Abstract
Summary
Background and objectives
Hypertension is an important cause of chronic kidney disease (CKD). Identifying risk factors for progression to CKD in patients with normal kidney function and hypertension may help target therapies to slow or prevent decline of kidney function. Our objective was to identify risk factors for development of incident CKD and decline in estimated GFR (eGFR) in hypertensive patients.
Design, setting, participants, & measurements
Cox proportional hazards models were used to assess the relationship between incident CKD (defined as eGFR <60 ml/min per 1.73 m2) and potential risk factors for CKD from a registry of hypertensive patients.
Results
Of 43,305 patients meeting the inclusion criteria, 12.1% (5236 patients) developed incident CKD. Diabetes was the strongest predictor of incident CKD (hazard ratio, 1.96; 95% confidence interval, 1.84 to 2.09) and was associated with the greatest rate of decline in eGFR (−2.2 ml/min per 1.73 m2 per year). Time-varying systolic BP was associated with incident CKD with risk increasing above 120 mmHg; each 10-mmHg increase in baseline and time-varying systolic BP was associated with a 6% increase in the risk of developing CKD (hazard ratio, 1.06; 95% confidence interval, 1.04 to 1.08 for both). Time-weighted systolic BP was associated with a more rapid decline in eGFR of an additional 0.2 ml/min per 1.73 m2 per year decline for every 10-mmHg increase in systolic BP.
Conclusions
We found that time-varying systolic BP was associated with incident CKD, with an increase in risk above a systolic BP of 120 mmHg among individuals with hypertension.
Introduction
Hypertension is highly prevalent, affecting approximately one-third of adults in the United States (1). It is one of the leading causes of chronic kidney disease (CKD) (2), which affects almost one in seven people (3). Identifying risk factors for progression to CKD in patients with hypertension may help target therapies to slow or prevent decline of kidney function. To date, however, only a few risk factors for development of CKD have been identified (4–6). Blood pressure (BP) control in hypertensive CKD patients has been demonstrated to slow progression of kidney function decline in patients with established CKD (7). However, little is known about the effect of BP control in hypertensive patients without CKD, and there is ongoing controversy about the level of BP control needed to slow or prevent kidney disease progression (8).
Accordingly, in this study we identified risk factors associated with the development of incident CKD in hypertensive subjects with normal or near-normal kidney function at baseline in a large integrated healthcare delivery system. Second, we identified risk factors associated with the rate of decline of estimated GFR (eGFR). Finally, we also assessed the prospective relationship between BP levels over time and the development of incident CKD.
Materials and Methods
Setting and Patients
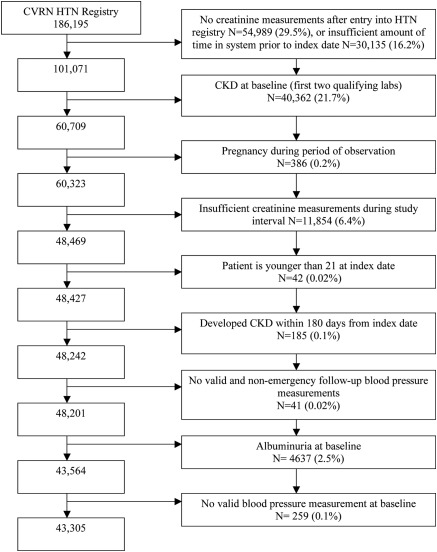
We conducted a retrospective cohort study of patients in a hypertension disease registry at Kaiser Permanente Colorado, a group model health maintenance organization that serves approximately 500,000 people primarily in the Denver metropolitan area. The registry contains demographic data, outpatient BP measurements, laboratory data, medications dispensed, and utilization information between January 1, 2000, and December 31, 2007. Patients were eligible for inclusion into the hypertension registry if they met one or more of the following criteria: (1) two or more diagnosis codes for hypertension; (2) one or more diagnosis codes for hypertension plus a prescription for an antihypertensive medication; (3) one or more diagnosis codes for hypertension plus one or more elevated BP readings (defined as >140/90 mmHg for patients without diabetes, >130/80 mmHg for those with diabetes); or (4) two or more elevated BP readings. This latter inclusion criterion assured that individuals with clinically undiagnosed hypertension would also be included. The validity of these criteria has been established previously (9). An index date was defined as the date of the first creatinine measurement at least 90 days after entry into the hypertension registry. Patients were required to be enrolled in the health plan for at least 90 days before the index date to ensure the ability to capture baseline comorbidity data. In addition, patients needed a minimum of three measurements of serum creatinine at least 1 month apart, with at least 1 year between the first measurement and the final measurement. The first two measurements were required to ensure appropriate categorization of their baseline eGFR, and the third measurement was required to insure sufficient period of observation for the outcome of interest. Only outpatient creatinine values were used to minimize the effect of temporary fluctuations in creatinine that can occur with acute illness. Patients were excluded if they had established CKD at baseline (defined as either eGFR <60 ml/min per 1.73 m2) or the presence of albuminuria (defined as urine albumin to creatinine ratio >0.030 in mg/mg). Figure 1 depicts the number of individuals excluded at each step.
Figure 1.
Inclusion criteria for analysis. CKD, chronic kidney disease.
Definitions
We defined baseline comorbidities (diabetes, coronary heart disease, cerebrovascular disease, congestive heart failure, and peripheral vascular disease) using combinations of ICD-9 codes and laboratory data. These diagnoses were considered present at baseline if they were first recorded at visits before or up to 180 days after the index date.
We defined eGFR using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (10). The CKD-EPI equation was chosen over the widely used Modification of Diet in Renal Disease equation because of the reported improved performance at higher GFR values. We repeated our analysis using the four-variable abbreviated Modification of Diet in Renal Disease study equation (11); this analysis provided similar results and is not described further. Nonphysiologic eGFR values were set to a maximum of 200 ml/min per 1.73 m2 (12).
The primary outcome of interest, time to incident CKD, was defined using the National Kidney Foundation Kidney Disease Outcome Quality Initiative (NKF-K/DOQI) definition of eGFR <60 ml/min per 1.73 m2) persisting for at least 3 months (11). We considered CKD as being present if there was at least one eGFR <60 ml/min per 1.73 m2) at least 3 months after the first eGFR <60 ml/min per 1.73 m2), with no intervening values ≥60 ml/min per 1.73 m2).
To assess the effect of longitudinal BP measurements with incident CKD, time-varying BP was included as a covariate. To improve the precision of the estimate of the exposed BP, BP values were averaged in 6-month intervals. If there were no values for an individual in the 6-month block, the last value was carried forward (13,14).
To assess the effect of longitudinal BP measurements and eGFR decline, time-weighted average systolic BP (SBP) for all measurements before the follow-up date was included as a covariate rather than a simple mean of all BP readings. A time-weighted average approximates the area under the curve for BP over time and was calculated by multiplying each SBP reading by the proportion of the entire time period of observation between that reading and the next, or the follow-up date for the last measurement used. The sum of these values constitutes the time-weighted SBP. Time weighting of SBPs in a series improves the precision of the measurement and allows accounting for all values in the data set but minimizes the upward bias of a simple average that can occur when there are frequent follow-up appointments for uncontrolled hypertension (15).
Statistical Methods
We compared baseline characteristics of those who developed CKD with those who did not using Wilcoxon rank-sum tests for continuous variables and chi-squared tests for dichotomous or categorical variables. Cox proportional hazards models were utilized to identify risk factors for incident CKD. Covariates that were included in the model included age, gender, race/ethnicity, baseline eGFR, baseline and time-varying SBP, HDL cholesterol, body mass index, and baseline comorbidities. In the subset of patients with diabetes at baseline, hemoglobin A1c was added to the model in a separate analysis. For the 187 patients with missing hemoglobin A1c data (1.9% of the diabetic population), a single median value was used for imputation. Model discrimination was assessed with the c-statistic. We performed a competing risk analysis to determine whether censoring these patients at the time of death biased our results. There were no substantive differences in the hazard ratios in the models comparing patients who developed CKD and those who developed CKD or died, suggesting that our modeling was not confounded by the competing risk of death (data not shown) (16).
We used general linear mixed-effects models (random slope and random intercept) to estimate the rate of decline in eGFR and the degree to which the baseline covariates predicted eGFR. Repeated measures within each patient were modeled as linear time trend (growth curve) model. The intercept coefficients represent the within subgroup difference in baseline eGFR. The slope coefficients (time, or time × variable interaction in the statistical model) represent effects on the rate of change of eGFR per year, where a negative number represents a greater decline in kidney function.
To better understand the relationship between time-varying SBP and time to incident CKD, we first assessed the relationship of quartiles of time-varying SBP and cumulative incidence of CKD. Time-varying SBP was divided into quartiles rather than arbitrary categories, because these were observed values (rather than goal or target BP values). Second, a penalized smoothing spline basis function (with three degrees of freedom for the nonlinear part and one degree of freedom for the linear part) was used to show the unadjusted smoothed centered log hazard function of time-varying BP and time to incident CKD.
All analyses were conducted with SAS, version 9.1 (SAS Institute, Cary, NC), with the exception of the penalized smoothing spline, which was performed with R, version 2.8.1 (R Development Core Team, 2008, R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0; http://www.R-project.org).
Results
There were 43,305 patients who met our entry criteria (Figure 1). These individuals were followed for a median of 44.0 (interquartile range [IQR], 28.6 to 60.1) months and had a median of six serum creatinine measurements (IQR, 4 to 9) during the study period. The median baseline SBP was 132 mmHg, diastolic BP was 80 mmHg, the median baseline eGFR was 82.0 ml/min per 1.73 m2 (IQR, 72.1 to 92.9) (Table 1). There were 2590 patients (5.9% of the cohort) who died during the follow-up period (data not shown). As shown in Table 1, 5236 patients, or 12.1% of the cohort, developed CKD.
Table 1.
Baseline characteristics
| Total (n = 43,305) | No CKD (n = 38,069, 87.9%) | CKD (n = 5236, 12.1%) | P Value for Comparison | |
|---|---|---|---|---|
| Age, years | 60 (52, 69) | 59 (51, 68) | 69 (62, 75) | <0.01 |
| Male gender | 20,010 (46.2%) | 18,139 (47.7%) | 1871 (35.7%) | <0.01 |
| Race/ethnicity | ||||
| African American | 2352 (5.4%) | 2128 (5.6%) | 224 (4.3%) | <0.01 |
| Hispanic/Latino | 2573 (5.9%) | 2285 (6.0%) | 288 (5.5%) | 0.14 |
| Caucasian | 23,326 (53.9%) | 20,057 (52.7%) | 3269 (62.4%) | <0.01 |
| other | 1297 (3.0%) | 1155 (3.0%) | 142 (2.7%) | 0.19 |
| missing | 13,757 (31.8%) | 12,444 (32.7%) | 1313 (25.1%) | <0.01 |
| Period of observation, months | 44.0 (28.6, 60.1) | 45.8 (30.0, 61.4) | 33.2 (21.0, 47.3) | <0.01 |
| Number of creatinine measurements | 6 (4, 9) | 5 (4, 8) | 11 (8, 17) | <0.01 |
| Number of BP measurements per year of follow-up | 3.2 (2.0, 4.9) | 3.1 (2.0, 4.8) | 3.7 (2.4, 5.5) | <0.01 |
| Number of primary care visits per year of follow-up | 3.5 (2.3, 5.4) | 3.3 (2.2, 5.1) | 4.9 (3.1, 7.6) | <0.01 |
| Number of hospitalizations per year of follow-up | 0 (0, 0.4) | 0 (0, 0.4) | 0 (0, 0.6) | <0.01 |
| Comorbidities | ||||
| diabetes | 8154 (18.8%) | 6753 (17.7%) | 1401 (26.8%) | <0.01 |
| coronary heart disease | 5419 (12.5%) | 4408 (11.6%) | 1011 (19.3%) | <0.01 |
| cerebrovascular disease | 1480 (3.4%) | 1201 (3.2%) | 279 (5.3%) | <0.01 |
| congestive heart failure | 1872 (4.3%) | 1432 (3.8%) | 440 (8.4%) | <0.01 |
| peripheral vascular disease | 56 (0.1%) | 44 (0.1%) | 12 (0.2%) | 0.05 |
| eGFR, baseline (ml/min per 1.73 m2) | 82.0 (72.1, 92.9) | 83.6 (73.6, 94.2) | 71.6 (65.7, 80.1) | <0.01 |
| Baseline systolic BP | 132 (122, 145) | 132 (122, 144) | 138 (126, 150) | <0.01 |
| Baseline diastolic blood pressure BP | 80 (76, 90) | 81 (76, 90) | 80 (72, 88) | <0.01 |
| Systolic blood pressure BP, time-weighted | 132.1 (125.0, 139.8) | 131.7 (124.7, 139.2) | 135.7 (128.0, 144.0) | <0.01 |
| ACEI or ARB at baseline | 15,945 (36.8%) | 13,518 (35.5%) | 2427 (46.4%) | <0.01 |
| LDL cholesterol (n = 41822) | 113.4 (94.5, 132.4) | 113.8 (94.8, 132.7) | 110.4 (92.7, 130.5) | <0.01 |
| HDL cholesterol (n = 37083) | 51.2 (42.7, 62.5) | 51.2 (42.7, 62.5) | 52.0 (42.9, 63.0) | 0.18 |
| A1c-DM subpopulation (n = 9796) | 7.1 (6.5, 8.1) | 7.1 (6.5, 8.1) | 7.3 (6.6, 8.2) | <0.01 |
The data are presented as medians (interquartile range) or n values (%). Chi-squared likelihood ratio test was used for categorical variable tests of significance. Wilcoxon rank-sum test was used for continuous variable tests of significance. CKD, chronic kidney disease; eGFR, estimated GFR; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; DM, diabetes mellitus.
The results of the multivariable regression analysis of predictors of time to incident CKD are shown in Table 2. Each 10-mmHg increase in baseline and time-varying SBP was associated with a 6% increase in the risk of developing incident CKD (hazard ratio [HR], 1.06; 95% confidence interval [CI], 1.04 to 1.08; P < 0.01 for both baseline and time-varying systolic BP). Diabetes was the strongest predictor of incident CKD (HR, 1.96; 95% CI, 1.84 to 2.09; P < 0.01). In the subset of patients with diabetes, the HR associated with each 1.0-increase in hemoglobin A1c was 1.32 (95% CI, 1.26 to 1.37; P < 0.01). The c-statistic for the adjusted Cox proportional hazards model was 0.79, indicating good model discrimination. A subanalysis of the 4716 patients who were not prescribed antihypertensive therapy during the period of observation was performed and did not demonstrate any substantive differences (data not shown).
Table 2.
Multivariable Cox proportional hazards analysis of predictors of incident CKD (n = 43,305)
| Unadjusted HR (95% CI) | Unadjusted P Value | Adjusted HR (95% CI) | Adjusted P Value | |
|---|---|---|---|---|
| Age (in decades) | 1.98 (1.93, 2.03) | <0.01 | 1.60 (1.55, 1.65) | <0.01 |
| Gender | ||||
| <0.01 | <0.01 | |||
| female (versus male) | 1.53 (1.45, 1.62) | 1.60 (1.51, 1.70) | ||
| Race/ethnicity | ||||
| African American | 0.63 (0.55, 0.72) | <0.01 | 0.99 (0.86, 1.13) | 0.88 |
| Hispanic/Latino | 0.81 (0.72, 0.92) | <0.01 | 1.04 (0.92, 1.18) | 0.50 |
| Caucasian (referent) | 1.00 | 1.00 | ||
| other | 0.79 (0.66, 0.93) | 0.01 | 1.01 (0.86, 1.20) | 0.88 |
| missing | 0.83 (0.78, 0.89) | <0.01 | 0.92 (0.86, 0.98) | 0.01 |
| Comorbidities | ||||
| diabetes | 1.57 (1.48, 1.67) | <0.01 | 1.96 (1.84, 2.09) | <0.01 |
| coronary heart disease | 1.44 (1.33, 1.55) | <0.01 | 1.24 (1.15, 1.33) | <0.01 |
| cerebrovascular disease | 1.42 (1.25, 1.60) | <0.01 | 1.08 (0.95, 1.22) | 0.25 |
| congestive heart failure | 1.90 (1.71, 2.11) | <0.01 | 1.51 (1.36, 1.67) | <0.01 |
| peripheral vascular disease | 1.54 (0.87, 2.72) | 0.14 | 1.82 (1.03, 3.22) | 0.04 |
| eGFR, baseline (per 10 ml/min per 1.73 m2) | 0.50 (0.49, 0.52) | <0.01 | 0.55 (0.54, 0.57) | <0.01 |
| Systolic BP, baseline (per 10 mmHg) | 1.11 (1.09, 1.12) | <0.01 | 1.06 (1.04, 1.08) | <0.01 |
| Systolic BP, time-varying (per 10 mmHg) | 1.13 (1.11, 1.15) | <0.01 | 1.06 (1.04, 1.08) | <0.01 |
| HDL <40 mg/dl | 0.93 (0.87, 1.00) | 0.06 | 1.17 (1.08, 1.26) | <0.01 |
| Body mass index | 0.98 (0.98, 0.99) | <0.01 | 1.01 (1.01, 1.02) | <0.01 |
| A1c-DM subpopulation (n = 9982, imputed missing) | 1.15 (1.11, 1.19) | <0.01 | 1.32 (1.26, 1.37) | <0.01 |
CKD, chronic kidney disease; HR, hazard ratio; CI, confidence interval; eGFR, estimated GFR; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; DM, diabetes mellitus.
Predictors of eGFR at baseline (intercept) and of the change of eGFR over time (slope) are shown in Table 3. The slope of eGFR for the reference group (Caucasian, male, 60 years of age, with time-weighted SBP 133 mmHg, HDL 53.6 mg/dl without diabetes or congestive heart failure) was −1.4 ml/min per 1.73 m2 per year. Diabetes was associated with a higher baseline eGFR but a greater rate of decline (0.8 ml/min per 1.73 m2 per year additional decline or a slope of −2.2 ml/min per 1.73 m2 per year). Time-weighted SBP was also associated with a more rapid decline in eGFR over time of an additional 0.2 ml/min per 1.73 m2 per year decline for every 10 mmHg (for example, −1.6 ml/min per 1.73 m2 per year for an individual with a time-weighted SBP of 143 mmHg).
Table 3.
Coefficients of the intercepts and slopes of the eGFR progression analysis
| Coefficient (95% CI) | P | |
|---|---|---|
| Variables that affect the intercept | ||
| Intercept | 80.9 (80.7, 81.1) | <0.01 |
| Age (decades) | −5.1 (−5.2, −5.0) | <0.01 |
| Female gender (versus male) | 1.2 (1.0, 1.4) | <0.01 |
| Race/ethnicity | ||
| African American | 4.1 (3.6, 4.6) | <0.01 |
| Hispanic/Latino | 3.7 (3.2, 4.2) | <0.01 |
| Caucasian (referent) | 1.0 | |
| other | 1.7 (1.0, 2.3) | <0.01 |
| missing | 1.0 (0.7, 1.2) | <0.01 |
| Diabetes | 2.9 (2.6, 3.2) | <0.01 |
| Systolic BP, time-weighted (per 10 mmHg) | 0.2 (0.2, 0.3) | <0.01 |
| HDL, time-weighted (per 10 mg/dl) | 0.2 (0.1, 0.3) | <0.01 |
| Variables that affect the slope | ||
| Time (years) | −1.4 (−1.4, −1.3) | <0.01 |
| Age (decades) | −0.2 (−0.2, −0.2) | <0.01 |
| Female gender (versus male) | −0.8 (−0.8, −0.7) | <0.01 |
| Diabetes | −0.8 (−0.9, −0.7) | <0.01 |
| Congestive heart failure | −0.3 (−0.5, −0.2) | <0.01 |
| Systolic BP, time-weighted (per 10 mmHg) | −0.2 (−0.2, −0.2) | <0.01 |
| HDL, time-weighted (per 10 mg/dl) | 0.1 (0.0, 0.1) | <0.01 |
The intercept coefficients represent the within-subgroup difference in baseline estimated GFR (eGFR). The slope coefficients represent the rate of change of eGFR per year, where a negative number represents a decline in kidney function. CI, confidence interval.
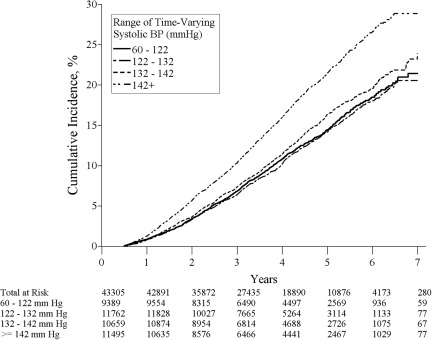
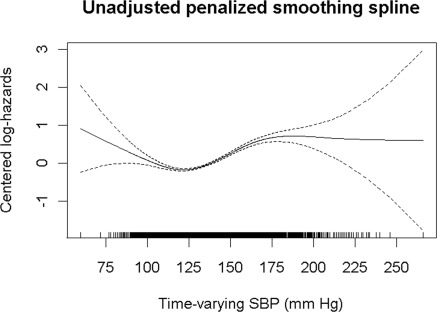
To better understand the relationship between SBP and kidney function decline, we examined the adjusted cumulative incidence of CKD by quartile of time-varying SBP, which is shown in Figure 2. We found little difference in the cumulative incidence of CKD in the first two quartiles (representing SBP 60 to 122 mmHg and 122 to 132 mmHg); however, the rates of incident CKD increase with higher time-varying SBP. To assess the inflection point above which the log hazard of time to incident CKD increases, an unadjusted penalized smoothing spline model was performed (Figure 3). This model showed that time-varying SBP values above approximately 120 mmHg were associated with a steady increase in the risk of incident CKD.
Figure 2.
Adjusted cumulative incident of chronic kidney disease by time-varying systolic BP.
Figure 3.
Unadjusted penalized smoothing spline of the risk of incident chronic kidney disease by time-varying systolic BP.
Discussion
In a large community-based registry of patients with hypertension, we found that time-varying SBP was associated with incident CKD, with a steady increase in risk of incident CKD above an SBP of 120 mmHg. Time-weighted SBP was associated with a more rapid decline of kidney function. Diabetes was the strongest predictor of incident CKD, and more rapid decline of kidney function and worse glycemic control were associated with greater risk. This study provides further evidence supporting the role of BP and other traditional risk factors like diabetes in the initiation and progression of kidney function decline in hypertensive patients with normal kidney function at baseline.
Our study was unique in its focus on patients with normal or near-normal kidney function at baseline and incident CKD as the outcome of interest. CKD is an important outcome because it is associated with increased risk for cardiovascular disease, and it is a precursor to end-stage renal disease. Inclusion of eGFR slope as an outcome is also important because predicting those with a faster decline in kidney function is more important than the dichotomous outcome of achieving a threshold value of eGFR. Therefore, identification of patients who are more likely to progress to CKD or to have kidney function decline may provide opportunities for early intervention to reduce the risk of kidney disease progression.
Although many of the predictors for incident CKD that we identified were similar to those identified in prior studies (4–6), none of them had the extensive BP data available as did our study, and they only included baseline BP in their analyses. In a cohort of 722 hypertensive male veterans with eGFR >60 ml/min per 1.73 m2, Vupputuri et al. (17) found that higher treated BP was associated with early kidney function decline (defined as a rise in serum creatinine ≥0.6 mg/dl). They found that a 1-SD increase in SBP (or about 18 mmHg) was associated with a relative risk of 1.81 (95% CI 1.29 to 2.55) for early kidney function decline. They found a similar eGFR change of −1.34 ml/min per 1.73 m2 per year, but a larger effect of SBP on eGFR decline (multivariable adjusted decline of 0.92 ml/min per 1.73 m2) per year for a 1-SD increase in SBP (or about 18 mmHg), although they did not evaluate the risk of incident CKD, and a threshold SBP for incident CKD was not identified.
The patients in this cohort who developed CKD were more likely to be hospitalized than the comparison group. Hospital-related acute kidney injury is an important potential risk factor for future development of CKD, and this finding supports the need for further investigation in this area. In addition, patients who developed CKD had a lower eGFR at baseline, which suggests that they were possibly already on the continuum of progression to CKD, although not yet at stage 3 CKD (the stage at which NKF-K/DOQI guidelines suggest that one can diagnose CKD without evidence of renal injury). Improved accuracy in measurement of eGFR by use of the CKD-EPI equation at higher levels of eGFR may help to more accurately identify patients at risk for decline in kidney function (10).
Although the ability of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers to blunt the decline in eGFR is an important clinical question, evaluating this question in an observational study is susceptible to substantial “confounding by indication,” because patients at higher risk of decline in kidney function caused by their comorbid health conditions are more likely to receive these medications. In fact, the patients in our cohort who developed CKD were more likely to be on angiotensin-converting enzyme inhibitors and angiotensin receptor blockers at baseline. Because our goal was to assess the effect of SBP control, however achieved, on eGFR, we did not assess the comparative effectiveness of different approaches to treating hypertension on our findings.
Our study has limitations. First, our study may be subject to ascertainment bias, because the patients who developed CKD had more contact with the system (more primary care visits) and more measurements of BP and serum creatinine (Table 1). This greater degree of contact may have been a cause of the observed associations, because those who had greater contact and more measurements had a greater opportunity to have their progression to CKD detected. Greater system contact could also have been a result of the higher risk of CKD, because patients with a greater tendency to develop CKD because of their comorbidities could have been followed more closely, resulting in a greater number of measurements. These alternative explanations cannot be fully explored in this observational study. Although this study is more susceptible to ascertainment bias that could be reduced by a carefully controlled, prospective observational study, it is less susceptible to selection bias, because it evaluates the experience of a large and more representative sample of community members. Finally, as with all retrospective studies, unmeasured confounding is likely to have been present.
The strengths of our study include the large sample size, long duration of follow-up, and representative sample including individuals with untreated hypertension (defined as >140/90 for patients without diabetes, >130/80 for those with diabetes). In addition to describing predictors of decline in kidney function in patients with hypertension, our data suggest that even treated BP above approximately 120 mmHg is associated with increased risk of incident CKD. This is an observational study, and therefore its results should not be viewed as conclusive, but it supports the need for well designed randomized controlled trials to test the best targets for BP therapy to decrease risk of important consequences of hypertension including CKD. The target SBP to prevent or delay the complications of hypertension is an important question and not fully assessed (8); observational studies such as ours (18,19) and post hoc analyses of achieved BP in randomized controlled trials (20,21) suggest an increase in complications in patients with SBP >120 mmHg, whereas other studies have suggested there may be an increase in risk or no benefit at lower levels of achieved BP (22–24). Randomized trials performed to date have not fully answered the question of optimal target BP because of small differences in achieved BP between treatment arms, failure to achieve low targets, or limitation of the study population to patients with advanced CKD (8). Because there exists a discrepancy between observational studies, which support lower targets and randomized trials, which fail to support lower targets in patients without proteinuria, the National Institutes of Health has funded the Systolic Blood Pressure Intervention Trial (SPRINT), designed to test the benefit and risk of intensive SBP control (<120 mmHg) versus standard treatment (<140 mmHg) and its effect on cardiovascular outcomes and kidney disease progression in patients with and without kidney disease. We hope that this large randomized control trial will answer this important question.
Disclosures
Dr. Ho is supported by a Veterans Affairs Health Services Research and Development Career Development Award 05-026 and serves as a consultant for Wellpoint, Inc.
Acknowledgments
This study was conducted within the Cardiovascular Research Network, a consortium of research organizations affiliated with the HMO Research Network and sponsored by the National Heart Lung and Blood Institute (U19 HL91179-01). The results presented in this paper have not been published previously in whole or part, except in abstract form at the 43rd Annual American Society of Nephrology Conference.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Hajjar I, Kotchen TA: Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA 290: 199–206, 2003 [DOI] [PubMed] [Google Scholar]
- 2. U.S. Renal Data System, USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda MD, 2009 [Google Scholar]
- 3. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Fox CS, Larson MG, Leip EP, Culleton B, Wilson PWF, Levy D: Predictors of new-onset kidney disease in a community-based population. JAMA 291: 844–850, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Kahirsagar AV, Bang H, Bomback AS, Vupputuri S, Shoham DA, Kern LM, Klemmer PJ, Mazumdar M, August PA: A simple algorithm to predict incident kidney disease. Arch Intern Med 168: 2466–2473, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hanratty R, Chonchol M, Dickinson LM, Beaty BL, Estacio RO, MacKenzie TD, Hurley LP, Linas SL, Steiner JF, Havranek EP: Incident chronic kidney disease and the rate of kidney function decline in individual with hypertension. Nephrol Dial Transplant 25: 801–807, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, Striker G: The effects of dietary protein restriction and blood pressure control on the progression of chronic renal disease. N Engl J Med 330: 877–884, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Arguedas JA, Perez MI, Wright JM. Treatment blood pressure targets for hypertension. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No.: CD004349. DOI: 10.1002/14651858.CD004349.pub2 [DOI] [PubMed] [Google Scholar]
- 9. Selby JV, Peng T, Karter AJ, Alexander M, Sidney S, Lian J, Arnold A, Pettitt D: High rates of co-occurrence of hypertension, elevated low-density lipoprotein cholesterol, and diabetes mellitus in a large managed care population. Am J Manag Care 10: 163–170, 2004 [PubMed] [Google Scholar]
- 10. Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J: A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification and stratification. Am J Kidney Dis 39: S1–S266, 2002 [PubMed] [Google Scholar]
- 12. Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS: Prevalence of chronic kidney disease and decresed kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 41: 1–12, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Zager PG, Nikolic J, Brown RH, Campbell MA, Hunt WC, Peterson D, Van Stone J, Levey A, Meyer KB, Klag MJ, Johnson HK, Clark E, Sadler JH, Teredesai P: “U” curve association of blood pressure and mortality in hemodialysis patients. Kidney Int 54: 561–569, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Kalanter-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD: Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 70: 771–780, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Neutra RR, Stason WB, Solomon HS, Gillum RF: Techniques for characterizing a series of blood pressure measurements over time. Prev Med 9: 144–149, 1980 [DOI] [PubMed] [Google Scholar]
- 16. Allison PD: Survival Analysis Using SAS: A Practical Guide, 1st Edition SAS Publishing, Cary, North Carolina: 1995, pp 185–209 [Google Scholar]
- 17. Vupputuri S, Batuman V, Muntner P, Bazzano LA, Lefante JJ, Whelton PK, He J: Effect of blood pressure on early decline in kidney function among hypertensive men. Hypertension 42: 1144–1149, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Haroun MK, Jaar BG, Hoffman SC, Comstock GW, Klag MJ, Coresh J: Risk factors for chronic kidney disease: A prospective study of 23,534 men and women in Washington County, Maryland. J Am Soc Nephrol 14: 2934–2941, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Hsu C, McCulloch CE, Darbinian J, Go AS, Iribarren C: Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch Intern Med 165: 923–928, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Berl T, Hunsicker LG, Lewis JB, Pfeffer MA, Porush JG, Rouleau J, Drury PL, Esmatjes E, Hricik D, Pohl M, Raz I, Vanhille P, Wiegmann TB, Wolfe BM, Locatelli F, Goldhaber SZ, Lewis EJ: Impact of achieved blood pressure on cardiovascular outcomes in irbesartan diabetic nephropathy trial. J Am Soc Nephrol 16: 2170–2179, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, de Jong PE, de Zeeuw D, Shahinfar S, Toto R, Levey AS: Progression of chronic kidney disease: The role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition. Ann Intern Med 139: 244–252, 2003 [DOI] [PubMed] [Google Scholar]
- 22. The ACCORD Study Group: Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 362: 1575–1585, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wright JT, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG: Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease. JAMA 288: 2421–2431, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Weiner DE, Tighiouart H, Levey AS, Elsayed E, Griffith JL, Salem DN, Sarnak MJ: Lowest systolic blood pressure is associated with stroke in stages 3 to 4 chronic kidney disease. J Am Soc Nephrol 18: 960–966, 2007 [DOI] [PubMed] [Google Scholar]