on cardiac status. All study subjects were at least 2 years post menarche. Results may not be generalizable to premenarchal girls. Lastly, our adolescent subjects were moderately malnourished (median BMI, 15.8). While this sample accurately reflects those patients deemed ill enough to require acute inpatient medical care at our institution, future studies will need to validate our findings in more severely malnourished populations (BMI ≤14.0).
Decreased emphasis on bed rest during hospitalization may be beneficial. Our patients continued to have evidence of orthostatic intolerance throughout their hospitalization. In addition, these patients had evidence of skeletal deficits. There are ample data that bed rest exacerbates both of these observations.5,33 These associations reinforce the implication that AN is a multisystem disorder, further emphasizing the need for creative, multidisciplinary treatment plans.
In conclusion, in a group of acutely malnourished and medically compromised adolescents with AN, few truly pathologic cardiac findings were identified. Most subjects demonstrated sinus bradycardia, which resolved for most with successful refeeding. Mild reductions in LV mass and LV function are seen both at baseline and at follow-up, suggesting early sparing of cardiac muscle in the face of moderate malnutrition as well as a relative delay of muscle restoration. No clinically important arrhythmias were identified. Measurements of spinal aBMD were inversely related to hemodynamic parameters, suggesting a link between skeletal health and the cardiovascular system. While limited, these data are concordant with other studies of the moderately malnourished patient with AN, particularly the careful study by Mont et al.12 Our findings suggest that in the patient with moderate malnutrition, less intensive cardiac monitoring and a more permissive approach to graded physical activity may be reasonable. However, individualized care plans may be needed depending on patient stability and behavioral issues.
Acknowledgments
Financial Disclosure: Dr DiVasta has received research grant funding for a clinical research trial from Wyeth Pharmaceuticals, Abbott Pharmaceuticals, and Duramed Pharmaceuticals. Dr Gordon is a consultant for Gilead Sciences and is associate director for the Pfizer-Merck–sponsored Clinical Investigator Training Program with Harvard and Massachusetts Institute of Technology.
Funding/Support: This study was supported by the John and Mary McCarthy Family Foundation, the Children’s Hospital Boston General Clinical Research Center, the William F. Milton Fund, project 5-T71-MC-00009-14 from the Maternal and Child Health Bureau, and the Sean Roy Johnson Fund through the Department of Cardiology, Children’s Hospital Boston.
Additional Contributions: We gratefully acknowledge our patients and their families; the expert nursing care of the Clinical and Translational Study Unit; and the adolescent medicine physicians at our institution.
Footnotes
Author Contributions: Study concept and design: DiVasta, Woods, Gordon, and Alexander. Acquisition of data: DiVasta, Quach, Gordon, and Alexander. Analysis and interpretation of data: DiVasta, Walls, Feldman, Woods, Gordon, and Alexander. Drafting of the manuscript: DiVasta, Walls, Feldman, and Alexander. Critical revision of the manuscript for important intellectual content: DiVasta, Walls, Feldman, Quach, Woods, Gordon, and Alexander. Statistical analysis: Walls and Feldman. Obtained funding: DiVasta and Alexander. Administrative, technical, and material support: DiVasta and Quach. Study supervision: DiVasta, Feldman, Woods, Gordon, and Alexander.
REFERENCES
- 1.Schwartz BI, Mansbach JM, Marion JG, Katzman DK, Forman SF. Variations in admission practices for adolescents with anorexia nervosa: a North American sample. J Adolesc Health. 2008;43(5):425–431. doi: 10.1016/j.jadohealth.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Casiero D, Frishman WH. Cardiovascular complications of eating disorders. Cardiol Rev. 2006;14(5):227–231. doi: 10.1097/01.crd.0000216745.96062.7c. [DOI] [PubMed] [Google Scholar]
- 3.Sylvester CJ, Forman SF. Clinical practice guidelines for treating restrictive eating disorder patients during medical hospitalization. Curr Opin Pediatr. 2008;20(4):390–397. doi: 10.1097/MOP.0b013e32830504ae. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Treatment of patients with eating disorders, third edition. Am J Psychiatry. 2006;163(7) suppl:4–54. [PubMed] [Google Scholar]
- 5.DiVasta AD, Feldman HA, Quach AE, Balestrino M, Gordon CM. The effect of bed rest on bone turnover in young women hospitalized for anorexia nervosa: a pilot study. J Clin Endocrinol Metab. 2009;94(5):1650–1655. doi: 10.1210/jc.2008-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lands L, Pavilanis A, Charge TD, Coates AL. Cardiopulmonary response to exercise in anorexia nervosa. Pediatr Pulmonol. 1992;13(2):101–107. doi: 10.1002/ppul.1950130208. [DOI] [PubMed] [Google Scholar]
- 7.Kahn D, Halls J, Bianco JA, Perlman SB. Radionuclide ventriculography in severely underweight anorexia nervosa patients before and during refeeding therapy. J Adolesc Health. 1991;12(4):301–306. doi: 10.1016/0197-0070(91)90003-5. [DOI] [PubMed] [Google Scholar]
- 8.Keys A, Henschel A, Taylor HL. The size and function of the human heart at rest in semi-starvation and in subsequent rehabilitation. Am J Physiol. 1947;150(1):153–169. doi: 10.1152/ajplegacy.1947.150.1.153. [DOI] [PubMed] [Google Scholar]
- 9.St John Sutton MG, Plappert T, Crosby L, Douglas P, Mullen J, Reichek N. Effects of reduced left ventricular mass on chamber architecture, load, and function: a study of anorexia nervosa. Circulation. 1985;72(5):991–1000. doi: 10.1161/01.cir.72.5.991. [DOI] [PubMed] [Google Scholar]
- 10.de Simone G, Scalfi L, Galderisi M, et al. Cardiac abnormalities in young women with anorexia nervosa. Br Heart J. 1994;71(3):287–292. doi: 10.1136/hrt.71.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moodie DS, Salcedo E. Cardiac function in adolescents and young adults with anorexia nervosa. J Adolesc Health Care. 1983;4(1):9–14. doi: 10.1016/s0197-0070(83)80221-6. [DOI] [PubMed] [Google Scholar]
- 12.Mont L, Castro J, Herreros B, et al. Reversibility of cardiac abnormalities in adolescents with anorexia nervosa after weight recovery. J Am Acad Child Adolesc Psychiatry. 2003;42(7):808–813. doi: 10.1097/01.CHI.0000046867.56865.EB. [DOI] [PubMed] [Google Scholar]
- 13.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 14.World Health Organization. Physical Status: The Use and Interpretation of Anthropometry. Geneva, Switzerland: World Health Organization; 1995. [Google Scholar]
- 15.Colan SD, Borow KM, Neumann A. Left ventricular end-systolic wall stress-velocity of fiber shortening relation: a load-independent index of myocardial contractility. J Am Coll Cardiol. 1984;4(4):715–724. doi: 10.1016/s0735-1097(84)80397-6. [DOI] [PubMed] [Google Scholar]
- 16.Kelly TL. Bone mineral density reference databases for American men and women. J Bone Miner Res. 1990;5 suppl 2:S249. [Google Scholar]
- 17.Looker AC, Wahner HW, Dunn WL, et al. Proximal femur bone mineral levels of US adults. Osteoporos Int. 1995;5(5):389–409. doi: 10.1007/BF01622262. [DOI] [PubMed] [Google Scholar]
- 18.Zemel BS, Leonard MB, Kalkwarf HJ, et al. Reference data for the whole body, lumbar spine, and proximal femur for American children relative to age, gender, and body size. J Bone Miner Res. 2004;1 suppl:S231. [Google Scholar]
- 19.Soldin SJ, Brugnara C, Wong EC, editors. Pediatric Reference Ranges. Washington, DC: AACC Press; 2003. [Google Scholar]
- 20.Moodie DS. Anorexia and the heart: results of studies to assess effects. Postgrad Med. 1987;81(8):46–48. 51–52, 55. doi: 10.1080/00325481.1987.11699858. [DOI] [PubMed] [Google Scholar]
- 21.Olivares JL, Vazquez M, Fleta J, Moreno LA, Perez-Gonzalez JM, Bueno M. Cardiac findings in adolescents with anorexia nervosa at diagnosis and after weight restoration. Eur J Pediatr. 2005;164(6):383–386. doi: 10.1007/s00431-005-1647-6. [DOI] [PubMed] [Google Scholar]
- 22.Kollai M, Bonyhay I, Jokkel G, Szonyi L. Cardiac vagal hyperactivity in adolescent anorexia nervosa. Eur Heart J. 1994;15(8):1113–1118. doi: 10.1093/oxfordjournals.eurheartj.a060636. [DOI] [PubMed] [Google Scholar]
- 23.Roche F, Barthelemy JC, Mayaud N, et al. Refeeding normalizes the QT rate dependence of female anorexic patients. Am J Cardiol. 2005;95(2):277–280. doi: 10.1016/j.amjcard.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Swenne I, Larsson PT. Heart risk associated with weight loss in anorexia nervosa and eating disorders: risk factors for QTc interval prolongation and dispersion. Acta Paediatr. 1999;88(3):304–309. doi: 10.1080/08035259950170079. [DOI] [PubMed] [Google Scholar]
- 25.Cooke RA, Chambers JB, Singh R, et al. QT interval in anorexia nervosa. Br Heart J. 1994;72(1):69–73. doi: 10.1136/hrt.72.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krantz MJ, Donahoo WT, Melanson EL, Mehler PS. QT interval dispersion and resting metabolic rate in chronic anorexia nervosa. Int J Eat Disord. 2005;37(2):166–170. doi: 10.1002/eat.20082. [DOI] [PubMed] [Google Scholar]
- 27.Galetta F, Franzoni F, Cupisti A, Belliti D, Prattichizzo F, Rolla M. QT interval dispersion in young women with anorexia nervosa. J Pediatr. 2002;140(4):456–460. doi: 10.1067/mpd.2002.122726. [DOI] [PubMed] [Google Scholar]
- 28.Bachrach LK, Guido D, Katzman D, Litt IF, Marcus R. Decreased bone density in adolescent girls with anorexia nervosa. Pediatrics. 1990;86(3):440–447. [PubMed] [Google Scholar]
- 29.Grinspoon S, Thomas E, Pitts S, et al. Prevalence and predictive factors for regional osteopenia in women with anorexia nervosa. Ann Intern Med. 2000;133(10):790–794. doi: 10.7326/0003-4819-133-10-200011210-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rigotti NA, Neer RM, Skates SJ, Herzog DB, Nussbaum SR. The clinical course of osteoporosis in anorexia nervosa: a longitudinal study of cortical bone mass. JAMA. 1991;265(9):1133–1138. [PubMed] [Google Scholar]
- 31.Romano C, Chinali M, Pasanisi F, et al. Reduced hemodynamic load and cardiac hypotrophy in patients with anorexia nervosa. Am J Clin Nutr. 2003;77(2):308–312. doi: 10.1093/ajcn/77.2.308. [DOI] [PubMed] [Google Scholar]
- 32.Galetta F, Franzoni F, Prattichizzo F, Rolla M, Santoro G, Pentimone F. Heart rate variability and left ventricular diastolic function in anorexia nervosa. J Adolesc Health. 2003;32(6):416–421. doi: 10.1016/s1054-139x(03)00048-x. [DOI] [PubMed] [Google Scholar]
- 33.Guinet P, Schneider SM, Macias BR, et al. WISE-2005: effect of aerobic and resistive exercises on orthostatic tolerance during 60 days bed rest in women. Eur J Appl Physiol. 2009;106(2):217–227. doi: 10.1007/s00421-009-1009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]