Abstract
In obesity, adipocytes distant from vasculature become hypoxic and dysfunctional. This hypoxic response is mediated by hypoxia inducible factors (Hif1α, Hif2α, and Hif3α), and their obligate partner Hif1β (Arnt). We show that mice lacking Hif1β in fat (FH1βKO) are lean, exhibit reduced adipocyte size, and are protected from age and diet-induced glucose intolerance. There is also reduced Vegf and vascular permeability in FH1βKO fat, but diet-induced inflammation and fibrosis is unchanged. Adipocytes from FH1βKO mice have reduced glucose uptake due to decreased Glut1 and Glut4, which is mirrored in 3T3-L1 adipocytes with Hif1β knockdown. Hif1β knockdown cells also fail to respond appropriately to hypoxia with reduced cellular respiration and reduced mitochondrial gene expression. Some, but not all, of these effects are reproduced by Hif1α knockdown. Thus, Hif1β/Arnt regulates glucose uptake, mitochondrial gene expression, and vascular permeability to control adipose mass and function, providing a novel target for obesity therapy.
Keywords: Obesity, Adipocyte, Vegf, Oxidative Phosphorylation, Glucose Uptake
Introduction
Over the past 30 years the prevalence of obesity dramatically increased, along with associated diseases, including type 2 diabetes, coronary heart disease, hypertension, hepatosteotosis, and even cancer (Bray, 2004). With obesity, adipocytes distant from the vasculature become hypoxic, and this has been proposed to be an important contributor to adipose tissue dysfunction leading to insulin resistance and diabetes (Trayhurn and Wood, 2004).
The main sensor and mediator of the hypoxic response is the Hif family of proteins. Hifs are basic helix-loop-helix transcription factors which function as α and β heterodimers. Hif proteins are essential for normal development, and whole-body Hif1α and Hif2α knockout mice die in mid-gestation with developmental defects of the cardiovascular system (Iyer et al., 1998; Scortegagna et al., 2003; Compernolle et al., 2002). All Hif-α isoforms require Hif1β as an obligate heterodimeric partner to function as transcriptional regulators. Hif1β is also known as Arnt (the aryl hydrocarbon receptor Ahr nuclear translocator), since it acts as the transcriptional partner for the aryl hydrocarbon receptor (Ahr) and single-minded gene1 (Sim1) (Swanson et al., 1995). Ablation of Hif1β therefore results in an absence of Hif- and Ahr-induced signaling. Whole body Hif1β knockout mice die at mid-gestation due to numerous developmental defects (Maltepe et al., 1997). Ablation of Hif1β in the liver is sufficient to lead to lack of target gene induction of Hif1α, Hif2α and Ahr (Tomita et al., 2000; Rankin et al., 2005). In addition, we have previously demonstrated that type 2 diabetes subjects have lower Hif1β levels in both the β cells and liver contributing to impaired glucose-stimulated insulin secretion and aberrant expression of key lipogenic and gluconeogenic enzymes (Gunton et al., 2005; Wang et al., 2009).
Recently, all Hif-α subunits have been shown to have roles in adipocyte biology. Over-expression of constitutively active Hif1α in mouse adipose tissue induces fibrosis and insulin resistance (Halberg et al., 2009). Conversely, expression of a dominant negative Hif1α in adipose tissue perturbs brown fat function leading to increased obesity and insulin resistance (Zhang et al., 2010). Hif2α and Hif3α have both been shown to have roles in adipogenesis and adipocyte function (Peng et al., 2000; Hatanaka et al., 2009). Finally, insulin resistance is observed in 3T3-L1 adipocytes over-expressing Hif1α or Hif2α (Regazzetti et al., 2009).
In the present study, we have evaluated the role of the Hif transcriptional system by creation of a mouse lacking Hif1β (Arnt) in adipose tissue. We find that these mice are resistant to age and diet-induced obesity and its associated glucose intolerance. This occurs through effects on varied processes, including effects on adipocyte cell size, glucose uptake, control of vascular permeability, and regulation of the hypoxic response. The effects are mediated in part through the actions of Hif1α, and involve both cell autonomous and cell non-autonomous changes mediated via Vegf. Taken together, these data demonstrate a critical role for Hif1β in the control of adipose tissue mass and function.
Results
aP2-Cre effectively ablates Hif1β from adipocytes
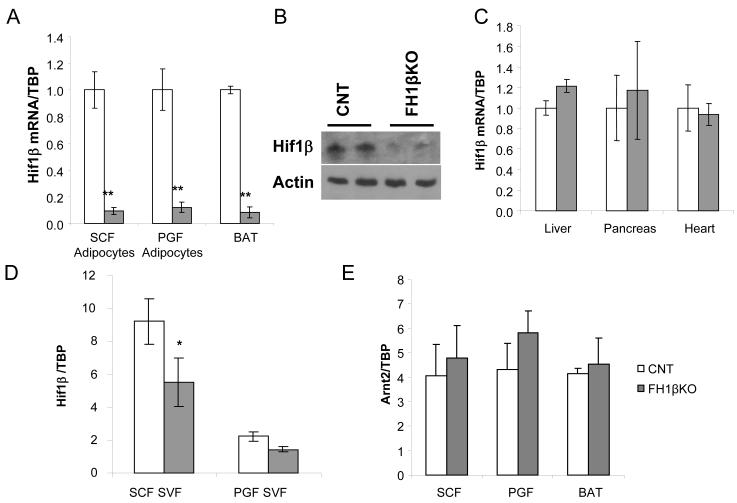
We created mice with an ablation of Hif1β in adipocytes by breeding aP2-Cre transgenic mice and mice carrying a Hif1β allele with loxP sites surrounding exon 5 (Abel et al., 2001). aP2-Cre; Hif1βf/f (termed fat-specific Hif1β knockout or FH1βKO) mice were born at the expected ratio and exhibited no overt phenotypic abnormalities. Efficient and specific ablation of Hif1β was confirmed by qPCR analysis. Hif1β mRNA was reduced >90% reduction in isolated subcutaneous and perigonadal adipocytes from fat and brown adipose tissue from 6 to 8 week old female mice (Figure 1a). Western blot analysis of perigonadal isolated adipocytes confirmed a corresponding decrease in Hif1β protein levels (Figure 1b). No changes in Hif1β mRNA levels were observed in liver, pancreas and heart (Figure 1c). In addition, qPCR analysis of the stromovascular fraction, which contains preadipocytes, from subcutaneous but not the perigonadal adipose tissue, showed a significant 40% reduction of Hif1β mRNA levels (Figure 1d). No compensatory upregulation of the related gene Arnt2 was noted (Figure 1e).
Figure 1. aP2-Cre effectively ablates Hif1β in adipocytes.
A) Expression level of Hif1β mRNA was compared using quantitative real-time PCR (qPCR) between adipocytes isolated from the subcutaneous fat (SCF) and perigonadal fat (PGF) and whole brown adipose tissue (BAT) in female control and FH1βKO mice. Mice were 6-8 weeks of age. Data are shown as mean ± SEM of five samples. Asterisks indicate p<0.05 in all panels.
B) Western blot of Hif1β from isolated PGF adipocytes of control and FH1βKO mice at 12 weeks of age. Actin served as a loading control. Data are representative of four samples.
C) Expression level of Hif1β mRNA by qPCR in liver, pancreas and heart of female control and FH1βKO mice. Mice were 6-8 weeks old. Data are shown as mean ± SEM of four samples.
D) Expression level of Hif1β mRNA by qPCR of stromovascular fraction (SVF) isolated from subcutaneous or perigonadal in control and FH1βKO mice. Mice were 6-8 weeks of age. Data are shown as mean ± SEM of four samples.
E) Expression level of Arnt2 mRNA was compared using qPCR in samples from
Ablation of Hif1β attenuates age and diet-induced obesity
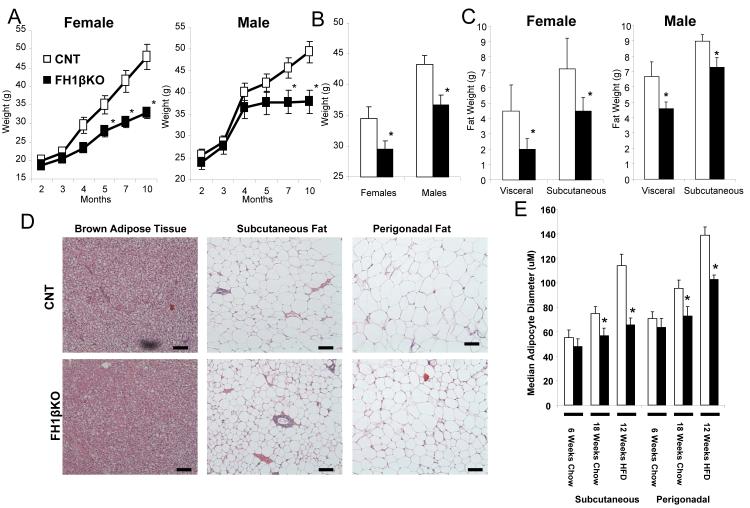
FH1βKO and littermate control floxed mice were fed a standard chow diet (22% of calories as fat) or subjected to high fat diet (HFD) (60% of calories as fat) starting at 6 weeks of age. On a chow diet, both male and female FH1βKO exhibited reduced weight gain compared to controls, by 10 months of age male FH1βKO mice had a 24% reduction in weight gain, (Figure 2a) and females FH1βKO mice were also 32% lighter (Figure S1a). Male FH1βKO also exhibited a ~16% reduction in weight gain when fed a HFD for 10 weeks compared to controls. Similar results were observed in female FH1βKO mice (Figure 2b). This weight gain difference was observed as early as eight weeks of HFD (Figure S1a).
Figure 2. Ablation of Hif1β attenuates age and diet induced obesity.
A) Body weight of female and male control and FH1βKO mice on chow diet. Data are shown as mean ± SEM of 5-6 animals/group. Asterisks indicate a significant difference (p<0.05) in all panels.
B) Body weight of female and male control and FH1βKO after 10 weeks of high fat diet (HFD) starting at 6 weeks of age. Data are shown as mean ± SEM of 8-10 animals/group.
C) Analysis of body composition by DEXA scan of control and FH1βKO at 5 months of age. Data are shown as mean ± SEM of 4-5 animals/group.
D) Hematoxylin and eosin stained representative sections of fat pads and FH1βKO mice at 20 weeks of age on chow diet. Pictures were taken at 20x.
E) Median adipocyte size in adipocytes. Perigonadal and subcutaneous fat from 8 wk chow fed, 20 week chow fed, and 12 weeks of HFD (started at 6 weeks) mice (n = 4/group) were hematoxylin and eosin stained with five digital images (20x) from non-overlapping fields were taken from each slide (total 20 fields per group), and adipocyte diameters were calculated using Image J software. Values are median ± SEM of 4 animals.
DEXA scans of chow fed animals at 20 weeks of age showed that male and female FH1βKO mice had 31% and 55% decreases of visceral fat mass and 20% and 33% decreases in subcutaneous fat mass, respectively (Figure 2c). Histological examination of the fat pads from 6-8 wk old animals showed no changes in adipose tissue morphology. By 20 weeks of age, there was reduced lipid droplet size in subcutaneous and perigonadal adipocytes in FH1βKO mice, as well as, reduced triglyceride accumulation in the BAT (Figures 2d and S2b). Median diameters of subcutaneous and perigonadal adipocytes were decreased by 42% and 26%, respectively, in FH1βKO animals relative to controls after 20 weeks of chow diet (Figures 2e and S2b-c). Similar results were seen after 12 weeks of HFD exposure, with a ~24% decrease in median adipocyte diameter in both white fat depots (Figures 2e and S2c). Consistent with the reduced adipose tissue mass on HFD, FH1βKO female and male mice had 41% and 34% decreases in leptin levels compared to controls. No changes in serum adiponectin levels were observed (Figures S1d,e). Together, these data show that FH1βKO mice are partially protected against age and diet-induced obesity due to a decrease in the ability of adipocytes to undergo hypertrophy.
Physiological parameters in FH1βKO mice
Young (8-10 week old) FH1βKO mice had no abnormality on any measured metabolic parameter, including fasting and fed glucose, fasting and fed insulin levels, serum free fatty acids, or glucose and insulin tolerance tests (Table S1; Figure S2a). However, with aging, metabolic parameters in FH1βKO mice differed from controls. Old FH1βKO female and male mice had improved glucose tolerance with 53% and 31% reductions of area under the curve on glucose tolerance testing as compared to controls. Old male FH1βKO mice also had lower fasting glucose levels (Table S1). Likewise, after HFD challenge, male and female FH1βKO mice had a 33% reduction under the curve on glucose tolerance testing as compared to controls (Table S1; Figure S2a). The reduction in weight gain and attenuation of obesity-induced glucose intolerance in the FH1βKO mice was not associated with a change in insulin tolerance (Table S1).
Hif1β detection had no discernable effect on other metabolically important tissues. In the liver, there were no changes in lipid accumulation, as assessed by Oil Red O staining and triglyceride analysis, or histological changes between FH1βKO and control mice, even after HFD exposure (Figures S2b-c). Additionally, western blot analysis of liver extracts showed no changes in phosphorylation of IRS-1, Akt, or MAP kinase in response to insulin, suggesting no major changes in insulin sensitivity in the liver of FH1βKO mice (Figure S2d). Finally, islet function, as assessed by glucose-stimulated insulin secretion was not affected in FH1βKO mice (Figure S3e).
To better understand the basis for differences in weight gain and fat mass, indirect calorimetry was performed on young FH1βKO and controls mice. No differences were observed when animal have been fed with chow diet. However, after 10 weeks of HFD feeding, FH1βKO had a significant 11-15% increase in average oxygen consumption during both the light phase (8508 + 370 in FH1βKO versus 7417 + 398 mL/kg lean mass/hr, p<0.05) and dark phase of the diurnal cycle (9365 + 416 versus 8476 + 408 mL/kg lean mass/hr, p<0.05) (Table S2). In addition, FH1βKO consumed similar amount of calories and had comparable levels of activity as controls. Together these data suggest that the decreased weight gain exhibited by FH1βKO mice was due to increased energy expenditure.
Ablation of Hif1β does not affect fibrosis and macrophage infiltration
Over-expression of Hif1α has been reported to increase fibrosis in adipocytes (Halberg et al., 2009). In control mice, expression of known markers of fibrosis mLox and Col1a1 were increased >50% and 200%, respectively, in the subcutaneous fat after 12 weeks of HFD, while Col6a1 expression was increased by over 80% in perigonadal fat (Figures S1f-h). Similar increases in markers of fibrosis were observed in the FH1βKO mouse indicating that these effects occurred independently of Hif signaling.
Determination of the expression levels of inflammation markers in adipose tissue revealed a significant increase in pro-inflammatory cytokines tumor necrosis factor α (Tnfα) and macrophage chemo-attractant protein-1 (Mcp1) in both subcutaneous and perigonadal fat of control mice after HFD exposure (Figures S2i-j). Mcp1 was also increased in the brown fat of control mice on HFD. Likewise, expression of macrophage marker, CD68 was significantly increased in the subcutaneous and perigonadal fat pads of control mice, and interleukin-6 (Il6) was significantly increased in the subcutaneous fat of control mice on HFD (Figures S2k-l). Immunohistochemical staining of the macrophage marker F4/80 in control mice showed a marked increase after HFD exposure. All of these markers of inflammation and macrophage infiltration showed almost identical increases in the fat depots of FH1βKO mice (Figures 3Si-m). Therefore, although Hif1β ablation leads to decreased adipose cell size, induction of makers of fibrosis and macrophage infiltration, classically observed in adipose tissue with obesity, were not reduced in FH1βKO mice after challenge with a HFD.
Hif1β ablation decreases glucose uptake
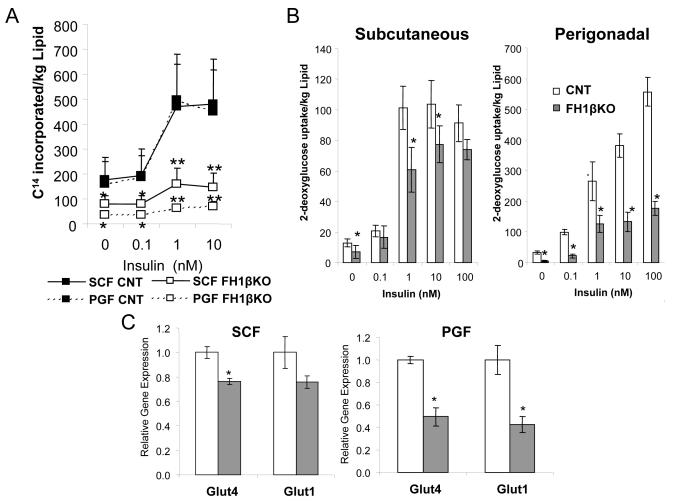
The decreased adipocyte size observed in FH1βKO mice could be the result of increased lipolysis, decreased lipogenesis, and/or a defect in adipocyte differentiation. qPCR analysis from subcutaneous and perigonadal fat of FH1βKO and littermate control animals revealed no significant differences in the mRNA levels of regulators and markers of adipogenesis, including Cebpβ, Cebpδ, Pparγ, FoxO1, and aP2 (Figures S3a,b) suggesting that Hif1β ablation does not impair adipocyte differentiation. Additionally, no differences in basal or isoproterenol-stimulated lipolysis were observed (Figure S3g). However, both basal and insulin-stimulated lipogenesis rates were markedly reduced in isolated subcutaneous (~60%) and perigonadal (~80%) adipocytes from male FH1βKO mice (Figure 3a). Likewise, in subcutaneous fat, uptake of [H3] 2-deoxyglucose was reduced by >40% in the basal state and by 19-40% in the insulin-stimulated state in FH1βKO mice compared to controls (Figure 3b). This reduction was even greater in perigonadal adipocytes, with an 86% decrease in basal glucose uptake and 52-78% decreases in insulin-stimulated glucose uptake over the concentration range of 0.1 nM to 100 nM insulin (Figure 3b).
Figure 3. FH1βKO adipocytes display defects in lipogenesis, glucose uptake, and glucose transporter expression.
A) [14C] D-glucose incorporation into lipids in subcutaneous and perigonadal adipocytes isolated from control and FH1βKO animals at 12 weeks of age. Data are represented as 14C counts and normalized to lipid content. Data shown as mean ± SEM of 4 animals/group and are representative of two independent experiments. Asterisks in all panels indicate p<0.05, and double asterisks indicate p<0.01.
B) [3H] 2-deoxyglucose uptake into subcutaneous and perigonadal adipocytes isolated from control and FH1βKO animals at 15 weeks of age. Data are represented as 3H counts and normalized to lipid content. Data are mean ± SEM of 4 animals/group. The entire experiment was repeated twice. Asterisks in all panels indicate p<0.05.
C) Expression levels of Glut1 and Glut4 mRNA were compared using qPCR between subcutaneous and perigonadal in male control and FH1βKO mice. Mice were 6-8 weeks old. Data are mean ± SEM of four samples.
In agreement with these defects in adipocyte glucose uptake and lipogenesis, gene expression levels of the glucose transporter Glut4 and Glut1 were reduced by >50% and 24%, respectively, in both perigonadal and subcutaneous fat of 6-8 week old in FH1βKO mice (Figure 3c). Thus, ablation of Hif1β leads to decreased levels of glucose transport proteins, which results in decreases in glucose transport and, subsequently, decreased lipogenesis.
qPCR analysis of the key lipogenic genes, including sterol-regulatory-element-binding protein 1c (Srebp1C), acetyl-CoA carboxylase (Acc1) and fatty acid synthase (Fas), in fat of 6 week-old male FH1βKO males showed no changes in expression compared with controls (Figures S3c,d). After exposure to HFD for 12 weeks, qPCR analysis revealed significantly lower levels of Srebp1C, Acc1, Fas, and stearoyl-coenzyme A desaturase 1 (Scd1) mRNA in the fat depots of FH1βKO mice (Figures S3e,f).
Knockdown of Hif1β reduces glucose uptake
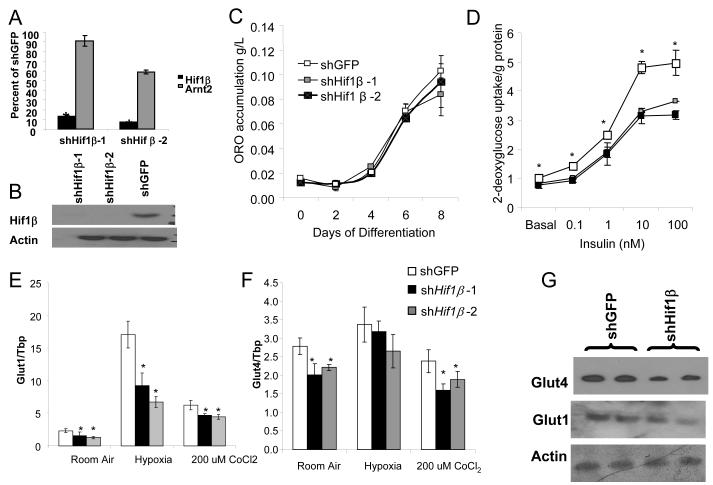
To further investigate the mechanisms underlying the effects of Hif1β in adipocytes, we utilized shRNA lentiviruses directed against Hif1β to create two independent stable 3T3-L1 knockdown cell lines, shHif1β–1 and shHif1β–2, with 87 % and 93% reductions in Hif1β expression (Figure 4a). This decrease was specific; the highly homologous Arnt2 was decreased by only 9% and 41% in these two cell lines, respectively (Figure 4a). Western blot analysis showed no detectible Hif1β protein levels in either shHif1β cell lines (Figure 4b). These shHif1β 3T3-L1 adipocyte cell lines displayed no difference in lipid accumulation, as visualized by Oil Red O staining or triglyceride quantitation (Figures S4a, 4c). No changes in the expression of the major regulators and markers of adipose differentiation, including Pparγ, Cebpα, Cebpβ, Cebpβ, Foxo1, and aP2 were observed (Figure S4b). Thus, knockdown of Hif1β does not affect adipocyte differentiation.
Figure 4. shRNA knockdown of Hif1β decreases glucose uptake.
A) Expression level of Hif1β and Arnt2 mRNA was compared using qPCR between 3T3-L1 preadipocytes stably transfected with shHif1β-1 and shHif1β-2. Data are normalized by relative expression of Hif1β and Arnt2 mRNA in shGFP cells and are shown as mean ± SEM of triplicate samples, repeated five times. Asterisks indicate p<0.05 in all panels.
B) Western blot of Hif1β from protein extracts from 3T3-L1 preadipocytes stably transfected with shHif1β-1, shHif1β-2, and shGFP cells. Actin was used as a loading control.
C) Quantification of Oil Red-O staining in shHif1β-1, shHif1β-2, and shGFP adipocytes. Data are shown as mean ± SEM of triplicate samples repeated twice. Asterisks indicate a significant difference (p<0.05).
D) Glucose uptake, as assessed by acute uptake of 3H-labeled 2-deoxyglucose into shHif1β-1, shHif1β-2, and shGFP stably transfected adipocytes. Data are represented as 3H count normalized to protein content, and are shown as mean ± SEM of triplicate samples repeated three times.
E) Expression of Glut1 and Glut4 as measured by qPCR. In cells with stable Knockdown of Hif1β-1, Hif1β-2 and shGFP. After eight days of differentiation, cells were subjected to normoxia, hypoxia (0.1% O2 for 16hrs), or 200 nM CoCl2 for 16 hrs. Data shown as mean ± SEM of triplicate samples and repeated three times.
F) Western blot of Glut1 and Glut4 from protein extracts from shHif1β-2 and shGFP 3T3-L1 adipocytes after eight days of differentiation. Actin was used as a loading control.
However, basal and insulin-stimulated uptake of 2-deoxyglucose were decreased by 20-30% in both shHif1β–1 and shHif1β–2 adipocytes compared to controls (Figure 4c). This correlated with 28 and 32% decreases in Glut1 and Glut4 mRNA levels in shHif1β–1 cells, and 45% and 21% decreases in shHif1β–2 cells under normoxic conditions (Figures 4d, e).
These shHif1β knockout cells showed altered responses to hypoxia and the hypoxia mimetic CoCl2. Thus, in control cells Glut1 expression was increased approximately 7-fold by hypoxia (16 hours at 0.1% O2) and 3-fold by following treatment with CoCl2 (200 μM for 16h), whereas in shHif1β cells, the induction of Glut1 was blunted by 47% to 60% (Figure 4d). In these cells, Western blot analysis demonstrated that CoCl2 was sufficient to also strongly increased Hif1α protein (Figure S4d). Western blot analysis confirmed the reduced Glut1 and Glut4 levels in shHif1β adipocytes under normoxic conditions (Figure 4f). Glut4 mRNA levels were not significantly changed by hypoxia or CoCl2 treatment in either shHif1β or control cells, and expression levels remain significantly different between shHif1β and control adipocytes after treatment with CoCl2 (Figure 4e).
As previously reported, osmotic shock can stimulate Glut4 translocation in 3T3-L1 adipocytes in an insulin independent manner (Chen et al., 1997). shHif1β cells exhibited a decrease of ~25% in 2-deoxygucose uptake when subjected to osmotic shock with 600 mM sorbitol (Figure S4c). By contrast, no change in fatty acid uptake, measured by 14C-labelled palmitic acid incorporation, was observed in shHif1β cells (Figure S4e). Thus, the decreased levels of glucose transport proteins that result in decreases in glucose transport are cell autonomous effects caused by the ablation of Hif1β.
Hif1β ablation decreases vascular permeability
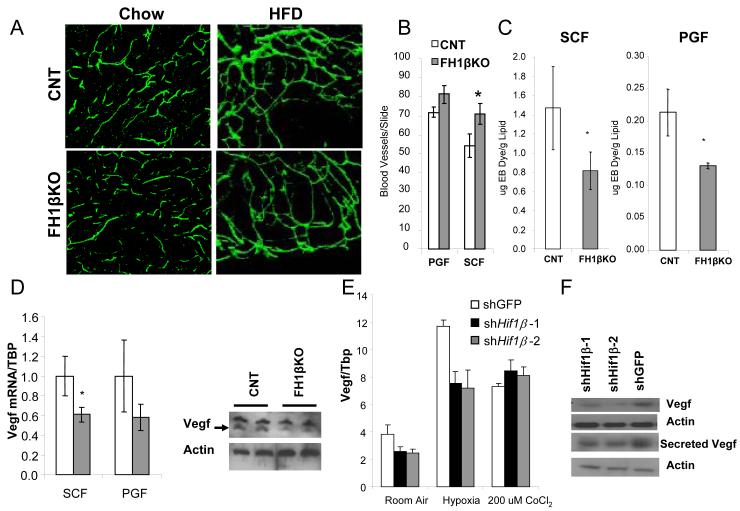
Many tissues have been shown to undergo remodeling of vascular structure in response to hypoxia (Kaluz et al., 2008). To determine if ablation of Hif1β in adipocytes would cause a difference in vascular structure in fat, we visualized blood vessels using CD31 immunofluorescence in whole mount fat pads from FH1βKO and control mice. Vascular density within individual fat pads exhibited heterogeneity, but there were no significant differences in blood vessel density in either subcutaneous or perigonadal fat pads of young or HFD-exposed FH1βKO mice compared to control mice (Figure 5a, S5a). Capillary density at eight weeks of age, as assessed by immunofluorescence for GSL I-isolectin, was also unchanged in FH1βKO males (Figure S5c,d). After HFD, capillary density was increased by 31% the subcutaneous fat and several fold in brown adipose tissue of FH1βKO males (Figure 5b, S6e). However, these increases are most likely due to the difference in adipose cell size, which leads to an apparent difference in cellular density in microscopic fields between controls and FH1βKO.
Figure 5. Hif1β ablation decreases vascular permeability and Vegf expression.
A) Left panels: CD31 immunohistochemistry on whole mounts of subcutaneous fat from control and FH1βKO male mice at 8 weeks of age. The photograph was taken at 100x. Right panels: CD31 immunohistochemistry on whole mounts of subcutaneous fat from control and FH1βKO male mice after 12 weeks of exposure to high fat diet.
B) Quantitation of capillary density in the perigonadal and subcutaneous fat pads after 12 weeks on HFD, starting at 6 weeks of age (n = 4/group). Slides were stained for GSL I - isolectin B4. Capillaries were counted from three digital images (20×) from non-overlapping fields were taken from each slide (total 12 fields per group) from control and FH1βKO males. Data are shown as mean ± SEM of three samples. Asterisks indicate p<0.05.
C) Quantitation of the amount of Evan’s blue in fat pads 10 minutes after intra-cardiac injection and perfusion into control and FH1βKO male mice at 8 weeks of age. Data are normalized by gram of lipid from fat pads and are shown as mean ± SEM of three samples. Asterisks indicate a significant difference in all panels (p<0.05).
D) Levels of Vegf mRNA were compared using qPCR between adipocytes isolated from the subcutaneous fat (SCF) and perigonadal fat (PGF). Mice were 6-8 weeks old. Data are shown as mean ± SEM of five samples. Asterisks indicate p<0.05. Western blot of Vegf from isolated PGF adipocytes of female control and FH1βKO− mice at 12 weeks of age. Western blot for actin is a loading control. Data are representative of four samples.
E) mRNA was isolated from shHif1β-1, shHif1β-2, and shGFP stably transfected adipocytes after eight days of differentiation, and subjected to normoxia, hypoxia (0.1% O2 for 16hrs), or 200 nM CoCl2 for 16 hrs. Expression of Vegf was measured by qPCR. Data shown as mean ± SEM of triplicate samples and repeated three times.
F) Western blot of Vegf from protein extracts from 3T3-L1 adipocytes stably transfected with shHif1β-1, shHif1β-2, and shGFP adipocytes, or media secreted by shHif1β-1, shHif1β-2, and shGFP adipocytes after eight days of differentiation. Actin was used as a loading control.
Staining with pimonidazole to identify hypoxic regions in fat pads from eight-week-old mice showed similar results. Under chow diet, young FH1βKO and control animals had very little pimonidazole staining in either the subcutaneous or perigonadal adipose tissue. After HFD, areas of scattered pimonidazole positivity were found in fat pads of both FH1βKO and control mice with no apparent differences between genotypes (Figure S5b).
Eight week old FH1βKO mice exhibited no changes in body weight or fat pad weight compared to controls. However, at this age, extravasation of Evans Blue dye into the subcutaneous and perigonadal fat pads revealed a ~40% reduction in vascular permeability in adipose tissue of FH1βKO mice (Figure 5c, S5f). Thus, FH1βKO mice do not have reduced vasculature, but do have a significant reduction in vascular permeability in fat.
Changes in vascular permeability during hypoxia are primarily mediated by vascular endothelial growth factor (Vegf). In all fat depots of FH1βKO mice Vegf mRNA expression was decreased by 40%, and this was confirmed by a corresponding decrease in Vegf protein level in isolated adipocytes from perigonadal fat (Figure 5c). Likewise, Vegf mRNA levels were decreased by 37% in both shHif1β knockdown cell lines under normoxic conditions (Figure 5e). In both control and knockdown cell lines, hypoxia led to a 3-fold increase in Vegf mRNA although Vegf expression remained proportionately lower in the shHif1β cells. These results were confirmed by western blot analysis of Vegf protein from whole cell lysates and surrounding tissue culture media (Figure 5f) mirroring the in vivo finding that loss of Hif1β leads to a decrease in Vegf and vascular permeability.
Role of Hif1β in hypoxia sensing
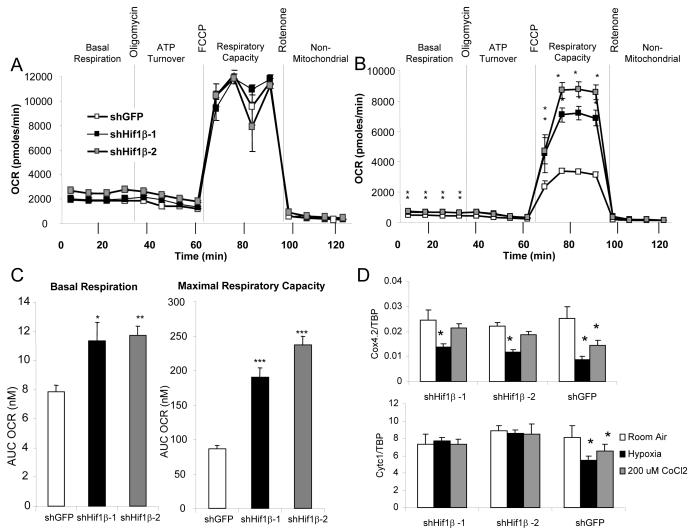
To assess the consequences of Hif1β ablation on cellular respiration, we used a Seahorse extracellular flux analyzer to determine basal and maximal mitochondrial respiration, ATP turnover, and non-mitochondrial respiration by measuring oxygen consumption rate (OCR) of differentiated control and Hif1β knockdown 3T3-L1 cells. Under normoxic conditions, no significant differences were seen in the bioenergetics profiles of control and shHif1β 3T3-L1 adipocytes (Figure 6a). Pretreatment of the control cells for 16 hrs with the hypoxia mimetic CoCl2 (200 μM) led to a ~33% reduction of basal respiration and a striking 67% reduction in maximal respiratory capacity. By comparison, shHif1β 3T3-L1 adipocytes showed no decrease in basal or maximal respiration after CoCl2 treatment (Figure 6a-c). No significant differences were found between control and knockdown cells in the proton leak or non-mitochondrial respiration. In order to investigate the molecular mechanisms that are changed in hypoxia sensing in the shHif1β cells, we performed qPCR analysis on genes encoding mitochondrial proteins involved in oxidative phosphorylation. Numerous genes were unchanged by Hif1β ablation either in normoxic conditions or after pre-treatment with CoCl2 (Figures S7a,b). Likewise, under normoxic conditions, no changes were observed in cytochrome c1 (Cytc1) or cytochrome c oxidase, subunit IV, isoform 2 (Cox4.2), both components of Complex III. However, while in control adipocytes hypoxia induced a 32% and 66% decrease in Cytc1 and Cox4.2 expression respectively, there was no change in Cytc1 expression, and the repression of Cox4.2 was reduced to 44% in shHif1β cells (Figure 6d). Likewise, in control adipocytes, treatment with CoCl2 produced a 20% decrease in Cytc1 expression and a 44% decrease in Cox4.2 expression, and these reductions were lost in the shHif1β adipocytes (Figure 6d). Thus, Hif1β is critical for the transition into the hypoxic state, and this involves repression of components of mitochondrial Complex III.
Figure 6. Hif1 β shRNA knockdown increases respiratory capacity during CoCl2 treatment.
A) Bioenergetics profile, as measured by oxygen consumption rate with a Seahorse X24 extracellular flux analyzer, of shHif1β-1, shHif1β-2, and shGFP 3T3-L1 adipocytes under normoxic conditions.
B) Bioenergetics profile, as measured by oxygen consumption rate with a Seahorse X24 extracellular flux analyzer, of shHif1β-1, shHif1β-2, and shGFP 3T3-L1 adipocytes after pre-treatment with CoCl2.
C) Basal respiration and maximal respiratory capacity of shHif1β-1, shHif1β-2, and shGFP 3T3-L1 adipocytes was determined by calculating the area under the curve (AUC) in the basal phase and after uncoupling with FCCP. Values are means ± SEM of 6-7 replicates of three separate experiments. Asterisks indicate a significant difference compared to shGFP control in all panels (p<0.05).
D) mRNA was isolated from shHif1β-1, shHif1β-2, and shGFP stably transfected adipocytes after eight days of differentiation, and subjected to normoxia, hypoxia (0.1% O2 for 16hrs), or 200 nM CoCl2 for 16 hrs. Expression of Cytc1 and Cox4.2 was measured by qPCR. Data shown as mean ± SEM of triplicate samples and repeated three times.
The roles of Hif1β in adipocytes are partially mediated by Hif1α
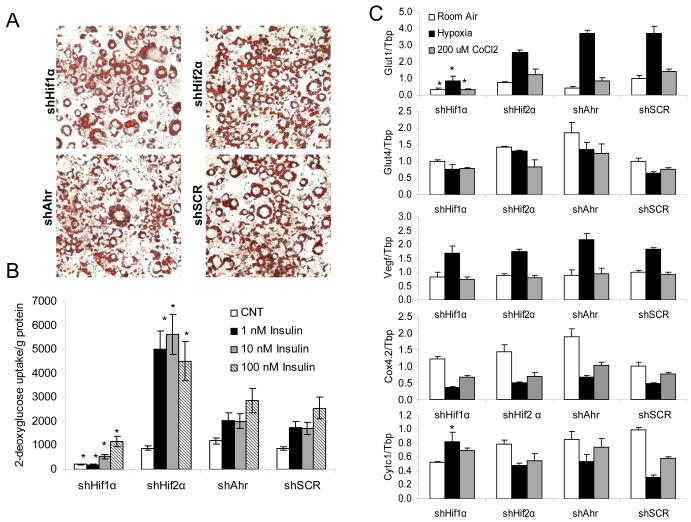
The data above show that ablation of Hif1β in adipocytes in vivo and in vitro leads to changes in the expression of glucose transporters, Vegf, and components of the mitochondrial electron transport chain. Since Hif1β acts as an obligate heterodimeric partner for all Hif-α isoforms, as well as Ahr, we created stable knockdown 3T3-L1 cell lines for the two dominant forms of Hif-α (1 and 2) and Ahr to elucidate which of these factors might be acting with Hif1β to regulate these effects. qPCR analysis of differentiated shHif1α, shHif2α, shAhr, and shSCR (control) adipocytes, demonstrated specific knockdowns of each transcription factor by 75-88% (Figure S6a), although knockdown of Hif1α did lead to a 3.5-fold compensatory increase in Hif2α levels. Western blot analysis of cell lines under hypoxic conditions confirmed ablation of Hif1α and Ahr in shHif1α and shAhr cell lines, respectively (Figure S6b).
shSCR, shHif1α, shHif2α, and shAhr 3T3-L1 adipocytes differentiated using a standard induction protocol including 10 nM rosiglitizone displayed no difference in lipid accumulation as visualized by Oil Red O staining (Figure 7a) or quantitation of cellular triglycerides (Figure S6d). However, there was a 50% reduction in Cebpα, Cebpβ, and aP2, but no differences in Pparγ, in differentiated shHif1α adipocytes. No changes in these adipocyte differentiation markers were found in differentiated shHif2α adipocytes, and shAhr cell lines had a significant ~2-fold increase in Cebpα and aP2 compared to controls (Figure S6c).
Figure 7. Ablation of Hif1α recapitulates a subset of the effects of Hif1β ablation.
A) Triglyceride accumulation after 8 days of differentiation was visualized by Oil Red-O staining in shHif1α, shHif2α, shAhr, and shScr (control) adipocytes after 8 days of differentiation.
B) 3H-labeled 2-deoxyglucose uptake into shHif1α, shHif2α, shAhr, and shScr stably transfected adipocytes after eight days of differentiation. Data are represented as 3H counts normalized to protein content. Data shown as mean ± SEM of four samples and repeated twice. Values are means ± SEM, and asterisks indicate a significant difference compared to shSCR control with the same treatment in all panels (p<0.05).
C) mRNA was isolated from shHif1α, shHif2α, shAhr, and shScr stably transfected adipocytes after eight days of differentiation, and subjected to normoxia, hypoxia (0.1% O2 for 16h), or 200 nM CoCl2 for 16 hrs. Expression of Glut1, Glut4, Vegf, Cytc1 and Cox4.2 was measured by qPCR. Data shown as mean ± SEM of four samples and repeated twice.
shHif1α adipocytes also had a defect in glucose uptake, with a 76% decrease in basal glucose uptake and a 55% to 88% decrease in insulin-stimulated glucose uptake (Figure 7b) that as similar to the decrease following Hif1β ablation (Figures 3b, 4d). By contrast, insulin-stimulated glucose uptake was unchanged in the shAhr adipocytes and was increased 3-fold in shHif2α adipocytes (Figure 7b). Interestingly, neither Glut4 nor Vegf expression levels were significantly changed in any of the knockdown cell lines (Figure 7c). As with shHif1α cells, Glut1 expression in the shHif1α cell line was decreased by 67% to 75% in all conditions. Likewise, the repression of Cytc1 during hypoxia and CoCl2 treatment that was lost upon Hif1β knockdown was also absent upon knockdown of Hif1α, but was normal in Hif2α or Ahr cells (Figure 7c). On the other hand,,Cox4.2 expression was efficiently repressed by both hypoxia and CoCl2 treatment in all knockdown cell lines. These data demonstrate that signaling through Hif1α is critical for some, but not all, processes controlled by Hif1β in adipocytes.
Discussion
In obesity, adipose tissue expands cells further from vessels become hypoxic, and this contributes to adipose tissue dysfunction (Trayhurn et al., 2008). Hypoxia in adipocytes has been demonstrated in several rodent models of obesity (Rausch et al., 2008; Ye et al., 2007; Hosogai et al., 2007), and is also present in humans, as shown by multiple lines of evidence. First, obesity leads to hypoperfusion of adipose tissue (Stuart, I et al., 2009). Secondly, Hif-1α expression is increased in the adipose tissue of obese individuals, and this can be reversed by weight loss (Cancello et al., 2005). Finally, all Hif-α subunits have been shown to have roles in adipocyte differentiation and function (Halberg et al., 2009; Regazzetti et al., 2009; Shimba et al., 2004; Hatanaka et al., 2009).Understanding the roles of individual Hif proteins, however, is complicated by the ability of different Hif-α subunits to at least partially compensate for one another, as illustrated by the partially overlapping target gene sets of Hif1α and Hif2α (Hu et al., 2003). Therefore, in the present study, to explore the role of the integrated Hif signaling system in adipose tissue we utilized a fat-specific ablation of the obligate heterodimeric partner for all Hif-α subunits, Hif1β.
We find that both male and female mice lacking Hif1β in fat (FH1βKO mice) are protected against age and diet-induced obesity. Indirect calorimetry revealed that this is associated with increased energy expenditure. FH1βKO mice also exhibit striking protection against obesity-related glucose intolerance and insulin resistance. Interestingly, despite the fact they are leaner, the inflammatory and fibrotic response in fat after HFD is unchanged in FH1βKO mice. Thus, deletion of Hif1β in adipocytes protects against some, but not all, of the changes induced by HFD.
Ablation of Hif1β in fat protects against obesity by causing reduced adipocyte cell size. The primary sources of triglyceride synthesis, which determines adipocyte cell size, are de novo lipogenesis and fatty acid uptake from plasma. Based on cellular models, uptake of radiolabeled C14 palmitate is unchanged by loss of Hif1β Thus, the primary defect leading to a reduction in adipose tissue size following Hif1β ablation is a defect in de novo lipogenesis. Both in vivo and in vitro, loss of Hif1β causes a reduction in the expression of the Glut1 and Glut4 glucose transporters. Glut1 is a well-known target of the hypoxia response and has been shown to be dependent on signaling by the Hif1a/Hif1β complex (Hayashi et al., 2004). In 3T3-L1cells, Hif 2a can also regulate Glut1 and Glut4 (Shimba et al., 2004). In FH1KO adipocytes and Hif1β knockdown 3T3-L1 adipocytes, this reduction in glucose transporters leads to decreased glucose uptake and a reduction of de novo lipogenesis, resulting in decreased adipocytes cell size.
It is likely that the ~50% reduction of glucose transporters observed when Hif1β is ablated plays an important role in the reduced glucose uptake and the smaller adipocytes in the FH1βKO mice. Ablation of Glut4 alone in adipose tissue results in a marked decrease in insulin stimulated glucose uptake, leading to glucose intolerance and insulin resistance, but without change in adipose cell size (Abel et al., 2001). By contrast, whole body haploinsuffiency of Glut1 leads to defects in development (Wang et al., 2006) and an altered cellular response to hypoxic stress (Heilig et al., 2003). Stable knockdown of Glut4 in 3T3-L1 adipocytes leads to decreased lipid accumulation, and the knockdown of Glut1 and Glut4 in combination leads to an almost complete absence of basal and insulin mediated glucose uptake (Liao et al., 2006).
In addition to the reduced glucose uptake, there is a significant down regulation in expression of key lipogenic enzymes in FH1βKO fat during HFD exposure. To what extent this is cause or effect of reduced adipose mass is difficult to assess, since previous studies have demonstrated that smaller adipocytes have lower levels of lipogenic gene expression (Bluher et al., 2004). Therefore, it is likely that Hif1β ablation causes a primary defect in glucose uptake which, in turn, leads to reduced adipocyte cell size and reduced lipogenic gene expression, since in 6-week FH1βKO mice, there is no decrease in fat mass and lipogenic gene expression in adipose tissue is normal.
In general, the transcription of Hif-1 target genes serves to alter cellular responses and the local microenvironment to alleviate the hypoxia stress. A key component of this response is to promote angiogenesis through increased expression of the Hif-1 target gene Vegf (Forsythe et al., 1996). Ablation of Hif1β both in vivo and in vitro leads to a significant decrease of Vegf mRNA and protein. This reduction in Vegf does not lead to a reduction of vessel or capillary density in the fat in either chow or HFD-challenged animals FH1βKO mice. However, prior to any change in body or fat mass, the reduction in Vegf leads to a significantly decreased vascular permeability, as assessed by Evans Blue extravasation of dye.. Additionally, increased vascular permeability, has been shown to be central to the adipose tissue expansion induced by thiazolidinediones treatment (Sotiropoulos et al, 2006). On the other hand, previous studies have shown that increases in adipocyte mass can be altered by angiogenesis inhibitors (Rupnick et al., 2002). Likewise, blocking Vegf action by treating mice with antibodies to the Vegf receptor has been shown to reduce weight gain during diet induced obesity (Tam et al., 2009). Thus, the changes we observe in Vegf and vascular permeability could contribute to the reduction of adipocyte hypertrophy in the FH1βKO animals.
There is a well-established link between mitochondrial activity and hypoxia. Previous studies have demonstrated that reactive oxygen species produced by the mitochondria (mtROS) are essential for oxygen sensing and Hif1α/Hif2α stabilization during hypoxia. In particular, mtROS generated from Complex III has been shown to be necessary for regulating Hif1α and Hif2α (Guzy et al., 2005; Bell et al., 2007), and loss of the Complex III protein, cytochrome c impairs cellular oxygen sensing and Hif1α activation in response to hypoxia (Mansfield et al., 2005). In this study, we find that Hif1β is necessary for the down-regulation of Cytc1 which occurs during hypoxia and after treatment with the hypoxia mimetic CoCl2. Interestingly, adipose tissue from mice with fat specific knockout of the insulin receptor (FIRKO mice) exhibits lower levels of Hif1α and higher levels of Cytc1 (Katic et al., 2007). It is likely that the smaller adipocytes present in the FIRKO mice have lower levels of hypoxia, and thus retain higher levels of mitochondrial activity. The results presented here further elucidate that relationship by demonstrating the coordination of these hypoxia and mitochondrial activity in an entirely different model system.
In the lung and liver, Hif1α has been shown to regulate Cox4.1 and Cox4.2 expression (Fukuda et al., 2007). Reduced oxygen availability leads to an increase in transcription of Cox4.2 and Lon, the mitochondrial protease required for Cox4.1 degradation and optimization of respiratory efficiency. In contrast to these findings, in adipocytes from normal mice there is a decrease in expression of Cox4.2 after exposure to hypoxia or treatment with CoCl2 treatment and no change in Cox4.1 expression, while expression of Lon is increased by hypoxia, but not CoCl2. In adipocytes, this repression of Cox4.2, but not the increased expression of Lon, is dependent on the presence of Hif1β (Figure S7c,d). Thus, the regulation of Cox4.1 and Cox4.2 expression in response to hypoxia is different in adipocytes than in the liver or lung.
The relationship of these changes in gene expression to other markers of hypoxic stress is complex. Immunohistochemical staining for pimonidazole adducts, which mark hypoxic areas, show that hypoxia within the fat pads of young control and FH1βKO animals is relatively low. However, in HFD-induced obesity, hypoxic regions can be found scattered through both the subcutaneous and perigonadal fat pads of control mice, and also found in FH1βKO mice despite their protection from adipocytes hypertrophy and obesity. Levels of the pro-inflammatory cytokines Tnfα and Mcp1 and macrophage infiltration are increased by HFD in control mice as expected, and again this was unchanged in FH1βKO mice despite the smaller adipocytes and fat pad weight. Additionally, consistent with previous studies in the ob/ob mouse (Halberg et al., 2009), we observed increased expression of numerous markers of fibrosis including mLox, Col6a1, and Col1a1, after 12 weeks of HFD, and these markers were unchanged by Hif1β ablation. Thus, despite protection from adipocytes enlargement and obesity, FH1βKO mice still exhibit evidence of increased fibrosis and inflammation in response to HFD.
FH1βKO mice display normal brown fat morphology on normal and HFD, but show increased capillary density compared to controls after HFD exposure. This is in contrast to effects of adipose specific expression of a dominant negative Hif1α which results decreases in mitochondrial content and vascular density in the BAT leading to obesity, glucose intolerance, and insulin resistance (Zhang et al., 2010). An important difference between these two models is that ablation of Hif1β not only inhibits Hif1α signaling, but also inhibits the actions of Hif2α and Ahr. This is in keeping with our findings that in cultured cells, we find that Hif1α and Hif2α have distinct roles in adipocyte biology. Ablation of Hif1α reduces glucose uptake, whereas ablation of Hif2α enhances glucose uptake. Interestingly, Hif2α is upregulated in Hif1α knockout cells. These data suggest an important role for Hif2α in adipocytes. Hif1β may also exert effects by partnering with the aryl hydrocarbon receptor Ahr. Knockdown of Ahr in 3T3-L1 adipocytes leads to an increase in the differentiation markers Cebpα and aP2, supporting previous experiments that demonstrate Ahr is a negative regulator of adipogenesis that affects expression of Cebpα, and subsequently, aP2 (Alexander et al., 1998; Shimba et al., 2001). Thus, attenuation of Ahr activity by ablation of Hif1β in the adipose tissue of FH1βKO may lead to an enhanced differentiation capacity that rescues differentiation defect observed in the BAT of dominant negative Hif1α mice.
Both shHif1β and shHif1α adipocytes exhibit reduced expression of Glut1, reduced glucose uptake and a lack of repression of Cytc1 in response to hypoxia. However, other changes molecular changes, including reduced Glut4 and Vegf expression, as well as, the loss of Cox4.2 repression observed in the shHif1β adipocytes are not observed after ablation of the interacting partners. These data suggest that Hif1β alters signaling through other pathways or that there is redundancy between these interacting partners.
In conclusion, Hif1β (Arnt) plays a pivotal role in adipose tissue by controlling glucose uptake, expression of Vegf, and regulation of the hypoxia in a cell autonomous manner response. The altered Vegf expression results in secondary cell, or non-autonomous, changes in vascular permeability. Together, these effects control adipose growth and triglyceride accumulation. Thus, reducing Hif1β in adipose tissue limits the effects of hypoxia on adipose tissue in the obese state and may represent a novel therapeutic target for treatment of obesity.
Supplementary Material
Highlights.
ice lacking Hif1β in fat are protected from obesity and glucose intolerance.
Mice lacking Hif1β in fat have reduced VEGF and vascular permeability in fat.
Hif1β regulates glucose uptake in fat both in vivo and in vitro.
Hif1β regulates cellular respiration and mitochondrial genes in response to hypoxia.
Acknowledgements
The authors would like to thank Dr. Frank J. Gonzalez (National Cancer Institute) for the Hif1β floxed mice. We are grateful to M. Rourk and G. Smyth for animal care; C. Cahill (Joslin DERC Advanced Microscopy Core); and A. Clermont, M. Poillucci (DERC Physiology Core), H. Li (DERC Specialized Assay Core), and J. Schroeder (DERC Genomics Core) for technical assistance. This work was supported by the Joslin Training Grant (T32DK007260), NIH grants DK 60837 and DK 82655, an American Diabetes Association mentor-based award, and the Mary K. Iacocca Professorship to C.R. Kahn.
APPENDIX
Material and Methods
Further details on many of the experimental methods including the maintenance of mice, metabolic analysis, glucose and palmitate uptake, lipogensis, lipolysis, cell culture, cloning, histology, antibodies utilized, and the oxygen consumption assays can be found in the Supplemental Experimental Procedures.
Animals and diets
aP2-cre and Hif1β floxed animals have previously been described (Abel et al., 2001; Tomita et al., 2000). Animal care and study protocols were approved by the Animal Care Committee of Joslin Diabetes Center.
Body composition and metabolic analysis
Body composition was measured using a Lunar PIXImus2 densitometer (GE Medical Systems). Adipocyte diameters from FH1βKO and control mice at 6-8 weeks old, 20 weeks old, and after 12 weeks of HFD exposure were calculated using Image J software Activity was measured using the OPTO-M3 sensor system (Comprehensive Laboratory Animal Monitoring System, CLAMS; Columbus Instruments). Indirect calorimetry was measured on the same mice using an open-circuit Oxymax system (Columbus Instruments). Intraperitoneal glucose (2 g/kg weight) and insulin tolerance (1.25 unit/kg) tests were performed after a 16- and 4-h fast, respectively. Insulin, free fatty acids, leptin, adiponectin were measured by ELISA (Crystal Chem). Adipocyte diameters were calculated using Image J software.
Analysis of gene expression
Total RNA was isolated using an RNeasy minikit (Qiagen), and cDNA was synthesized with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Gene expression was determined by RT-qPCR performed on a ABI Prism 7900HT sequence.
Lentiviral infection and 3T3-L1 differentation
Hif1β, Hif1α, Hif2α, and Ahr were stably knocked-down in 3T3-L1 cells by shRNA delivered by lentiviral infection and selected with puromycin. Differentiation was induced by exposing the cells 1 μM dexamethasone, 500 μM isobutylmethylxanthine and 100 nM insulin for two days. Except for the assessment of differentiation capacity, the PPARγ agonist 1 μM rosiglitazone was added in all experiments. Cells were then grown for six days in 100 nM insulin. Medium was replaced every other day.
Histology
Vascularity in the fat pads was determined by whole mount CD31 immunofluorescence and staining of GSL I – isolectin B4. For permeability analysis, Evans blue dye was administered by intra-cardiac injection followed by perfusion with phosphate buffered saline. Lipids from liver samples and differentiated culture plates were visualized and quantified by Oil Red O staining.
Oxygen consumption assay
Oxygen consumption was measured using the XF24 Extracellular Flux Analyzer from Seahorse Bioscience. A bioenergetics profile was determined by sequential injections of oligomycin, FCCP, and rotenone.
Statistics
All differences were analyzed by Student’s t-test. Results were considered significant if p<0.05.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, Minnemann T, Shulman GI, Kahn BB. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409:729–733. doi: 10.1038/35055575. [DOI] [PubMed] [Google Scholar]
- Alexander DL, Ganem LG, Fernandez-Salguero P, Gonzalez F, Jefcoate CR. Aryl-hydrocarbon receptor is an inhibitory regulator of lipid synthesis and of commitment to adipogenesis. J Cell Sci. 1998;111(Pt 22):3311–3322. doi: 10.1242/jcs.111.22.3311. [DOI] [PubMed] [Google Scholar]
- Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89:2583–9. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- Bell EL, Klimova TA, Eisenbart J, Moraes CT, Murphy MP, Budinger GR, Chandel NS. The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J Cell Biol. 2007;177:1029–1036. doi: 10.1083/jcb.200609074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluher M, Patti ME, Gesta S, Kahn BB, Kahn CR. Intrinsic heterogeneity in adipose tissue of fat-specific insulin receptor knock-out mice is associated with differences in patterns of gene expression. J Biol Chem. 2004;279:31891–31901. doi: 10.1074/jbc.M404569200. [DOI] [PubMed] [Google Scholar]
- Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, Bouloumie A, Barbatelli G, Cinti S, Svensson PA, Barsh GS, Zucker JD, Basdevant A, Langin D, Clement K. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- Chen D, Elmendorf JS, Olson AL, Li X, Earp HS, Pessin JE. Osmotic shock stimulates GLUT4 translocation in 3T3L1 adipocytes by a novel tyrosine kinase pathway. J Biol Chem. 1997;272:27401–27410. doi: 10.1074/jbc.272.43.27401. [DOI] [PubMed] [Google Scholar]
- Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, Nemery B, Dewerchin M, Van Veldhoven P, Plate K, Moons L, Collen D, Carmeliet P. Loss of HIF-2 and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med. 2002;7:702–10. doi: 10.1038/nm721. [DOI] [PubMed] [Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129:111–122. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Gunton JE, Kulkarni RN, Yim S, Okada T, Hawthorne WJ, Tseng YH, Roberson RS, Ricordi C, O’Connell PJ, Gonzalez FJ, Kahn CR. Loss of ARNT/HIF1beta mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell. 2005;3:337–49. doi: 10.1016/j.cell.2005.05.027. 2005. [DOI] [PubMed] [Google Scholar]
- Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, Brekken RA, Scherer PE. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29:4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka M, Shimba S, Sakaue M, Kondo Y, Kagechika H, Kokame K, Miyata T, Hara S. Hypoxia-inducible factor-3alpha functions as an accelerator of 3T3-L1 adipose differentiation. Biol Pharm. Bull. 2009;32:1166–1172. doi: 10.1248/bpb.32.1166. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Sakata M, Takeda T, Yamamoto T, Okamoto Y, Sawada K, Kimura A, Minekawa R, Tahara M, Tasaka K, Murata Y. Induction of glucose transporter 1 expression through hypoxia-inducible factor 1alpha under hypoxic conditions in trophoblast-derived cells. J Endocrinol. 2004;183:145–154. doi: 10.1677/joe.1.05599. [DOI] [PubMed] [Google Scholar]
- Heilig C, Brosius F, Siu B, Concepcion L, Mortensen R, Heilig K, Zhu M, Weldon R, Wu G, Conner D. Implications of glucose transporter protein type 1 (GLUT1)-haplodeficiency in embryonic stem cells for their survival in response to hypoxic stress. Am J Pathol. 2003;163:1873–1885. doi: 10.1016/S0002-9440(10)63546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361–9374. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes. Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaluz S, Kaluzova M, Stanbridge EJ. Regulation of gene expression by hypoxia: integration of the HIF-transduced hypoxic signal at the hypoxia-responsive element. Clin Chim. Acta. 2008;395:6–13. doi: 10.1016/j.cca.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katic M, Kennedy AR, Leykin I, Norris A, McGettrick A, Gesta S, Russell SJ, Bluher M, Maratos-Flier E, Kahn CR. Mitochondrial gene expression and increased oxidative metabolism: role in increased lifespan of fat-specific insulin receptor knock-out mice. Aging. Cell. 2007;6:827–839. doi: 10.1111/j.1474-9726.2007.00346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith B, Adelman DM, Simon MC. Targeted mutation of the murine arylhydrocarbon receptor nuclear translocator 2 (Arnt2) gene reveals partial redundancy with Arnt. Proc Natl. Acad Sci U S. A. 2001;98:6692–6697. doi: 10.1073/pnas.121494298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Nguyen MT, Imamura T, Singer O, Verma IM, Olefsky JM. Lentiviral short hairpin ribonucleic acid-mediated knockdown of GLUT4 in 3T3-L1 adipocytes. Endocrinology. 2006;147:2245–52. doi: 10.1210/en.2005-1638. [DOI] [PubMed] [Google Scholar]
- Maltepe E, Schmidt JV, Baunoch D, Bradfield CA, Simon MC. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature. 1997;386:403–407. doi: 10.1038/386403a0. [DOI] [PubMed] [Google Scholar]
- Mansfield KD, Guzy RD, Pan Y, Young RM, Cash TP, Schumacker PT, Simon MC. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab. 2005;1:393–399. doi: 10.1016/j.cmet.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Zhang L, Drysdale L, Fong GH. The transcription factor EPAS-1/hypoxia-inducible factor 2alpha plays an important role in vascular remodeling. Proc Natl. Acad Sci U S. A. 2000;97:8386–8391. doi: 10.1073/pnas.140087397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin EB, Higgins DF, Walisser JA, Johnson RS, Bradfield CA, Haase VH. Inactivation of the arylhydrocarbon receptor nuclear translocator (Arnt) suppresses von Hippel-Lindau disease-associated vascular tumors in mice. Mol Cell Biol. 2005;25:3163–3172. doi: 10.1128/MCB.25.8.3163-3172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int. J Obes. (Lond.) 2008;32:451–463. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- Regazzetti C, Peraldi P, Gremeaux T, Najem-Lendom R, Ben Sahra I, Cormont M, Bost F, Marchand-Brustel Y, Tanti JF, Giorgetti-Peraldi S. Hypoxia decreases insulin signaling pathways in adipocytes. Diabetes. 2009;58:95–103. doi: 10.2337/db08-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R, Folkman MJ. Adipose tissue mass can be regulated through the vasculature. Proc Natl. Acad Sci U S. A. 2002;99:10730–10735. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, Yan LJ, Marck BT, Matsumoto AM, Shelton JM, Richardson JA, Bennett MJ, Garcia JA. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. Nat Genet. 2003;4:331–40. doi: 10.1038/ng1266. [DOI] [PubMed] [Google Scholar]
- Sekine H, Mimura J, Yamamoto M, Fujii-Kuriyama Y. Unique and overlapping transcriptional roles of arylhydrocarbon receptor nuclear translocator (Arnt) and Arnt2 in xenobiotic and hypoxic responses. J Biol Chem. 2006;281:37507–37516. doi: 10.1074/jbc.M606910200. [DOI] [PubMed] [Google Scholar]
- Shimba S, Wada T, Hara S, Tezuka M. EPAS1 promotes adipose differentiation in 3T3-L1 cells. J Biol Chem. 2004;279:40946–40953. doi: 10.1074/jbc.M400840200. [DOI] [PubMed] [Google Scholar]
- Shimba S, Wada T, Tezuka M. Arylhydrocarbon receptor (AhR) is involved in negative regulation of adipose differentiation in 3T3-L1 cells: AhR inhibits adipose differentiation independently of dioxin. J Cell Sci. 2001;114:2809–2817. doi: 10.1242/jcs.114.15.2809. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos KB, Clermont A, Yasuda Y, Rask-Madsen C, Mastumoto M, Takahashi J, Della Vecchia K, Kondo T, Aiello LP, King GL. Adipose-specific effect of rosiglitazone on vascular permeability and protein kinase C activation: novel mechanism for PPARgamma agonist’s effects on edema and weight gain. FASEB J. 2006;8:1203–5. doi: 10.1096/fj.05-4617fje. [DOI] [PubMed] [Google Scholar]
- Stuart W,I, de Heredia FP, Wang B, Trayhurn P. Cellular hypoxia and adipose tissue dysfunction in obesity. Proc Nutr Soc. 2009;68:370–377. doi: 10.1017/S0029665109990206. [DOI] [PubMed] [Google Scholar]
- Swanson HI, Chan WK, Bradfield CA. DNA binding specificities and pairing rules of the Ah receptor, ARNT, and SIM proteins. J Biol Chem. 1995;270:26292–26302. doi: 10.1074/jbc.270.44.26292. [DOI] [PubMed] [Google Scholar]
- Tam J, Duda DG, Perentes JY, Quadri RS, Fukumura D, Jain RK. Blockade of VEGFR2 and not VEGFR1 can limit diet-induced fat tissue expansion: role of local versus bone marrow-derived endothelial cells. PLoS. One. 2009;4:e4974. doi: 10.1371/journal.pone.0004974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL. The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes. Dev. 1998;12:3320–3324. doi: 10.1101/gad.12.21.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Sinal CJ, Yim SH, Gonzalez FJ. Conditional disruption of the aryl hydrocarbon receptor nuclear translocator (Arnt) gene leads to loss of target gene induction by the aryl hydrocarbon receptor and hypoxia-inducible factor 1alpha. Mol Endocrinol. 2000;14:1674–1681. doi: 10.1210/mend.14.10.0533. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Wang B, Wood IS. Hypoxia and the endocrine and signalling role of white adipose tissue. Arch. Physiol. Biochem. 2008;114:267–276. doi: 10.1080/13813450802306602. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br. J Nutr. 2004;92:347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- Wang D, Pascual JM, Yang H, Engelstad K, Mao X, Cheng J, Yoo J, Noebels JL, De Vivo DC. A mouse model for Glut-1 haploinsufficiency. Hum. Mol Genet. 2006;15:1169–1179. doi: 10.1093/hmg/ddl032. [DOI] [PubMed] [Google Scholar]
- Wang XL, Suzuki R, Lee K, Tran T, Gunton JE, Saha AK, Patti ME, Goldfine A, Ruderman NB, Gonzalez FJ, Kahn CR. Ablation of ARNT/HIF1beta in liver alters gluconeogenesis, lipogenic gene expression, and serum ketones. Cell Metab. 2009;5:428–39. doi: 10.1016/j.cmet.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol. Endocrinol Metab. 2007;293:E1118–E1128. doi: 10.1152/ajpendo.00435.2007. [DOI] [PubMed] [Google Scholar]
- Zhang X, Lam KS, Ye H, Chung SK, Zhou M, Wang Y, Xu A. Adipose tissue-specific inhibition of hypoxia-inducible factor 1{alpha} induces obesity and glucose intolerance by impeding energy expenditure in mice. J Biol Chem. 2010;285:32869–32877. doi: 10.1074/jbc.M110.135509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.