Abstract
Summary
Background and objectives
Osteoprotegerin (OPG), a cytokine that regulates bone resorption, has been implicated in the process of vascular calcification and stiffness.
Design, setting, participants, & measurements
Serum OPG was measured in 351 participants with chronic kidney disease (CKD) from one site of the Chronic Renal Insufficiency Cohort Study. Cortical bone mineral content (BMC) was measured by quantitative computed tomography in the tibia. Multivariable linear regression was used to test the association between serum OPG and traditional cardiovascular risk factors, measures of abnormal bone and mineral metabolism, and pulse wave velocity.
Results
Higher serum OPG levels were associated with older age, female gender, greater systolic BP, lower estimated GFR, and lower serum albumin. OPG was not associated with measures of abnormal bone or mineral metabolism including serum phosphorus, albumin-corrected serum calcium, intact parathyroid hormone, bone-specific alkaline phosphatase, or cortical BMC. Among 226 participants with concurrent aortic pulse wave velocity measurements, increasing tertiles of serum OPG were associated with higher aortic pulse wave velocity after adjustment for demographics, traditional vascular risk factors, and nontraditional risk factors such as estimated GFR, albuminuria, serum phosphate, corrected serum calcium, presence of secondary hyperparathyroidism, serum albumin, and C-reactive protein or after additional adjustment for cortical BMC in a subset (n = 161).
Conclusions
These data support a strong relationship between serum OPG and arterial stiffness independent of many potential confounders including traditional cardiovascular risk factors, abnormal bone and mineral metabolism, and inflammation.
Introduction
Osteoprotegerin (OPG), a soluble member of the TNF receptor superfamily, plays a critical role in the regulation of bone turnover (1). OPG is expressed by a variety of tissues, but predominant sources are osteoblasts and vascular endothelium (2). Within bone, OPG binds and neutralizes receptor activator of NF-κB ligand (RANKL), a potent promoter of osteoclast differentiation and survival, thereby inhibiting bone resorption (3). In addition to regulating bone turnover, OPG may inhibit vascular calcification, inhibit apoptosis, and modulate inflammation in the vascular wall (2,4,5).
Animal models suggest that OPG may be an important link between bone loss and vascular disease. OPG null mice develop severe osteoporosis and medial calcification of the aorta and renal arteries (6). This phenotype can be rescued through transgenic overexpression of OPG during gestational development and into adulthood (7). These findings suggest a physiologic role for OPG in maintaining bone mass and a healthy vascular wall. The connection of bone and vascular health is of particular interest in patients with chronic kidney disease (CKD), whose disease is often complicated by medial arterial calcification (8,9) and a constellation of bone and mineral metabolism abnormalities known as Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). The causes of medial arterial calcification in patients with CKD are not fully understood but serum phosphate and other retained uremic toxins likely play a pathologic role (10).
Distinct from intimal calcification, medial calcification does not result in arterial luminal stenosis, but may contribute to cardiovascular disease by decreasing the distensibility of the great vessels. This subsequent aortic stiffness results in increased left ventricular afterload and increased myocardial oxygen demand, promoting left ventricular hypertrophy and subendocardial ischemia (11). Aortic pulse wave velocity is an indirect, noninvasive measurement of aortic stiffness that is associated with adverse cardiovascular outcomes in patients with CKD (12,13).
In contrast to knockout animal studies that suggest OPG may be vasculoprotective, human studies have demonstrated associations between higher serum OPG levels and adverse cardiovascular outcomes in many patient groups, including those with CKD (14–23). It is unknown whether these associations result from a role of OPG in the vascular wall, if the observed associations are secondary to the effects of OPG in modulating bone turnover and mass, or if OPG rises in response to vascular injury. The objectives of this study are (1) to identify correlates of serum OPG levels in patients with moderate to severe CKD with particular interest in vascular risk factors and measures of CKD-MBD and (2) to determine if OPG levels are associated with aortic pulse wave velocity, independent of these vascular risk factors and measures of CKD-MBD.
Materials and Methods
Patients and Study Design
The Chronic Renal Insufficiency Cohort (CRIC) Study is a multicenter cohort study of adult participants with CKD (about 50% with diabetes) recruited between 2003 and 2008. By design, the cohort represents a wide spectrum of kidney dysfunction, and is racially and ethnically diverse (24). An ancillary study of bone structure that included measures of serum OPG levels was performed at the University of Pennsylvania site. The closest ancillary study visit within 1 year was linked to a CRIC main study visit. Of the 380 ancillary study participants, 351 had a serum OPG level available at the time of the first CRIC bone study visit and were used to study correlates of OPG. Two hundred twenty-six participants had a measurement of aortic pulse wave velocity available for analyses of the association between OPG and pulse wave velocity. The study was approved by the Institutional Review Board at the University of Pennsylvania and all participating clinical sites. All participants provided written informed consent.
Data Collection and Measurement
Serum OPG levels were measured by ELISA (ALPCO, Salem, NH) with an interassay coefficient of variation (CV) of 11.3% and an intra-assay CV of 2.7%. Bone-specific alkaline phosphatase was measured by ELISA (QUIDEL, San Diego, CA) with an interassay CV of 5.6% and intra-assay CV of 3.8%. Plasma parathyroid hormone was measured using a total intact assay (Scantibodies, Santee, CA).
Bone measures in the left tibia were obtained by peripheral quantitative computed tomography (pQCT) using a Stratec XCT2000 device (Orthometrix, White Plains, NY) with a 12-detector unit, voxel size of 0.4 mm, slice thickness of 2.3 mm, and scan speed of 25 mm/s. Scans were analyzed with Stratec software version 5.50. Cortical bone mineral content (g) was assessed at 38% of tibia length proximal to the distal physis using Cortmode 2 (threshold, 711 mg/cm3). The manufacturer's hydroxyapatite phantom was scanned daily for quality assurance. The CV for short-term precision ranged from 0.5% to 1.6% for pQCT.
Aortic pulse wave velocity was measured in CRIC participants at the second annual follow-up visit using the SphygmoCor PVx System (AtCor Medical, West Ryde, Australia) by trained personnel. Measurements were made using the right carotid and femoral arteries with patients in the supine position after a 5-minute rest period. An adjusted measure of aortic pulse wave velocity was created after accounting for waist circumference–induced error in the measurement of the distance between the sternal notch and femoral artery. Detailed description of the protocol for measurement and adjustment of the pulse wave velocity have been described previously (25). Aortic pulse wave velocity measurements and serum OPG measurements occurred on the same day in 88% of participants, within 3 months in 95% of participants, and all within 1 year.
Routine laboratories including serum creatinine, albumin, calcium, phosphate, total alkaline phosphatase, high-sensitivity C-reactive protein, and urine albumin were performed using standard assays in a central laboratory. Urinary albumin and serum phosphate were available at baseline only; however, other laboratory values were available annually. Eighty percent of serum phosphorus and urinary albumin measures were collected on the same day as serum OPG with 87% and 86% performed within 3 months, respectively. Corrected serum calcium levels were calculated as follows: corrected calcium (mg/dl) = serum calcium (mg/dl) + 0.8 [4 − serum albumin (g/dl)] (26). GFR was estimated (eGFR) from serum creatinine using the abbreviated Modification of Diet in Renal Disease (MDRD) study equation for calibrated serum creatinine (27). The presence of secondary hyperparathyroidism was defined in two ways, as intact parathyroid hormone level greater than the upper limit of normal reported for the assay (≥66 pg/ml), and as exceeding 2003 Kidney Disease Outcome Quality Initiative (KDOQI) clinical practice guideline targets based on CKD stage (>70 pg/ml for stage 3 CKD; >110 pg/ml for stage 4 CKD; and >300 pg/ml for stage 5 CKD) (26). Demographics, medical history, updated medication information, and physical examination (i.e., BP, anthropometric measures) was obtained at annual CRIC visits. Calcitriol, doxercalciferol, and paricalcitol were classified as active vitamin D sterols. Phosphorus binding medications include calcium- and noncalcium-based binding agents. Diabetes was defined using fasting glucose measurements and/or the use of insulin or oral hypoglycemic medications. Hypertension was defined as a systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg, or use of antihypertensive medications. Prevalent cardiovascular disease was defined by self-report.
Statistical Analyses
Serum OPG levels were log-transformed to approximate a normal distribution and also analyzed categorically in tertiles. Characteristics of the study population were compared across tertiles of serum OPG using ANOVA (continuous variables) or Pearson's chi-squared test (categorical variables).
First, we assessed the relationship between candidate predictor variables and natural log (serum OPG) using scatterplots, and linear regression. In these models, age, systolic BP, body mass index, waist circumference, eGFR, serum phosphate, corrected serum calcium, serum albumin, total alkaline phosphatase, bone-specific alkaline phosphatase, and cortical bone mineral content were treated as continuous variables. C-reactive protein was treated as a log-transformed continuous variable. Demographics and traditional vascular risk factors that were hypothesized to be associated with serum OPG levels based on previous literature, biologic rationale, and/or observed univariate associations (P < 0.10) were included in a multivariable linear regression model to identify cardiovascular risk factors that were independently associated with serum OPG levels. CKD-MBD–specific risk factors including serum phosphate, corrected serum calcium, presence of secondary hyperparathyroidism, total alkaline phosphatase, bone-specific alkaline phosphatase, and cortical bone mineral content were tested for association with serum OPG levels after adjustment for age, gender, and race. Regression coefficients were exponentiated to obtain estimates of the percentage difference in serum OPG per 1 unit difference in predictor variables.
Subsequently, we assessed the relationship between OPG and aortic pulse wave velocity. Aortic pulse wave velocity was analyzed as a natural log-transformed variable to approximate a normal distribution. Linear regression was used to determine the association between increasing tertiles of serum OPG and natural log (aortic pulse wave velocity). Models were sequentially adjusted for demographics and traditional vascular risk factors (age, gender, race, diabetes, prevalent cardiovascular disease, history of hypertension, current/former smoking, and systolic BP), followed by nontraditional risk factors (eGFR, albuminuria, serum albumin, C-reactive protein, serum phosphate, corrected serum calcium, and the presence of secondary hyperparathyroidism), and finally for cortical bone mineral content obtained by pQCT in the subset for which this measure was available (n = 161). Interactions were tested between serum OPG tertiles and gender, race, diabetes, and presence of secondary hyperparathyroidism (≥66 pg/ml), and also categories of cortical bone mineral content and C-reactive protein (above versus below median) in models adjusted for demographics and traditional and nontraditional risk factors. Regression coefficients were exponentiated to obtain estimates of the ratio of aortic pulse wave velocity compared with the reference across serum OPG tertiles. The fit of linear models was assessed graphically through examination of residual plots. All analyses were performed using STATA Special Edition 10.0 (College Station, TX, 2008). Hypotheses were tested using a two-sided type 1 error rate of 0.05.
Results
A total of 351 individuals had measurements of serum OPG available at the first CRIC bone study visit and comprise the study population. The population was 30% female, 57% black, and 52% had diabetes. Median age was 61 years (range 21 to 77 years). Mean eGFR was 47.6 ml/min per 1.73 m2 (SD 14.1). Seventeen percent of participants had an eGFR ≥60 ml/min per 1.73 m2, 72% had an eGFR of 30 to 59 ml/min per 1.73 m2, 11% had an eGFR of 15 to 29 ml/min per 1.73 m2, and <1% had an eGFR <15 ml/min per 1.73 m2. Macroalbuminuria (≥300 mg of albumin per day) was present in 26% of participants. Serum OPG levels ranged from 1.21 to 22.31 pmol/L with a median value of 6.06 pmol/L (interquartile range 4.56 to 8.11 pmol/L).
Baseline clinical characteristics and measures of CKD-MBD stratified by tertile of serum OPG are presented in Tables 1 and 2, respectively. Increasing tertiles of serum OPG were significantly associated with greater age, female gender, diabetes, prior cardiovascular disease, prior hypertension, higher systolic BP, lower eGFR, lower serum albumin, higher serum phosphate, and use of active vitamin D sterols. Notably, increasing tertiles of OPG were not associated with markers of metabolic bone disease, such as intact parathyroid hormone, total and bone-specific alkaline phosphatase, or measures of bone mineral content by pQCT.
Table 1.
General clinical characteristics of the study population stratified by tertiles of serum osteoprotegerin
| Characteristic, Mean (±SD) or n (%) | Tertile 1 (1.21 to 5.03 pmol/L), n = 117 | Tertile 2 (5.05 to 7.45 pmol/L), n = 117 | Tertile 3 (7.46 to 22.31 pmol/L), n = 117 | Pa |
|---|---|---|---|---|
| Demographics | ||||
| age (years) | 56.4 (±11.6) | 60.2 (±9.3) | 63.6 (±9.1) | <0.01 |
| female gender | 29 (24.8) | 31 (26.5) | 47 (40.2) | 0.02 |
| black race | 56 (47.9) | 70 (59.8) | 73 (62.4) | 0.06 |
| Comorbid conditions | ||||
| diabetes | 44 (37.6) | 66 (56.4) | 73 (62.4) | <0.01 |
| cardiovascular disease | 26 (22.2) | 44 (37.6) | 53 (45.3) | <0.01 |
| hypertension | 99 (84.6) | 99 (84.6) | 110 (94.0) | 0.04 |
| current/former smoker | 52 (44.4) | 65 (55.6) | 69 (59.0) | 0.07 |
| Examination | ||||
| body mass index (kg/m2) | 31.7 (±7.0) | 31.9 (±7.2) | 30.9 (±6.2) | 0.54 |
| waist circumference (cm) | 107.5 (±17.1) | 107.8 (±17.3) | 105.2 (±16.9) | 0.44 |
| systolic BP (mmHg) | 124 (±21) | 131 (±21) | 136 (±21) | <0.01 |
| diastolic BP (mmHg) | 75 (±15) | 76 (±14) | 73 (±14) | 0.43 |
| aortic pulse wave velocity (m/s)b,c | 8.0 (7.0, 9.8) | 9.4 (7.9, 11.9) | 10.6 (9.0, 12.2) | <0.01 |
| Laboratory | ||||
| albuminuria | ||||
| <30 mg/day | 50 (52.1) | 40 (41.7) | 45 (45.0) | |
| 30 to 299 mg/day | 24 (25.0) | 31 (32.3) | 27 (27.0) | 0.62 |
| ≥300 mg/day | 22 (22.9) | 25 (26.0) | 28 (28.0) | |
| eGFR (ml/min per 1.73 m2) | 51.5 (±13.9) | 48.3 (±13.6) | 42.8 (±13.5) | <0.01 |
| serum albumin (g/dl) | 4.0 (±0.37) | 3.9 (±0.40) | 3.8 (±0.36) | <0.01 |
| hs-CRP (mg/L)b | 2.2 (1.0, 6.1) | 2.5 (0.9, 5.5) | 2.4 (1.0, 6.8) | 0.63 |
| Medications | ||||
| ACE inhibitors | 61 (52.6) | 57 (49.6) | 51 (44.0) | 0.41 |
| lipid-lowering medications | 62 (53.5) | 76 (66.1) | 73 (62.9) | 0.12 |
eGFR, estimated GFR; hs-CRP, high-sensitivity C-reactive protein; ACE, angiotensin-converting enzyme.
P value using ANOVA (continuous variables) or Pearson's chi-squared test (categorical variables).
Values presented as median (interquartile range). P-values calculated from log-transformed variable.
Among n = 226 with concurrent pulse wave velocity available.
Table 2.
Chronic Kidney Disease Mineral and Bone Disorder (CKD-MBD)–related characteristics of the study population stratified by tertiles of serum osteoprotegerin
| Characteristic, Mean (±SD) or n (%) | Tertile 1 (1.21 to 5.03 pmol/L), n = 117 | Tertile 2 (5.05 to 7.45 pmol/L), n = 117 | Tertile 3 (7.46 to 22.31 pmol/L), n = 117 | Pa |
|---|---|---|---|---|
| Laboratory | ||||
| serum phosphate (mg/dl) | 3.4 (±0.55) | 3.5 (±0.64) | 3.6 (±0.57) | 0.05 |
| corrected serum calcium (mg/dl) | 9.2 (±0.39) | 9.1 (±0.46) | 9.2 (±0.43) | 0.54 |
| intact PTH ≥66 pg/ml | 33 (29.2) | 41 (36.6) | 40 (35.1) | 0.46 |
| intact PTH above KDOQI guideline | 27 (23.9) | 33 (29.5) | 34 (29.8) | 0.54 |
| bone-specific alkaline phosphatase (U/L)b | 28 (22, 36) | 29 (20, 40) | 27 (21, 35) | 0.18 |
| total alkaline phosphatase (U/L)b | 78 (68, 92) | 81 (65, 106) | 81 (68, 102) | 0.23 |
| Bone structure by pQCT | ||||
| cortical bone mineral content (g)c | 409.7 (±81.7) | 406.7 (±82.3) | 387.9 (±82.3) | 0.14 |
| Medications | ||||
| phosphorus binders | 9 (7.8) | 15 (13.0) | 19 (16.4) | 0.13 |
| active vitamin D sterols | 0 (0) | 4 (3.5) | 9 (7.8) | <0.01 |
PTH, parathyroid hormone; KDOQI, Kidney Disease Outcomes Quality Initiative; pQCT, peripheral quantitative computed tomography.
P value using ANOVA (continuous variables) or Pearson's chi-squared test (categorical variables).
Values presented as median (interquartile range). P-values calculated from log-transformed variable.
280 of 351 participants had cortical bone mineral content available.
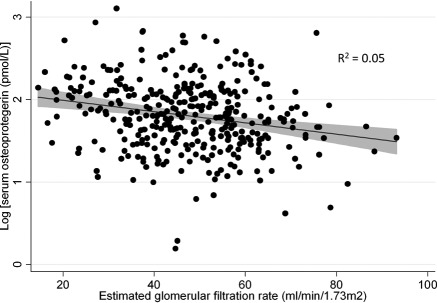
Table 3 presents adjusted associations between serum OPG and other clinical measures. After adjustment for age, gender, and race, serum OPG levels were not associated with clinical measures of CKD-MBD. Figure 1 presents the unadjusted association between eGFR and log-transformed serum OPG, demonstrating progressively higher serum OPG with lower eGFR. In multivariable models including demographics and vascular risk factors (age, gender, race, diabetes, cardiovascular disease, hypertension, systolic BP, former/current smoking, eGFR, and serum albumin), serum OPG levels remained strongly associated with lower eGFR, and also older age, female gender, greater systolic BP, and lower serum albumin (Table 3).
Table 3.
Association between clinical measurements and percentage difference in serum osteoprotegerin levels
| Percentage Difference (95% CI) | P | |
|---|---|---|
| Chronic Kidney Disease-Mineral and Bone Disorder measuresa | ||
| serum phosphate (per 1 mg/dl) | 6.5% (−1.5%, 15%) | 0.11 |
| corrected serum calcium (per 1 mg/dl) | −1.7% (−11.2%, 8.8%) | 0.74 |
| calcium X phosphorus product | 0.2% (−0.6%, 1.0%) | 0.58 |
| intact PTH ≥66 pg/ml | 3.4% (−6.1%, 13.9%) | 0.49 |
| intact PTH above KDOQI guidelines | 5.4% (−4.8%, 16.6%) | 0.31 |
| total alkaline phosphatase (per 10 U/L) | 1.5% (0%, 3.1%) | 0.05 |
| bone-specific alkaline phosphatase (per 10 U/L) | −1.5% (−4.5%, 1.5%) | 0.32 |
| cortical bone mineral content (per 50 g) | 1.6% (−2.5%, 5.9%) | 0.45 |
| Demographics and vascular risk factorsb | ||
| age (per 10 years) | 7.1% (2.6%, 11.8%) | <0.01 |
| female gender | 10.2% (0.2%, 21.3%) | 0.05 |
| black race | 1.0% (−7.7%, 10.5%) | 0.83 |
| diabetes | 8.1% (−0.8%, 17.8%) | 0.08 |
| cardiovascular disease | 9.3% (−0.1%, 19.6%) | 0.05 |
| hypertension | 5.2% (−8.2%, 20.5%) | 0.46 |
| current/former smoker | 4.3% (−4.5%, 14.0%) | 0.35 |
| systolic BP (per 10 mmHg) | 2.7% (0.6%, 4.9%) | 0.01 |
| eGFR (per 10 ml/min per 1.73 m2) | −3.9% (−6.8%, −0.8%) | 0.01 |
| serum albumin (per 1 mg/dl) | −17.0% (−25.8%, −7.2%) | <0.01 |
PTH, parathyroid hormone; KDOQI, Kidney Disease Outcomes Quality Initiative; eGFR, estimated GFR.
Percentage difference values were adjusted for age, gender, and race.
Percentage difference values were adjusted for all other demographics and vascular risk factors.
Figure 1.
Unadjusted scatterplot of estimated GFR and log(serum osteoprotegerin) (n = 351) with fitted regression line. Shaded area represents 95% confidence interval.
Among the 351 individuals in the study, 226 (64%) had a concurrent aortic pulse wave velocity measurement available and were included in analyses of the association between OPG and pulse wave velocity. Aortic pulse wave velocity ranged from 4.2 to 20.8 m/s with a median of 9.3 m/s. Participants who did not have a concurrent pulse wave velocity performed were less likely to have cardiovascular disease (45% versus 55%; P = 0.004) and had lower eGFR (45.1 versus 48.9 ml/min per 1.73 m2; P = 0.02), but otherwise had similar clinical characteristics to those of the full study population.
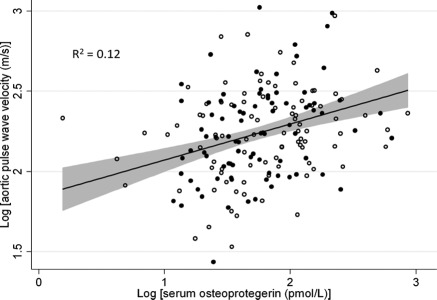
With use of linear regression, higher serum OPG was associated with faster pulse wave velocity (P < 0.001). Furthermore, the association was not modified by categories of bone mineral content (P = 0.52; Figure 2). Increasing tertiles of serum OPG were associated with faster pulse wave velocity (P = 0.001), with a 30% higher aortic pulse wave velocity seen in patients in the highest tertile of serum OPG compared with the lowest (P < 0.001). The association between increasing tertiles of serum OPG and the relative increase in aortic pulse wave velocity compared with the lowest tertile is shown in Table 4 in models adjusted for demographics and traditional vascular risk factors, and then further adjusted for nontraditional risk factors and cortical bone mineral content by pQCT. The association between serum OPG and aortic pulse wave velocity persisted despite these adjustments (P = 0.04). In the fully adjusted model, participants with the highest serum OPG levels had a 10% higher aortic pulse wave velocity compared with those with the lowest levels (P = 0.04). There was no evidence of interactions between serum OPG levels and gender (P = 0.44), race (P = 0.42), diabetes (P = 0.87), secondary hyperparathyroidism (intact parathyroid hormone ≥66 pg/ml; P = 0.60), cortical bone mineral content above versus below the median (P = 0.31), or C-reactive protein levels above versus below the median (P = 0.30). Results were unchanged in a sensitivity analysis including only those participants whose measurements of serum OPG and aortic pulse wave velocity occurred within 3 months of each other (n = 214; data not shown).
Figure 2.
Unadjusted scatterplot of log(serum osteoprotegerin) and log(aortic pulse wave velocity) (n = 226) with fitted regression line. Shaded area represents 95% confidence interval. Open circles represent participants with cortical bone mineral content below the median and closed circles represent participants with cortical bone mineral content above the median.
Table 4.
Ratio of pulse wave velocity compared with reference tertile (95% confidence interval) by tertiles of serum osteoprotegerin (n = 226)
| Modela | Tertile 1 (1.21 to 5.03 pmol/L) | Tertile 2 (5.05 to 7.45 pmol/L) | Tertile 3 (7.46 to 22.31 pmol/L) | P Trend |
|---|---|---|---|---|
| Unadjusted | Refc | 1.19d (1.10, 1.30) | 1.30d (1.20, 1.42) | <0.001 |
| Traditional vascular risk factor adjusted | Refc | 1.09d (1.01, 1.17) | 1.15d (1.06, 1.25) | 0.001 |
| Traditional/nontraditional risk factor adjusted | Refc | 1.06 (0.98, 1.14) | 1.11d (1.02, 1.20) | 0.01 |
| Cortical bone mineral content adjustedb | Refc | 1.06 (0.97, 1.15) | 1.10d (1.01, 1.20) | 0.04 |
Traditional vascular risk factor adjusted model adjusted for age, gender, race, diabetes, history of cardiovascular disease, history of hypertension, current/former smoking, and systolic blood pressure; traditional/nontraditional risk factor adjusted model adjusted for traditional risk factors above plus estimated GFR, albuminuria, serum albumin, log(C-reactive protein), serum phosphate, corrected serum calcium, and presence of secondary hyperparathyroidism.
Cortical bone mineral content adjusted model adjusted for traditional/nontraditional risk factors above plus and cortical bone mineral content in the subset with quantitative computed tomography available (n = 161).
Note reference = 1.0.
P < 0.05.
Discussion
In a cohort of patients with CKD, we demonstrated a graded association between serum OPG levels and aortic stiffness that was independent of eGFR, clinical measures of CKD-MBD, and markers of generalized inflammation. This association did not differ by categories of bone mineral content or by the presence or absence of secondary hyperparathyroidism. We observed a 10% higher aortic pulse wave velocity between the lowest and highest tertile of serum OPG, which corresponds to an absolute difference in aortic pulse wave velocity of about 0.7 to 1 m/s, on average, in this study population. Previous work in patients with kidney disease has demonstrated a 39% increase in odds of mortality for each 1 m/s increase in pulse wave velocity (28), suggesting that this is a clinically meaningful difference.
In this study, we used pQCT in the tibia diaphysis to assess bone mineral content. Measuring bone with pQCT is superior to measuring with dual-energy x-ray absorptiometry (DXA) because of its ability to differentiate cortical from trabecular bone and lack of artifact from overlying vascular calcification. Additionally, a cortical site was chosen because cortical bone is particularly sensitive to the effects of hyperparathyroidism (29,30). We found no evidence for a relationship between serum OPG and measures of bone mineral content, bone turnover, or mineral metabolism abnormalities. Additionally, there was no evidence that cortical bone mass above or below median values modified the association of serum OPG with aortic stiffness. We believe these findings argue against the hypothesis that the relationship between OPG and pulse wave velocity is secondary to effects of circulating OPG levels on bone resorption, but instead argues for a direct role in the vascular wall.
Our findings are consistent with other human studies that have demonstrated associations between higher serum OPG levels and various adverse cardiovascular outcomes (14,15,17,20,31,32). These findings in humans are in contrast to studies in OPG-deficient mice who exhibit diffuse medial calcification of the aorta and renal arteries because of low OPG levels (6). Several possibilities may explain this discrepancy. It is possible that the medial calcification present in OPG-deficient mice is secondary to the severe osteoporotic phenotype and not a direct result of OPG deficiency on the vasculature. It is also possible that OPG is a calcification inhibitor, as suggested by animal models, and acts as a marker of underlying vascular disease. In several studies, treatment with either recombinant OPG or the RANKL inhibitor, denosumab, inhibited or regressed the development of vascular calcification (4,6,7,33–35). Under this scenario, OPG levels may rise in patients with vascular disease as a compensatory response to mitigate further injury, by inhibiting a remodeling process in the vascular wall that resembles bone formation (4,7). Additionally, OPG is known to bind and neutralize the proapoptotic factor TNF-related apoptosis inducing ligand (TRAIL) (36). As apoptotic debris is a possible nucleus for hydroxyapatite deposition in the vascular wall, serum OPG expression in the endothelium may be upregulated to counteract apoptosis (37). Finally, it is also possible that OPG is a noncausal bystander in the process of medial calcification. Recent work has demonstrated that medial arterial calcification is an active process whereby vascular smooth muscle cells differentiate into osteoblast-like cells which express bone-specific proteins and actively deposit bone matrix (38). OPG, which is normally secreted by osteoblasts, may also be secreted by these osteoblast-like cells nonspecifically, making it a potent biomarker of this remodeling process. Because of the cross-sectional nature of our study, we cannot determine the temporal sequence of the rise in serum OPG and the development of arterial stiffness. For this reason we cannot distinguish between the possibilities that serum OPG levels rise in response to the development of arterial stiffness versus that elevated levels of serum OPG precede the development of arterial stiffness.
Previous cross-sectional work has also demonstrated that serum OPG levels are associated with aortic pulse wave velocity (21,32,39–42). However, in contrast to these studies, we have collected extensive phenotypic information about the study participants including careful measures of vascular risk factors and, most importantly, of bone mineral content. Our study extends these findings by evaluating these factors simultaneously, and implies that these associations may not be secondary to effects of circulating OPG on bone mass or the confounding effects of other strong vascular risk factors.
In interpreting these study results, it is important to recognize several limitations. Although we have carefully collected several measures of CKD-MBD, definitive diagnosis of renal osteodystrophy requires bone histomorphometry which was not available in this study. Additionally, some of these measurements were not available in the full study population, limiting our power to detect associations. Finally, this study was cross-sectional and not able to evaluate the association between serum OPG and longitudinal changes in measures of CKD-MBD or pulse wave velocity.
In conclusion, our data support a strong relationship between serum OPG and arterial stiffness, a major risk factor for mortality in patients with CKD. In addition, it is independent of many potential confounders including traditional cardiovascular risk factors, measures of CKD-MBD, and inflammation. Circulating levels of OPG were not associated with clinical measures of CKD-MBD, suggesting that circulating levels may not be indicative of active bone resorption. Given that serum levels of OPG are higher among those with lower eGFR, the OPG signaling pathway may be involved in the vascular disease associated with CKD independent of CKD-MBD.
Disclosures
S.E.R. and R.S.P. receive research funding from Abbott Laboratories to examine vascular calcification in kidney disease. S.L.S. receives grant funding from Amgen.
Acknowledgments
These studies were supported by NIH/NIDDK grants R01-DK-067390, U01-DK-060984, R01 DK064966, and K24 DK076808 and NIH/NCRR grant UL1-RR024134. Additionally, J.J.S. was supported by NIH grants T32 DK 00732–14 and 5KL2RR025006. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. This work was presented in abstract form at the 50th Cardiovascular Disease Epidemiology and Prevention Meeting of the American Heart Association from March 2, 2010 to March 5, 2010.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ: Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 89: 309–319, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Collin-Osdoby P: Regulation of vascular calcification by osteoclast regulatory factors RANKL and osteoprotegerin. Circ Res 95: 1046–1057, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ: Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93: 165–176, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Morony S, Tintut Y, Zhang Z, Cattley RC, Van G, Dwyer D, Stolina M, Kostenuik PJ, Demer LL: Osteoprotegerin inhibits vascular calcification without affecting atherosclerosis in ldlr(/) Mice. Circulation 117: 411–420, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zauli G, Corallini F, Bossi F, Fischetti F, Durigutto P, Celeghini C, Tedesco F, Secchiero P: Osteoprotegerin increases leukocyte adhesion to endothelial cells both in vitro and in vivo. Blood 110: 536–543, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS: Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Development 12: 1260–1268, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Min H, Morony S, Sarosi I, Dunstan CR, Capparelli C, Scully S, Van G, Kaufman S, Kostenuik PJ, Lacey DL, Boyle WJ, Simonet WS: Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J Exp Med 192: 463–474, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goodman W, London GM, Amann K, Block GA, Giachelli CM, Hruska KA, Ketteler M, Levin A, Massy Z, McCarron DA, Raggi P, Shanahan CM, Yorioka N: Vascular calcification in chronic kidney disease. Am J Kidney Dis 43: 572–579, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Moe SM, Drueke T: Improving global outcomes in mineral and bone disorders. Clin J Am Soc Nephrol 3: S127–S130, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moe SM, Duan D, Doehle BP, O'Neill KD, Chen NX: Uremia induces the osteoblast differentiation factor Cbfa1 in human blood vessels. Kidney Int 63: 1003–1011, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Dart AM, Kingwell BA: Pulse pressure–a review of mechanisms and clinical relevance. J Am Coll Cardiol 37: 975–984, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Pannier B, Guerin AP, Marchais SJ, Safar ME, London GM: Stiffness of capacitive and conduit arteries: Prognostic significance for end-stage renal disease patients. Hypertension 45: 592–596, 2005 [DOI] [PubMed] [Google Scholar]
- 13. London GM, Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME: Arterial wave reflections and survival in end-stage renal failure. Hypertension 38: 434–438, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Anand DV, Lahiri A, Lim E, Hopkins D, Corder R: The relationship between plasma osteoprotegerin levels and coronary artery calcification in uncomplicated type 2 diabetic subjects. J Am Coll Cardiol 47: 1850–1857, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Jono S, Otsuki S, Higashikuni Y, Shioi A, Mori K, Hara K, Hashimoto H, Ikari Y: Serum osteoprotegerin levels and long-term prognosis in subjects with stable coronary artery disease. J Thrombosis Haemostasis 8: 1170–1175, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Browner WS, Lui L-Y, Cummings SR: Associations of serum osteoprotegerin levels with diabetes, stroke, bone density, fractures, and mortality in elderly women. J Clin Endocrinol Metab 86: 631–637, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Kiechl S, Schett G, Wenning G, Redlich K, Oberhollenzer M, Mayr A, Santer P, Smolen J, Poewe W, Willeit J: Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation 109: 2175–2180, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Abedin M, Omland T, Ueland T, Khera A, Aukrust P, Murphy SA, Jain T, Gruntmanis U, McGuire DK, de Lemos JA: Relation of osteoprotegerin to coronary calcium and aortic plaque (from the Dallas Heart Study). Am J Cardiol 99: 513–518, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Omland T, Drazner MH, Ueland T, Abedin M, Murphy SA, Aukrust P, de Lemos JA: Plasma osteoprotegerin levels in the general population: Relation to indices of left ventricular structure and function. Hypertension 49: 1392–1398, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Semb AG, Ueland T, Aukrust P, Wareham NJ, Luben R, Gullestad L, Kastelein JJP, Khaw K-T, Boekholdt SM: Osteoprotegerin and soluble receptor activator of nuclear factor-{kappa}B ligand and risk for coronary events: A nested case-control approach in the prospective EPIC-Norfolk Population Study 1993–2003. Arterioscler Thromb Vasc Biol 29: 975–980, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Nakashima A, Carrero JJ, Qureshi AR, Hirai T, Takasugi N, Ueno T, Taniguchi Y, Lindholm B, Yorioka N: Plasma osteoprotegerin, arterial stiffness, and mortality in normoalbuminemic Japanese hemodialysis patients. Osteoporos Int 22: 1695–1701, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Morena M, Terrier N, Jaussent I, Leray-Moragues H, Chalabi L, Rivory J-P, Maurice F, Delcourt C, Cristol J-P, Canaud B, Dupuy A-M: Plasma osteoprotegerin is associated with mortality in hemodialysis patients. J Am Soc Nephrol 17: 262–270, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Sigrist MK, Levin A, Er L, McIntyre CW: Elevated osteoprotegerin is associated with all-cause mortality in CKD stage 4 and 5 patients in addition to vascular calcification. Nephrol Dial Transplant 24: 3157–3162, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu C-y, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER, III, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT: The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and methods. J Am Soc Nephrol 14: S148–S153, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Townsend RR, Wimmer NJ, Chirinos JA, Parsa A, Weir M, Perumal K, Lash JP, Chen J, Steigerwalt SP, Flack J, Go AS, Rafey M, Rahman M, Sheridan A, Gadegbeku CA, Robinson NA, Joffe M: Aortic PWV in chronic kidney disease: A CRIC ancillary study. Am J Hypertens 23: 282–289, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eknoyan G, Levin A, Levin NW: Bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42: 1–201, 2003. 12500213 [Google Scholar]
- 27. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F: Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM: Impact of aortic stiffness on survival in end-stage renal disease. Circulation 99: 2434–2439, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Nickolas TL, Leonard MB, Shane E: Chronic kidney disease and bone fracture: A growing concern. Kidney Int 74: 721–731, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jamal SA, Gilbert J, Gordon C, Bauer DC: Cortical PQCT measures are associated with fractures in dialysis patients. J Bone Mineral Res 21: 543–548, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Lieb W, Gona P, Larson MG, Massaro JM, Lipinska I, Keaney JF, Jr., Rong J, Corey D, Hoffmann U, Fox CS, Vasan RS, Benjamin EJ, O'Donnell CJ, Kathiresan S: Biomarkers of the osteoprotegerin pathway: Clinical correlates, subclinical disease, incident cardiovascular disease, and mortality. Arterioscler Thromb Vasc Biol 30: 1849–1854, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zagura M, Serg M, Kampus P, Zilmer M, Zilmer K, Eha J, Unt E, Lieberg J, Kals J: Association of osteoprotegerin with aortic stiffness in patients with symptomatic peripheral artery disease and in healthy subjects. Am J Hypertens 23: 586–591, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Helas S, Goettsch C, Schoppet M, Zeitz U, Hempel U, Morawietz H, Kostenuik PJ, Erben RG, Hofbauer LC: Inhibition of receptor activator of NF-{kappa}B ligand by denosumab attenuates vascular calcium deposition in mice. Am J Pathol 175: 473–478, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Price PA, June HH, Buckley JR, Williamson MK: Osteoprotegerin inhibits artery calcification induced by warfarin and by vitamin D. Arterioscler Thromb Vasc Biol 21: 1610–1616, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Panizo S, Cardus A, Encinas M, Parisi E, Valcheva P, Lopez-Ongil S, Coll B, Fernandez E, Valdivielso JM: RANKL increases vascular smooth muscle cell calcification through a RANK-BMP4-dependent pathway. Circ Res 104: 1041–1048, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Vitovski S, Phillips JS, Sayers J, Croucher PI: Investigating the interaction between osteoprotegerin and receptor activator of NF-kappa B or tumor necrosis factor-related apoptosis-inducing ligand. J Biol Chem 282: 31601–31609, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Shroff RC, McNair R, Figg N, Skepper JN, Schurgers L, Gupta A, Hiorns M, Donald AE, Deanfield J, Rees L, Shanahan CM: Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation 118: 1748–1757, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Jono S, Shioi A, Ikari Y, Nishizawa Y: Vascular calcification in chronic kidney disease. J Bone Mineral Metab 24: 176–181, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Schnabel R, Larson MG, Dupuis J, Lunetta KL, Lipinska I, Meigs JB, Yin X, Rong J, Vita JA, Newton-Cheh C, Levy D, Keaney JF, Jr., Vasan RS, Mitchell GF, Benjamin EJ: Relations of inflammatory biomarkers and common genetic variants with arterial stiffness and wave reflection. Hypertension 51: 1651–1657, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Frost M, Grella R, Millasseau S, Jiang B-y, Hampson G, Fogelman I, Chowienczyk P: Relationship of calcification of atherosclerotic plaque and arterial stiffness to bone mineral density and osteoprotegerin in postmenopausal women referred for osteoporosis screening. Calcified Tissue Int 83: 112–120, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Shroff RC, Shah V, Hiorns MP, Schoppet M, Hofbauer LC, Hawa G, Schurgers LJ, Singhal A, Merryweather I, Brogan P, Shanahan C, Deanfield J, Rees L: The circulating calcification inhibitors, fetuin-A and osteoprotegerin, but not Matrix Gla protein, are associated with vascular stiffness and calcification in children on dialysis. Nephrol Dial Transplant 23: 3263–3271, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Speer G, Fekete BC, El Hadj Othmane T, Szabo T, Egresits J, Fodor E, Kiss I, Logan AG, Nemcsik J, Szabo A, Nemeth ZK, Szathmari M, Tisler A: Serum osteoprotegerin level, carotid-femoral pulse wave velocity and cardiovascular survival in haemodialysis patients. Nephrol Dial Transplant 23: 3256–3262, 2008 [DOI] [PubMed] [Google Scholar]