Abstract
Diabetic retinopathy (DR) is a common cause of blindness. Although many studies have indicated an association between homocysteine and DR, the results so far have been equivocal. Amongst the many determinants of homocysteine, B-vitamin status was shown to be a major confounding factor, yet very little is known about its relationship to DR. In the present study, we, therefore, investigated the status of B-vitamins and homocysteine in DR. A cross-sectional case–control study was conducted with 100 normal control (CN) subjects and 300 subjects with type-2 diabetes (T2D). Of the 300 subjects with T2D, 200 had retinopathy (DR) and 100 did not (DNR). After a complete ophthalmic examination including fundus fluorescein angiography, the clinical profile and the blood levels of all B-vitamins and homocysteine were analyzed. While mean plasma homocysteine levels were found to be higher in T2D patients compared with CN subjects, homocysteine levels were particularly high in the DR group. There were no group differences in the blood levels of vitamins B1 and B2. Although the plasma vitamin-B6 and folic acid levels were significantly lower in the DNR and DR groups compared with the CN group, there were no significant differences between the diabetes groups. Interestingly, plasma vitamin-B12 levels were found to be significantly lower in the diabetes groups compared with the CN group; further, the levels were significantly lower in the DR group compared with the DNR group. Higher homocysteine levels were significantly associated with lower vitamin-B12 and folic acid but not with other B-vitamins. Additionally, hyperhomocysteinemia and vitamin-B12 deficiency did not seem to be related to subjects' age, body mass index, or duration of diabetes. These results thus suggest a possible association between vitamin-B12 deficiency and hyperhomocysteinemia in DR. Further, the data indicate that vitamin-B12 deficiency could be an independent risk factor for DR.
Introduction
Diabetic retinopathy (DR) is one of the most common microvascular complications of diabetes and ranks as a common cause of blindness worldwide [1], [2]. Diabetic retinopathy could become a major threat to public health in the future due to the global prevalence of diabetes, which is projected to affect 438 million people by 2030 [3]. Both the duration of diabetes and its metabolic control have been identified as the risk factors most strongly associated with the development of DR [4], [5]. Diabetic retinopathy occurs in 70% of all persons having diabetes for more than 15 years. While the prevalence of DR has varied (20%–60%) in different studies, a recent study indicated that the estimated prevalence was 28.5% among US adults [6]. The prevalence of DR among urban subjects with diabetes in India was reported to be about 17% [7], whereas in a clinical study it was found to be 34% among patients with type 2 diabetes (T2D) [8]. The prevalence of DR in South India was reported to be 22.4% based on the Andhra Pradesh Eye Disease Study (APEDS) of self-reported diabetes [9]. The prevalence of DR was 0.5% in the general rural populations of South India and 10.5% among patients with diabetes [10].
Diabetic retinopathy is characterized by the appearance of vascular lesions of increasing severity, culminating in the growth of new vessels. Early or nonproliferative DR (NPDR) is marked by retinal vascular microaneurysms, blot hemorrhages, cotton-wool spots, loss of retinal pericytes, increased vascular retinal permeability, alterations in regional blood flow, and abnormal retinal microvasculature, all of which lead to retinal ischemia. Proliferative DR (PDR), the more severe state, is marked by the formation of abnormal, fragile new blood vessels that are prone to hemorrhage [11], [12].
Although the prevalence of DR increases with the duration of diabetes, studies have shown that intensive glycemic control can delay its development [4], [5]. In principle, all patients with diabetes might be expected to develop diabetic microvascular complications if hyperglycemia alone were the triggering factor for diabetic complications. It is, however, noteworthy that some patients may still develop DR even with good glycemic control. Conversely, some patients with poor glycemic control avoid this complication, notably long-surviving patients with type-1 diabetes (T1D). Therefore, the impact of strict glycemic control on prevention of diabetic complications is not that scrupulous [4], [5], [13]. Multiple factors are likely to be involved in predisposing diabetes subjects to complications, as evidenced by many but not all patients with diabetes developing one or more microvascular complications. If the predisposing factors are known, it may be possible to delay the onset and progression of these complications. Hence, there is an obvious need to understand the risk factors that are associated with diabetic complications.
Although genetic susceptibility appears to be the primary predisposing factor for DR (reviewed by Ng, 2010 [14] and Fu et al, 2010 [15]; [16]), the role of environmental factors like nutritional and dietary factors are not to be discounted. Vitamins and mineral supplementation for the management of T2D has been reported [17], but its role in the prevention and development of T2D in general and diabetic complications in particular has not been established clearly. Further, diabetes itself can alter the nutritional status [18], [19], and experiments suggest that patients with diabetes are prone to deficiency of micronutrients such as magnesium, zinc, copper, manganese, and chromium. It was observed that serum ascorbic acid, B-vitamins, and possibly 1,25-dihydroxycholcalciferol concentrations are low in diabetic patients. Studies indicate low plasma thiamine (vitamin B1) in both T1D and T2D patients [20], and high-dose thiamine and its derivatives such as benfotiamine can prevent the development of microvascular complications [21]. However, the levels of vitamin B1 in DR have not been investigated so far.
Low concentrations of folic acid and other B vitamins are associated with increased risk of vascular damage through homocysteine. Homocysteine has been extensively studied in recent years as a biomarker as well as a risk factor for vascular diseases, including vaso-occlusive diseases of the eye [22]–[26]. Homocysteine is a by-product of transmethylation reactions and detoxified by methionine synthetase, which is dependent on vitamin B12 and folate as coenzymes for its proper function [27], [28]. Determinants of hyperhomocysteinemia such as low concentrations of folate and B-vitamin coenzymes and altered activities of enzymes involved in the breakdown of homocysteine are also associated with increased risk of cardiovascular complications [26]. Nevertheless, B-vitamin status and its contribution to hyperhomocysteinemia in DR have not been examined. Therefore, we investigated the status of B-vitamins (B1, B2, B6, B12, folic acid) and homocysteine in DR.
Results
This is a hospital-based case-control study consisting of T2D subjects with (DR) and without retinopathy (DNR) along with normal control (CN) subjects. The characteristics of the CN, DNR, and DR groups are shown in Table 1. The sex distribution was approximately the same in all the groups (male and female were, respectively, 58% and 42% in CN, 56% and 44% in DNR, and 55% and 45% in DR). Further, there was no significant difference between male and female subjects in all three groups with respect to demographics and measured parameters. Therefore, the data for both men and women were pooled as a whole in the respective groups. Mean age, body mass index (BMI), and hemoglobin levels were comparable between the groups. The duration of diabetes was matched for DNR and DR (p>0.05). Amongst diabetes groups, glycosylated hemoglobin (HbA1c) levels were higher in DR compared to DNR (p<0.05). While plasma total cholesterol and low-density lipoprotein (LDL) were comparable between the groups, triglyceride levels were higher and high-density lipoprotein (HDL) was lower in the diabetes groups compared with the CN group (Table 1). Nevertheless, the levels of triglycerides and HDL were comparable between the DNR and DR groups.
Table 1. Clinical and demographic profile of control (CN) and diabetes patients without (DNR) and with retinopathy (DR).
| Parameter | Normal(n = 100) | DNR(n = 100) | DR(n = 194) | F –Value | P –Value |
| Age (years) | 53.99±9.22a | 54.76±9.29a | 55.75±8.24a | 2.1 | 0.128 |
| BMI | 23.63±3.04a | 25.21±4.19a | 24.32±4.45a | 1.6 | 0.212 |
| Hb (g/dL) | 14.89±1.73a | 14.31±2.16a | 14.11±2.32a | 1.4 | 0.255 |
| Duration (years) | - | 10.16±6.92a | 11.03±6.92a | 2.2 | 0.098 |
| Glucose (mg/dL) | 99.16±18.86a | 209.97±85.10b | 221.28±91.36b | 146.8 | 0.000 |
| HbA1c (%) | 5.64±1.14a | 8.94±2.49b | 10.33±2.94c | 86.0 | 0.000 |
| Insulin (µU/mL) | 29.14±9.94a | 38.05±22.71a | 34.75±19.58a | 1.4 | 0.255 |
| Total Cholesterol (mg/dL) | 169.39±39.65a | 167.03±53.56a | 178.01±58.47a | 0.6 | 0.552 |
| Triglycerides (mg/dL) | 129.78±63.57a | 151.99±65.43b | 163.00±77.90b | 4.3 | 0.015 |
| HDL (mg/dL) | 35.02±8.81a | 30.09±9.21b | 28.70±7.41b | 8.8 | 0.000 |
| LDL (mg/dL) | 112.69±30.13a | 110.66±29.01a | 117.11±34.05a | 0.6 | 0.570 |
Note: 1. Values are Mean ± SD.
2. Variables of glucose, HbA1c and TC were transformed into logarithmic values due to heterogeneity of variances across groups and mean values across groups were compared by oneway ANOVA ‘F’ test with post hoc test of Tukey's multiple comparisons.
3. Significant differences (p<0.05) of mean values between the groups are indicated by different superscript letters.
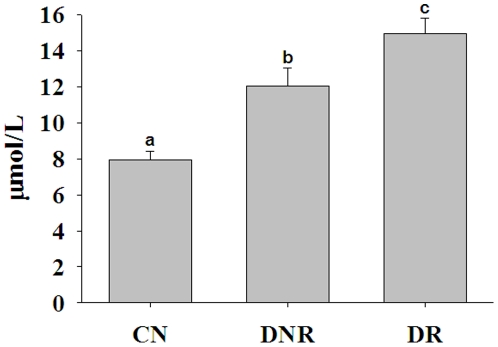
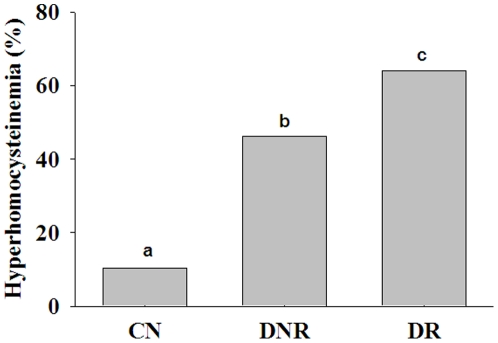
The mean plasma homocysteine levels were significantly higher in the DNR group compared with the CN group, and a further increase (p<0.05) was found in the DR group compared with the DNR group (Figure 1). However, there was a considerable overlap of hyperhomocysteinemia between the DNR and DR groups. Hence, we determined the prevalence of hyperhomocysteinemia (>12 µmol/L), which was significantly different (p<0.01) between the groups: 65% in DR, 46% in DNR, and 11% in CN (Figure 2). Further, we also compared the homocysteine levels in DR patients with a shorter duration of diabetes (<5 years) with those of DNR patients with a long duration of diabetes (>5 years). The mean value of homocysteinemia in DNR of >5 years duration was 12.4 µmol/L, while it was 14.2 µmol/L in DR of <5 years duration. In addition, when duration was controlled for in both DNR and DR groups, DR patients had significantly higher levels of homocysteine than DNR patients. Together, these results suggest an association between hyperhomocysteinemia and DR.
Figure 1. Plasma homocysteine levels.
Data represent mean ± SE in control (CN; n = 75) and diabetes patients without (DNR; n = 75) and with retinopathy (DR; n = 150). Data were transformed into log values and compared mean values across groups by oneway ANOVA ‘F’ test with post hoc test of Tukey's multiple comparisons. Significant differences (p<0.05) of mean values between the groups are indicated by different letters on the bars after adjusting the duration of diabetes between DNR and DR.
Figure 2. Prevalence (%) of hyperhomocysteinemia (>12 µmol/L) in control (CN) and diabetes patients without (DNR) and with retinopathy (DR).
Data indicate percent of subjects above 12 µmol/L of the respective group. Significant differences (p<0.05) of mean values between the groups are indicated by different letters on the bars after adjusting the duration of diabetes between DNR and DR.
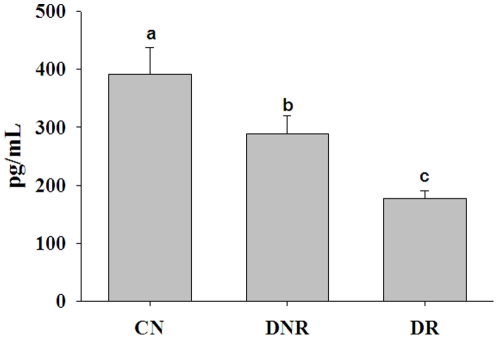
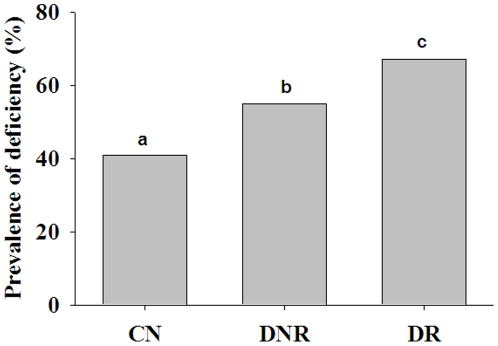
Since B-vitamins are linked to homocysteine metabolism and homeostasis, we then determined the B-vitamin levels. The vitamin B1 levels were marginally (but statistically significantly) higher in DNR compared with the CN group, but there was no significant difference between the DNR and DR groups. Furthermore, vitamin B1 levels were comparable between the DR and CN groups. While the blood levels of vitamin B2 were comparable between the groups, plasma vitamin B6 levels were significantly lower (p<0.05) in the diabetes groups (DNR and DR) compared with the CN group (Table 2). In spite of the deficiency of vitamin B6 in the diabetes groups, there was no significant difference in the mean levels of vitamin B6 between the DNR and DR groups (Table 2). Similarly, plasma folic acid levels were lower but not deficient in the diabetes groups (DNR and DR) compared with the CN group; however, the levels were comparable between the DNR and DR groups (Table 2). Plasma vitamin B12 levels were significantly lower (p<0.01) in diabetes patients (DNR and DR) compared with the CN subjects (Figure 3). Interestingly, in this study significantly lower (p<0.05) plasma vitamin B12 levels were observed in DR patients compared with DNR patients (Figure 3). The mean vitamin B12 levels in the DR group were below the normal range (200–1000 pg/mL). However, the vitamin B12 levels ranged from 20 to 1500 pg/mL with considerable overlap between the groups. Therefore, we examined the data for prevalence of vitamin B12 deficiency in the three groups. With 200 pg/mL as the cut-off level for deficiency [29]–[31], the prevalence of vitamin B12 deficiency was significantly different (p<0.001) between the groups; 67% in DR, 54% in DNR, and 41% in CN (Figure 4). The ratio of vitamin B12 to folic acid was also significantly different (p<0.05) between CN and DR groups, but not between the DNR and DR groups (data not shown). However, there was no significant difference in homocysteine and vitamin B12 levels between NPDR and PDR patients (14.72 vs. 14.11 µmol/L and 182 vs. 177 pg/mL, respectively). These results suggest that DR is associated with a higher prevalence of vitamin B12 deficiency.
Table 2. Blood and plasma levels of vitamins B1, B2, B6 and folic acid of control (CN) and diabetes patients without (DNR) and with retinopathy (DR).
| Vitamin | CN | DNR | DR | F Value | P-values |
| B1 (ng/mL) | 67.6±3.1an = 45 | 70.1±3.8bn = 45 | 67.2±3.0abn = 45 | 3.9 | 0.024 |
| B2 (ng/mL) | 231±9.3an = 45 | 248±8.2an = 45 | 238±7.0an = 45 | 1.99 | 0.143 |
| B6 (ng/mL) | 20.6±1.3an = 45 | 13.0±1.1bn = 45 | 14.6±1.0bn = 60 | 10.1 | 0.000 |
| Folic acid (ng/mL) | 10.0±0.9an = 75 | 7.8±0.6abn = 75 | 7.2±0.4bn = 150 | 4.7 | 0.009 |
Note: 1. Values are Mean ± SD.
2. Mean values across groups were compared by oneway ANOVA ‘F’ test with post hoc test of Tukey's multiple comparisons.
3. Significant differences (p<0.05) of mean values between the groups are indicated by different superscript letters.
Figure 3. Plasma vitamin-B12 levels.
Data represent mean ± SE in control (CN; n = 75) and diabetes patients without (DNR; n = 75) and with retinopathy (DR; n = 150). Data were transformed into log values and compared the mean values across groups by oneway ANOVA ‘F’ test with post hoc test of Tukey's multiple comparisons. Significant differences (p<0.05) of mean values between the groups are indicated by different letters on the bars.
Figure 4. Prevalence (%) of vitamin-B12 deficiency in control (CN) and diabetes patients without (DNR) and with retinopathy (DR).
Data indicate percent of subjects below 200 pg/mL of the respective group. Proportion Z test was done to compare prevalence between groups. Significant differences (p<0.001) of mean values between the groups are indicated by different letters on the bars.
Homocysteine levels were inversely related (p<0.05) to vitamin B12 and folic acid but not to vitamins B1, B2, and B6, when data of all the groups were considered (Table 3). Interestingly, irrespective of groups, homocysteine levels were significantly (p<0.05) associated with glucose and HbA1c levels but not related to subjects' age, BMI, or duration of diabetes (Table 3). Likewise, vitamin B12 levels were significantly (p<0.05) but inversely associated with glucose, HbA1c, and homocysteine and positively related with folic acid but not with age, BMI, or duration (Table 3). Considering that plasma folic acid levels were low but not deficient and glucose levels in diabetes groups were comparable irrespective of the presence of DR, the odds ratio for vitamin B12 is twice (95% CI, 1.1–3.7) in the DR group compared with the DNR group after adjusting for age and duration of diabetes.
Table 3. Correlations of vitamin-B12 and homocysteine with demographic and other biochemical parameters.
| Parameter | Vitamin-B12 | Homocysteine | ||
| r-value | p-value | r-value | p-value | |
| Age | 0.111 | 0.055 | 0.088 | 0.328 |
| Duration | 0.074 | 0.263 | 0.162 | 0.113 |
| BMI | 0.082 | 0.292 | 0.083 | 0.493 |
| Glucose | −0.177 | 0.016 | 0.444 | 0.000 |
| HbA1c | −0.192 | 0.034 | 0.247 | 0.047 |
| Vitamin -B1 | −0.064 | 0.571 | 0.294 | 0.066 |
| Vitamin -B2 | −0.062 | 0.577 | 0.284 | 0.056 |
| Vitamin -B6 | 0.047 | 0.614 | −0.137 | 0.383 |
| Folic acid | 0.473 | 0.000 | −0.323 | 0.002 |
| Vitamin -B12 | - | - | −0.485 | 0.000 |
| Homocysteine | −0.485 | 0.000 | - | - |
Correlations (r-value) were assessed by Spearman rank correlation. While positive r-value indicates direct correlation, negative r-value indicates inverse relationship between the variables.
Discussion
Approximately 5% of the global prevalence of blindness is considered to be due to DR, with estimates of 15%–17% in developed countries [1]. Nutritional status, particularly micronutrients, may affect the risk of DR by influencing the biochemical mechanisms underlying DR. A biochemical indicator that has generated considerable interest as a risk factor for many vascular diseases, including DR, is homocysteine. Although many studies have evaluated the association between homocysteine and DR, the results are varied and inconsistent [22], [25], [32]–[34]. Amongst the many determinants of homocysteine, B-vitamin status is a major confounding factor [23], [28], [35], [36]. However, very little is known about the extent to which B-vitamin status is associated with DR vis-à-vis homocysteine. Considering the general prevalence of micronutrient deficiency and its contribution to many metabolic disorders, such as intrauterine growth retardation, diabetes, and cardiovascular diseases in India [29]–[31], determining the status of micronutrients with regard to the prevalence and pathogenesis of DR is critical. Therefore, we evaluated the association of B-vitamin status and homocysteine with DR in a systematic way. To the best of our knowledge, a relationship between B-vitamin status, homocysteine, and DR has so far not been reported.
The results of the present study show that while lower levels of folic acid and vitamins B6 and B12 were observed in patients with diabetes irrespective of the presence of retinopathy, only vitamin B12 deficiency was associated with DR. Interestingly, a significant association was revealed between DR and hyperhomocysteinemia based on plasma homocysteine levels. Hyperhomocysteinemia has several causes, including dietary deficiencies of folic acid and vitamins B6 and B12. Furthermore, supplementation of folic acid and vitamin B12 is known to reduce homocysteine levels [23], [26]. Lack of vitamin B12 is thought to be a more important determinant for increased homocysteine, particularly in older people, and it becomes the limiting nutrient for maintaining normal plasma concentrations once folate levels are optimized. It should be noted in the present study that the mean age of the subjects was 55 years and the folate levels were still in the normal range, although lower mean levels were found in patients with diabetes. In addition, plasma homocysteine in diabetes varies depending on the presence or absence of nephropathy. However, in this study the increased levels of homocysteine were independent of renal failure because we excluded DNR and DR patients with renal complications. Therefore, lower vitamin B12 status appears to be a determining factor for increased homocysteine in DR patients in this population. Further, vitamin B12 levels did not seem to be influenced by patients' age, BMI, or duration of diabetes because the associations between vitamin B12 and these variables were not statistically significant. Moreover, vitamin B12 deficiency is two times as likely in the DR group compared with the DNR group after adjusting for age and duration of diabetes. Together, these results also suggest that vitamin B12 deficiency could be an independent risk factor for DR.
The Age-Related Eye Disease Study (AREDS) has shown that antioxidant and trace element micronutrients can reduce the risk of developing age-related macular degeneration [37]–[39]. Supplementation with some antioxidants and micronutrients, including vitamin B1 (benfotiamine), has shown encouraging results in experimental models of DR and human studies, though the findings of clinical trials with these antioxidant micronutrients have been less conclusive [40]–[42]. Interestingly, a study showed that AREDS-based micronutrients proven to be beneficial in ameliorating the lesions associated with DR in experimental rats [43]. Although we have yet to analyze the association of micronutrients other than B-vitamins with DR, the results reported here imply that a deficiency or inadequacy of vitamin B12 may predispose diabetes patients to DR. Lowered levels of cobalt, an integral component of vitamin B12 (cyanocobalamine), paired with decreased dietary intake of vitamin B12 in the DR group compared with the DNR group further supports the above implication (GBR unpublished data). It should be noted that low levels of vitamin B12 have been recognized in Indians for a long time and recent studies confirm low concentrations of vitamin B12 and the implications for diabetes and cardiovascular diseases in India [29], [30], [44]. Our study also further substantiates the general prevalence of vitamin B12 deficiency in India; about 40% adults above 50 years are deficient and the prevalence is much higher in patients with diabetes. This is the first study to show an association of B-vitamins with DR, and more controlled prospective studies are warranted to confirm the role of vitamin B12 deficiency in the development of DR.
Methods
Study design, subjects, and sample collection
This is a hospital-based case–control study conducted during April 2008 to March 2010 consisting of 300 T2D patients either with or without retinopathy (DR, n = 200 and DNR, n = 100, respectively). In addition, we recruited 100 control (CN) subjects consisting of partners, relatives, and friends of patients and employees of the National Institute of Nutrition matched for similar socioeconomic status of the T2D patients. The CN group consisted of asymptomatic subjects of age 50 years and above without any history of cardiovascular and renal complications. Subjects with T2D with and without retinopathy were recruited from patients attending the Pushpagiri Vitreo Retina Institute and were matched for duration of diabetes. Control and diabetes subjects on nutritional supplements for the last 6 months and those with a history of nephropathy (based standard renal function tests) and complications other than DR were excluded. However, many subjects with diabetes (DR and DNR) used antidiabetic medication per their physician's advice. History or presence of diabetic complications other than DR was assessed by clinical as well as biochemical methods. All patients with diabetes underwent a complete ophthalmic examination consisting of best corrected visual acuity, slit-lamp biomicroscopy, indirect ophthalmoscopy, and fundus fluorescein angiography. Diabetic retinopathy grading was done using the Early Treatment Diabetic Retinopathy Study adaptation of the modified Airlie House classification system, and DR was further categorized as NPDR and PDR [11], [45], [46]. The study was carried out in accordance with the guidelines of the Helsinki Declaration of 1975 and approved by the Institutional Ethics Committees of Pushpagiri Vitreo Retina Institute and National Institute of Nutrition. After obtaining written informed consent from all participants, venous blood samples were collected in EDTA tubes in the morning following an overnight fast. An aliquot of each whole blood sample was kept, while the remainder was separated into plasma and red blood cells [46].
Biochemical estimations
Fasting blood glucose was estimated in plasma by the glucose oxidase–peroxidase method using a kit (BioSystems, Barcelona, Spain). Glycosylated hemoglobin (HbA1c) was estimated in whole blood by ion-exchange chromatography using a kit (BioSystems). While plasma insulin was estimated by a radioimmunoassay kit (Board of Radiation and Isotope Technology-Department of Atomic Energy, Mumbai, India), the lipid profile (total cholesterol, triglycerides, HDL) was analyzed using commercially available kits (BioSystems).
Estimation of homocysteine and B-vitamins
We employed HPLC to estimate vitamins B1, B2, and B6. The levels of vitamin B1 and B2 in whole blood and B6 in plasma were measured as total thiamine pyrophosphate, flavin adenine dinucleotide, and pyridoxal-5′-phosphate, respectively, based on previously reported methods [47] using commercially available HPLC kits (Recipe Chemicals and Instruments GmbH, Germany). Plasma levels of vitamin B12 and folic acid were measured by a solid phase radioimmunoassay method using a commercially available kit designed for simultaneous measurement of these vitamins (Siemens Medical Solutions Diagnostics, Los Angeles, CA, USA). Radioactivity was measured by a gamma counter with a dual channel for determining 57Co and 125I simultaneously (Perkin Elmer, 3 wizard 1480, USA). Plasma total homocysteine levels were measured by HPLC using a Supelcosil™ LC-18-DB (150 mm by 4.6 mm) column according to methods reported previously [48], [49].
Statistical analysis
Statistical analysis was performed using SPSS for Windows version 15.0. Mean and SD or SE values of vitamins and homocysteine, BMI, age, and duration of disease were calculated. No outliers were found in the data based on Grubb test. Comparison of mean values of these variables across groups was done by one-way ANOVA F test with post hoc Tukey test. Log transformations were also performed to stabilize the normality of the skewed variables for glucose, HbA1c, HDL, homocysteine, and vitamin B12. Proportion Z test was used for comparison of prevalence of vitamin B12 deficiency. The relationship between homocysteine and vitamin B12 with age, duration of disease, BMI, glucose, and vitamins B1, B2, B6, and folic acid was calculated by Spearman rank correlation coefficients, and risk was estimated by odds ratio with logistic regression model. Two-tailed test was considered for all statistical tests. The level of significance was p<0.05.
Sample size for the estimation of vitamins and homocysteine
Although the data for demographic and clinical parameters were collected for all 400 subjects, the actual minimum required sample size for vitamins and homocysteine was determined assuming 95% CI and 80% power and using SD of respective vitamins and homocysteine. Hence the data on vitamins and homocysteine were obtained on a subsample. Nevertheless, the sample size was increased for homocysteine and vitamin B12, for strengthening their association with DR.
Acknowledgments
The authors are grateful to all the participants for their cooperation in this study. We thank Drs. Nilanjana Deb-Joardar, O. Muralidhar, and P. Sunitha, Pushpagiri Vitreo Retina Institute, Hyderabad, for their service in the recruitment and medical examination of study patients. The author also acknowledges the help of Ms. C. Akileshwari in the manuscript preparation. Part of this work was presented at the 19th Biennial Meeting of the International Society for Eye Research, July 18–23, 2010, Montreal, Canada, and a part of the work was presented at the ARVO (Association for Research in Vision and Ophthalmology) Annual Meeting, May 1–5, 2011, Fort Lauderdale, FL, USA.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: GBR received grants from the Department of Science and Technology (SR/SO/HS/0055/2008), Government of India; AS received a research fellowship from the Indian Council of Medical Research, India. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–851. [PMC free article] [PubMed] [Google Scholar]
- 2.Kempen JH, O'Colmain BJ, Leske MC, Haffner SM, Klein R, et al. Eye Diseases Prevalence Research Group: The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122:552–563. doi: 10.1001/archopht.122.4.552. [DOI] [PubMed] [Google Scholar]
- 3.International Diabetes Federation. The Diabetes Atlas. 4th edition. Brussels: 2009. [Google Scholar]
- 4.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 5.Turner R. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 6.Zhang X, Jinan B, Saaddine, Chou CF, Cotch MF, et al. Prevalence of diabetic retinopathy in the United States, 2005–2008. JAMA. 2010;304:649–656. doi: 10.1001/jama.2010.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rema M, Premkumar S, Anitha B, Deepa R, Pradeepa R, et al. Prevalence of diabetic retinopathy in urban India: the Chennai Urban Rural Epidemiology Study (CURES) eye study. Invest Ophthalmol Vis Sci. 2005;46:2328–33. doi: 10.1167/iovs.05-0019. [DOI] [PubMed] [Google Scholar]
- 8.Rema M, Ponnaiya M, Mohan M. Prevalence of retinopathy in non-insulin dependent diabetes mellitus at a diabetes center in southern India. Diab Res Clin Pract. 1996;34:29–36. doi: 10.1016/s0168-8227(96)01327-7. [DOI] [PubMed] [Google Scholar]
- 9.Dandona L, Dandona R, Naduvilath TJ, McCarty CA, Rao GN. Population based assessment of diabetic retinopathy in an urban population in southern India. Br J Ophthalmol. 1999;83:937–40. doi: 10.1136/bjo.83.8.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nirmalan PK, Katz J, Robin AL, Tielsch JM, Namperumalsamy P, et al. Prevalence of vitreoretinal disorders in a rural population of Southern India: the Aravind Comprehensive Eye Study. Arch Ophthalmol. 2004;122:581–586. doi: 10.1001/archopht.122.4.581. [DOI] [PubMed] [Google Scholar]
- 11.Viswanath K, Murray McGavi DD. Diabetic retinopathy: Clinical findings and management. Community Eye Health. 2003;16:21–24. [PMC free article] [PubMed] [Google Scholar]
- 12.Moss SE, Klein R, Klein BE. The 14-year incidence of visual loss in a diabetic population. Ophthalmol. 1998;105:998–1003. doi: 10.1016/S0161-6420(98)96025-0. [DOI] [PubMed] [Google Scholar]
- 13.Keenan HA, Costacou T, Sun JK, Doria A, Cavellerano J, et al. Clinical factors associated with resistance to microvascular complications in diabetic patients of extreme disease duration: the 50-year medalist study. Diabetes Care. 2007;30:1995–1997. doi: 10.2337/dc06-2222. [DOI] [PubMed] [Google Scholar]
- 14.Ng DP. Human genetics of diabetic retinopathy-current perspectives. J Ophthalmol, 2010. 2010;pii:172593. doi: 10.1155/2010/172593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu YP, Hallman DM, Gonzalez VH, Klein BE, Klein R, et al. Identification of diabetic retinopathy genes through a genome-wide association study among Mexican-Americans from Starr County, Texas. J Ophthalmol, 2010. 2010;pii:861291. doi: 10.1155/2010/861291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rema M, Saravanan G, Deepa R, Mohan V. Familial clustering of diabetic retinopathy in South Indian type 2 diabetic patients. Diab Med. 2002;19:910–916. doi: 10.1046/j.1464-5491.2002.00820.x. [DOI] [PubMed] [Google Scholar]
- 17.Martini LA, Catania AS, Ferreira SR. Role of vitamins and minerals in prevention and management of type 2 diabetes mellitus. Nutr Rev. 2010;68:341–54. doi: 10.1111/j.1753-4887.2010.00296.x. [DOI] [PubMed] [Google Scholar]
- 18.Failla ML, Kiser RA. Altered tissue content and cytosol distribution of trace metals in experimental diabetes. J Nutr. 1981;11:1900–1909. doi: 10.1093/jn/111.11.1900. [DOI] [PubMed] [Google Scholar]
- 19.Mooradian AD, Morley JE. Micronutrient status in diabetes mellitus. Am J Clin Nutr. 1987;45:877–895. doi: 10.1093/ajcn/45.5.877. [DOI] [PubMed] [Google Scholar]
- 20.Thornalley PJ, Babaei-Jadidi R, Ali AH. High prevalence of low plasma thiamine concentration in diabetes linked to a marker of vascular disease. Diabetologia. 2007;50:2164–2170. doi: 10.1007/s00125-007-0771-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thornalley PJ. The potential role of thiamin (vitamin B1) in diabetic complications. Curr Diab Rev. 2005;1:287–298. doi: 10.2174/157339905774574383. [DOI] [PubMed] [Google Scholar]
- 22.Brazoins L, Rowley K, Itsiopoulos K, Harper CA, O'Dea K. Homocysteine and diabetic retinopathy. Diabetes Care. 2008;31:50–56. doi: 10.2337/dc07-0632. [DOI] [PubMed] [Google Scholar]
- 23.Wright AD, Martin N, Dodson PM. Homocysteine, folates and the eye. Eye. 2008;22:989–993. doi: 10.1038/sj.eye.6703061. [DOI] [PubMed] [Google Scholar]
- 24.Coral K, Angayarkanni N, Gomathy N, Bharathselvi M, Pukhraj R, et al. Homocysteine levels in the vitreous of proliferative diabetic retinopathy and rhegmatogenous retinal detachment: its modulating role on lysyl oxidase. Invest Ophthalmol Vis Sci. 2009;50:3607–12. doi: 10.1167/iovs.08-2667. [DOI] [PubMed] [Google Scholar]
- 25.Hoogeveen EK, Kostense PJ, Eysink PE, Polak BC, Beks PJ, et al. Hyperhomocysteinemia is associated with the presence of retinopathy in type 2 diabetes mellitus: the Hoorn Study. Arch Intern Med. 2000;160:2984–2990. doi: 10.1001/archinte.160.19.2984. [DOI] [PubMed] [Google Scholar]
- 26.Stanger O, Herrmann W, Pietrzik K, Fowler B, Geisel J, et al. DACH-LIGA homocystein (german, austrian and swiss homocysteine society): consensus paper on the rational clinical use of homocysteine, folic acid and B-vitamins in cardiovascular and thrombotic diseases: guidelines and recommendations. Clin Chem Lab Med. 2003;41:1392–403. doi: 10.1515/CCLM.2003.214. [DOI] [PubMed] [Google Scholar]
- 27.Chang PK, Gordon RK, Tal J, Zeng GC, Docot BP, et al. S-Adenosylmethionine and methylation. FASEB J. 1996;10:471–480. [PubMed] [Google Scholar]
- 28.Wijekoon EP, Brosnan ME, Brosnan JT. Homocysteine metabolism in diabetes. Biochem Soc Trans. 2007;35 doi: 10.1042/BST0351175. [DOI] [PubMed] [Google Scholar]
- 29.Misra A, Vikram NK, Pandey RM, Dwivedi M, Ahmad FU, et al. Hyperhomocysteinemia and low intakes of folic acid and vitamin B 12 in urban North India. Eur J Nutr. 2002;41:68–77. doi: 10.1007/s003940200010. [DOI] [PubMed] [Google Scholar]
- 30.Yajnik CS, Deshpande SS, Lubree HG, Naik SS, Bhat DS, et al. Vitamin B12 deficiency and hyperhomocysteinemia in rural and urban Indians. J Assoc Physicians India. 2006;54:775–782. [PubMed] [Google Scholar]
- 31.Muthayya S, Kurpad AV, Duggan CP, Bosch RJ, Dwarkanath P, et al. Low maternal vitamin B12 status is associated with intrauterine growth retardation in urban South Indians. Eur J Clin Nutr. 2006;60:791–801. doi: 10.1038/sj.ejcn.1602383. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein M, Leibovitch I, Yeffimov I, Gavendo S, Sela BA, et al. Hyperhomocysteinemia in patients with diabetes mellitus with and without diabetic retinopathy. Eye. 2004;18:460–465. doi: 10.1038/sj.eye.6700702. [DOI] [PubMed] [Google Scholar]
- 33.Looker HC, Fagot-Campagna A, Gunter EW, Pfeiffer CM, Narayan KM, et al. Homocysteine as a risk factor for nephropathy and retinopathy in type 2 diabetes. Diabetologia. 2003;46:766–772. doi: 10.1007/s00125-003-1104-x. [DOI] [PubMed] [Google Scholar]
- 34.Neugebauer S, Baba T, Kurokawa K, Watanabe T. Defective homocysteine metabolism as a risk factor for diabetic retinopathy. Lancet. 1997;349:473–474. doi: 10.1016/S0140-6736(05)61185-3. [DOI] [PubMed] [Google Scholar]
- 35.Selhub J, Jacques PF, Wilson PWF, Rash D, Rosenberg IH. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. JAMA. 1993;270:2693–2698. doi: 10.1001/jama.1993.03510220049033. [DOI] [PubMed] [Google Scholar]
- 36.Vermeulen EGJ, Stehouwer CDA, Twisk JDR, Van den Berg M, de Jong SC, et al. Effect of homocysteine lowering treatment with folic acid plus vitamin B6 on progression of subclinical atherosclerosis: a randomized, placebo-controlled trial. Lancet. 2000;355:517–522. doi: 10.1016/s0140-6736(99)07391-2. [DOI] [PubMed] [Google Scholar]
- 37.Chiu CJ, Milton RC, Gensler G, Taylor A. Association between dietary glycemic index and age-related macular degeneration in nondiabetic participants in the Age-Related Eye Disease Study. Am J Clin Nutr. 2007;86:180–188. doi: 10.1093/ajcn/86.1.180. [DOI] [PubMed] [Google Scholar]
- 38.Giovanni JPS, Chew EY, Clemons TE. Age-Related Eye Disease Study Research Group. The relationship of dietary lipid intake and age-relatedmacular degeneration in a case-control study: AREDS report No. 20. Arch Ophthalmol. 2007;125:671–679. doi: 10.1001/archopht.125.5.671. [DOI] [PubMed] [Google Scholar]
- 39.Giovanni JPS, Chew EY, Clemons TE. Age-Related Eye Disease StudyResearch Group. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS report No. 22. Arch Ophthalmol. 2007;125:1225–1232. doi: 10.1001/archopht.125.9.1225. [DOI] [PubMed] [Google Scholar]
- 40.Hammes HP, Du X, Edelstein D. Benfotiamine blocks three major path-ways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med. 2003;9:294–299. doi: 10.1038/nm834. [DOI] [PubMed] [Google Scholar]
- 41.Lee CTC, Emma I, Gayton, Chir MB, Beulens JW, et al. Micronutrients and diabetic retinopathy: A systematic review. Ophthalmol. 2010;117:71–78. doi: 10.1016/j.ophtha.2009.06.040. [DOI] [PubMed] [Google Scholar]
- 42.Mayer-Davis EJ, Bell RA, Reboussin BA, Rushing J, Marshall JA, et al. The San Luis Valley Study: Antioxidant nutrient intake and diabetic retinopathy. Ophthalmol. 1998;105:2264–2270. doi: 10.1016/S0161-6420(98)91227-1. [DOI] [PubMed] [Google Scholar]
- 43.Kowluru RA, Kanwar M, Chan P, Zhang JP. Inhibition of retinopathy and retinal metabolic abnormalities in diabetic rats with AREDS-based micronutrients. Arch Ophthalmol. 2008;126:266–1272. doi: 10.1001/archopht.126.9.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yajnik CS, Deshpande SS, Jackson AA, Refsum H, Rao S, et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia. 2008;51:29–38. doi: 10.1007/s00125-007-0793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Early Treatment of Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic colour fundus photographs - an extension of the modified Airlie House Classification. ETDRS Report 10. Ophthalmol. 1991;98:786–806. [PubMed] [Google Scholar]
- 46.Reddy GB, Satyanarayana A, Balakrishna N, Ayyagari R, Padma M, et al. Erythrocyte aldose reductase activity and sorbitol levels in diabetic retinopathy. Mol Vis. 2008;14:593–601. [PMC free article] [PubMed] [Google Scholar]
- 47.Botticher B, Botticher D. Determination of B1, B2, B6 Vitamers in blood. Int J Vit Nutr Res. 1987;57:273–278. [PubMed] [Google Scholar]
- 48.Pitla S, Nagalla B. Gender-related differences in the relationship between plasma homocysteine, anthropometric and conventional biochemical coronary heart disease risk factors in middle-aged Indians. Ann Nutr Metab. 2009;54:1–6. doi: 10.1159/000199452. [DOI] [PubMed] [Google Scholar]
- 49.Ubbink JB, Hayward Vermaak WJ, Bissbort S. Rapid high-performance liquid chromatographic assay for total homocysteine levels in human serum. J Chromatogr. 1991;565:441–446. doi: 10.1016/0378-4347(91)80407-4. [DOI] [PubMed] [Google Scholar]