Abstract
Summary
Background and objectives
Fibroblast growth factor 23 (FGF23) is an independent risk factor for mortality in patients with ESRD. Before FGF23 testing can be integrated into clinical practice of ESRD, further understanding of its determinants is needed.
Design, setting, participants, & measurements
In a study of 67 adults undergoing peritoneal dialysis, we tested the hypothesis that longer dialysis vintage and lower residual renal function and renal phosphate clearance are associated with higher FGF23. We also compared the monthly variability of FGF23 versus parathyroid hormone (PTH) and serum phosphate.
Results
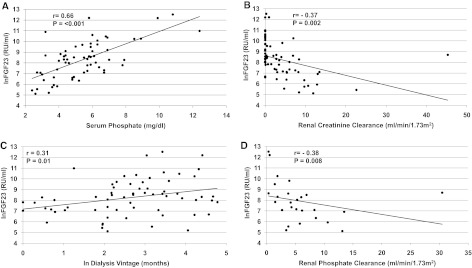
In unadjusted analyses, FGF23 correlated with serum phosphate (r = 0.66, P < 0.001), residual renal function (r = −0.37, P = 0.002), dialysis vintage (r = 0.31, P = 0.01), and renal phosphate clearance (r = −0.38, P = 0.008). In adjusted analyses, absence of residual renal function and greater dialysis vintage associated with higher FGF23, independent of demographics, laboratory values, peritoneal dialysis modality and adequacy, and treatment with vitamin D analogs and phosphate binders. Urinary and dialysate FGF23 clearances were minimal. In three serial monthly measurements, within-subject variability accounted for only 10% of total FGF23 variability compared with 50% for PTH and 60% for serum phosphate.
Conclusions
Increased serum phosphate, loss of residual renal function, longer dialysis vintage, and lower renal phosphate clearance are associated with elevated FGF23 levels in ESRD patients undergoing peritoneal dialysis. FGF23 may be a more stable marker of phosphate metabolism in ESRD than PTH or serum phosphate.
Introduction
Disordered phosphorus metabolism is a common complication of kidney disease that contributes to the development of arterial calcification, myocardial hypertrophy, and endothelial dysfunction (1–3). Multiple observational studies have reported an independent association between hyperphosphatemia and mortality in patients with chronic kidney disease (CKD) and in the general population (4–6). However, isolated serum phosphate levels provide an imprecise assessment of disordered phosphorus metabolism because serum phosphate levels exhibit circadian and postprandial excursions of up to 1.0 mg/dl (7,8). Similar diurnal fluctuations have been reported in patients undergoing dialysis (9). As a result, lower serum phosphate levels measured during daily nadirs can belie more severe derangements in overall phosphorus balance and thus, likely underestimate the actual risk attributable to disordered phosphorus metabolism. Translating observations of phosphate-related risk of cardiovascular disease and mortality into improved patient care requires more sensitive tools for risk stratification than the serum phosphate.
Emerging data suggest that combining serum phosphate levels with measurements of its primary hormonal regulator, fibroblast growth factor 23 (FGF23), may enhance assessment of phosphorus-related cardiovascular risk. FGF23 levels increase early in the course of CKD and help maintain normal serum phosphate levels despite reduced renal function (10). Unlike parathyroid hormone (PTH), which is also involved in regulating serum phosphate but which fluctuates diurnally, in the context of meals, and acutely in response to changes in serum calcium, FGF23 levels exhibit minimal circadian and postprandial variation even in CKD (8,11–13). Prospective studies found elevated FGF23 to independently associate with mortality in incident hemodialysis patients, kidney transplant recipients, CKD stage 2 to 4, and individuals with a history of coronary artery disease (14–17). Furthermore, when directly compared with phosphate and PTH, FGF23 was the strongest predictor of adverse outcomes (14,15,17). These data highlight the potential of FGF23 as a sensitive biomarker to help clinicians detect the presence of disordered phosphorus metabolism and perhaps, to more effectively discriminate risk of related adverse outcomes.
Before FGF23 can transition from research tool to biomarker for use in mainstream management of patients with ESRD, determinants of its marked interindividual variation in ESRD must be characterized. FGF23 levels in this setting are often the highest encountered in clinical practice, and levels can range from 10- to 1000-fold above the normal range. Chronic peritoneal dialysis is preferable to hemodialysis as a model to study determinants of FGF23 levels in ESRD because it is a form of renal replacement therapy in which a steady state is achieved. In addition, patients undergoing peritoneal dialysis often maintain some amount of residual renal function that is systematically quantified on a quarterly basis as part of standard care. Therefore, we used the peritoneal dialysis model to test our hypotheses that FGF23 levels would be greater in patients with longer dialysis vintage, reduced or absent residual renal function, and lower phosphate clearance and that short-term serial FGF23 measurements would exhibit less variability within individual patients over time than contemporaneous measurements of phosphate or PTH.
Materials and Methods
Study Population
We studied 67 consecutive patients aged 18 years or older who had been undergoing chronic peritoneal dialysis (continuous ambulatory peritoneal dialysis [CAPD] or continuous cyclic peritoneal dialysis) for the treatment of ESRD for at least 3 months at three participating institutions: University of Miami, Massachusetts General Hospital, and Vanderbilt University Medical Center. All willing participants were included; there were no exclusion criteria. The study protocol was approved by the human research committee at each institution, and all participants provided written informed consent.
Procedures
The study consisted of three or more consecutive monthly visits, which coincided with routine follow-up visits at the participating peritoneal dialysis clinics. Dialysate calcium prescription remained unchanged, and use of active vitamin D analogs, cinacalcet, and oral phosphate binders remained the same during the 3-month study period. The only changes to therapy that took place were switches in phosphate binder class in a minority of patients. We collected blood samples at all visits, and during the quarterly visits when dialysis adequacy was measured, we collected urine and dialysate samples from the 24-hour collections. A subset of 29 patients completed a single 4-day food record to estimate average daily dietary intake of phosphorus as analyzed by Nutrition Data System for Research, developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis, Minnesota (18).
Assays
Routine laboratory testing of blood, dialysate, and 24-hour urinary samples was performed by local laboratories using standard procedures. A central laboratory at the University of Miami measured urinary and dialysate concentrations of phosphate, plasma PTH, and FGF23. In an exploratory analysis, we also measured FGF23 in urine and dialysate samples in a subset of 10 participants with residual renal function who were selected based on their plasma FGF23 to be representative of the spectrum of blood levels in the study sample. Dialysate and 24-hour urinary phosphate concentrations were measured using standard autoanalyzers. We measured intact plasma PTH using a chemiluminescent immunoassay (Roche Diagnostics, Indianapolis, Indiana; coefficient of variation <3%) and measured plasma FGF23 using a second-generation C-terminal assay (Immutopics, San Clemente, California) in duplicate with a mean intra-assay coefficient of variation <5%. The upper limit of detection for this assay is 1500 reference units (RU)/ ml; samples greater than 1500 RU/ml required serial dilutions to obtain a measurement. We measured urinary and dialysate FGF23 levels using the same assay and methods. To ascertain whether pretest handling would affect stability of FGF23 and PTH measurements, we compared FGF23 measurements before and after storage and shipping and compared PTH measurements obtained locally within the context of clinical care with measurements obtained at a central research laboratory after storage and shipping. Both analyses yielded high correlation (>0.7), indicating that both PTH and FGF23 tests were minimally affected by pretest handling.
Calculations
We measured parameters of dialysis adequacy, including weekly total, renal and peritoneal Kt/V, and creatinine clearance, using standard methods (19). We calculated normalized protein equivalent nitrogen appearance (nPNA) normalized to body weight (20). We analyzed renal creatinine clearance (in ml/min per 1.73 m2) as the measure of residual renal function. Presence of residual renal function was defined as renal creatinine clearance greater than zero. Anuric patients were defined as having zero residual renal function.
We quantified the daily phosphate removal by dialysis or urinary excretion (mg/d) as follows:
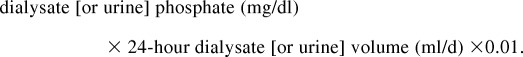 |
Total daily phosphate removal equaled the sum of daily peritoneal and renal phosphate removal.
Peritoneal and renal phosphate clearances (L/d per 1.73 m2) were calculated as follows:
 |
Total phosphate clearance equaled the sum of peritoneal and renal phosphate clearance.
In the subset of 10 participants for whom dialysate and urine FGF23 levels were measured, we calculated peritoneal and renal FGF23 clearances (ml/min/1.73 m2) as follows:
 |
Statistical Analysis
We compared laboratory and clinical characteristics in patients with and without residual renal function using t test, Wilcoxon, or chi-squared tests, as appropriate. The distributions of FGF23, PTH, and dialysis vintage were right-skewed, requiring natural log (ln)-transformation. To assess the univariate relationships of serum phosphate, residual renal function, dialysis vintage, and phosphate clearance with lnFGF23, we examined scatter plots and Pearson's correlations. To determine if these associations were independent of demographics, laboratory values, peritoneal dialysis modality (CAPD or continuous cyclic peritoneal dialysis) and adequacy (Kt/V), and use of active vitamin D and phosphate binders, we used separate linear regression models with lnFGF23 as the dependent variable and residual renal function (dichotomous variable) and dialysis vintage (naturally log-transformed) as primary predictors. Next, we examined the within-subject range of variation for serum phosphate, PTH, and FGF23. For each marker, we calculated the intraclass correlation from estimates of between-subject (σ2b) and within-subject variance (σ2w), derived from mixed linear models, using the following formula: σ2b/(σ2b + σ2w) (21). P values <0.05 were considered significant. Analyses were performed with SAS 9.2 (SAS Institute, Cary, North Carolina).
Results
Demographic, Clinical, and Laboratory Characteristics by Residual Renal Function
The study population consisted of 67 patients with mean (±SD) age of 47 ± 14 years; 52% were men, 15% were black, and 51% were Hispanic. Hypertension and diabetes were present in 84% and 31% of participants, respectively. Active vitamin D and phosphate binders were being used by approximately 85% of participants. Residual renal function was present in 64% of participants, and the median dialysis vintage was 15 months. Differences in demographic, clinical, and laboratory characteristics between participants with (n = 43) and without (n = 24) residual renal function are shown in Table 1. Patients with residual renal function had lower dialysis vintage and serum phosphate levels and used phosphate binders less frequently than anuric patients. Dietary phosphorus intake estimates trended higher in the patients with residual renal function, and the nPNA was significantly higher in this group compared with anuric patients. Despite greater total phosphate removal, the median FGF23 levels were 3.7-fold higher in patients without compared with those with residual renal function. PTH levels and use of active vitamin D analogs did not differ significantly between the groups.
Table 1.
Demographic, clinical, and laboratory characteristics by residual renal function
| With RRF (n = 43) | Without RRF (n = 24) | P | |
|---|---|---|---|
| Demographics | |||
| age (years)a | 49 ± 13 | 44 ± 15 | 0.2 |
| male (%) | 56 | 46 | 0.4 |
| black (%) | 9 | 25 | 0.1 |
| Hispanic (%) | 49 | 54 | 0.7 |
| Clinical data | |||
| current smoker (%) | 16 | 8 | 0.6 |
| body mass indexab | 27 ± 5 | 28 ± 6 | 0.8 |
| systolic BP (mmHg)a | 128 ± 18 | 128 ± 24 | 0.9 |
| hypertension (%) | 85 | 82 | 0.8 |
| diabetes (%) | 34 | 25 | 0.5 |
| etiology of ESRD | 0.5 | ||
| diabetes (%) | 28 | 21 | |
| hypertension (%) | 8 | 10 | |
| glomerulonephritis (%) | 10 | 5 | |
| renovascular (%) | 3 | 16 | |
| polycystic kidney disease (%) | 10 | 5 | |
| other (%) | 41 | 42 | |
| 24-hour urine volume (ml)c | 800 (300 to 1450) | 0 | — |
| Peritoneal dialysis vintage (months)c | 9 (3 to 28) | 23 (14 to 69) | <0.01 |
| Continuous cycling peritoneal dialysis (%) | 51 | 83 | 0.01 |
| Laboratory values | |||
| FGF23 (RU/ml)c | 2381 (1010 to 5486) | 8895 (3602 to 30,071) | <0.01 |
| albumin (g/dl)a | 4.0 ± 0.3 | 3.8 ± 0.5 | 0.2 |
| calcium (mg/dl)a | 9.1 ± 0.7 | 9.0 ± 0.7 | 0.5 |
| phosphate (mg/dl)c | 4.8 (3.4 to 5.9) | 5.8 (4.5 to 6.8) | 0.03 |
| PTH (pg/ml)c | 241 (77 to 364) | 252 (122 to 575) | 0.3 |
| Indices of dialysis adequacy | |||
| dialysate weekly (Kt/V)a | 1.6 ± 0.4 | 2.1 ± 0.6 | <0.01 |
| residual renal function weekly (Kt/V) | 0.98 ± 0.9 | 0 | — |
| total weekly (Kt/V)a | 2.6 ± 0.9 | 2.1 ± 0.6 | 0.048 |
| dialysate creatinine clearance (ml/min per 1.73 m2)a | 3.7 ± 1.2 | 4.7 ± 1.1 | <0.01 |
| residual renal creatinine clearance (ml/min per 1.73 m2)a | 6.4 ± 7.7 | 0 | — |
| total creatinine clearance (ml/min per 1.73 m2)a | 10.1 ± 7.5 | 4.7 ± 1.1 | 0.03 |
| Phosphate clearanced | |||
| dialysate phosphate clearance (L/d per 1.73 m2)c | 7 (5 to 10) | 16 (9 to 22) | <0.01 |
| residual renal phosphate clearance (L/d per 1.73 m2)c | 4 (2 to 7) | 0 | — |
| total phosphate clearance (L/d per 1.73 m2)c | 12 (9 to 16) | 16 (9 to 22) | 0.2 |
| renal daily phosphate removal (mg/d)c | 233 (88 to 342) | 0 | — |
| dialysate daily phosphate removal (mg/d)c | 334 (268 to 537) | 860 (579 to 1137) | <0.01 |
| total daily phosphate removal (mg/d)c | 619 (529 to 769) | 860 (579 to 1137) | 0.047 |
| Diet and medicationsd | |||
| nPNA (g/kg per day)a | 0.94 ± 0.27 | 0.81 ± 0.17 | 0.04 |
| dietary phosphate (mg/d)c | 947 (748 to 1053) | 797 (702 to 885) | 0.1 |
| phosphate binder use (%) | 77 | 96 | 0.04 |
| active vitamin D use (%) | 84 | 87 | 0.7 |
RRF, residual renal function; FGF23, fibroblast growth factor 23; RU, reference units; PTH, parathyroid hormone; nPNA, normalized protein equivalent nitrogen appearance.
Values are expressed as mean ± SD.
Calculated as weight in kilograms divided by height in meters squared (kg/m2).
Values are medians with interquartile range in parentheses. —, not available.
Phosphate clearances were available in 40 participants (24 with and 16 without residual renal function). Dietary intake was available in 29 participants (21 with and eight without residual renal function).
Associations of Residual Renal Function, Dialysis Vintage, and Phosphate Clearance with FGF23
In unadjusted analyses (Figure 1), lnFGF23 correlated with serum phosphate (r = 0.66, P < 0.001), residual renal function (r = −0.37, P = 0.002), and dialysis vintage (r = 0.31, P = 0.01). After adjusting for demographics, other mineral metabolites (serum albumin, calcium, phosphate, and PTH), dialysis adequacy (peritoneal dialysis Kt/V), peritoneal dialysis modality, and treatment with active vitamin D and phosphate binders, higher serum phosphate (β = 0.50, P < 0.001), absence of residual renal function (β = −0.89, P = 0.03), and longer dialysis vintage (β = 0.43, P = 0.002) remained independently associated with higher FGF23 levels. Similarly, the log-linear relationship between lnFGF23 (on a continuous scale) and residual renal function (β = −0.07, P = 0.02) persisted among those with residual renal function after multivariable adjustment. Moreover, inclusion of both residual renal function and dialysis vintage together in the same multivariable model demonstrated that each was significantly associated with lnFGF23 in patients with residual renal function.
Figure 1.
Relationships between fibroblast growth factor 23 (FGF23) and residual renal function, dialysis vintage, serum phosphate, and phosphate clearance. Scatter plots depict the unadjusted associations of lnFGF23 with (A) serum phosphate; (B) residual renal function; (C) dialysis vintage; and (D) phosphate clearance. For each relationship, linear regression lines and Pearson correlation coefficients with their respective P values are shown.
To explore whether intensity of phosphate clearance associated with FGF23 levels, we examined univariate associations between renal and peritoneal phosphate clearances and FGF23 in patients with residual renal function. In anuric patients, we correlated peritoneal clearance with FGF23 levels. As shown in Figure 1D, lnFGF23 correlated inversely with renal phosphate clearance (r = −0.38, P = 0.008) in patients with residual renal function. This relationship persisted after adjustment for demographic, laboratory values, peritoneal dialysis modality and adequacy, and treatment with vitamin D and phosphate binders. In contrast, there was no significant relationship between peritoneal clearance and lnFGF23 in patients with (r = 0.02, P = 0.92) or without (r = −0.14, P = 0.53) residual renal function.
FGF23 Clearance
In a subset of 10 participants with residual renal function (average renal creatinine clearance of 6.6 ml/min per 1.73 m2) in whom we measured FGF23 levels in dialysate and urine, plasma FGF23 levels correlated strongly with urinary FGF23 (r = 0.92, P < 0.001) and to a lesser extent with dialysate FGF23 (r = 0.60, P = 0.06). The median (interquartile range) renal FGF23 clearance was 0.2 (0.1 to 0.3) ml/min. The median (interquartile range) peritoneal FGF23 clearance was 0.8 (0.5 to 1.8) ml/min.
Variability of FGF23 versus PTH and Phosphate
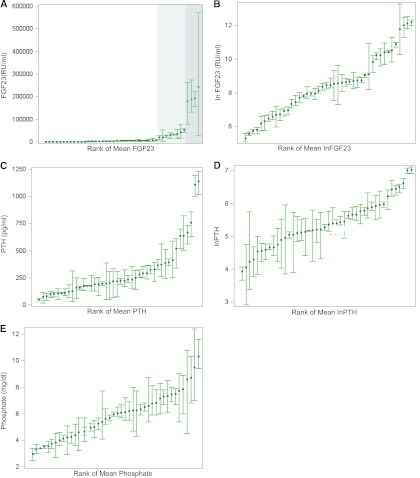
Forty-four participants had 3-month repeated measurements of FGF23, PTH, and phosphate, and we used these values to quantify the components of variation for each mineral metabolite. Total variation is composed of the within-and between-subject variation, with the former further subdivided into analytic variation, arising from measurement error, and true biologic variation. For the three repeated tests of each of the markers of mineral metabolism, we first examined the within-subject mean and range of variation, rank-ordered by individual participants' means (Figure 2). For FGF23 and PTH both the measured scale and ln-transformed values are shown. Compared with PTH and phosphate, the overall range of within-subject variation was less pronounced for FGF23, and only became substantial at the highest values above the threshold when serial dilutions were required, and likely introduced greater analytic variation (Figure 2A, shaded areas). Table 2 presents the sources of variation in the three analytes and their intraclass correlations (ICC), which quantify the percentage of total variation explained by between-subject variation. The remainder is explained by within-subject variation. A high ICC indicates that most of the observed variability in a given assay is explained by between-subject variation, implying greater stability within individual patients upon repeated measurements over time. In contrast, a low ICC implies that the within-subject variation is high and that repeated measurements in the same individuals over time will yield more disparate results. The ICC for FGF23 was significantly higher (based on nonoverlapping 95% confidence intervals [CI]) than for either PTH or phosphate, indicating that among the three, FGF23 had the largest component of variability explained by between-subject variation and thus, the lowest within-subject variation of 10%. Moreover, when we repeated these analyses in participants in the highest quartiles of FGF23 and PTH values, we found that the ICC for FGF23 was 0.7 (95% CI: 0.3 to 0.9) and the ICC for PTH was 0.3 (95% CI: 0.2 to 0.5). This suggests that FGF23 measurements are more stable than PTH within-individuals even at the highest end of the spectrum of each.
Figure 2.
Range of variation in mineral metabolism markers. The plots depict mean (blue squares) and range of variation (green whiskers for lower and upper range values) in (A) fibroblast growth factor 23 (FGF23), (B) lnFGF23, (C) parathyroid hormone (PTH), (D) lnPTH, and (E) phosphate. Values for each individual participant are plotted in rank order of their individual means. In (A), the area shaded in light gray encompasses FGF23 values >15,000 RU/ml, and the area shaded in dark gray included FGF23 values >150,000 RU/ml.
Table 2.
Variance components for FGF23, PTH, and phosphate
| Variable | Mean | Variance |
ICC Estimateb | ||
|---|---|---|---|---|---|
| Between-Subject σ2ba | Within-Subject σ2wa | Total Variance σ2b + σ2wa | |||
| lnFGF23 | 8.2 | 2.5 (0.4) | 0.3 (0.03) | 2.8 (0.4) | 0.9 (0.82 to 0.95 |
| lnPTH | 5.6 | 0.4 (0.1) | 0.5 (0.06) | 0.9 (0.1) | 0.5 (0.32 to 0.60) |
| Phosphate | 5.7 | 2.4 (0.5) | 1.4 (0.2) | 3.8 (0.5) | 0.6 (0.39 to 0.76) |
FGF23, fibroblast growth factor 23; PTH, parathyroid hormone; ICC, intraclass correlation.
Values are expressed as mean with SEM in parentheses.
Values in parentheses are 95% confidence intervals. The ICC was calculated from estimates of between-subject (σ2b) and within-subject variance (σ2w), derived from mixed linear models, using the following formula: σ2b/(σ2b + σ2w).
Discussion
We confirm that FGF23 levels are markedly elevated in patients with ESRD and associate with hyperphosphatemia. The new findings of this study are that longer dialysis vintage and lower residual renal function and renal phosphate clearance are also important determinants of higher FGF23 levels. Interestingly, despite controversy surrounding what is the ideal FGF23 assay, FGF23 demonstrated significantly less within-subject variability over a 3-month period compared with contemporaneous PTH and phosphate measurements. This suggests that single measurements of FGF23 may provide a more accurate assessment of disordered phosphate metabolism than either marker currently in use clinically. When considered alongside reports of elevated FGF23 independently associating with increased risk of cardiovascular disease and mortality (14,22), these data lend further support in favor of using FGF23 testing as a stable biomarker of disordered phosphate metabolism.
Studies in patients with chronic kidney disease not yet on dialysis, transplant donors, and animal experiments all demonstrate that reduced kidney function is a leading determinant of FGF23 levels (23–25). Although data on FGF23 levels in patients undergoing peritoneal dialysis are limited (11,26,27), one report linked absence of residual renal function with higher FGF23 levels in children (26). We report similar findings in adults and extend this observation by showing that among those with residual renal function, there is a continuous relationship between higher FGF23 and lower residual renal function and that longer dialysis vintage also independently associates with higher FGF23. These relationships were independent of other classic regulators of FGF23, including serum phosphate and use of active vitamin D and phosphate binders. Thus, in addition to differences in serum phosphate, differences in dialysis vintage and residual renal function contribute to the heterogeneity in FGF23 in the dialysis population.
Prior reports suggest that enhanced total phosphate clearance driven by residual renal function (28,29) may reduce FGF23 secretion, leading to the lower FGF23 levels we observed in this group compared with the anuric patients. However, we found that the anuric patients had higher FGF23 levels but greater total daily phosphorus removal. We can speculate that more severe hyperphosphatemia in this group maintained the concentration gradient for phosphate removal that drove their higher peritoneal removal of phosphate. Additionally, greater convective removal of phosphate in the anuric group, whose ultrafiltration requirements are higher than in patients who continue to make urine, may have contributed to their greater peritoneal phosphate removal. Yet, the anuric group had higher FGF23 levels in the setting of greater phosphate removal and despite lower dietary phosphorus intake and greater use of phosphate binders. This discrepancy could be explained by a direct stimulatory effect of their more severe hyperphosphatemia or their greater net positive phosphate balance due to longer dialysis vintage. Balance studies are needed to measure differences in phosphate balance between patients with and without residual renal function and their relation to FGF23 and serum phosphate levels.
In addition to increased FGF23 production by bone (30), it has been proposed that accumulation of FGF23 due to decreased renal clearance is an important mechanism of elevated FGF23 levels in patients with chronic kidney disease (31). Our pilot data suggest otherwise. We found that FGF23 clearance by the kidney or peritoneal dialysis is minimal, only 4% to 5% of the corresponding clearances of creatinine. Our findings are in agreement with the one previous report in the literature of simultaneous FGF23 measurements in the blood (110 RU/ml) and urine (87.6 RU/ml) from a healthy volunteer (11). Assuming a urine volume of 2 L/d, the healthy individual's FGF23 clearance was approximately 1.1 ml/min, which is in a comparably low range that we observed in patients with ESRD (median renal FGF23 clearance, 0.2 ml/min; median total FGF23 clearance, 1.2 ml/min). These data suggest that differences in FGF23 clearance, in its purest definition, contribute negligibly to FGF23 levels in health and in CKD. Alternatively, CKD could contribute to FGF23 “accumulation” through reduced cellular uptake, impaired proteolytic degradation, or aberrant processing of intact FGF23 in renal tubular cells, as has been described for other peptide hormones (32). Interestingly, a prior report showed that both the intact FGF23 and its C-terminal fragments were detected in the urine of dialysis patients with residual renal function (11). Additional studies are needed to identify mechanisms of FGF23 degradation and how these are affected by kidney disease.
Strengths of our study include an ethnically diverse population of patients receiving peritoneal dialysis across three academic centers in the United States, quantification of residual renal function and peritoneal and renal phosphate and FGF23 clearance, and availability of 3-month repeated measurements of mineral metabolism markers. However, our study also has limitations. We were not able to study longitudinal changes in residual renal function and their association with FGF23 over longer durations, and our ascertainment of residual renal function relied on renal creatinine clearance, which may have overestimated the values due to tubular secretion of creatinine. Our assessment of dietary intake was limited to use of 4-day food records that provide only an estimate of short-term intake, and we were unable to study the components of phosphate balance antecedent to our evaluation. Finally, we did not take into account peritoneal membrane characteristics that were recently noted to play a role in peritoneal phosphate clearance (33).
Ideally, a clinically useful biomarker provides prognostic utility for outcomes of interest, is easily and reproducibly measured, and manifests minimal variability diurnally and longitudinally. These appear to be characteristics of FGF23. In addition to associating with outcomes and varying minimally across the day and with relation to meals, we report minimal within-subject variability during 3-month serial measurements of FGF23. Indeed, the variability was higher only in participants with the highest values when 1:100 or greater dilutions were required, which likely introduced significant (human) measurement error more than true biologic scatter. Despite this variation at the high range, FGF23 demonstrated significantly less overall within-subject variation than serum phosphate or PTH, which have known circadian patterns (7,8,13). In support of our findings, a recent report estimated that 26 PTH measurements would be needed to accurately estimate an individual hemodialysis patient's true homeostatic set point for PTH (34). Although comparable studies are needed in peritoneal dialysis patients, these results suggest that FGF23 is a more stable marker of phosphate metabolism than PTH or phosphate, which could help explain its stronger association with outcomes and support the further development of FGF23 testing for clinical practice.
Disclosures
None.
Acknowledgments
This study was supported in part by the Clinical Translational Science Award 1UL-1RR024975 from the National Center for Research Resources (T.A.I.), and by grants K24 DK 62849 (T.A.I.), K23DK087858 (T.I.), R01DK076116 (M.W.), and R01DK081374 (M.W.) from the National Institute of Diabetes and Digestive and Kidney Diseases and the Center for D-Receptor Activation Research (T.A.I.).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Giachelli CM: The emerging role of phosphate in vascular calcification. Kidney Int 75: 890–897, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Neves KR, Graciolli FG, dos Reis LM, Pasqualucci CA, Moyses RM, Jorgetti V: Adverse effects of hyperphosphatemia on myocardial hypertrophy, renal function, and bone in rats with renal failure. Kidney Int 66: 2237–2244, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Shuto E, Taketani Y, Tanaka R, Harada N, Isshiki M, Sato M, Nashiki K, Amo K, Yamamoto H, Higashi Y, Nakaya Y, Takeda E: Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol 20: 1504–1512, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G, for the C, Recurrent Events Trial I: Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 112: 2627–2633, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Block GA, Hulbert-Shearon TE, Levin NW, Port FK: Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis 31: 607–617, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL: Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 16: 520–528, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Markowitz M, Rotkin L, Rosen JF: Circadian rhythms of blood minerals in humans. Science 213: 672–674, 1981 [DOI] [PubMed] [Google Scholar]
- 8. Isakova T, Gutierrez O, Shah A, Castaldo L, Holmes J, Lee H, Wolf M: Postprandial mineral metabolism and secondary hyperparathyroidism in early CKD. J Am Soc Nephrol 19: 615–623, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ring T, Sanden AK, Hansen HH, Halkier P, Nielsen C, Fog L: Ultradian variation in serum phosphate concentration in patients on haemodialysis. Nephrol Dial Transplant 10: 59–63, 1995 [PubMed] [Google Scholar]
- 10. Isakova T, Wahl P, Vargas GS, Gutierrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M: Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79: 1370–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB: Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int 64: 2272–2279, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Carpenter TO, Insogna KL, Zhang JH, Ellis B, Nieman S, Simpson C, Olear E, Gundberg CM: Circulating levels of soluble klotho and FGF23 in X-linked hypophosphatemia: Circadian variance, effects of treatment, and relationship to parathyroid status. J Clin Endocrinol Metab 95: E352–E357, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. el-Hajj Fuleihan G, Klerman EB, Brown EN, Choe Y, Brown EM, Czeisler CA: The parathyroid hormone circadian rhythm is truly endogenous—A general clinical research center study. J Clin Endocrinol Metab 82: 281–286, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Juppner H, Wolf M: Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359: 584–592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wolf M, Molnar MZ, Amaral AP, Czira ME, Rudas A, Ujszaszi A, Kiss I, Rosivall L, Kosa J, Lakatos P, Kovesdy CP, Mucsi I: Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol 22: 956–966, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parker BD, Schurgers LJ, Brandenburg VM, Christenson RH, Vermeer C, Ketteler M, Shlipak MG, Whooley MA, Ix JH: The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: The Heart and Soul Study. Ann Intern Med 152: 640–648, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutierrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M: Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305: 2432–2439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schakel SF, Sievert YA, Buzzard IM: Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc 88: 1268–1271, 1988 [PubMed] [Google Scholar]
- 19. Nolph KD, Moore HL, Twardowski ZJ, Khanna R, Prowant B, Meyer M, Ponferrada L: Cross-sectional assessment of weekly urea and creatinine clearances in patients on continuous ambulatory peritoneal dialysis. ASAIO J 38: M139–M142, 1992 [DOI] [PubMed] [Google Scholar]
- 20. Randerson DH, Chapman GV, Farrell PC: Amino acid and dietary status in CAPD patients. In: Peritoneal Dialysis, edited by Atlkins RC, Farrell PC, Thompson N. Edinburgh, Scotland: Churchill-Livingstone, 1981, pp 180–191 [Google Scholar]
- 21. Ockene IS, Matthews CE, Rifai N, Ridker PM, Reed G, Stanek E: Variability and classification accuracy of serial high-sensitivity C-reactive protein measurements in healthy adults. Clin Chem 47: 444–450, 2001 [PubMed] [Google Scholar]
- 22. Jean G, Terrat J-C, Vanel T, Hurot J-M, Lorriaux C, Mayor B, Chazot C: High levels of serum fibroblast growth factor (FGF)-23 are associated with increased mortality in long haemodialysis patients. Nephrol Dial Transplant 24: 2792–2796, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Juppner H, Wolf M: Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 16: 2205–2215, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Westerberg PA, Ljunggren O, Larsson TE, Wadstrom J, Linde T: Fibroblast growth factor-23 and mineral metabolism after unilateral nephrectomy. Nephrol Dial Transplant 25: 4068–4071, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Hasegawa H, Nagano N, Urakawa I, Yamazaki Y, Iijima K, Fujita T, Yamashita T, Fukumoto S, Shimada T: Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int 78: 975–980, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Wesseling-Perry K, Pereira RC, Wang H, Elashoff RM, Sahney S, Gales B, Juppner H, Salusky IB: Relationship between plasma fibroblast growth factor-23 concentration and bone mineralization in children with renal failure on peritoneal dialysis. J Clin Endocrinol Metab 94: 511–517, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shimada T, Urakawa I, Isakova T, Yamazaki Y, Epstein M, Wesseling-Perry K, Wolf M, Salusky IB, Juppner H: Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. J Clin Endocrinol Metab 95: 578–585, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang AY, Woo J, Sea MM, Law MC, Lui SF, Li PK: Hyperphosphatemia in Chinese peritoneal dialysis patients with and without residual kidney function: What are the implications? Am J Kidney Dis 43: 712–720, 2004 [PubMed] [Google Scholar]
- 29. Kuhlmann MK: Phosphate elimination in modalities of hemodialysis and peritoneal dialysis. Blood Purif 29: 137–144, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Pereira RC, Juppner H, Azucena-Serrano CE, Yadin O, Salusky IB, Wesseling-Perry K: Patterns of FGF-23, DMP1, and MEPE expression in patients with chronic kidney disease. Bone 45: 1161–1168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Filler G, Liu D, Huang SH, Casier S, Chau LA, Madrenas J: Impaired GFR is the most important determinant for FGF-23 increase in chronic kidney disease. Clin Biochem 44: 435–437, 2011 [DOI] [PubMed] [Google Scholar]
- 32. Waldmann TA, Strober W, Mogielnicki RP: The renal handling of low molecular weight proteins. II. Disorders of serum protein catabolism in patients with tubular proteinuria, the nephrotic syndrome, or uremia. J Clin Invest 51: 2162–2174, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bernardo AP, Contesse SA, Bajo MA, Rodrigues A, Del Peso G, Ossorio M, Cabrita A, Selgas R: Peritoneal membrane phosphate transport status: A cornerstone in phosphate handling in peritoneal dialysis. Clin J Am Soc Nephrol 6: 591–597, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gardham C, Stevens PE, Delaney MP, LeRoux M, Coleman A, Lamb EJ: Variability of parathyroid hormone and other markers of bone mineral metabolism in patients receiving hemodialysis. Clin J Am Soc Nephrol 5: 1261–1267, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]