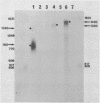
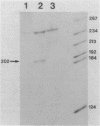
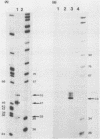
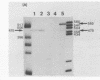
Abstract
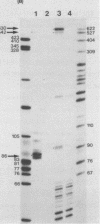
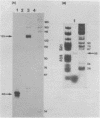
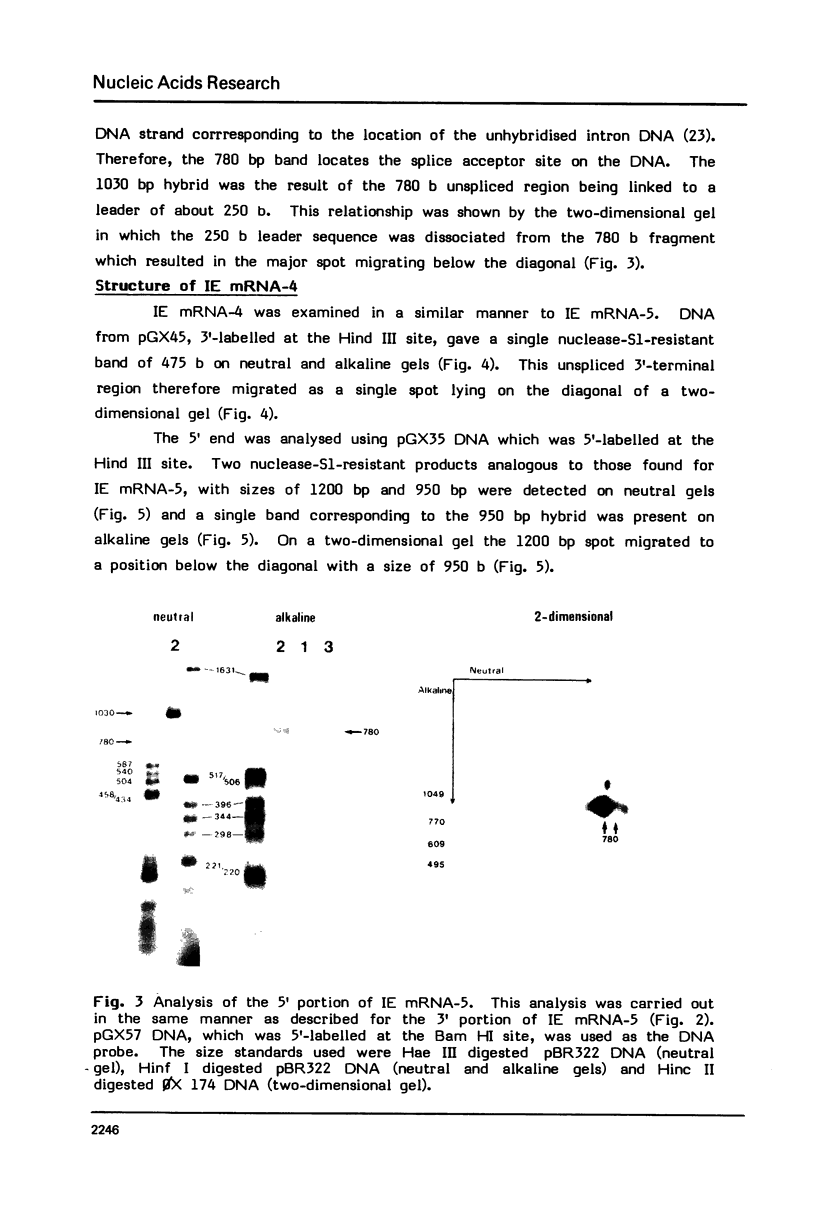
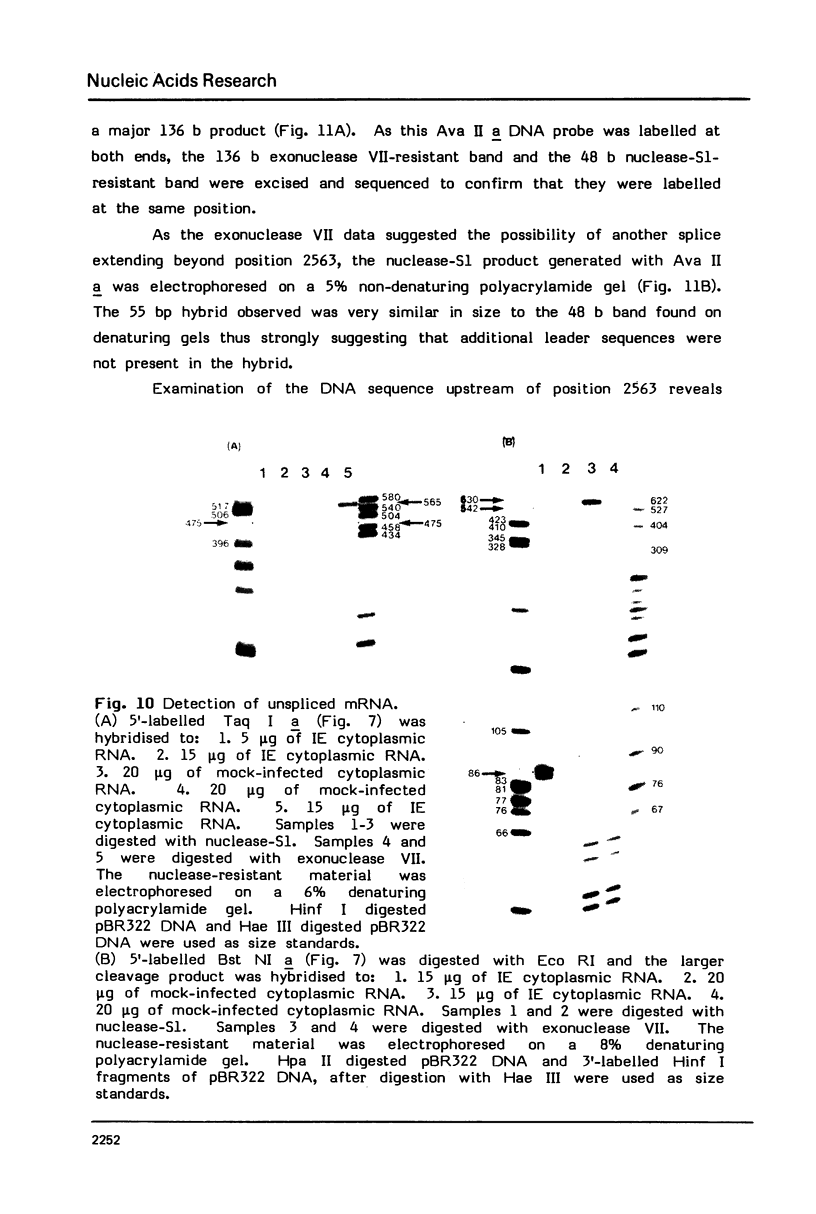
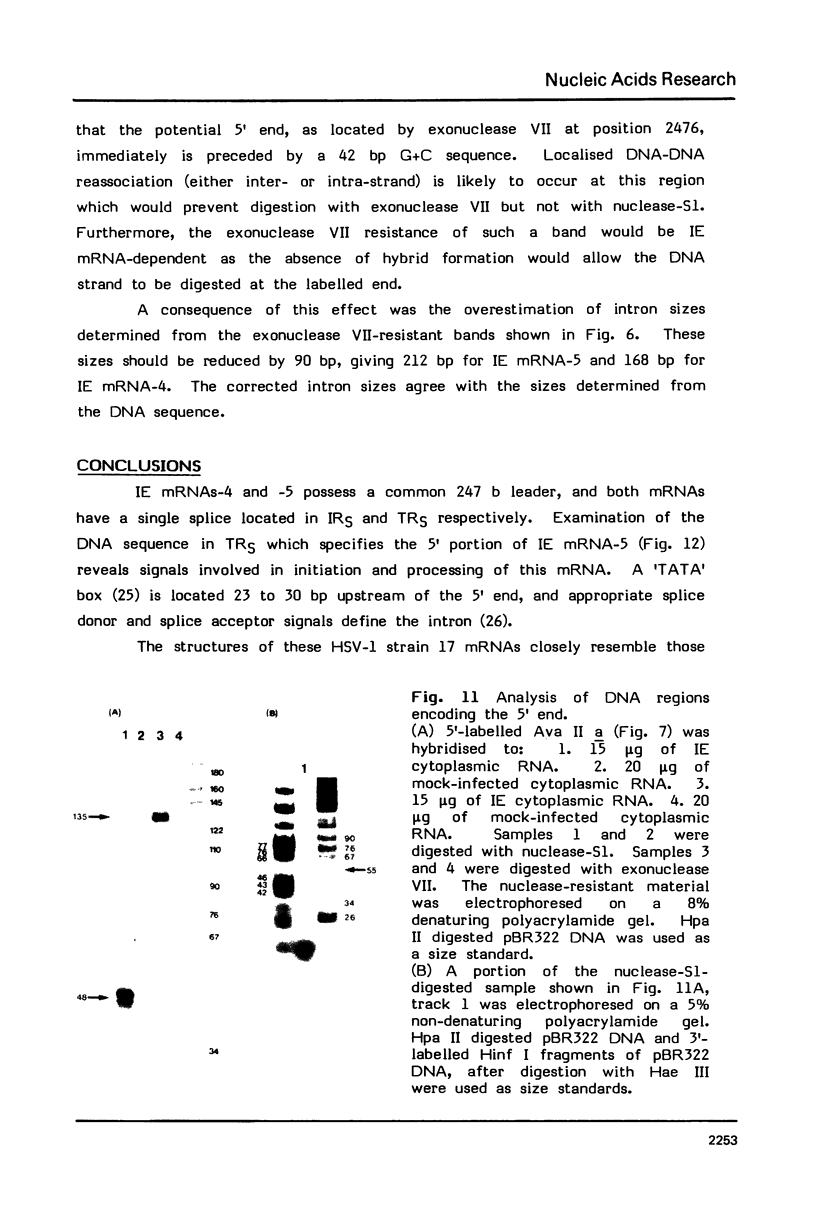
The structures of two HSV-1 immediate-early mRNAs have been determined by nuclease-digestion procedures using 5' and 3' end-labelled DNA probes. These mRNAs, which map across the junctions between the short unique (US) and short repeat (IRS and TRS) genome regions, have common 5' portions located in IRS and TRS. The 3' portions, which extend into opposite ends of US, and unique. The DNA sequence encoding the common 5' portions largely comprises a 247 base pair (bp) leader region and a single intron of variable size. The variation in intron length is due to different copy numbers of a 22 bp tandem reiteration. A small proportion of the mRNA population is unspliced, but otherwise is identical to the more abundant spliced species.
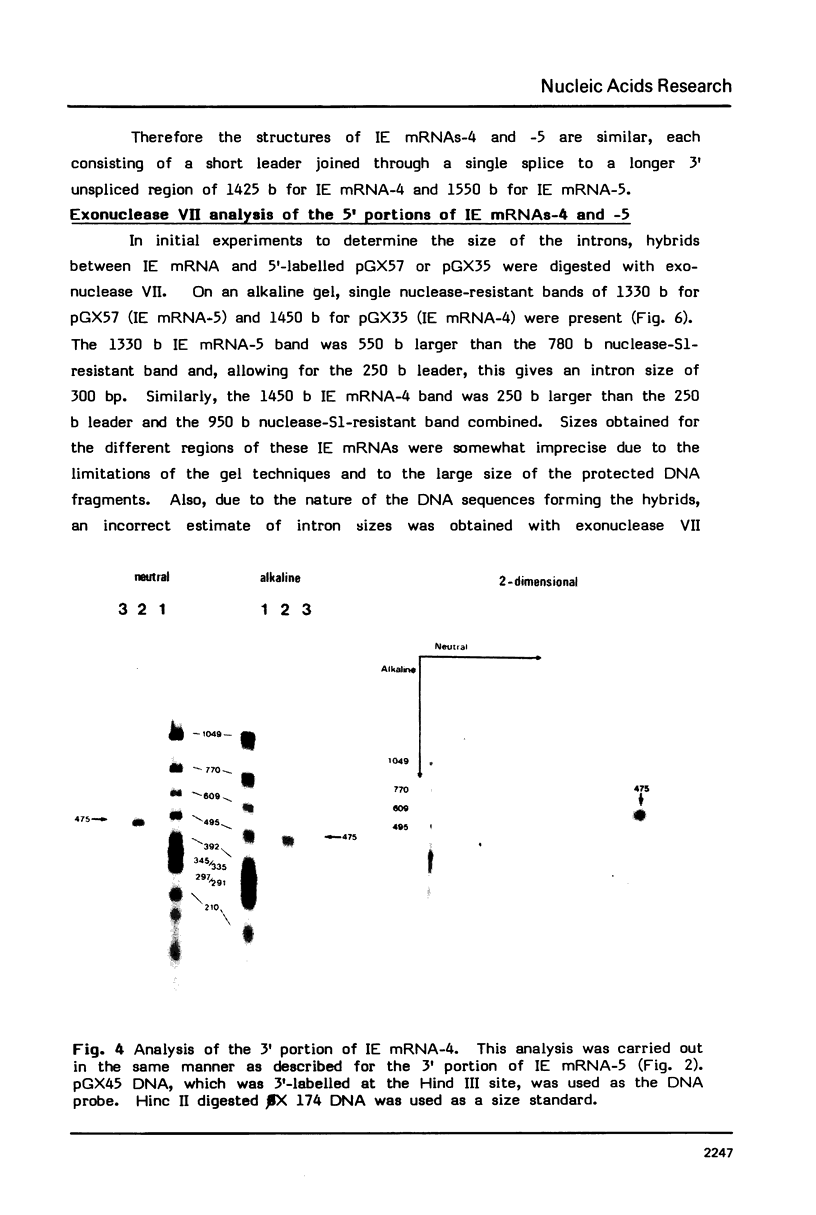
Full text
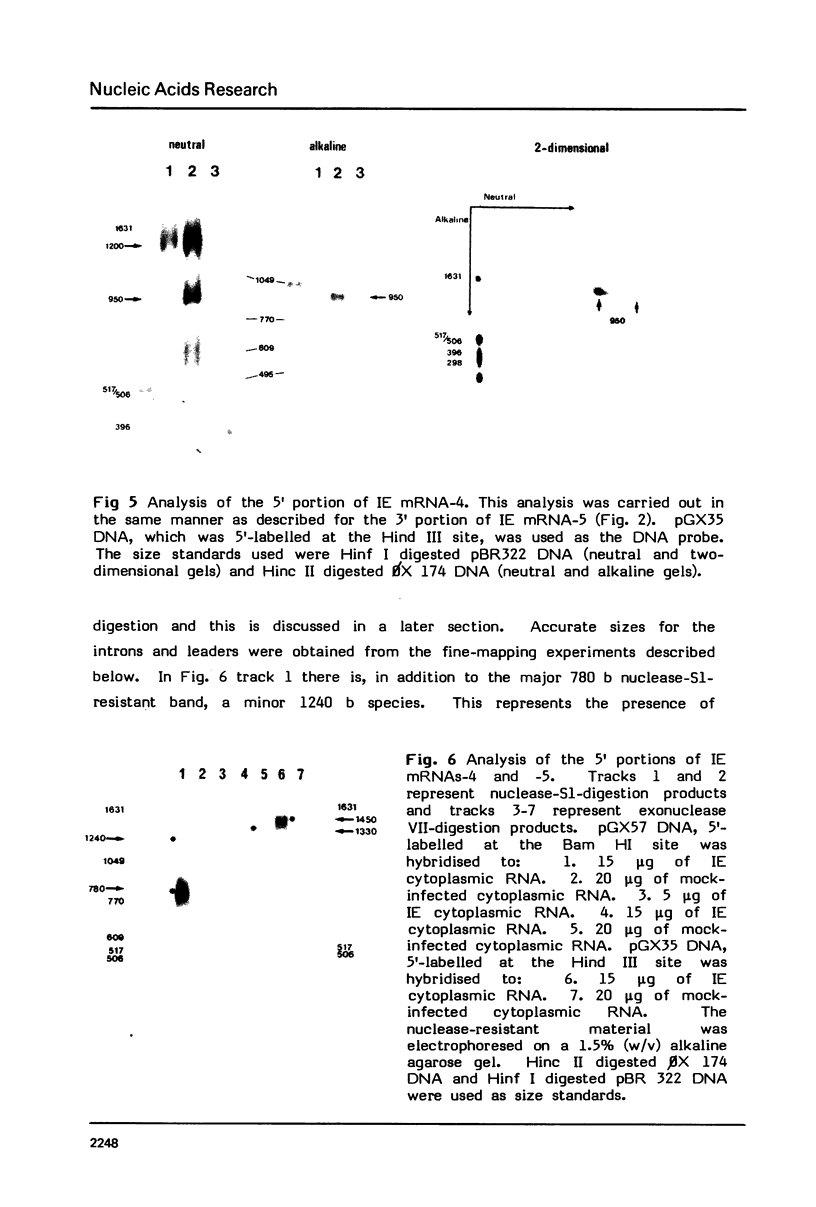
PDF



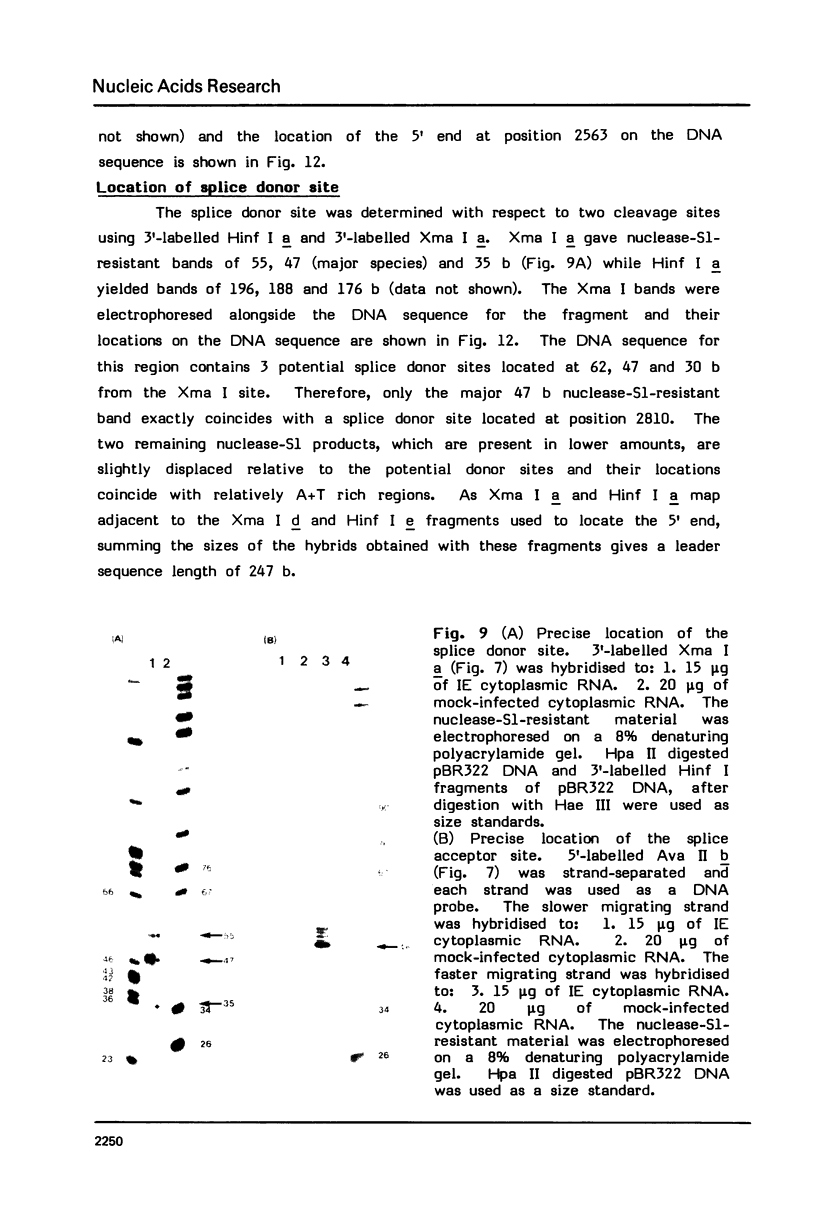




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. P., Frink R. J., Devi G. B., Gaylord B. H., Costa R. H., Wagner E. K. Detailed characterization of the mRNA mapping in the HindIII fragment K region of the herpes simplex virus type 1 genome. J Virol. 1981 Mar;37(3):1011–1027. doi: 10.1128/jvi.37.3.1011-1027.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlock L., Jones N. C. Synthesis of an unspliced cytoplasmic message by an adenovirus 5 deletion mutant. Nature. 1981 Dec 10;294(5841):572–574. doi: 10.1038/294572a0. [DOI] [PubMed] [Google Scholar]
- Chase J. W., Richardson C. C. Exonuclease VII of Escherichia coli. Mechanism of action. J Biol Chem. 1974 Jul 25;249(14):4553–4561. [PubMed] [Google Scholar]
- Clements J. B., McLauchlan J., McGeoch D. J. Orientation of herpes simplex virus type 1 immediate early mRNA's. Nucleic Acids Res. 1979 Sep 11;7(1):77–91. doi: 10.1093/nar/7.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. B., Watson R. J., Wilkie N. M. Temporal regulation of herpes simplex virus type 1 transcription: location of transcripts on the viral genome. Cell. 1977 Sep;12(1):275–285. doi: 10.1016/0092-8674(77)90205-7. [DOI] [PubMed] [Google Scholar]
- Costa R. H., Devi B. G., Anderson K. P., Gaylord B. H., Wagner E. K. Characterization of a major late herpes simplex virus type 1 mRNA. J Virol. 1981 May;38(2):483–496. doi: 10.1128/jvi.38.2.483-496.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo F., Campadelli-Fiume G., Foa-Tomasi L., Cassai E. Evidence that herpes simplex virus DNA is transcribed by cellular RNA polymerase B. J Virol. 1977 Mar;21(3):996–1001. doi: 10.1128/jvi.21.3.996-1001.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Wilkie N. M. Nucleotide sequences of the joint between the L and S segments of herpes simplex virus types 1 and 2. J Gen Virol. 1981 Aug;55(Pt 2):315–331. doi: 10.1099/0022-1317-55-2-315. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Schaffer P. A. Fine-structure mapping and functional analysis of temperature-sensitive mutants in the gene encoding the herpes simplex virus type 1 immediate early protein VP175. J Virol. 1980 Oct;36(1):189–203. doi: 10.1128/jvi.36.1.189-203.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton A. J., Clements J. B. Temporal regulation of herpes simplex virus type 2 transcription and characterization of virus immediate early mRNA's. Nucleic Acids Res. 1980 Jun 25;8(12):2627–2645. doi: 10.1093/nar/8.12.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Frink R. J., Anderson K. P., Wagner E. K. Herpes simplex virus type 1 HindIII fragment L encodes spliced and complementary mRNA species. J Virol. 1981 Aug;39(2):559–572. doi: 10.1128/jvi.39.2.559-572.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon F., O'Hare K., Perrin F., LePennec J. P., Benoist C., Cochet M., Breathnach R., Royal A., Garapin A., Cami B. Organisation and sequences at the 5' end of a cloned complete ovalbumin gene. Nature. 1979 Mar 29;278(5703):428–434. doi: 10.1038/278428a0. [DOI] [PubMed] [Google Scholar]
- Ghosh P. K., Roy P., Barkan A., Mertz J. E., Weissman S. M., Lebowitz P. Unspliced functional late 19S mRNAs containing intervening sequences are produced by a late leader mutant of simian virus 40. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1386–1390. doi: 10.1073/pnas.78.3.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward G. S., Jacob R. J., Wadsworth S. C., Roizman B. Anatomy of herpes simplex virus DNA: evidence for four populations of molecules that differ in the relative orientations of their long and short components. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4243–4247. doi: 10.1073/pnas.72.11.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. C., Roizman B. Regulation of herpesvirus macromolecular synthesis. VIII. The transcription program consists of three phases during which both extent of transcription and accumulation of RNA in the cytoplasm are regulated. J Virol. 1979 Aug;31(2):299–314. doi: 10.1128/jvi.31.2.299-314.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M., Roizman B. Regulation of herpesvirus macromolecular synthesis: nuclear retention of nontranslated viral RNA sequences. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4322–4326. doi: 10.1073/pnas.71.11.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Lindberg U. Characterization of messenger ribonucleoprotein and messenger RNA from KB cells. Proc Natl Acad Sci U S A. 1972 Mar;69(3):681–685. doi: 10.1073/pnas.69.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdale D. M., Brown S. M., Lang J., Subak-Sharpe J. H., Koprowski H., Warren K. G. Variations in herpes simplex virus isolated from human ganglia and a study of clonal variation in HSV-1. Ann N Y Acad Sci. 1980;354:291–308. doi: 10.1111/j.1749-6632.1980.tb27973.x. [DOI] [PubMed] [Google Scholar]
- Mackem S., Roizman B. Regulation of herpesvirus macromolecular synthesis: transcription-initiation sites and domains of alpha genes. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7122–7126. doi: 10.1073/pnas.77.12.7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McLauchlan J., Clements J. B. A 3' co-terminus of two early herpes simplex virus type 1 mRNAs. Nucleic Acids Res. 1982 Jan 22;10(2):501–512. doi: 10.1093/nar/10.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif I., Khoury G., Dhar R. BKV splice sequences based on analysis of preferred donor and acceptor sites. Nucleic Acids Res. 1979 Jul 25;6(10):3387–3398. doi: 10.1093/nar/6.10.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick P., Berthelot N. Inverted repetitions in the chromosome of herpes simplex virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):667–678. doi: 10.1101/sqb.1974.039.01.080. [DOI] [PubMed] [Google Scholar]
- Swanstrom R. I., Wagner E. K. Regulation of synthesis of herpes simplex type 1 virus mRNA during productive infection. Virology. 1974 Aug;60(2):522–533. doi: 10.1016/0042-6822(74)90346-8. [DOI] [PubMed] [Google Scholar]
- Treisman R., Novak U., Favaloro J., Kamen R. Transformation of rat cells by an altered polyoma virus genome expressing only the middle-T protein. Nature. 1981 Aug 13;292(5824):595–600. doi: 10.1038/292595a0. [DOI] [PubMed] [Google Scholar]
- Wagner M. J., Sharp J. A., Summers W. C. Nucleotide sequence of the thymidine kinase gene of herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1441–1445. doi: 10.1073/pnas.78.3.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. J., Clements J. B. A herpes simplex virus type 1 function continuously required for early and late virus RNA synthesis. Nature. 1980 May 29;285(5763):329–330. doi: 10.1038/285329a0. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Clements J. B. Characterization of transcription-deficient temperature-sensitive mutants of herpes simplex virus type 1. Virology. 1978 Dec;91(2):364–379. doi: 10.1016/0042-6822(78)90384-7. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Preston C. M., Clements J. B. Separation and characterization of herpes simplex virus type 1 immediate-early mRNA's. J Virol. 1979 Jul;31(1):42–52. doi: 10.1128/jvi.31.1.42-52.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. J., Sullivan M., Vande Woude G. F. Structures of two spliced herpes simplex virus type 1 immediate-early mRNA's which map at the junctions of the unique and reiterated regions of the virus DNA S component. J Virol. 1981 Jan;37(1):431–444. doi: 10.1128/jvi.37.1.431-444.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. J., Umene K., Enquist L. W. Reiterated sequences within the intron of an immediate-early gene of herpes simplex virus type 1. Nucleic Acids Res. 1981 Aug 25;9(16):4189–4199. doi: 10.1093/nar/9.16.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziff E. B. Transcription and RNA processing by the DNA tumour viruses. Nature. 1980 Oct 9;287(5782):491–499. doi: 10.1038/287491a0. [DOI] [PubMed] [Google Scholar]