Abstract
The most recently discovered collagen gene, COL27A1, codes for type XXVII collagen. The COL27A1 gene is strongly expressed in developing cartilage and weakly expressed in many other tissue types. The present study was undertaken to identify transcriptional regulatory mechanisms that govern the expression of COL27A1 in cartilage, and in particular to determine whether SOX9, a key regulator of chondrogenesis, could activate COL27A1. The first intron of COL27A1 was examined to identify sites with homology to the Sox consensus sequence A/TA/TCAAA/TG. Three 50-bp regions that contained paired Sox sites arranged in opposite orientation to each other and separated by 3 or 4 bp were targeted for further analysis. The elements were tested by transient transfection of reporter plasmids, and two of the three elements showed enhancer activity in chondrocytic cells. The same two elements bound SOX9 in electrophoretic mobility shift assays (EMSA). They were not transcriptionally active in fibroblasts, but cotransfection with a SOX9 expression plasmid resulted in activation. The independent mutation of either Sox site in a pair prevented SOX9 binding to the enhancers in EMSA experiments, indicating that SOX9 binds these enhancers only as a dimer. Mutation of either site in a pair also abolished enhancer activity in chondrocytes, indicating that dimeric binding of SOX9 is required for transcriptional activation of the two new enhancers. In summary, these results suggest that SOX9 may play an important role in the transcriptional activation of the newest collagen gene, COL27A1.
Keywords: COL27A1, SOX9, cartilage, chondrocyte, enhancer, transcription
INTRODUCTION
The high mobility group (HMG)1 protein SOX9 is emerging as a key regulator of chondrogenesis (de Crombrugghe, et al., 2000). Heterozygous mutation of the SOX9 gene in humans results in a syndrome known as campomelic dysplasia (CD), which is characterized by severe skeletal anomalies as well as male-to-female sex reversal in about two-thirds of XY cases (Foster, et al., 1994; Houston, et al., 1983; Mansour, et al., 1995; Wagner, et al., 1994). In mice, targeted inactivation of the Sox9 gene renders embryonic cells incapable of differentiating into chondrocytes (Bi, et al., 1999). SOX9-binding enhancer elements have been identified in the 5’ regions and/or the first introns of the cartilage collagen genes Col2a1, Col11a2, Col9a1, and Col9a2, a well as in the non-collagen genes CD-RAP and aggrecan, which are also expressed in cartilage (Bernard, et al., 2003; Bridgewater, et al., 1998; Bridgewater, et al., 2003; Lefebvre, et al., 2001; Lefebvre, et al., 1997; Liu, et al., 2000; Sekiya, et al., 2000; Xie, et al., 1999; Zhang, et al., 2003; Zhou, et al., 1998).
Each of the enhancers in the Col2a1, Col11a2, and Col9a2 genes has been shown to contain paired SOX9-binding sites, with the two sites separated by three or four base pairs and arranged in opposite orientation to each other (Bernard, et al., 2003; Bridgewater, et al., 1998; Bridgewater, et al., 2003; Zhou, et al., 1998). Mutation of either of the two SOX-9 binding sites in a pair eliminates the transcriptional activity of the Col2a1 enhancer and of each of the three Col11a2 enhancers (Bridgewater, et al., 2003; Zhou, et al., 1998). Furthermore, the spacing between the paired Sox sites is critical for the transcriptional activity of the Col11a2 enhancers, suggesting that Sox9 binds these enhancers as a dimer in order to activate transcription (Bernard, et al., 2003; Bridgewater, et al., 2003). Electrophoretic mobility shift assays (EMSA) have also indicated that Sox9 binds the Col11a2 and Col9a2 enhancers as a dimer (Bernard, et al., 2003; Bridgewater, et al., 2003). Finally, recent work has demonstrated that SOX9 contains a DNA-dependent dimerization domain that, when mutated, can result in CD with the typical skeletal anomalies but without sex reversal, suggesting that dimerization of SOX9 is necessary for the activation of cartilage genes but not sex determination genes (Bernard, et al., 2003; Sock, et al., 2003).
A new fibrillar collagen gene, COL27A1, was recently identified (Boot-Handford, et al., 2003; Pace, et al., 2003). This gene is highly expressed in developing skeletal cartilage, with lower levels of expression noted in developing lungs, eye, ear, colon, stomach and several other tissues as demonstrated by RT-PCR and in situ hybridization experiments (Boot-Handford, et al., 2003; Pace, et al., 2003). The high level of Col27a1 expression in mouse cartilage suggested the possibility that Col27a1 may be regulated in cartilage by Sox9, as are Col2a1, Col11a2, Col9a1, and Col9a2.
To test this possibility the sequence of the human COL27A1 gene first intron was examined, and regions containing paired sites with homology to the heptameric Sox consensus sequence (A/TA/TCAAA/TG) were identified for further study. Candidate enhancer elements were analyzed, and two distinct elements were found to activate transcription in cultured chondrocytes but not fibroblasts. Each COL27A1 enhancer contains two sites with homology to the Sox consensus sequence, oriented oppositely to each other and separated by 3 base pairs (bp). The enhancers both bind SOX9, and mutation of either binding site in a pair prevents both SOX9 binding and transcriptional activation by the enhancers.
These results reveal that the newest collagen gene, COL27A1, contains enhancers that are activated by SOX9 binding, as are enhancers from several other cartilage collagen genes. This work, therefore, provides information about a likely mechanism of transcriptional regulation of the newest cartilage collagen gene while also expanding the list of cartilage genes potentially regulated by SOX9. Furthermore, this work demonstrates that SOX9 dimerization is required for transcriptional activation of the COL27A1 enhancers, consistent with the hypothesis that SOX9 dimerization is necessary for the activation of all cartilage collagen genes in chondrocytes.
RESULTS
Three candidate enhancer elements are present in the first intron of COL27A1
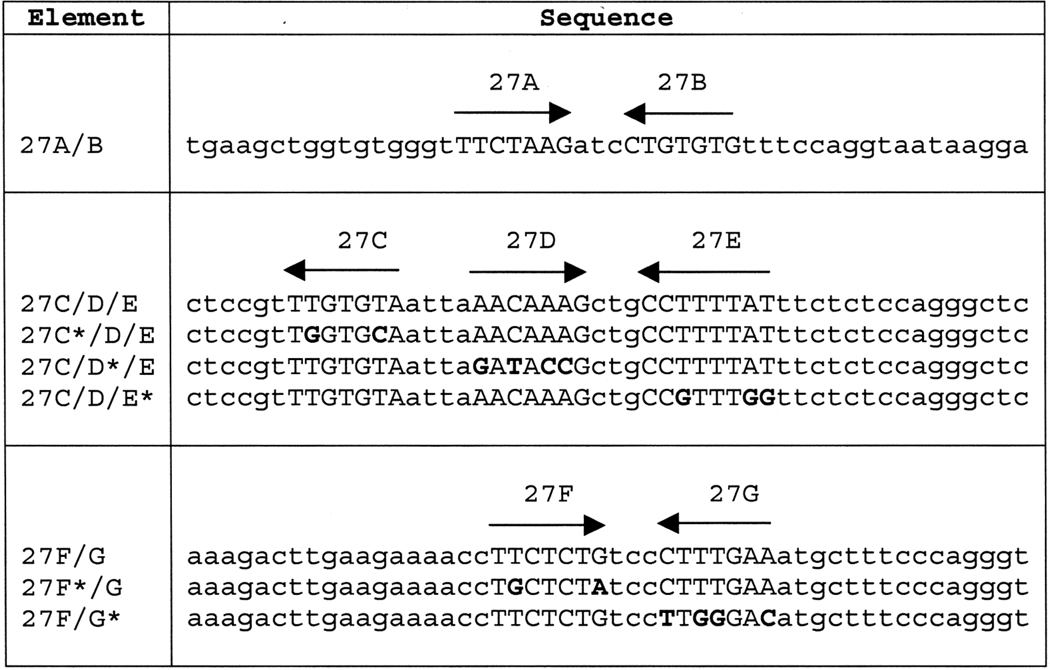
The first intron of the COL27A1 gene was searched for oppositely oriented pairs of the Sox consensus sequence ATATCAAATG, allowing a maximum of a 2-bp mismatch from this consensus. Three candidate sequences were identified (Table 1). Sequence 27A/B, located from +2271 to +2320, contained a 1-bp mismatch (27A) and a 2-bp mismatch (27B) to the Sox consensus sequence. Sequence 27C/D/E, located from +3823 to +3872, contained a 2-bp mismatch (27C), a perfect match (27D), and a 2-bp mismatch (27E). Four base pairs downstream of 27D is another site with a 1-bp mismatch. Because this site overlaps the 27E site by six of the seven bp, all eight bp encompassing both sites were considered part of the 27E site. Sequence 27F/G, located from +5209 to +5258, contained a 2-bp mismatch (27F) and a perfect match (27G).
Table 1.
Sequence of wild-type and mutant elements tested for enhancer activity. Sites with homology to the Sox consensus sequence are shown in upper case. Substituted nucleotides are shown in bold. Arrows indicate the orientation of each consensus sequence. All three elements are located in the first intron of the human COL27A1 gene, with A/B located from +2271 to +2320, C/D/E located from +3823 to +3872, and F/G located from +5209 to +5258.
 |
Elements 27C/D/E and 27F/G exhibit enhancer activity in RCS cells
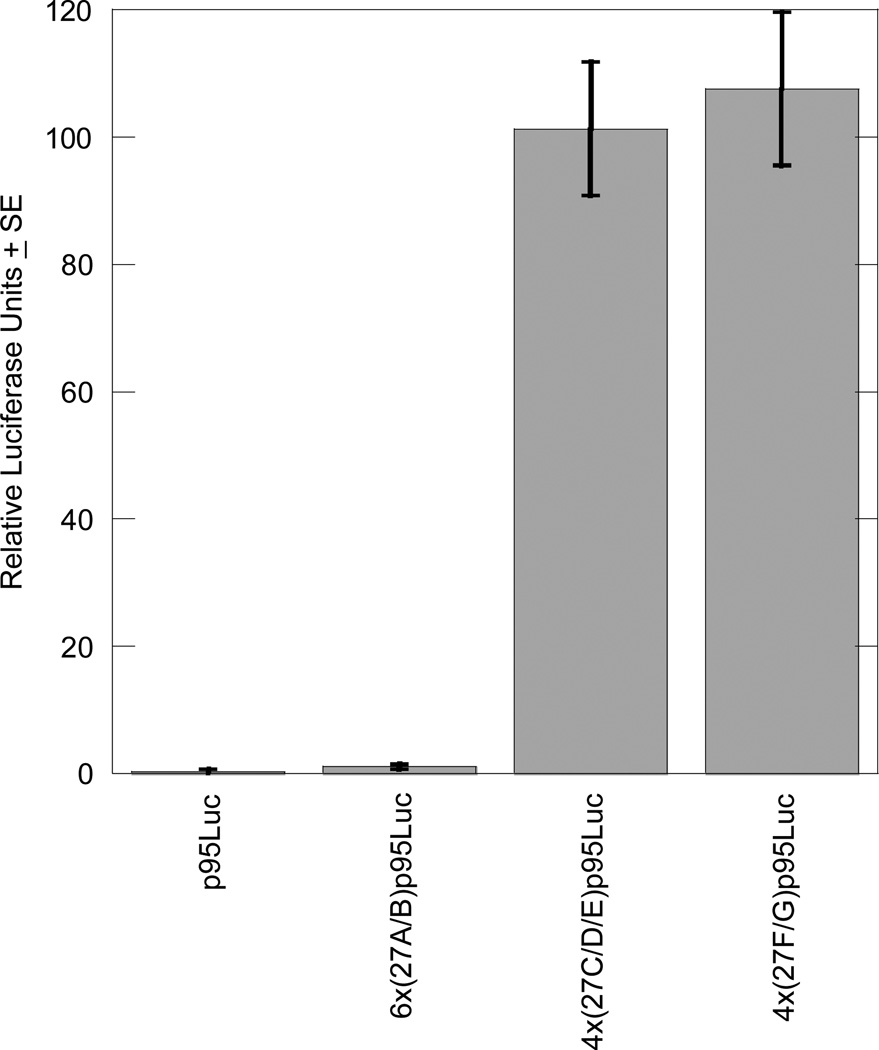
Using transient transfections of reporter plasmids, the three candidate sequences 27A/B, 27C/D/E and 27F/G were tested for their ability to act as independent enhancers in chondrocytic RCS cells. Six tandem copies of the 27A/B sequence cloned upstream of a minimal promoter and a luciferase reporter gene in the 6x(27A/B)p95Luc plasmid showed no significant activity compared to the negative control, indicating that the 27A/B sequence does not function as an independent enhancer. Four tandem copies of the 27C/D/E sequence cloned upstream of the minimal promoter and luciferase reporter in the 4x(27C/D/E)p95Luc plasmid, however, significantly activated transcription above the negative control to 101.3 relative luciferase units (RLU). Similarly, the 4x(27F/G)p95Luc plasmid significantly activated transcription to 107.6 RLU (Figure 1).
Figure 1. The 27C/D/E and 27F/G sequences exhibit enhancer activity in a chondrocytic cell line.
Transient transfections of luciferase reporter plasmids containing the three candidate COL27A1 enhancer elements 27A/B, 27C/D/E, and 27F/G, as well as a no-enhancer control plasmid (p95Luc) containing only the minimal promoter and luciferase reporter were performed in RCS cells. Each transfection reaction included 1.5 µg luciferase reporter plasmid and 0.5 µg pSV-β-galactosidase as an internal control for transfection efficiency. The graph includes the results of three independent experiments, each performed in triplicate, and results are presented as RLU (luciferase units per β-galactosidase unit) ± standard error (SE). Each experiment included a parallel transfection with the chondrocyte-specific reporter plasmid (4xD/E)p95Luc, which contains the D/E enhancer from Col11a2, and results were normalized to its activity (Bridgewater, et al., 1998).
Ectopic expression of SOX9 activates 27C/D/E and 27F/G in fibroblasts
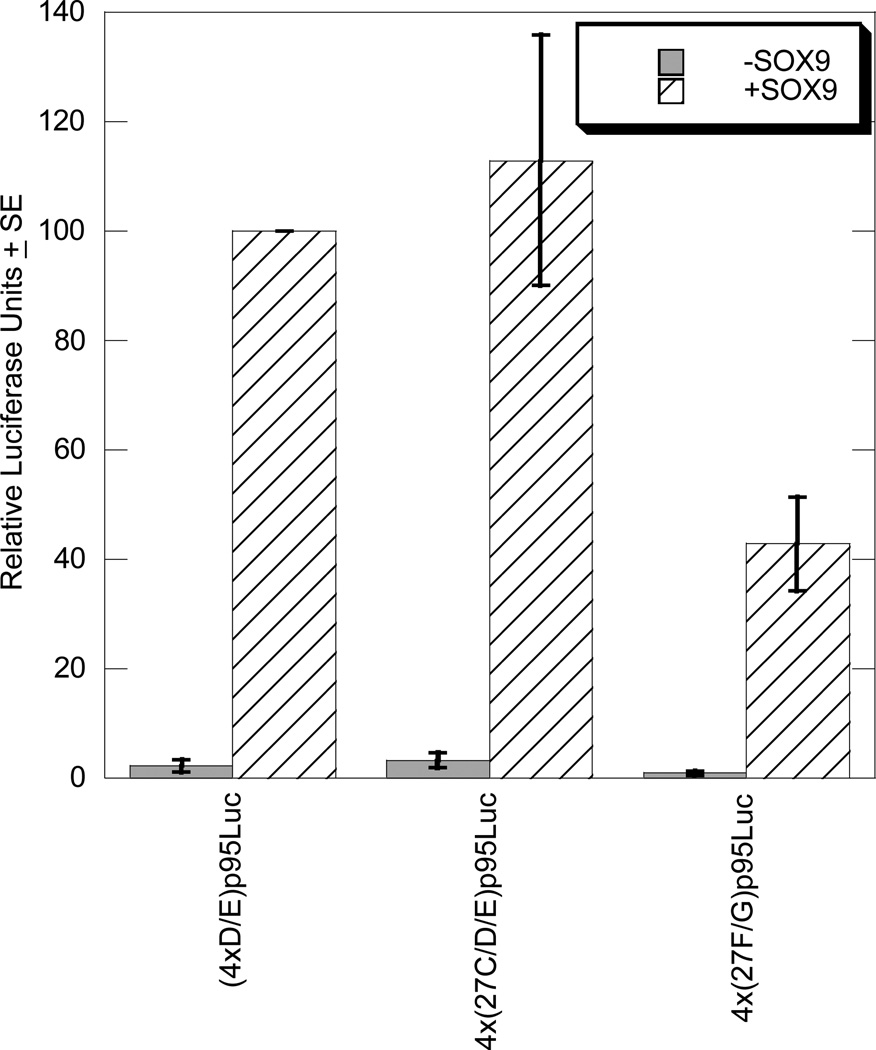
When the 4x(27C/D/E)p95Luc and 4x(27F/G)p95Luc plasmids were transfected into BALB/3T3 fibroblasts that do not produce SOX9 endogenously, no enhancer activity was detected. Cotransfection with the SOX9 expression plasmid, however, activated both of the enhancers (Figure 2). The Col11a2 enhancer (4xD/E)p95Luc construct, which is strongly activated by SOX9, served as a positive control (Bridgewater, et al., 1998). SOX9 activated 4x(27C/D/E)p95Luc and 4x(27F/G)p95Luc to 112.0% and 45.8% of (4xD/E)p95Luc activity, respectively.
Figure 2. SOX9 activates the 27C/D/E and 27F/G enhancers in BALB/3T3 fibroblasts.
Transient transfection reactions included 1.0 µg luciferase reporter plasmid, 0.5 µg SOX9 expression plasmid where noted, and 0.5 µg pSVβ-gal to control for transfection efficiency. Results are normalized to the activity of (4xD/E)p95Luc activated by SOX9. The graph includes results of at least three independent experiments performed in duplicate.
SOX9 binds to the 27C/D/E and 27F/G enhancers in vitro
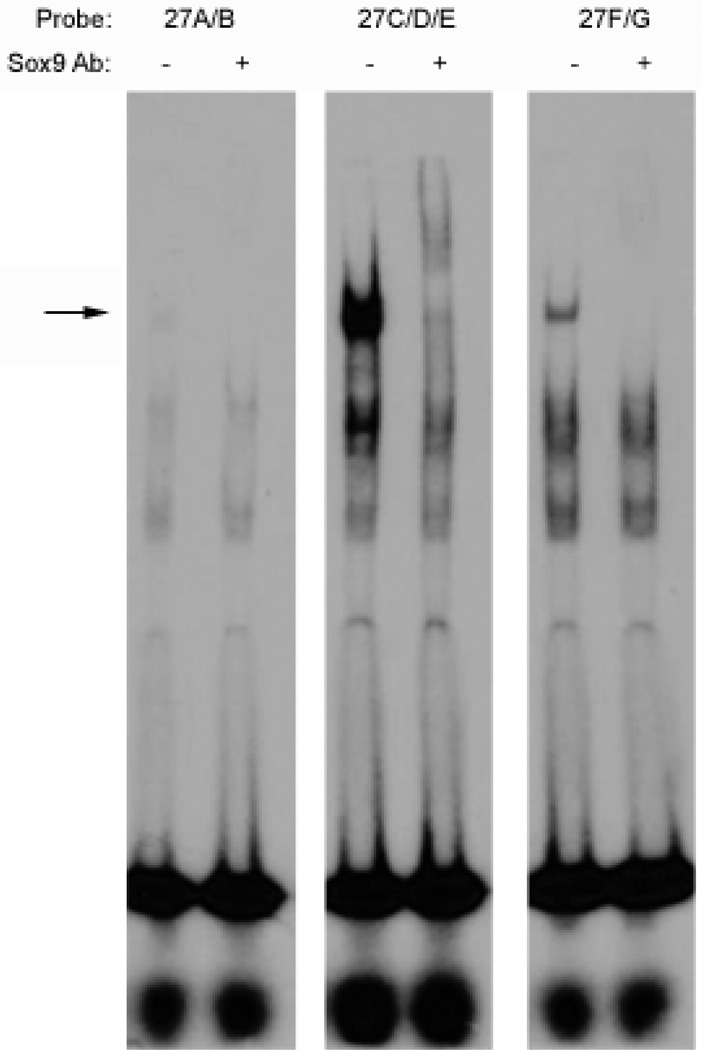
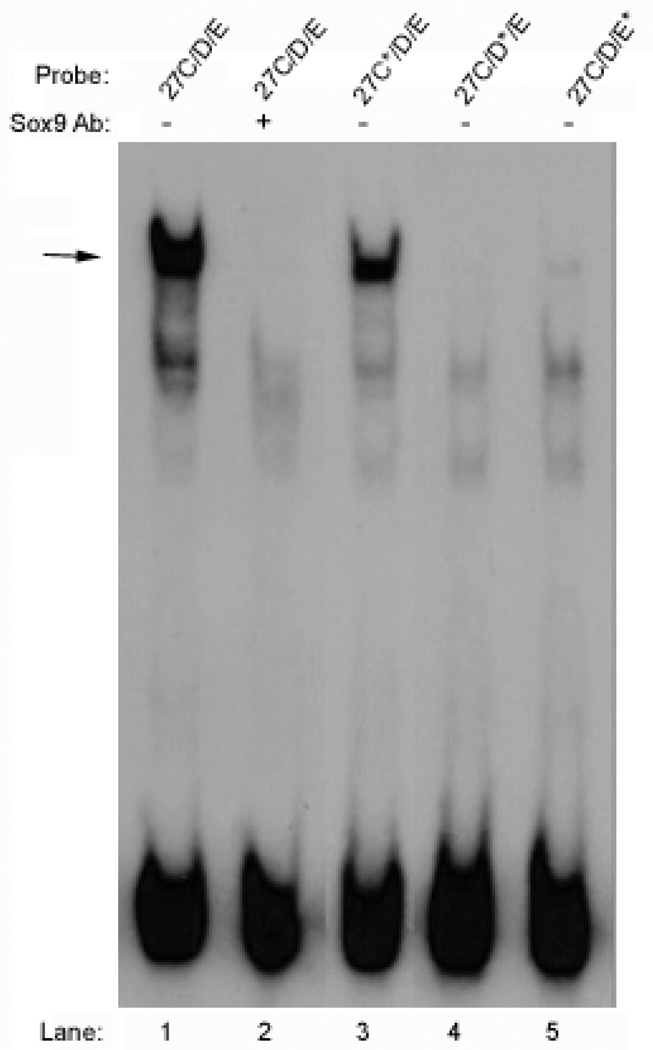
The radiolabeled 27A/B, 27C/D/E, and 27F/G probes were incubated with in vitro transcribed and translated SOX9 protein, with or without antibody against SOX9. Both the 27C/D/E and 27 F/G probes bound SOX9, as indicated by the supershift or blockshift of a single DNA-protein complex when antibody against SOX9 was included (Figure 3, arrow). The 27A/B probe, however, did not bind SOX9.
Figure 3. SOX9 binds the 27C/D/E and 27F/G sequences but not the 27A/B sequence.
EMSA experiments were performed using the 27A/B, 27C/D/E, and 27F/G probes as indicated, with in vitro synthesized SOX9, and with or without antibodies against SOX9. Probes 27C/D/E and 27F/G each formed a complex with SOX9 (arrow) that was supershifted or blockshifted by antibodies against SOX9. The 27B/C probe did not bind SOX9.
Mutating the 27D, 27E, 27F, or 27G sites decreased enhancer activity
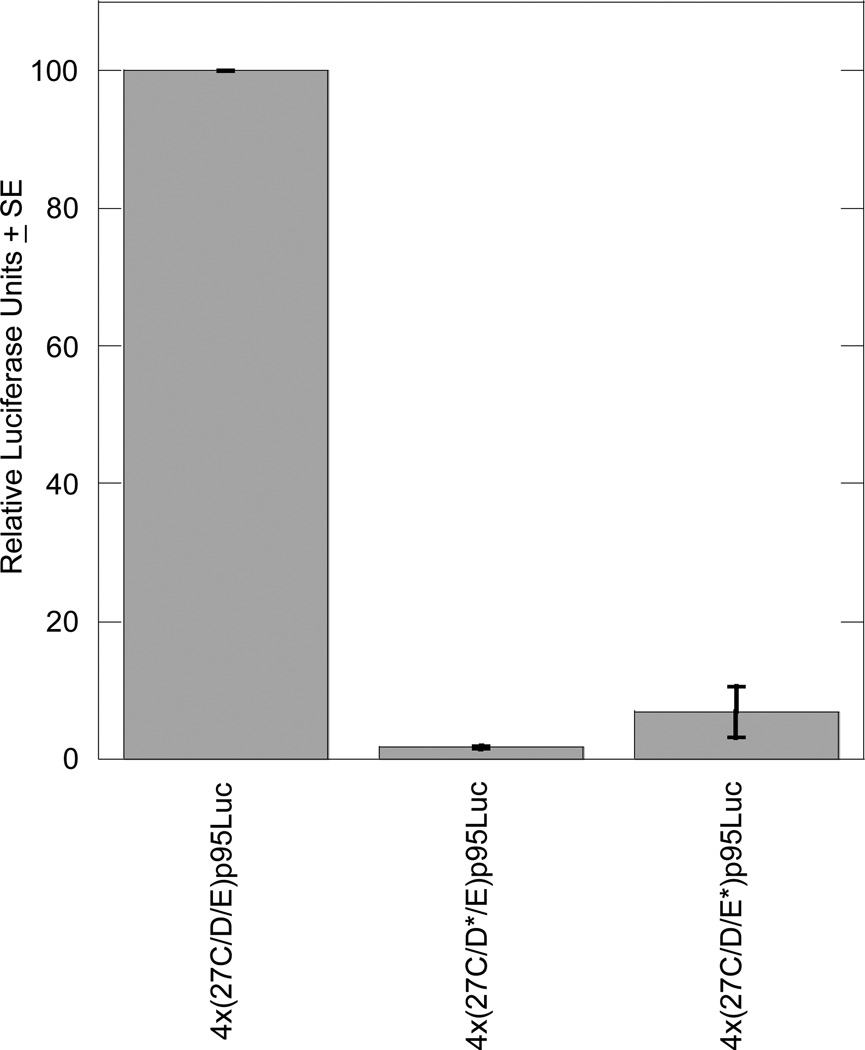
Each of the putative SOX9 binding sites in the 27C/D/E and 27F/G enhancers was mutated individually to contain a total of four mismatches to the Sox consensus sequence (Table 1). Reporter plasmids containing these mutant enhancers were transiently transfected into both chondrocytic RCS cells and BALB/3T3 fibroblasts. When transfected into RCS cells, construct 4x(27C*/D/E)p95Luc showed no decrease in enhancer activity compared to the positive control. In fact, transcription was increased, indicating that the 27C site is not important for transcriptional activation (data not shown).
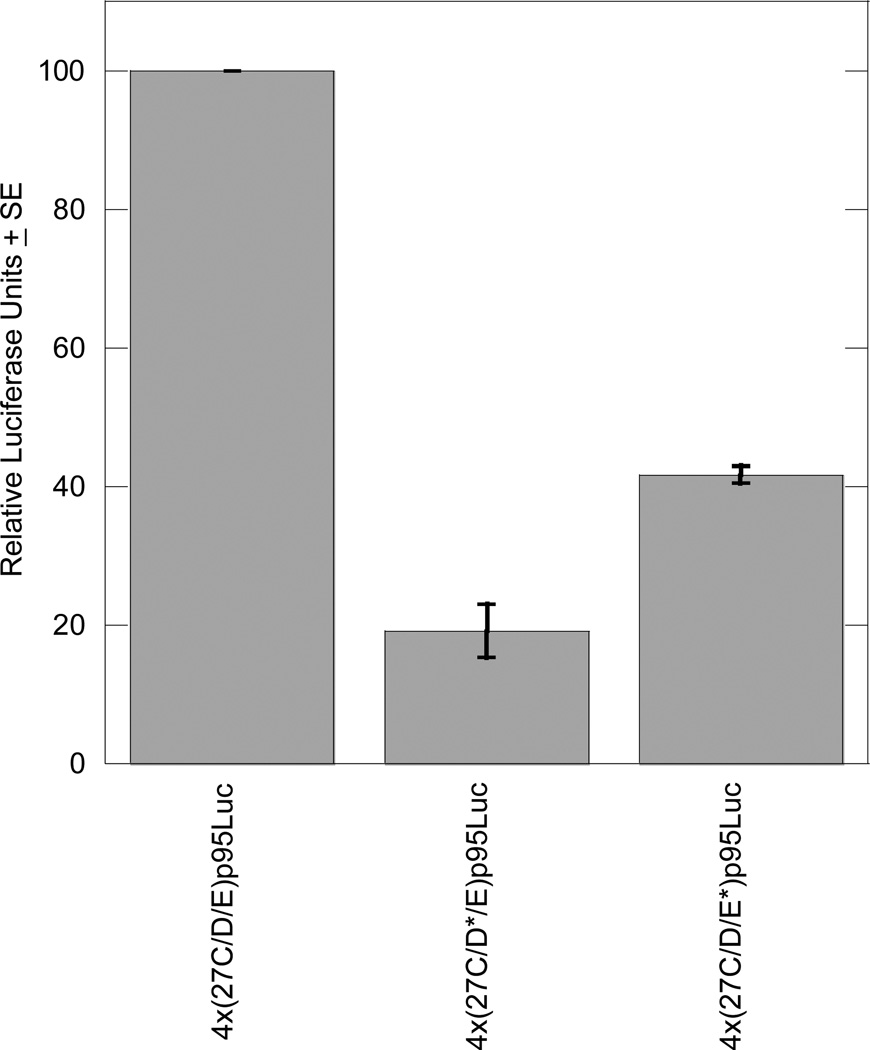
In RCS cells, mutation of the 27D site in construct 4x(27C/D*/E)p95Luc abolished transcriptional activation in transient transfections; activity was 1.7% of the wild-type 4x(27C/D/E)p95Luc. Similarly, mutation of the 27E site in construct 4x(27C/D/E*)p95Luc reduced activation to only 6.8% of wild-type in RCS cells (Figure 4).
Figure 4. Mutating the 27D or 27E site eliminates enhancer activity in RCS cells.
Each transient transfection reaction included 1.5 µg luciferase reporter plasmid and 0.5 µg pSV-β-galactosidase plasmid as an internal control for transfection efficiency. The graph includes results from three independent experiments, each performed in duplicate, and results are normalized to the activity of the wild-type 4x(27C/D/E)p95Luc plasmid.
When the mutated 27C/D/E enhancer constructs were cotransfected with the SOX9 expression plasmid into BALB/3T3 fibroblasts, the mutations reduced enhancer responsiveness to SOX9. The 27D and 27E site mutations reduced transcriptional activity to 19.2% and 41.7% of wild-type, respectively (Figure 5).
Figure 5. Mutating the 27D or 27E site reduces SOX9 responsiveness in BALB/3T3 fibroblasts.
Each transient transfection reaction included 1.0 µg luciferase reporter plasmid, 0.5 µg SOX9 expression plasmid, and 0.5 µg pSV-β-galactosidase as in internal control for transfection efficiency. The graph includes results from three independent experiments, each performed in duplicate, and results are normalized to the activity of the wild-type 4x(27C/D/E)p95Luc plasmid.
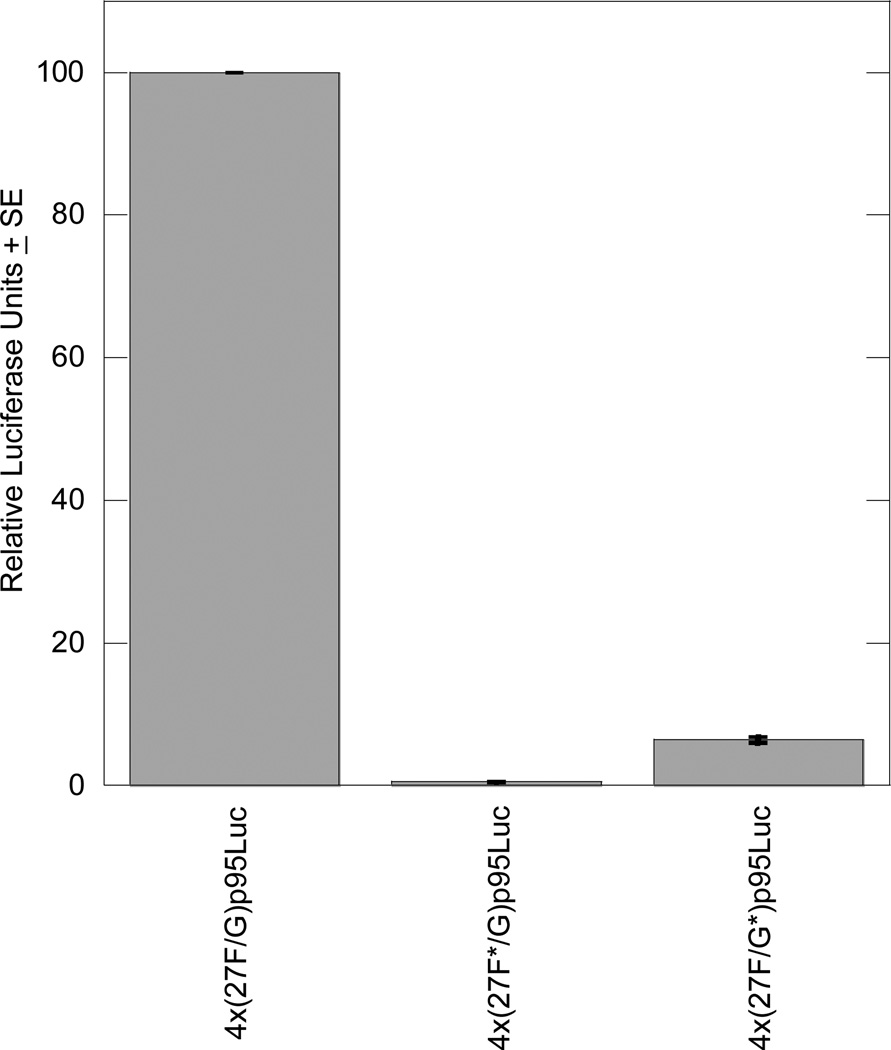
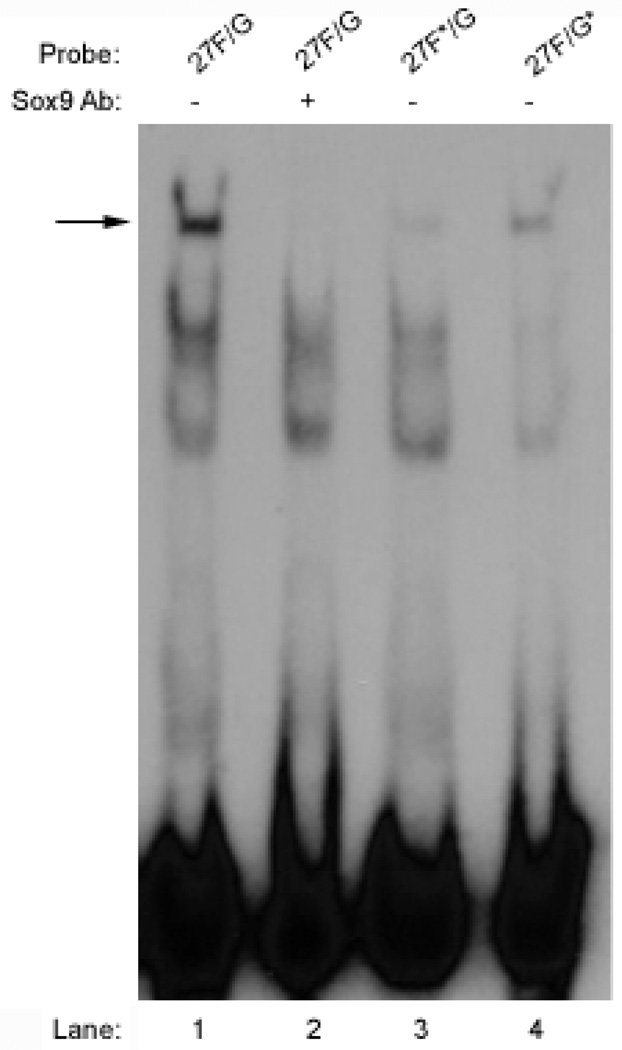
In RCS cells, mutation of the 27F site in construct 4x(27F*/G)p95Luc prevented transcriptional activation in transient transfections; activity was only 0.5% of the wild-type 4x(27F/G)p95Luc. Similarly, mutation of the 27G site in construct 4x(27F/G*)p95Luc reduced transcriptional activation to 6.4% of wild-type in RCS cells (Figure 6).
Figure 6. Mutating the 27F or 27G site eliminates enhancer activity in RCS cells.
Each transient transfection reaction included 1.5 µg luciferase reporter plasmid and 0.5 µg pSV-β-galactosidase to control for transfection efficiency. The graph includes results from three independent experiments, each performed in duplicate, and results are normalized to the activity of the wild-type 4x(27F/G)p95Luc plasmid.
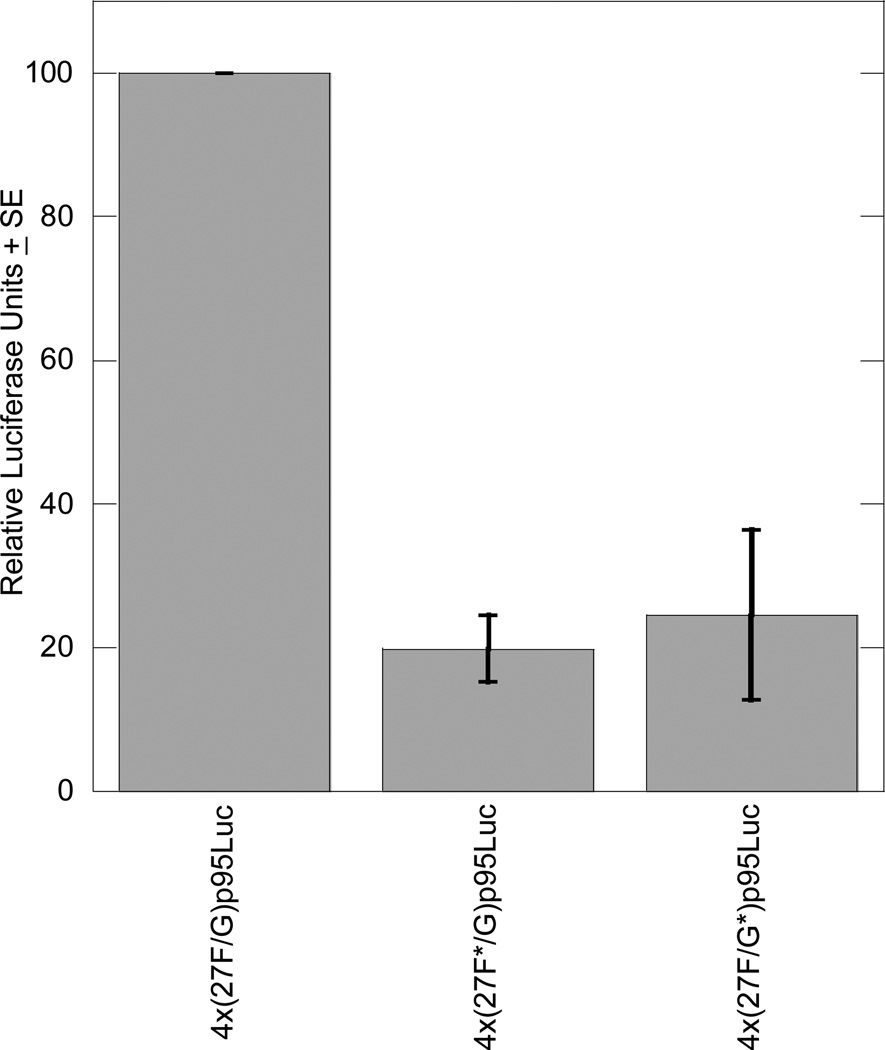
When the mutated 27F/G enhancer constructs were cotransfected with the SOX9 expression plasmid into BALB/3T3 fibroblasts, these mutations were also found to reduce responsiveness to SOX9. The 27F mutation reduced transcription to 19.9% of the wild-type control, and the 27G mutation reduced transcription to 24.6% (Figure 7).
Figure 7. Mutating the 27F or 27G site reduces SOX9 responsiveness in BALB/3T3 cells.
Each transient transfection reaction included 1.0 µg luciferase reporter plasmid, 0.5 µg SOX9 expression plasmid, and 0.5 µg pSV-β-galactosidase to control for transfection efficiency. The graph includes results from three independent experiments, each performed in duplicate, and results are normalized to the activity of the wild-type 4x(27F/G)p95Luc plasmid.
Mutating the 27D, 27E, 27F, or 27G sites prevents SOX9 binding in EMSA
When EMSAs were performed using the mutated 27C/D/E probes, mutation of the 27C site was found to have little effect on SOX9 binding, consistent with the transient transfection results showing that the 27C site was not necessary for enhancer activity (Figure 8, lane 3). Mutation of the 27D entirely prevented SOX9 binding to DNA (Figure 8, lane 4), and mutation of the 27E site prevented the majority of SOX9 binding (Figure 8, lane 5).
Figure 8. Mutating the 27D or 27E site prevents SOX9 binding to the 27C/D/E enhancer.
In vitro synthesized SOX9 was preincubated with or without antibody against SOX9 and then combined with 27C/D/E wild-type and mutant probes as indicated. Mutation of the 27C site did not prevent binding of SOX9 to 27C/D/E, but mutation of either the 27D or 27E sites did prevent binding.
Mutating the 27F site in the 27F/G probe prevented the majority of SOX9 binding seen with the wild-type 27F/G probe (Figure 9, lane 3). Similarly, mutating the 27G site reduced SOX9 binding markedly (Figure 9, lane 4). Interestingly, no higher mobility complexes increased as the primary SOX9 complexes with 27C/D/E and 27 F/G decreased. Such a result would have suggested the binding of SOX9 as a monomer when one of the sites in a pair was mutated to prevent dimer formation. Instead, mutation of either site in a pair prevented SOX9 binding, suggesting that SOX9 binds to the 27C/D/E and 27F/G enhancers as a dimer or not at all.
Figure 9. Mutating the 27F or 27G site prevents binding to the 27F/G enhancer.
In vitro synthesized SOX9 was preincubated with or without antibody against SOX9 and then combined with 27F/G wild-type and mutant probes as indicated. Mutation of either the 27F or 27G sites prevented binding of SOX9 to the 27F/G enhancer.
DISCUSSION
We demonstrate here that the newest cartilage collagen gene, COL27A1, contains two enhancer elements that bind SOX9. The enhancers resemble chondrocyte-specific enhancer elements from three other cartilage collagen genes, Col2a1, Col11a2, and Col9a2, in that they feature paired binding sites for the transcription factor SOX9 arranged in opposite orientation facing each other and separated by 3 or 4 bp (Bernard, et al., 2003; Bridgewater, et al., 1998; Bridgewater, et al., 2003; Zhou, et al., 1998). The identification of these two elements has significance beyond its relevance to COL27A1 transcription because it expands the list of cartilage genes likely to be regulated by SOX9, and moreover, because it provides new evidence for the hypothesis that SOX9 functions as a dimer when activating cartilage genes.
The work presented here also demonstrates, however, that paired Sox consensus sequences are not alone sufficient to activate transcription in chondrocytes—even when they are oriented facing toward each other and separated by three or four base pairs. This point was demonstrated convincingly by the 27A/B element, which contains Sox sites arranged and paired like those in the 27C/D/E and 27F/G enhancers. The 27A/B element, however, has no enhancer activity and does not bind SOX9 in EMSA experiments. It is notable that the 27A/B element contains no perfect match to the Sox consensus sequence. Neither, however, do the B/C and F/G enhancers from the Col11a2 gene, and both are very strong chondrocyte-specific enhancer elements (Bridgewater, et al., 1998; Bridgewater, et al., 2003). A possible explanation for this paradox is that other proteins are required as cofactors of SOX9 in order to achieve stable DNA binding and transcriptional activation, and 27A/B contains no binding sites for those proteins. The observation, however, that enhancers 27C/D/E and 27F/G bind relatively pure in vitro synthesized SOX9 in EMSA experiments, apparently without the need for additional cofactors, argues against this possibility unless those cofactors are present in the in vitro transcription/translation reaction mix. An alternative explanation is that the actual binding site for SOX9 in a dimer is longer than the currently recognized heptameric consensus sequence. Unfortunately, an examination of the sequences surrounding the Sox sites in the 27A/B, 27C/D/E, and 27F/G elements reveals no obvious similarities between the two functional elements or differences between them and the non-functional 27A/B element.
The results presented here indicate that the paired Sox sites must be oriented oppositely and facing each other to produce transcriptional activation. This is illustrated by the observation that the 27C site in the 27C/D/E enhancer plays no role in SOX9 binding or in transcriptional activation. The 27C site is located four base pairs upstream of the 27D site, as close as the functional pair of sites in the Col11a2 B/C enhancer element. It is oriented oppositely to the 27D site, but they face away from instead of toward each other. This orientation apparently does not support SOX9 dimer binding. Mutation of the 27C site did not reduce enhancer activity or prevent SOX9 binding in EMSA experiments, and even when the 27E site was mutated, the 27C/D pair did not become functional. Instead, enhancer activity was abolished.
The necessity of paired sites for SOX9 binding was clearly illustrated by the fact that mutation of either site in a pair abolished enhancer activity of both 27C/D/E and 27F/G in RCS cells. Mutation of either site in a pair also prevented SOX9 dimer binding in EMSA experiments without producing a corresponding increase in a monomeric complex, suggesting that with the COL27A1 enhancers at least, SOX9 binds as a dimer or not at all. It is interesting to note, however, that the same mutations that eliminated enhancer activity in RCS cells reduced but did not eliminate the ability of the enhancers to be activated by ectopic expression of SOX9 in fibroblasts. This result is likely due the fact that the SOX9 expression plasmid produced levels of SOX9 in fibroblasts that were far higher than the endogenous levels present in RCS cells and other chondrocytes. It may be that in the presence of a large excess of SOX9, some unnatural monomeric binding and transcriptional activation can occur.
The observations presented here indicating that SOX9 must function as a dimer to activate the COL27A1 enhancers are consistent with results published regarding Sox10, another Sox family protein that is closely related to SOX9 (Peirano, et al., 2000). Sox10 was shown to have a DNA-dependent dimerization domain on the immediate N-terminal side of its HMG domain. Dimerization of Sox10 only occurred in the presence of paired DNA binding sites that were oriented oppositely toward each other (Peirano, et al., 2000). Alteration of the paired sites so that both were facing in the same orientation or so that they were facing away from each other abolished Sox10 dimer binding (Peirano, et al., 2000).
In 2003, two different groups demonstrated that SOX9 also has a DNA-dependent dimerization domain that is highly homologous to the Sox10 dimerization domain (Bernard, et al., 2003; Sock, et al., 2003). SOX9 has been shown to bind as a dimer to the three Col11a2 chondrocyte-specific enhancer elements, and changing the spacing between the SOX9-binding sites eliminates enhancer activity (Bernard, et al., 2003; Bridgewater, et al., 2003). SOX9 has also been shown to bind as a dimer to a Col9a2 enhancer element and to a CD-RAP enhancer element (Bernard, et al., 2003; Bridgewater, et al., 1998; Bridgewater, et al., 2003; Sock, et al., 2003). It bound as a monomer, however to the regulatory region of the sex-determining gene SF1 and to a target sequence from the promoter of the human anti-Mullerian hormone gene (Bernard, et al., 2003; Sock, et al., 2003). This, combined with the identification of two patients with SOX9 missense mutations in the dimerization domain that resulted in cartilage abnormalities but not sex reversal suggested the hypothesis that dimerization may be required for the regulation of chondrogenesis but not sex-determination genes (Bernard, et al., 2003; Sock, et al., 2003).
In summary, the work presented here has revealed a mechanism for the transcriptional regulation of the newest collagen gene, COL27A1, in chondrocytes. The new gene contains two enhancer elements that respond to SOX9, adding to the growing list of cartilage genes likely activated by this key regulator of chondrogenesis. Furthermore, this work has demonstrated that SOX9 must function as a dimer to activate the COL27A1 enhancers. This work, combined with previous studies on the function of SOX9 in cartilage gene activation, is instrumental in establishing a clearer understanding of the pattern of regulation in cartilage genes. A review of previous work reveals that SOX9 functions as a dimer in the activation of several cartilage collagen genes, and studies of other collagen genes have implicated SOX9 without examining its dimerization state. Still other cartilage collagen genes have never been examined as possible targets of SOX9. Finally, the observation that CD-RAP also contains binding sites for dimerized SOX9 supports the idea that non-collagen cartilage genes such as the aggrecan gene may also be regulated by dimerized SOX9. With this pattern emerging, a broad-scale search for paired Sox sites in the regulatory regions of a range of cartilage genes will likely yield interesting results.
EXPERIMENTAL PROCEDURES
Cell Types and Culture
Rat chondrosarcoma (RCS) cells and BALB/3T3 cells (714 line) were a gift from Dr. Benoit de Crombrugghe of the University of Texas M.D. Anderson Cancer Center. Cells were cultured at 37°C under 5% CO2 in Dulbecco's modified Eagle's medium (Cellgro), supplemented with penicillin/streptomycin (50 units/ml and 50 µg/ml, respectively; Gibco), L-glutamine (2 mM; Gibco) and 10% fetal bovine serum (Atlanta Biologicals).
Plasmid constructions
The first intron of the COL27A1 gene was searched to identify sites with homology to the heptameric Sox consensus sequence A/TA/TCAAA/TG. Sites that matched the consensus perfectly as well as 1-bp mismatches were identified. The sequences surrounding the perfect consensus sites and 1-bp mismatches were then searched to identify 2-bp mismatches within pairing range (3 to 4 bp). Three regions 50 bp long containing at least two Sox sites oriented oppositely toward each other and separated by 3 or 4 bp were selected for further analysis. The candidate SOX9 binding sites within these regions were named 27A, 27B, 27C, 27D, 27E, 27F and 27G, sequentially in the 5’ to 3’ direction. The candidate enhancer elements that included these potential Sox9 binding sites were therefore named 27A/B (located from +2271 to + 2320), 27C/D/E (located from +3823 to + 3872), and 27F/G (located from +5209 to +5258) (Table 1).
To generate mutations of the Sox sites, substitutions were introduced to produce a total of four mismatches to the Sox consensus sequence at each targeted site. Wild-type and mutant versions of each enhancer were synthesized as complementary oligonucleotides, purified by polyacrylamide gel electrophoresis, and annealed for use either as probes for electrophoretic mobility shift assay (EMSA) or to be cloned into luciferase reporter plasmids (Table 1).
The plasmids 6x(27A/B)p95Luc, 4x(27C/D/E)p95Luc, and 4x(27F/G)p95Luc, as well as the mutant versions of each plasmid, were constructed in the luciferase reporter vector pLuc4. Intermediate steps of construction and enhancer multimerization were performed in the p89Col2a1Bs plasmid as previously described (Bridgewater, et al., 1998; Bridgewater, et al., 2003). Briefly, double stranded oligonucleotides were cloned into p89Col2a1Bs at the BamHI/BglII site and multimerized to four or six tandem copies, and then the multimerized enhancers along with a minimal 89-bp promoter from the Col2a1 gene were transferred into pLuc4 upstream of the luciferase reporter. Each final construct, therefore, contained the multimerized enhancers immediately upstream of the minimal promoter, which was immediately upstream from the luciferase reporter. The p89Col2a1Bs plasmid as well as the SOX9 expression plasmid SOX9-pcDNA-5’-UT were kind gifts of Veronique Lefebvre of the Cleveland Clinic, Cleveland, OH.
Electrophoretic Mobility Shift Assays
DNA probes were radiolabeled by end-filling with Klenow fragment using α32P-dGTP. For binding reactions, 1 µl in vitro transcribed and translated SOX9 protein (STP3 kit; Novagen) and 0.1 µg of poly(dG-dC)·poly(dG-dC) nonspecific competitor were incubated with or without SOX9 antibody (Santa Cruz) at room temperature for 30 minutes. Radiolabeled DNA probe was added to the reaction and incubated another 30 minutes. Binding reactions were separated on native 4% polyacrylamide gels at 150 V for 2–3 hours, and results were visualized by autoradiography.
Transient transfections
Transfections were performed in triplicate, using the LipofectAMINE reagent (Life Technologies, Inc.) for RCS cells and the FUGENE-6 transfection reagent (Roche) for BALB/3T3 cells; both were used according to manufacturer's instructions. A total of 2 µg of DNA was used to transfect each 10-cm2 dish, including luciferase reporter plasmid, SOX9 expression plasmid, and the constitutive β-galactosidase producing plasmid pSV-β-galactosidase (Promega, Madison, WI) as an internal control for transfection efficiency. Cellular extracts were prepared as described (Mukhopadhyay, et al., 1995). Luciferase assays were performed using the Luciferase Assay Reagent (Promega), and β-galactosidase assays were performed using the Galacto-Light/Plus system (Tropix), both according to manufacturer’s instructions. Assay results were measured using a TD 20/20 Luminometer (Turner Designs). Results are reported as relative luciferase units (RLU), calculated as luciferase units per unit of the β-galactosidase internal control.
ACKNOWLEDGMENTS
We thank Dr. Benoit de Crombrugghe of the M.D. Anderson Cancer Center in Houston, Texas, for RCS cells and BALB/3T3 714 cells, and Dr. Veronique Lefebvre of the Cleveland Clinic in Cleveland, Ohio, for the p89Col2a1Bs and SOX9-pcDNA-5’-UT plasmids. This work was supported by NIH grant #AR48839 to L.C.B.
Footnotes
Abbreviations: HMG, high mobility group; CD, campomelic dysplasia; EMSA, electrophoretic mobility shift assay; bp, base pairs; RCS, rat chondrosarcoma; RLU, relative luciferase units; SE, standard error
REFERENCES
- Bernard P, Tang P, Liu S, Dewing P, Harley VR, Vilain E. Dimerization of SOX9 is required for chondrogenesis, but not for sex determination. Hum Mol Genet. 2003;12:1755–1765. doi: 10.1093/hmg/ddg182. [DOI] [PubMed] [Google Scholar]
- Bi W, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Sox9 is required for cartilage formation. Nat Genet. 1999;22:85–89. doi: 10.1038/8792. [DOI] [PubMed] [Google Scholar]
- Boot-Handford RP, Tuckwell DS, Plumb DA, Rock CF, Poulsom R. A novel and highly conserved collagen (pro(alpha)1(XXVII)) with a unique expression pattern and unusual molecular characteristics establishes a new clade within the vertebrate fibrillar collagen family. J Biol Chem. 2003;278:31067–31077. doi: 10.1074/jbc.M212889200. [DOI] [PubMed] [Google Scholar]
- Bridgewater LC, Lefebvre V, de Crombrugghe B. Chondrocyte-specific enhancer elements in the Col11a2 gene resemble the Col2a1 tissue-specific enhancer. J Biol Chem. 1998;273:14998–15006. doi: 10.1074/jbc.273.24.14998. [DOI] [PubMed] [Google Scholar]
- Bridgewater LC, Walker MD, Miller GC, Ellison TA, Holsinger LD, Potter JL, Jackson TL, Chen RK, Winkel VL, Zhang Z, McKinney S, de Crombrugghe B. Adjacent DNA sequences modulate Sox9 transcriptional activation at paired Sox sites in three chondrocyte-specific enhancer elements. Nucleic Acids Res. 2003;31:1541–1553. doi: 10.1093/nar/gkg230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Crombrugghe B, Lefebvre V, Behringer RR, Bi W, Murakami S, Huang W. Transcriptional mechanisms of chondrocyte differentiation. Matrix Biol. 2000;19:389–394. doi: 10.1016/s0945-053x(00)00094-9. [DOI] [PubMed] [Google Scholar]
- Foster JW, Dominguez-Steglich MA, Guioli S, Kowk G, Weller PA, Stevanovic M, Weissenbach J, Mansour S, Young ID, Goodfellow PN, et al. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994;372:525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- Houston CS, Opitz JM, Spranger JW, Macpherson RI, Reed MH, Gilbert EF, Herrmann J, Schinzel A. The campomelic syndrome: review, report of 17 cases, and follow-up on the currently 17-year-old boy first reported by Maroteaux et al in 1971. Am J Med Genet. 1983;15:3–28. doi: 10.1002/ajmg.1320150103. [DOI] [PubMed] [Google Scholar]
- Lefebvre V, Behringer RR, de Crombrugghe B. L-Sox5, Sox6 and Sox9 control essential steps of the chondrocyte differentiation pathway. Osteoarthritis Cartilage. 2001;(9 Suppl A):S69–S75. doi: 10.1053/joca.2001.0447. [DOI] [PubMed] [Google Scholar]
- Lefebvre V, Huang W, Harley VR, Goodfellow PN, de Crombrugghe B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol Cell Biol. 1997;17:2336–2346. doi: 10.1128/mcb.17.4.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Li H, Tanaka K, Tsumaki N, Yamada Y. Identification of an enhancer sequence within the first intron required for cartilage-specific transcription of the alpha2(XI) collagen gene. J Biol Chem. 2000;275:12712–12718. doi: 10.1074/jbc.275.17.12712. [DOI] [PubMed] [Google Scholar]
- Mansour S, Hall CM, Pembrey ME, Young ID. A clinical and genetic study of campomelic dysplasia. J Med Genet. 1995;32:415–420. doi: 10.1136/jmg.32.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay K, Lefebvre V, Zhou G, Garofalo S, Kimura JH, de Crombrugghe B. Use of a new rat chondrosarcoma cell line to delineate a 119-base pair chondrocyte-specific enhancer element and to define active promoter segments in the mouse pro-alpha 1(II) collagen gene. J Biol Chem. 1995;270:27711–27719. doi: 10.1074/jbc.270.46.27711. [DOI] [PubMed] [Google Scholar]
- Pace JM, Corrado M, Missero C, Byers PH. Identification, characterization and expression analysis of a new fibrillar collagen gene, COL27A1. Matrix Biol. 2003;22:3–14. doi: 10.1016/s0945-053x(03)00007-6. [DOI] [PubMed] [Google Scholar]
- Peirano RI, Wegner M. The glial transcription factor Sox10 binds to DNA both as monomer and dimer with different functional consequences. Nucleic Acids Res. 2000;28:3047–3055. doi: 10.1093/nar/28.16.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya I, Tsuji K, Koopman P, Watanabe H, Yamada Y, Shinomiya K, Nifuji A, Noda M. SOX9 enhances aggrecan gene promoter/enhancer activity and is up-regulated by retinoic acid in a cartilage-derived cell line, TC6. J Biol Chem. 2000;275:10738–10744. doi: 10.1074/jbc.275.15.10738. [DOI] [PubMed] [Google Scholar]
- Sock E, Pagon RA, Keymolen K, Lissens W, Wegner M, Scherer G. Loss of DNA-dependent dimerization of the transcription factor SOX9 as a cause for campomelic dysplasia. Hum Mol Genet. 2003;12:1439–1447. doi: 10.1093/hmg/ddg158. [DOI] [PubMed] [Google Scholar]
- Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Bricarelli FD, Keutel J, Hustert E, et al. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994;79:1111–1120. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Xie WF, Zhang X, Sakano S, Lefebvre V, Sandell LJ. Trans-activation of the mouse cartilage-derived retinoic acid-sensitive protein gene by Sox9. J Bone Miner Res. 1999;14:757–763. doi: 10.1359/jbmr.1999.14.5.757. [DOI] [PubMed] [Google Scholar]
- Zhang P, Jimenez SA, Stokes DG. Regulation of human COL9A1 gene expression. Activation of the proximal promoter region by SOX9. J Biol Chem. 2003;278:117–123. doi: 10.1074/jbc.M208049200. [DOI] [PubMed] [Google Scholar]
- Zhou G, Lefebvre V, Zhang Z, Eberspaecher H, de Crombrugghe B. Three high mobility group-like sequences within a 48-base pair enhancer of the Col2a1 gene are required for cartilage-specific expression in vivo. J Biol Chem. 1998;273:14989–14997. doi: 10.1074/jbc.273.24.14989. [DOI] [PubMed] [Google Scholar]