Abstract
Background
Lobectomy is considered the standard treatment for early-stage non–small cell lung cancer (NSCLC); however, more limited resections are commonly performed. We examined patient and surgeon factors associated with limited resection and compared postoperative and long-term outcomes between sublobar and lobar resections.
Methods
A population- and health system–based sample of patients newly diagnosed with stage I or II NSCLC between 2003 and 2005 in five geographically defined regions, five integrated health-care delivery systems, and 15 Veterans Affairs hospitals was observed for a median of 55 months, through May 31, 2010. Predictors of limited resection and postoperative outcomes were compared using unadjusted and propensity score–weighted analyses. All P values are from two-sided tests.
Results
One hundred fifty-five (23%) patients underwent limited resection and 524 (77%) underwent lobectomy. In adjusted analyses of patient-specific factors, smaller tumor size (P = .004), coverage by Medicare or Medicaid, no insurance or unknown insurance (P = .02), more severe lung disease (P < .001), and a history of stroke (P = .049) were associated with receipt of limited resection. In adjusted analyses of surgeon characteristics, thoracic surgery specialty (P = .02), non–fee-for-service compensation (P = .008), and National Cancer Institute cancer center designation (P = .006) were associated with higher odds of limited resection. Unadjusted 30-day mortality was higher with limited resection than with lobectomy (7.1% vs 1.9%, difference = 5.2%, 95% confidence interval [CI] = 1.5% to 10.8%, P = .003), and the adjusted difference was not statistically significant (6.5% vs 2.9%, difference = 3.6%, 95% CI = −.1% to 9.2%, P = .09). Postoperative complications did not differ by type of surgery (all P > .05). Over the course of the study, a non-statistically significant trend toward improved long-term survival was evident for lobectomy, compared with limited resection, in adjusted analyses (hazard ratio of death = 1.35 for limited resection, 95% CI = 0.99 to 1.84, P = .05).
Conclusions
Evidence is statistically inconclusive but suggestive that lobectomy, compared with limited resection, is associated with increased long-term survival for early-stage lung cancer. Clinical, socioeconomic, and surgeon factors appear to be associated with the choice of surgical resection.
CONTEXT AND CAVEATS
Prior knowledge
It is unclear whether lobectomy or limited resection is preferable for the surgical treatment of early-stage non–small cell lung cancer (NSCLC).
Study design
Data was collected for patients who were newly diagnosed with stage I or II NSCLC in 2003–2005 in a variety of regions and hospitals within the United States who either underwent limited resection (n = 155; 23%) or lobectomy (n = 524; 77%). The authors examined patient and surgeon characteristics associated with limited resection and perioperative complications associated with each resection type.
Contribution
Smaller tumor size, lack of insurance, and more severe lung disease, and having a surgeon who was a thoracic specialist or at an NCI cancer center each statistically significantly associated with limited resection. Unadjusted 30-day mortality was higher for limited resection than lobectomy; however, the adjusted values were not statistically significant.
Implication
Thirty-day and long-term survival were slightly, but not statistically significantly, greater for patients who underwent lobectomy compared with limited resection.
Limitations
This was a retrospective observational study, and patients who were chosen for limited resection had different characteristics from those chosen for lobectomy. Survival analyses were limited to overall survival and did not include disease-free survival or disease-specific mortality.
From the Editors
Lung cancer is the most common cause of cancer-related death for both men and women in the United States (1). In 2010, an estimated 222 520 people will be diagnosed and 157 300 will die of the disease (2). For the approximately 80% of lung cancer patients diagnosed with non–small cell histology, surgical resection provides the best chance for cure of localized disease (3).
Lobectomy, defined as the removal of one of the five lobes of the lung and associated lymph nodes within a single pleural membrane, is considered the standard treatment for surgical resection of early-stage non–small cell lung cancer (NSCLC) (4,5). Sublobar resections, including segmental or wedge resections, remove less uninvolved lung tissue and thus may benefit patients with less physiological reserve in lung function. Limited resections, however, require division of lung parenchyma across shared lobar vasculature, lymphatics, and bronchi, thus theoretically increasing the risk of local recurrence (4).
In 1995, the Lung Cancer Study Group’s randomized trial of limited resection vs lobectomy for T1N0 disease revealed a tripling of locoregional recurrence with limited resection and a trend of improved survival for lobectomy (6,7). The ideal extent of resection for early-stage NSCLC, however, remains controversial. Sublobar resection has been advocated for very small tumors (8,9) and high-risk patients including the elderly (10) or those with poor cardiac or respiratory function (11,12). Multiple nonrandomized studies have shown no difference in recurrence rate and overall survival between limited resection and lobectomy for specific subgroups (8,9,11–14).
In the 15 years since the Lung Cancer Study Group trial, surgical management has evolved with limited resections and lobectomy increasingly performed by minimally invasive techniques. Additionally, advances in medical imaging have resulted in better preoperative staging and the detection of smaller lesions (15–17). Improvements in perioperative management have increased patient safety (18,19). These changes may have altered the risk–benefit balance between sublobar resection and lobectomy. However, current rates and outcomes of limited resections for stage I and II NSCLC in the United States are unknown.
Therefore, we analyzed a large multiregional cohort of patients newly diagnosed with lung cancer to determine the relative frequency of different resection types and to examine whether patients’ clinical and sociodemographic characteristics were associated with receipt of a limited resection. In addition, we surveyed surgeons of these patients to assess surgeon and practice characteristics that may influence the choice of resection. Finally, to compare the effectiveness of limited resection and lobectomy, we analyzed rates of postoperative complications, mortality, and long-term survival.
Methods
Study Overview
This study was conducted through the Cancer Care Outcomes Research and Surveillance (CanCORS) Consortium, a large multiregional observational study of processes and outcomes of care for approximately 10 000 patients diagnosed with lung or colorectal cancer between 2003 and 2005. These patients were living in California, North Carolina, Iowa, or Alabama, or received their care in one of five large integrated delivery systems or health maintenance organizations (HMOs) (Group Heath Cooperative, Seattle, WA; Harvard Pilgrim Health Care, Boston, MA; Henry Ford Health System, Detroit, MI; Kaiser Permanente Hawaii, Honolulu, HI; Kaiser Permanente Northwest, Portland, OR) or at one of 15 hospitals in the Veterans Affairs (VA) health-care system (Harbor Healthcare System, Manhattan and Brooklyn, NY; Lakeside and Hines, Chicago, IL; Baltimore, MD; Tucson, AZ; Durham, NC; Biloxi, MS; Nashville, TN; Temple, TX; Minneapolis, MN; Atlanta, GA; Indianapolis, IN; Houston, TX; Seattle, WA). Details of the study design have been described previously (20). Briefly, eligible patients were identified through state or regional tumor registries or through hospital tumor registries in participating integrated delivery systems. Eligible patients were invited to participate by mail and phone. The study protocol was approved by the human subjects committees at all participating institutions, and patients provided written informed consent for participation.
Study Cohort
We included patients with stage I or II NSCLC as defined by the American Joint Commission on Cancer (21). To create a cohort of patients who could plausibly undergo either lobectomy or sublobar resection, we excluded patients who underwent pneumonectomy, extended resection (lung resection with en bloc removal of extrapulmonary tissue such as chest wall, diaphragm, or pericardium), local tumor destruction, or undefined surgery type. The limited resection group was defined as those who underwent wedge resection or segmentectomy. The lobectomy group included patients who underwent lobectomy or bilobectomy but not a bronchial sleeve resection (indicating a proximal tumor ineligible for limited resection).
Data Collection
A baseline telephone interview was conducted approximately 4–6 months after initial diagnosis, with either patients or their surrogates if patients were deceased or too ill to be interviewed. Interview topics included information about their care providers, income, education, and smoking history. Medical records were obtained from providers identified by patients or surrogates and abstracted by trained medical record abstractors to assess insurance coverage, tumor stage and histology, surgery type, comorbidities, and postoperative medical events. Severity of lung disease was defined as noted in the legend of Table 1. Postoperative complications included 24 possible medical events (Appendix) within 30 days after surgery. In addition, patient-identified surgeons were surveyed about their specialty training, practice type, mode of compensation, surgical volume, and involvement in teaching or clinical trials. Patients’ vital status was reported by participating sites through May 31, 2010.
Table 1.
Characteristics of patient study cohort by resection type*
| Patient characteristic | Limited resection, n = 155 (23%) | Lobectomy, n = 524 (77%) | P† |
| Male, No. (%) | 91 (59) | 293 (56) | .58 |
| Age, No. (%) | .91 | ||
| <65 y | 48 (31) | 172 (33) | |
| 65–74 y | 62 (40) | 205 (39) | |
| ≥75 y | 45 (29) | 147 (28) | |
| Race and/or ethnicity, No. (%)‡ | .11 | ||
| White | 119 (77) | 414 (79) | |
| Hispanic and/or Latino | 9 (6) | 10 (2) | |
| Black | 12 (8) | 44 (8) | |
| Other | 15 (10) | 56 (11) | |
| Marital status, No. (%) | .46 | ||
| Married or living with partner | 89 (57) | 315 (61) | |
| Single | 66 (43) | 203 (39) | |
| Insurance, No. (%) | .03 | ||
| Private | 31 (20) | 165 (32) | |
| Medicare and/or Medicaid | 83 (54) | 250 (48) | |
| Military-based insurance | 11 (7) | 36 (7) | |
| None, other, or unknown | 30 (19) | 73 (14) | |
| Education, No. (%) | .02 | ||
| Less than high school | 25 (18) | 99 (21) | |
| High school diploma | 77 (55) | 303 (63) | |
| College diploma or more | 38 (27) | 79 (16) | |
| Income level, No. (%) | .79 | ||
| <$20 000 | 33 (32) | 138 (35) | |
| $20 000–$39 999 | 30 (29) | 123 (31) | |
| $40 000–$59 999 | 22 (21) | 75 (19) | |
| >$60 000 | 19 (18) | 61 (15) | |
| Site, No. (%) | .21 | ||
| 5 HMOs§ | 27 (17) | 89 (17) | |
| 8 Northern California counties|| | 20 (13) | 73 (14) | |
| State of Alabama | 24 (16) | 77 (15) | |
| Los Angeles county | 28 (18) | 60 (11) | |
| State of Iowa | 25 (16) | 123 (24) | |
| VA hospitals¶ | 31 (20) | 102 (20) | |
| Smoking status, No. (%) | .91 | ||
| Non-smoker | 21 (14) | 70 (13) | |
| Former smoker | 87 (56) | 303 (58) | |
| Current smoker | 46 (30) | 147 (28) | |
| Comorbidity score, No. (%)# | <.001 | ||
| None | 17 (11) | 74 (14) | |
| Mild | 43 (28) | 232 (44) | |
| Moderate | 48 (31) | 121 (23) | |
| Severe | 47 (30) | 97 (19) | |
| Specific comorbidities, No. (%) | |||
| Lung disease** | <.001 | ||
| None | 57 (37) | 272 (52) | |
| Mild | 50 (32) | 179 (34) | |
| Moderate | 25 (16) | 51 (10) | |
| Severe | 23 (15) | 22 (4) | |
| Angina | 42 (27) | 113 (22) | .16 |
| History of myocardial infarction | 16 (10) | 43 (8) | .42 |
| Heart failure | 15 (10) | 20 (4) | .007 |
| Stroke | 20 (13) | 35 (7) | .02 |
| End stage renal disease | 6 (4) | 11 (2) | .24 |
| Diabetes | 23 (15) | 73 (14) | .79 |
| Obesity†† | 11 (7) | 15 (3) | .03 |
| Other cancer | 27 (17) | 59 (11) | .05 |
| Stage, No. (%) | <.001 | ||
| I | 142 (92) | 418 (80) | |
| II | 13 (8) | 106 (20) | |
| Tumor size in mm, median [IQR] | 22 [15,30] | 30 (20 to 40) | <.001 |
| Tumor histology, No. (%) | .15 | ||
| Adenocarcinoma | 82 (54) | 287 (55) | |
| Squamous cell carcinoma | 30 (20) | 131 (25) | |
| Other NSCLC type | 39 (26) | 100 (19) |
FEV = forced expiratory volume; IQR = interquartile range; HMO = health maintenance organization; NSCLC = non–small cell lung cancer. Sample size varies by amount of missing data per variable.
P values were generated by two-sided Fisher exact tests for categorical variables and two-sided Wilcoxon tests for continuous non-normal variables.
Race or ethnic group was self-reported.
Group Health Cooperative, Seattle, WA; Harvard Pilgrim Health Care, Boston, MA; Henry Ford Health System, Detroit, MI; Kaiser Permanente Hawaii, Honolulu, HI; Kaiser Permanente Northwest, Portland, OR.
Eight counties in San Jose, San Francisco, Oakland, and Sacramento areas.
Harbor Healthcare System, Manhattan and Brooklyn, NY; Lakeside and Hines, Chicago, IL; Baltimore, MD; Tucson, AZ; Durham, NC; Biloxi, MS; Nashville, TN; Temple, TX; Minneapolis, MN; Atlanta, GA; Indianapolis, IN; Houston, TX; Seattle, WA.
Comorbidity score was assigned based on medical abstraction data according to the highest ranked of 26 single ailments. If two or more ailments in different organ systems were scored as “moderate,” the score was designated as “severe.”.
Severity of lung disease was categorized as mild if the patient carried a diagnosis of restricted lung disease or chronic obstructive pulmonary disease that responded to treatment, recent dyspnea with activity, or recent FEV1 in the range of 66%–80%. Moderate lung disease defined as lung disease that limits activities, recent dyspnea on mild exertion, such as walking from chair to television, or a recent FEV1 from 51% to 65%. Severe disease included patients with any of the following: marked pulmonary insufficiency, dyspnea at rest despite treatment, recent supplemental oxygen requirement, recent carbon dioxide retention, as evidenced by arterial carbon dioxide value of greater than 50 torr, recent hypoxemia as evidenced by oxygen pressure less than 50 torr, or a recent FEV1 of less than 50%.
Obesity was defined as body mass index greater than 38 kg/m2 or a report of morbid obesity in the medical record.
Statistical Analysis
Patient and surgeon characteristics of the two resection groups were compared separately. Wilcoxon and t tests were used in univariate analyses to assess the relation between continuous variables and receipt of limited resection, as appropriate. Fisher exact test and χ2 test were used to assess associations with categorical variables. Univariate and multivariable analyses that included surgeon characteristics were adjusted for clustering by surgeon. Logistic regression models, including all variables with P less than .10 in univariate analyses, were used to examine the joint association of multiple predictor variables with receipt of limited resection.
Unadjusted and propensity score–adjusted analyses were performed to compare postoperative outcomes. Unadjusted Kaplan–Meier estimates for overall survival were generated for lobectomy and limited resection groups and were compared by the log-rank test. To adjust for differences between the limited resection and lobectomy cohorts using propensity score weighting, we fitted a logistic regression model with receipt of limited resection as the dependent variable and the patient characteristics listed in Table 1 as independent predictors and calculated the predicted probability of limited resection for each patient based on their characteristics. We then weighted the limited resection group by the propensity for lobectomy and the lobectomy group by the propensity for limited resection. The weighted distributions for all covariates in the model were consequently made equal for the two groups (22).
Adjusted comparisons of 30-day complications by resection type were performed by weighted χ2 tests. A doubly robust propensity score–adjusted Cox proportional hazards model was used to examine differences in risk of death of patients who received lobectomies vs limited resections. We used propensity score weighting to balance the treatment groups on all the patient characteristic variables in Table 1 and further adjusted the Cox model for variables chosen by forward selection of predictors with P < .05 in a preliminary Cox model without propensity score adjustment; these variables included study site, sex, age, education, respiratory status, history of myocardial infarction, history of congestive heart failure, and stage (23). Schoenfeld residuals were used to confirm the assumption of proportionality for the Cox model. Statistical analyses were conducted using SAS statistical software (Cary, NC). All statistical tests were two-sided, and P values less than .05 were considered statistically significant.
Results
Study Cohort
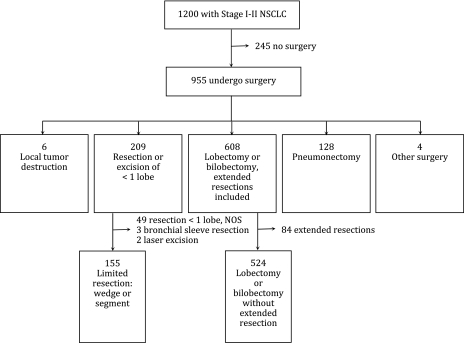
Among patients who were initially recorded as having stage I or II disease during the study enrollment process, the patient consent rate was 67.0%. Using a standard definition, the patient survey response rate was 64.3% and the surgeon response rate for the physician survey was 62.5% (24). Of the 1200 patients identified with confirmed stage I or II NSCLC, 955 (80%) underwent surgery. The types and numbers of surgical resections that were included in our study cohort are depicted in a flow chart (Figure 1). We excluded six (0.6%) patients who underwent local tumor destruction, 128 (13%) who underwent a pneumonectomy, and four (0.4%) who had other types of surgery, leaving 209 (22%) patients who underwent resection or excision of less than one lobe and 608 (64%) patients who underwent lobectomy or bilobectomy, including extended resections. From the former group, we excluded 49 patients with unspecified sublobar resections (to ensure the exclusion of sleeve resections or laser excisions), three patients with bronchial sleeve resections, and two patients with laser excisions; and from the latter group, we excluded 84 patients with extended resections. The final study cohort included 155 (23%) patients who underwent either a wedge (n = 120) or segmentectomy (n = 35) and 524 (77%) patients who had a lobectomy or bilobectomy without an extended resection.
Figure 1.
Flowchart of study participants from Cancer Care Outcomes Research and Surveillance (CanCORS) Study.
Patient Characteristics
The demographic characteristics of patients who underwent limited resection and lobectomy are shown (Table 1). The sample was largely elderly, male, white, and married or living with a partner. No statistically significant differences in age or income were noted between patients who received the two resection types. There was a statistically significant difference in insurance status between the two cohorts, with patients who underwent lobectomy more likely to have private insurance and less likely to have Medicare, Medicaid, an unknown type of insurance, or no insurance (P = .03). There was also a statistically significant difference in educational level between the two groups, with the limited resection group more likely to have a college degree (P = .02).
Greater than 85% of the patients in each group were current or former smokers. The overall comorbidity score was statistically significantly higher in the limited resection group than in the lobectomy group (P < .001). Patients in the limited resection cohort had greater severity of lung disease than the lobectomy group (P < .001) and were more likely to have a history of heart failure (P = .007), stroke (P = .02), and obesity (P = .03). Patients were more likely to undergo limited resection for pathological stage I disease than stage II disease (P < .001). Patients in the limited resection group had smaller tumors (for patients who received sublobar resections, median tumor diameter = 22 mm; interquartile range [IQR] = 15–30 mm; for patients who received lobectomies, median tumor diameter = 30 mm; IQR = 20–40 mm; P < .001). There was no difference in tumor histology between patients who underwent sublobar vs lobar resection; most patients in each group had adenocarcinoma.
Adjusted Patient-Specific Predictors of Limited Resection
We first examined multivariable predictors of receiving a limited resection (Table 2). After controlling for study site differences, insurance status remained statistically significantly associated with limited resection; patients with Medicare, Medicaid, or insurance categorized as none, other, or unknown had an increased odds of receiving a limited resection relative to patients with private insurance (P = .02). The likelihood of limited resection increased with more severe lung disease (P < .001) and with a history of a stroke (P = .049). Each centimeter increase in tumor size was associated with decreasing odds of receiving a limited resection (odds ratio [OR] = 0.82, P = .004). In the multivariable model, education, heart failure, and obesity were not statistically significantly associated with receipt of a limited resection.
Table 2.
Multivariable patient-specific predictors of limited resection*
| Patient Characteristic | OR (95% CI) | P† |
| Insurance (vs private) | .02 | |
| Medicare or Medicaid | 1.65 (1.00 to 2.75) | |
| Military-based insurance | 1.71 (0.67 to 4.44) | |
| None, other or unknown | 2.70 (1.42 to 5.13) | |
| Education (vs less than high school) | .20 | |
| High school diploma | 1.06 (0.62 to 1.83) | |
| College diploma or more | 1.78 (0.94 to 3.40) | |
| Lung disease (vs none)‡ | <.001 | |
| Mild | 1.20 (0.76 to 1.89) | |
| Moderate | 2.58 (1.41 to 4.73) | |
| Severe | 5.39 (2.67 to 10.88) | |
| Heart failure | 1.91 (0.88 to 4.17) | .10 |
| Stroke | 1.89 (1.00 to 3.55) | .049 |
| Obesity§ | 2.39 (0.99 to 5.76) | .05 |
| Tumor size, per cm increase | 0.82 (0.72 to 0.94) | .004 |
CI = confidence interval; FEV = forced expiratory volume; OR = Odds ratio.
Values are based on logistic regression using all variables with P less than .1 on univariate screen by two-sided Fisher exact tests for categorical variables and two-sided Wilcoxon tests for continuous non-normal variables, excluding collinear terms.
Severity of lung disease was categorized as mild if the patient carried a diagnosis of restricted lung disease or chronic obstructive pulmonary disease that responded to treatment, recent dyspnea with activity, or recent FEV1 in the range of 66%–80%. Moderate lung disease was defined as lung disease that limits activities, recent dyspnea on mild exertion, such as walking from chair to television, or a recent FEV1 from 51% to 65%. Severe disease included patients with any of the following: marked pulmonary insufficiency, dyspnea at rest despite treatment, recent supplemental oxygen requirement, recent carbon dioxide retention, as evidenced by arterial carbon dioxide value of greater than 50 torr, recent hypoxemia as evidenced by an oxygen pressure less than 50 torr, or a recent FEV1 of less than 50%.
Obesity was defined as body mass index greater than 38 kg/m2 or a report of morbid obesity in the medical record.
Surgeon Characteristics
Of the 679 patients who underwent either limited resection or lobectomy, 364 (54%) patients had a surgeon who completed a provider survey. Of these 364 patients, 35 had more than one surgeon who completed a survey, with a total of 400 surveys linked to patients in the cohort. The number of patients with multiple surgeon surveys was similar in the limited resection and lobectomy groups (16% vs 18%, P = .64). A total of 146 surgeons provided the survey responses for a median of two patients in the study cohort [IQR = 2–10, maximum 36].
Surgeons of patients in the lobectomy group were statistically significantly more likely to be male than those of patients in the limited resection group (95% vs 89%, respectively, P = .002). Surgeons of patients in the limited resection group tended more often to be thoracic surgeons compared with surgeons of patients in the lobectomy group (81% vs 72%, P = .09) and they tended to perform a higher number of lung resections per month (median: 6 [IQR = 3–10]) vs 4 [IQR = 2–10], P = .07).
Surgeons of patients in the limited resection group were statistically significantly less likely to be in a physician-owned practice than those of patients in the lobectomy group (P = .004) and they less commonly reported fee-for-service payment (P = .005) but had a similar percentage of patients in managed care (P = .75). Surgeons of patients in the limited resection group were more likely to practice at a National Cancer Institute (NCI)–designated cancer center than surgeons of patients in the lobectomy group (55% vs 27%, respectively, P < .001) and were also more likely to enroll patients in clinical trials (49% vs 36%, P = .048). No statistically significant difference in tumor board attendance (P = .06) or the amount of time teaching (P = .08) was evident between surgeons of patients in the limited resection and lobectomy groups.
Adjusted Surgeon-Specific Predictors of Limited Resection
In adjusted analyses, thoracic surgeons were more likely to perform limited resection than general surgeons (OR = 2.86, 95% CI = 1.36 to 6.00, P = .02). Surgeons who reported payment by fee-for-service were statistically significantly less likely to perform limited resection relative to those who reported other payment types (OR = 0.31, 95% CI = 0.13 to 0.73, P = .008). Surgeons at NCI-designated cancer centers were more likely than those at non–NCI-designated centers to perform limited resection (OR = 3.19, 95% CI = 1.55 to 6.58, P = .006). Sex, volume of lung resections per month, practice ownership, clinical trial involvement, and tumor board attendance were not statistically significant adjusted predictors of limited resection.
Clinical Outcomes
Twenty-eight (19%) patients in the limited resection and 85 (17%) in the lobectomy cohort had postoperative complications, with no statistically significant difference in either unadjusted or propensity score–adjusted analyses (all Ps > .05; Table 3). The unadjusted 30-day mortality rate was higher among patients who received limited resections than among the patients who received lobectomies (7.1% vs 1.9%, difference = 5.2%, 95% CI = 1.5% to 10.8%, P = .003), but after adjustment the difference was not statistically significant (6.5% vs 2.9%, difference = 3.6%, 95% CI = −.1% to 9.2%, P = .09).
Table 3.
Perioperative morbidity and mortality by resection type*
| Unadjusted analysis |
Propensity score–adjusted analysis† |
|||||
| Limited resection (%) | Lobectomy (%) | P‡ | Limited resection (%) | Lobectomy (%) | P‡ | |
| Any perioperative complication | 28 (19%) | 85 (17%) | .62 | 18 | 18 | .97 |
| Pulmonary§ | 19 (13%) | 67 (13%) | .89 | 13.0 | 15 | .58 |
| Cardiovascular‖ | 8 (5%) | 15 (3%) | .20 | 5 | 3 | .15 |
| Wound infection and/or sepsis | 2 (1%) | 6 (1%) | 1.00 | 1 | 2 | .43 |
| Other complication | 2 (1%) | 10 (2%) | 1.00 | 2 | 2 | .84 |
| 30-day mortality | 11 (7.1%) | 10 (1.9%) | .003 | 6.5 | 2.9 | .09 |
Twenty-nine patients had missing complications data.
Adjustment by weighted propensity score.
P values generated by two-sided Fisher exact tests for unadjusted and weighted two-sided χ2 tests for adjusted analyses.
Pulmonary complications include aspiration, bronchopleural fistula, new home oxygen requirement, pneumonia, and respiratory failure requiring intubation.
Cardiovascular complications include angina, congestive heart failure, stroke, cardiac arrest, deep vein thrombosis, indwelling venous catheter clot, myocardial infarction, and pulmonary embolus.
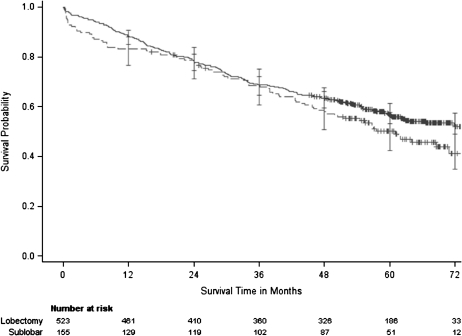
We next examined the unadjusted Kaplan–Meier estimate of overall survival by resection type, with a median follow-up of 55 months (Figure 2). The 5-year survival rate was somewhat lower for patients who received limited resections compared with that for patients who received lobectomies (5-year survival with limited resection = 49% [95% CI = 41% to 57%]; 5-year survival with lobectomy = 57% [95% CI = 52% to 61%]). A non-statistically significant trend toward improved survival for lobectomy was evident in unadjusted analyses (P = .09) and in analyses adjusted by propensity score, with double adjustment for study site, sex, age, education, respiratory status, history of myocardial infarction, history of congestive heart failure, and stage (hazard ratio = 1.35, 95% CI = 0.99 to 1.84, P = .05).
Figure 2.
Unadjusted overall survival by resection type. There was no statistically significant difference in overall survival between limited resection and lobectomy (for limited resection relative to lobectomy, unadjusted hazard ratio [HR] = 1.24, 95% confidence interval [CI] = 0.97 to 1.60, P = .09 by log-rank test; adjusted HR = 1.35, 95% CI = 0.99 to 1.84, P = .05). The solid line represents patients who received lobectomies and the broken line represents patients who received sublobar resection. Censored data is depicted with a plus symbol, and error bars depict 95% confidence intervals.
Discussion
Although lobectomy is considered the standard treatment for early-stage NSCLC, we found that limited resections were commonly performed in this study population, with sublobar resections representing greater than 20% of all procedures performed for patients with stage I or II disease. This relatively high frequency likely reflects both the extent of comorbidity seen in patients with lung cancer and ongoing disagreement concerning the appropriate role for limited resection in the treatment of NSCLC. In adjusted analyses of patient-specific factors, Medicare, Medicaid, lack of or unknown type of insurance, small tumor size, increasing severity of lung disease, and history of stroke were independently associated with receipt of a limited resection. Receipt of limited resection was not associated with age, race or ethnicity, or income. From the provider perspective, thoracic surgery specialty, practice at an NCI-designated cancer center, and non–fee-for-service compensation were all associated with higher odds of performing limited resection.
Previous literature exploring the impact of socioeconomic factors on lung cancer care processes and outcomes has primarily focused on survival differences or whether appropriate patients received any surgical treatment. Greenberg et al. (25) found that NSCLC patients with private insurance were more likely to be treated with surgery, whereas McDavid et al. (26) found a 10% decrease in 3-year survival after lung cancer diagnosis for patients with no insurance vs private insurance (13% vs 23%, respectively). Higher income has also been associated with receipt of surgical treatment of NSCLC and increased likelihood of achieving 5-year survival (27,28). Similarly, lower education levels have been found to be associated with a decreased likelihood of surgical treatment (29). In our study, insurance status, but not income or education, was associated with the type of surgical resection received in the adjusted analysis. This association could represent a provider bias against certain insurance types.
Use of limited resection for very small tumors has been extensively debated in the literature, with advocates both for and against consideration of sublobar resection for tumors smaller than a variety of size thresholds (6,9,13,30–33). This study confirmed that tumor size is an important determinant of the choice of resection type. A randomized controlled trial of sublobar resection vs lobectomy for stage IA tumors less than 2 cm in diameter is ongoing, with completion expected in 2012 (34).
Additionally, our results indicate that patients’ overall health and comorbid conditions affect the decision whether to use a limited resection. Increasing severity of lung disease and a history of stroke were associated with receipt of limited resection, indicating that sublobar resections can serve as an alternative approach for those unable to tolerate lobectomy. Whereas limited resections are also often advocated for the elderly (10,35,36), we did not find an association between age and resection type in this cohort.
The finding of higher frequency of limited resection by thoracic surgeons at NCI-designated cancer centers could reflect either a tendency for surgeons at those hospitals to perform sublobar resections rather than declaring a patient a nonsurgical candidate or a tendency for higher risk patients to be referred to tertiary treatment centers. The method of compensation is known to affect physician behavior, with evidence of earlier diagnosis of some cancers in the HMO relative to the fee-for-service setting (37–40). Our results suggest that surgeon payment method could also affect the type of resection performed, with limited resections performed less often in fee-for-service than salary-based practices. This finding may in part be due to lower Medicare reimbursement for some limited resections, depending on the procedure performed. National average reimbursement rates range from $808 for a thoracoscopic wedge to $1491 for a segmentectomy, in contrast to $1537 to $1642 for a lobectomy (41).
Comparing short-term outcomes after limited resection and lobectomy, we found no difference in postoperative complications, consistent with previous findings of the Lung Cancer Study Group (6). The higher 30-day mortality rate in the limited resection group likely reflects underlying comorbidity differences, as the discrepancy in rates became non-statistically significant after adjustment for baseline patient characteristics. Although we ascertained many postoperative complications, our morbidity data did not include some events that are traditionally monitored after thoracic surgery, including prolonged air leak or chest tube requirement, recurrent laryngeal nerve injury, or new atrial arrhythmias. Our complication rate is thus lower than reported in other series (42,43).
Like the Lung Cancer Study Group’s randomized controlled trial of lobectomy vs limited resection (6) and a recent Surveillance, Epidemiology, and End Results–Medicare analysis of patients with tumors sized 3 cm in diameter or less (13), this study showed a trend of improved survival with lobectomy; however, this trend was non-statistically significant before and after adjustment for differences in patient characteristics. Examination of all-cause mortality reflects the risk of death both due to cancer and from unrelated illnesses. Due to poorer baseline health, we expected a higher rate of death due to competing causes in the limited resection group, and persistence of this difference even after adjustment may be due to unobserved clinical factors.
Strengths of this study include the use of a large multiregional patient cohort. It encompassed a wide range of practice settings, extensive rigorously collected data, and a contemporary timeframe that reflects recent advances in medical and surgical care.
This study also had limitations inherent in retrospective analyses of observational data. Patients who underwent sublobar resections were different than those chosen to undergo lobectomy. Unmeasured selection effects in an observational study can make it difficult to determine definitely whether a potential survival benefit is a true consequence of resection type. We did not ascertain differences in cause of death or disease-free survival.
In summary, this evidence was statistically inconclusive; however, it suggested that lobectomy may be associated with greater long-term survival than limited resection in patients with early-stage lung cancer. Some clinical, socioeconomic, and surgeon factors were statistically significantly associated with the choice of surgical resection for early-stage NSCLC. We believe that providers should seek to reduce the impact of socioeconomic factors such as patient insurance status and surgeon compensation type on clinical decision making.
Funding
The Cancer Care Outcomes Research and Surveillance (CanCORS) Consortium was supported by grants from the National Cancer Institute (NCI) to the Statistical Coordinating Center (U01 CA093344) and the NCI-supported Primary Data Collection and Research Centers (Harvard Medical School/Northern California Cancer Center [U01 CA093324], Dana Farber Cancer Institute/Cancer Research Network [U01 CA093332], RAND/University of California Los Angeles [U01 CA093348], University of Alabama at Birmingham [U01 CA093329], University of Iowa [U01 CA093339], University of North Carolina [U01 CA093326]) and by a Department of Veterans Affairs grant to the Durham VA Medical Center (CDA093344 [MOU] and HARQ 03-438MO-03).
Appendix
List of possible postoperative complications
| Pulmonary |
| Aspiration |
| Bronchopleural fistula |
| Chylothorax |
| New home oxygen therapy |
| Pneumonia |
| Respiratory failure requiring intubation |
| Cardiovascular |
| Angina |
| Congestive heart failure |
| Cerebrovascular accident/Stroke |
| Cardiac arrest |
| Deep vein thrombosis |
| Indwelling venous catheter clot |
| Myocardial infarction |
| Pulmonary embolus |
| Infection/Sepsis |
| Deep wound infection |
| Sepsis |
| Other |
| Acute renal failure (new dialysis or serum creatinine >6) |
| Bowel obstruction |
| Hypercalcemia |
| Neuropathy |
| Seizure |
| Upper gastrointestinal bleeding |
| Lower gastrointestinal bleeding |
| Gastrointestinal bleeding, not otherwise specified |
Footnotes
The authors report no financial conflicts of interest. The study sponsors did not have any role in the design of the study, the collection, analysis, or interpretation of the data; the writing of the article, and the decision to submit the article for publication.
References
- 1.National Cancer Institute. A Snapshot of Lung Cancer: Incidence and Mortality Rate Trends. http://www.cancer.gov/aboutnci/servingpeople/snapshots/lung.pdf Accessed September 14, 2011. [Google Scholar]
- 2.Altekruse S, Kosary C, Krapcho M, Neyman N SEER Cancer Statistics Review. http://seer.cancer.gov/csr/1975_2007/. Accessed January 21, 2011. [Google Scholar]
- 3.Teeter SM, Holmes FF, McFarlane MJ. Lung carcinoma in the elderly population. Influence of histology on the inverse relationship of stage to age. Cancer. 1987;60(6):1331–1336. doi: 10.1002/1097-0142(19870915)60:6<1331::aid-cncr2820600628>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 4.Scott WJ, Howington J, Feigenberg S, Movsas B, Pisters K. Treatment of non-small cell lung cancer stage I and stage II: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 suppl):234S–242S. doi: 10.1378/chest.07-1378. [DOI] [PubMed] [Google Scholar]
- 5. Anonymous. NCCN Clinical Practice Guidelines in Oncology: Non-small Cell Lung Cancer, V.2.2010. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed January 21, 2011. [Google Scholar]
- 6.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60(3):615–622. doi: 10.1016/0003-4975(95)00537-u. discussion 622–623. [DOI] [PubMed] [Google Scholar]
- 7.Lederle FA. Lobectomy versus limited resection in T1 N0 lung cancer. Ann Thorac Surg. 1996;62(4):1249–1250. doi: 10.1016/0003-4975(96)85176-9. [DOI] [PubMed] [Google Scholar]
- 8.Koike T, Yamato Y, Yoshiya K, Shimoyama T, Suzuki R. Intentional limited pulmonary resection for peripheral T1 N0 M0 small-sized lung cancer. J Thorac Cardiovasc Surg. 2003;125(4):924–928. doi: 10.1067/mtc.2003.156. [DOI] [PubMed] [Google Scholar]
- 9.Okada M, Yoshikawa K, Hatta T, Tsubota N. Is segmentectomy with lymph node assessment an alternative to lobectomy for non-small cell lung cancer of 2 cm or smaller? Ann Thorac Surg. 2001;71(3):956–960. doi: 10.1016/s0003-4975(00)02223-2. [DOI] [PubMed] [Google Scholar]
- 10.Mery CM, Pappas AN, Bueno R, et al. Similar long-term survival of elderly patients with non-small cell lung cancer treated with lobectomy or wedge resection within the Surveillance, Epidemiology, and End Results Database. Chest. 2005;128(1):237–245. doi: 10.1378/chest.128.1.237. [DOI] [PubMed] [Google Scholar]
- 11.Keenan RJ, Landreneau RJ, Maley RH, et al. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg. 2004;78(1):228–233. doi: 10.1016/j.athoracsur.2004.01.024. discussion 228–233. [DOI] [PubMed] [Google Scholar]
- 12.Martin-Ucar AE, Nakas A, Pilling JE, West KJ, Waller DA. A case-matched study of anatomical segmentectomy versus lobectomy for stage I lung cancer in high-risk patients. Eur J Cardiothorac Surg. 2005;27(4):675–679. doi: 10.1016/j.ejcts.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Wisnivesky JP, Henschke CI, Swanson S, et al. Limited resection for the treatment of patients with stage IA lung cancer. Ann Surg. 2010;251(3):550–554. doi: 10.1097/SLA.0b013e3181c0e5f3. [DOI] [PubMed] [Google Scholar]
- 14.Kodama K, Doi O, Higashiyama M, Yokouchi H. Intentional limited resection for selected patients with T1 N0 M0 non-small-cell lung cancer: a single institution study. J Thorac Cardiovasc Surg. 1997;114(3):347–353. doi: 10.1016/S0022-5223(97)70179-X. [DOI] [PubMed] [Google Scholar]
- 15.Lardinois D, Weder W, Hany TF, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med. 2003;348(25):2500–2507. doi: 10.1056/NEJMoa022136. [DOI] [PubMed] [Google Scholar]
- 16.Pieterman RM, van Putten JW, Meuzelaar JJ, et al. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med. 2000;343(4):254–261. doi: 10.1056/NEJM200007273430404. [DOI] [PubMed] [Google Scholar]
- 17.Fischbach F, Knollmann F, Griesshaber V, et al. Detection of pulmonary nodules by multislice computed tomography: improved detection rate with reduced slice thickness. Eur Radiol. 2003;13(10):2378–2383. doi: 10.1007/s00330-003-1915-7. [DOI] [PubMed] [Google Scholar]
- 18.Okada M, Nishio W, Sakamoto T, et al. Evolution of surgical outcomes for nonsmall cell lung cancer: time trends in 1,465 consecutive patients undergoing complete resection. Ann Thorac Surg. 2004;77(6):1926–1930. doi: 10.1016/j.athoracsur.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Wu CL, Hurley RW, Anderson GF, et al. Effect of postoperative epidural analgesia on morbidity and mortality following surgery in medicare patients. Region Anesth Pain Med. 2004;29(6):525–533. doi: 10.1016/j.rapm.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Ayanian JZ, Chrischilles EA, Fletcher RH, et al. Understanding cancer treatment and outcomes: the Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol. 2004;22(15):2992–2996. doi: 10.1200/JCO.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. 6th ed. Springer-Verlag; 2002. [Google Scholar]
- 22.Hirano K, Imbens GW. Estimation of causal effects using propensity score weighting: an application to data on right heart catheterization. Health Serv Outcomes Res Methodol. 2001;2(3–4):259–278. [Google Scholar]
- 23.Bang H, Robins JM. Doubly robust estimation in missing data and causal inference models. Biometrics. 2005;61(4):962–973. doi: 10.1111/j.1541-0420.2005.00377.x. [DOI] [PubMed] [Google Scholar]
- 24. The American Association for Public Opinion Research. Standard Definitions—Final Dispositions of Case Codes and Outcomes Rates for Surveys, Revised 2011. http://www.aapor.org/AM/Template.cfm?Section=Standard_Definitions2&Template=/CM/ContentDisplay.cfm&ContentID=3156. Accessed April 18, 2011. [Google Scholar]
- 25.Greenberg E, Chute C, Stukel T, et al. Social and economic factors in the choice of lung cancer treatment. A population-based study in two rural states. N Engl J Med. 1988;318(10):612–617. doi: 10.1056/NEJM198803103181006. [DOI] [PubMed] [Google Scholar]
- 26.McDavid K, Tucker TC, Sloggett A, Coleman MP. Cancer survival in Kentucky and health insurance coverage. Arch Intern Med. 2003;163(18):2135–2144. doi: 10.1001/archinte.163.18.2135. [DOI] [PubMed] [Google Scholar]
- 27.Fry WA, Menck HR, Winchester DP. The National Cancer Data Base report on lung cancer. Cancer. 1996;77(9):1947–1955. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1947::AID-CNCR27>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 28.Greenwald HP, Polissar NL, Borgatta EF, McCorkle R, Goodman G. Social factors, treatment, and survival in early-stage non-small cell lung cancer. Am J Public Health. 1998;88(11):1681–1684. doi: 10.2105/ajph.88.11.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith TJ, Penberthy L, Desch CE, et al. Differences in initial treatment patterns and outcomes of lung cancer in the elderly. Lung Cancer. 1995;13(3):235–252. doi: 10.1016/0169-5002(95)00496-3. [DOI] [PubMed] [Google Scholar]
- 30.Landreneau RJ, Sugarbaker DJ, Mack MJ, et al. Wedge resection versus lobectomy for stage I (T1 N0 M0) non-small-cell lung cancer. J Thorac Cardiovasc Surg. 1997;113(4):691–698. doi: 10.1016/S0022-5223(97)70226-5. discussion 698–700. [DOI] [PubMed] [Google Scholar]
- 31.Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non–small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg. 2006;132(4):769–775. doi: 10.1016/j.jtcvs.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 32.Miller DL, Rowland CM, Deschamps C, et al. Surgical treatment of non-small cell lung cancer 1 cm or less in diameter. Ann Thorac Surg. 2002;73(5):1545–1551. doi: 10.1016/s0003-4975(02)03525-7. [DOI] [PubMed] [Google Scholar]
- 33.Ohta Y, Oda M, Wu J, et al. Can tumor size be a guide for limited surgical intervention in patients with peripheral non-small cell lung cancer? Assessment from the point of view of nodal micrometastasis. J Thorac Cardiovasc Surg. 2001;122(5):900–906. doi: 10.1067/mtc.2001.117626. [DOI] [PubMed] [Google Scholar]
- 34. Cancer and Leukemia Group B. Comparison of Different Types of Surgery in Treating Patients with Stage IA Non-small Cell Lung Cancer. http://clinicaltrials.gov/ct2/show/NCT00499330?term=non+small+cell+lung+cancer+and+lobectomy&rank=4. Accessed January 21, 2011. ClinicalTrials.gov. [Google Scholar]
- 35.Ishida T, Yokoyama H, Kaneko S, Sugio K, Sugimachi K. Long-term results of operation for non-small cell lung cancer in the elderly. Ann Thorac Surg. 1990;50(6):919–922. doi: 10.1016/0003-4975(90)91119-v. [DOI] [PubMed] [Google Scholar]
- 36.Sugarbaker DJ. Lung cancer: the case for limited surgical resection in non-small cell lung cancer. Thorax. 2003;58(7):639–641. doi: 10.1136/thorax.58.7.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gosden T, Forland F, Kristiansen IS, et al. Capitation, salary, fee-for-service and mixed systems of payment: effects on the behaviour of primary care physicians. Cochrane Database Syst Rev. 2000;(3) doi: 10.1002/14651858.CD002215. CD002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gosden T, Pedersen L, Torgerson D. How should we pay doctors? A systematic review of salary payments and their effect on doctor behaviour. QJM. 1999;92(1):47–55. doi: 10.1093/qjmed/92.1.47. [DOI] [PubMed] [Google Scholar]
- 39.Riley GF, Potosky AL, Lubitz JD, Brown ML. Stage of cancer at diagnosis for Medicare HMO and fee-for-service enrollees. Am J Public Health. 1994;84(10):1598–1604. doi: 10.2105/ajph.84.10.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riley GF, Potosky AL, Klabunde CN, Warren JL, Ballard-Barbash R. Stage at diagnosis and treatment patterns among older women with breast cancer: an HMO and fee-for-service comparison. JAMA. 1999;281(8):720–726. doi: 10.1001/jama.281.8.720. [DOI] [PubMed] [Google Scholar]
- 41.American Medical Association. CPT Code Relative Value Search. https://catalog.ama-assn.org/Catalog/cpt/cpt_search.jsp. Accessed January 21, 2011. [Google Scholar]
- 42.Deslauriers J, Ginsberg RJ, Piantadosi S, Fournier B. Prospective assessment of 30-day operative morbidity for surgical resections in lung cancer. Chest. 1994;106(6 suppl):329S–330S. doi: 10.1378/chest.106.6_supplement.329s. [DOI] [PubMed] [Google Scholar]
- 43.Allen MS, Darling GE, Pechet TTV, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 Trial. Ann Thorac Surg. 2006;81(3):1013–1020. doi: 10.1016/j.athoracsur.2005.06.066. [DOI] [PubMed] [Google Scholar]