Abstract
Background
Taxanes have been extensively used as adjuvant chemotherapy for the treatment of early or operable breast cancer, particularly in high risk, node-negative breast cancer. Previous studies, however, have reported inconsistent findings regarding their clinical efficacy and safety. We investigated disease-free survival (DFS), overall survival (OS), and drug-related toxicities of taxanes by a systematic review and meta-analysis.
Methodology and Principal Findings
We systematically searched PubMed, EMBASE, the Cochrane Center Register of Controlled Trials, proceedings of major meetings, and reference lists of articles for studies conducted between January 1980 and April 2011. Randomized controlled trials (RCTs) comparing chemotherapy with and without taxanes in the treatment of patients with early-stage or operable breast cancer were eligible for inclusion in our analysis. The primary endpoint was DFS. Nineteen RCTs including 30698 patients were identified, including 8426 recurrence events and 3803 deaths. Taxanes administration yielded a 17% reduction of hazard ratio (HR) for DFS (HR = 0.83, 95% CI 0.79–0.88, p<0.001) and a 17% reduction of HR for OS (HR = 0.83, 95% CI 0.77–0.90, p<0.001). For high risk, node-negative breast cancer, the pooled HR also favoured the taxane-based treatment arm over the taxane-free treatment arm (HR = 0.82, 95% CI 0.77–0.87, p = 0.022). A significantly increased rate of neutropenia, febrile neutropenia, fatigue, diarrhea, stomatitis, and oedema was observed in the taxane-based treatment arm.
Conclusions/Significance
Adjuvant chemotherapy with taxanes could reduce the risk of cancer recurrence and death in patients with early or operable breast cancer, although the drug-related toxicities should be balanced. Furthermore, we also demonstrated that patients with high risk, node-negative breast cancer also benefited from taxanes therapy, a result that was not observed in previous studies.
Introduction
Breast cancer (BC) is a leading cause of morbidity and mortality among women worldwide [1]–[2]. Most BCs (>75%) are diagnosed at an early stage or are operable [3]. For these patients, it is essential to administer adjuvant chemotherapy to reduce the risk of recurrence [4]–[5]. Taxanes(paclitaxel or docetaxel) are active cytotoxic agents that promote polymerization of tubulin and stabilization of microtubules by preventing their disassembly. Recently, several randomized trials have been conducted to identify the efficacy and safety of taxane-based adjuvant chemotherapy for early or operable BC, often with conflicting results. Additionally, the efficacy of taxanes for patients with high risk, node-negative BC remains uncertain. Two previous meta-analyses [6]–[7] have been conducted to determine the efficacy and safety of this agent in patients with BC although investigators did not present the efficacy of taxanes in node-negative BC. We undertook a meta-analysis to update the results and resolve the uncertain efficacy of taxanes in women with node-negative BC. Furthermore, we also reported the efficacy of taxanes treatment in some specific subgroups.
Methods
Search strategy and selection criteria
Randomized controlled trials (RCTs) and literatures trials resulted of taxane therapy were eligible for inclusion in our meta-analysis, with no restriction on language or publication status (i.e., published, unpublished, in press or in progress). The search process was initiated as follows:
Electronic databases (from January 1980 to April 2011): We retrieved literatures from PubMed, EmBase and the Cochrane Center Register of Controlled Trials, using the search terms of “early breast cancer,” “operable breast cancer,” “node-negative breast cancer,” “stage I or stage II breast cancer,” and “docetaxel or taxane or paclitaxel”.
Additional resources: Two important annual meetings including American Society of Clinical Oncology Annual Scientific Meeting (ASCO) and the San Antonio Breast Cancer Symposium (from 1995 to 2011), were manually searched. In addition, information about registered randomized controlled trials was obtained from the website http://clinicaltrials.gov/ (US NIH). Relevant reviews and meta-analyses regarding the role of taxane-based adjuvant chemotherapy in patients with early or operable BC were examined for potential trials.
This review was conducted and reported according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) Statement issued in 2009 (Checklist S1) [8].
The eligible RCTs should meet the following inclusion criteria: (a) early or operable BC; (b) high quality RCT comparing a taxane-based adjuvant chemotherapy arm with a taxane-free adjuvant chemotherapy arm; and (c) the primary outcome was either disease-free survival (DFS) or overall survival (OS). Search and selection of studies was conducted independently by 2 investigators (Y-YQ and X-JG).
Data extraction and quality assessment
Data extraction and quality assessment were conducted independently by 2 investigators (Y-YQ and HL) using a standardized data recording form and Jadad scale [9]. Information was examined and adjudicated independently by 2 additional investigators (X-FY and Y-HZ) referring to the original articles after data extraction and assessment.
The following information was extracted from each eligible study: study design, year of publication, number of patients, regimen details, median follow-up, median age, node status, main endpoint, the hazard ratios (HRs) and corresponding 95% confidence interval (CI), and the drug-related toxicities (WHO grades ≥3). For studies which reported HRs for the taxane-free treatment arm rather than the taxane-based treatment arm, HRs were recalculated by the exponential of negative ln(HR). If HRs and 95% CIs were not directly obtained from the original articles, they were estimated indirectly using reported events in each arm and the corresponding P value as suggested by Tierney et al [10]. If information could not be obtained from the original literature, direct communication with the authors was initiated. The quantitative 5-point Jadad scale was used as a gauge to assess the quality of the inclusive trials in our study.
Statistical analysis
The primary efficacy outcome of our meta-analysis was disease-free survival (DFS). DFS was defined as time from randomization to any recurrence of BC (local or distant), new primary BC, a second cancer, or death. The subgroup analyses were prospectively planned according to node status, drug dosage, schedule, observation period, menopausal status, hormone receptor status, and tumor size. Interaction tests were performed to compare differences between the 2 estimates [11]. The adverse events (AEs) of taxane-based treatment were analyzed as drug-related toxicities (WHO grades ≥3). The pooled estimation plotted as odd ratios (ORs) was obtained [12]. A pooled OR and 95% CI greater than 1 indicated a statistically significant result.
Heterogeneity between trials was evaluated by chi-square ( ) test and I-squared (I2) statistic [13]. These indices assess the percentage of variability across studies attributable to heterogeneity rather than chance. Statistical heterogeneity was considered significant when p<0.10 for the
) test and I-squared (I2) statistic [13]. These indices assess the percentage of variability across studies attributable to heterogeneity rather than chance. Statistical heterogeneity was considered significant when p<0.10 for the  test or I2>50%. Although fixed-effects model and random-effects model yielded similar conclusions, we chose to use the random-effects model, which assumed that the true underlying effect varied among included trials. Moreover, many investigators consider that the random-effects model to be a more natural choice than fixed effects model in medical decision-making contexts [14]–[15]. The probability of publication bias was assessed with the funnel plots and the Begg-Mazumdar test [16]. Additionally, the pooled HR estimates were recalculated after excluding low-scoring trials to test their sensitivity. All reported P values were two-sided and P values less than 0.05 were regarded as statistically significant. Statistical analyses were carried out using STATA 11.0 (Stata Corporation, Lakeway, Texas, USA).
test or I2>50%. Although fixed-effects model and random-effects model yielded similar conclusions, we chose to use the random-effects model, which assumed that the true underlying effect varied among included trials. Moreover, many investigators consider that the random-effects model to be a more natural choice than fixed effects model in medical decision-making contexts [14]–[15]. The probability of publication bias was assessed with the funnel plots and the Begg-Mazumdar test [16]. Additionally, the pooled HR estimates were recalculated after excluding low-scoring trials to test their sensitivity. All reported P values were two-sided and P values less than 0.05 were regarded as statistically significant. Statistical analyses were carried out using STATA 11.0 (Stata Corporation, Lakeway, Texas, USA).
Results
Trial characteristic
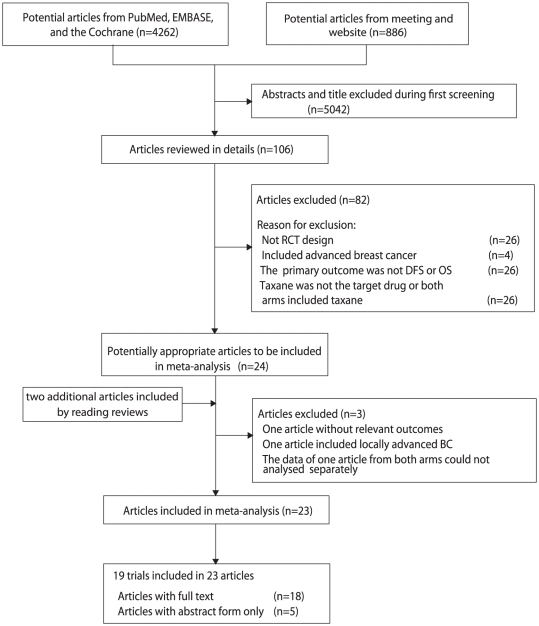
Twenty-two potential trials were identified and 3 trials [17]–[19] of them were excluded for specific reasons listed in flow chart (Figure 1 and Protocol S1 [8]). The remaining 19 trials [20]–[42] included 30698 women with early or operable BC. Two trails [30]–[31], [33] were published in abstracts and the remaining 17 trials [20]–[27], [29], [32], [34]–[37], [39]–[42] were published in full articles. All of the trials included were open-label, phase III, randomized trials. Concurrent regimens were conducted in 5 trials [20], [22], [25], [27], [37], while sequential regimens were tested in the remaining 14 trials [21], [23]–[24], [29]–[30], [32]–[36], [38], [40]–[42]. The GEICAM 9805 [20] recruited patients with node-negative breast cancer, and the ECTO trial [38] only recruited patients with tumor size >2 cm. Recurrence/relapse-free survival (RFS) was the main endpoint of FinHer and Boccardo et al [23], [34], and freedom from progression (FFP) was the main endpoint of the ECTO trial [38]. However, the definition of RFS and FFP of these 3 trials was similar to DFS, so we included them. Fourteen [20]–[21], [23]–[24], [27], [29]–[30], [32], [34], [37]–[38], [40]–[42] of the 19 trials had Jadad scores of 3, and 5 trials [22], [26], [33], [35]–[36] were assessed with scores of 2. Other detailed information from each trial was also listed in Table S1.
Figure 1. Flow diagram of the trials search and selection process.
Total effect of efficacy
Data for DFS were available from all 19 trials [20]–[25], [27], [29]–[30], [32]–[38], [40]–[42] with 8426 events reported. The taxane-based treatment arm was associated with a clinically and statistically significant 17% improvement in DFS when compared with the taxane-free treatment arm (HR = 0.83, 95% CI 0.79–0.88; p<0.001; Figure 2) under a random-effect model, and there was no evidence of significant heterogeneity among individual trials (p = 0.194, I2 = 21.4%). The taxane-based treatment arm had lower risk of recurrence in both concurrent and sequential regimens than the taxane-free treatment arm (p value 0.002 and 0.000, respectively; test for interaction, p = 0.046).
Figure 2. Taxane-based therapy versus non-taxane-based therapy: meta-analysis of disease-free survival (DFS).
NR: not report.
OS was reported in 17 trials [20]–[25], [27], [29]–[30], [32], [34]–[35], [37]–[38], [40]–[42] of the 19 trials (BIG 2–98 and NSABP B-27 [33], [36] did not reported OS data), including 25 407 patients who were recruited in the meta-analysis on the risk of death, resulting in 3803 deaths. The efficacy of taxenes on reducing the risk of death was presented more both in all trials (HR of overall 0.83, 95% CI 0.77–0.90) and the trials of different therapy regimens (Figure 3). We found no evidence of publication bias on either DFS or OS by the funnel plots and the Begg-Mazumdar test.
Figure 3. Taxane-based therapy versus non-taxane-based therapy: meta-analysis of overall survival (OS).
NR: not report.
In addition, the sensitivity analysis was conducted among 14 trials [20]–[21], [23]–[24], [27], [29]–[30], [32], [34], [37]–[38], [40]–[42] after excluding 5 trials [22], [26], [33], [35]–[36] with a low Jadad score (score<3). The estimated pooled HRs for DFS (HR 0.82, 95% CI 0.76–0.88) and OS (HR 0.85, 95% CI 0.78–0.92) all favoured the arm treated with taxanes when compared with arm without, and no evidence of significant heterogeneity was observed among individual trials.
Subgroup analysis of efficacy
Node status
Only 4 trials [20], [24], [26]–[27] reported HR for DFS of patients with node-negative BC. The pooled HR of DFS for these trials was 0.83 (95% CI 0.71–0.97, p = 0.022; Figure 4), which corresponds to a 17% reduction in the risk of recurrence among patients with node-negative BC who received taxanes (docetaxel). Among the 19 included trials, 10 trials [21], [23], [29]–[30], [32]–[33], [35], [37], [41]–[42] included only patients with node-positive disease, and the pooled HR of these trials for DFS also favoured taxane treatment (HR 0.82, 95% CI 0.77–0.87; Table 1).
Figure 4. Efficacy of taxanes in subgroup of node-negative, node = 1–3, node  4: meta-analysis of DFS.
4: meta-analysis of DFS.
NR: not report.
Table 1. Taxane-based therapy versus non-taxane-based therapy in subgroups: meta-analysis of disease-free survival (DFS).
| Trials | DFS | p value | Test of Heterogeneity | ||||
| HR | 95% CI |

|
I2 | p | |||
| Docetaxel | 20,21,22,24,25,27,30,33–37 | 0.83 | 0.77 to 0.90 | 0.000 | 22.85 | 43.1% | 0.044 |
 75 mg/m2
75 mg/m2
|
20,22,25,27,33,37 | 0.81 | 0.73 to 0.91 | 0.000 | 7.63 | 34.5% | 0.178 |
| 100 mg/m2 | 21,24,30,34–36 | 0.84 | 0.76 to 0.94 | 0.000 | 14.98 | 53.3% | 0.036 |
| Paclitaxel | 23,29,32,38,40–42 | 0.82 | 0.76 to 0.88 | 0.000 | 5.42 | 0.0% | 0.770 |
| Weekly sequential | 29 | 0.77 | 0.62 to 0.95 | 0.016 | . | . | . |
| Every 2 weeks | 32,40 | 0.84 | 0.67 to 1.04 | 0.109 | 0.10 | 0.0% | 0.752 |
| Every 3 weeks | 23,38,41,42 | 0.82 | 0.73 to 0.93 | 0.003 | 4.96 | 39.5% | 0.175 |
| Trials with N+ only | 21,23,29,30,32,33,35,37,41,42 | 0.82 | 0.77 to 0.87 | 0.000 | 5.69 | 23.8% | 0.080 |
| Observation Period | |||||||
Median Follow–up
 5 years
5 years
|
34,35,37,38 | 0.73 | 0.64 to 0.83 | 0.000 | 5.36 | 25.3% | 0.253 |
| Median Follow–up >5 years | 20–25,27,29,30,32,33,36,40–42 | 0.86 | 0.82 to 0.90 | 0.000 | 19.78 | 24.1% | 0.181 |
| Menopausal Status | |||||||
| Premenopausal | 20,21,23,30,35,37 | 0.78 | 0.65 to 0.94 | 0.010 | 9.63 | 48.1% | 0.086 |
| Postmenopausal | 20,21,23,30,35,37 | 0.78 | 0.68 to 0.90 | 0.001 | 5.47 | 8.5% | 0.362 |
| ER Status | |||||||
| ER+ | 20,21,22,24,27,29,30,35,37,40,41 | 0.83 | 0.76 to 0.90 | 0.000 | 13.74 | 27.2% | 0.185 |
| ER− | 20,21,22,24,27,29,30,35,37,40,41 | 0.80 | 0.73 to 0.88 | 0.000 | 5.88 | 0.0% | 0.826 |
Furthermore, 5 trials [21], [24], [31], [35], [37] reported HRs for DFS separately in the nodes 1–3 and nodes  4 subgroups. The pooled HRs also show greater efficacy in the taxane-based treatment arm of the subgroups with nodes 1–3 and nodes
4 subgroups. The pooled HRs also show greater efficacy in the taxane-based treatment arm of the subgroups with nodes 1–3 and nodes  4 (HR 0.73, 95% CI 0.59–0.90, and HR 0.89, 95% CI 0.80–0.98, respectively; Figure 4).
4 (HR 0.73, 95% CI 0.59–0.90, and HR 0.89, 95% CI 0.80–0.98, respectively; Figure 4).
Drug dosage, schedule, and observation period
The subgroup analysis of DFS was stratified to trials of different taxane agents (paclitaxel or docetaxel) with different dosage and schedule (docetaxel  75 mg/m2 or = 100 mg/m2 and paclitaxel weekly, every 2 weeks, or every 3 weeks) and different observation periods (median follow-up
75 mg/m2 or = 100 mg/m2 and paclitaxel weekly, every 2 weeks, or every 3 weeks) and different observation periods (median follow-up  5 years or >5 years). Most of the results showed that the taxane-based treatment arm provided greater efficacy on improving DFS among patients with early or operable BC (Table 1). An 18% HR reduction (95% CI 0.76–0.88) was observed for paclitaxel therapy, a 17% HR reduction (95% CI 0.77–0.90) was observed for docetaxel therapy, and a 14% HR reduction (95% CI 0.82–0.90) was observed in the treatment arm after follow-up of greater than 5 years. Not all taxane schedules might be equal, and table 1 also indicated that there was no significantly statistical difference between the paclitaxel every 2 weeks arm and control arm (HR 0.84, 95% CI 0.67 to 1.04), although analyses of remaining 2 paclitaxel schedules (weekly and every 3 weeks) favoured the taxane-based treatment arm.
5 years or >5 years). Most of the results showed that the taxane-based treatment arm provided greater efficacy on improving DFS among patients with early or operable BC (Table 1). An 18% HR reduction (95% CI 0.76–0.88) was observed for paclitaxel therapy, a 17% HR reduction (95% CI 0.77–0.90) was observed for docetaxel therapy, and a 14% HR reduction (95% CI 0.82–0.90) was observed in the treatment arm after follow-up of greater than 5 years. Not all taxane schedules might be equal, and table 1 also indicated that there was no significantly statistical difference between the paclitaxel every 2 weeks arm and control arm (HR 0.84, 95% CI 0.67 to 1.04), although analyses of remaining 2 paclitaxel schedules (weekly and every 3 weeks) favoured the taxane-based treatment arm.
Others
Subgroup analysis of patients according to their menopausal status, ER (oestrogen receptor) status, and tumor size was shown in table 1 and figure 5. Superior efficacy of taxanes was found in both premenopausal (HR 0.78, 95% CI 0.65–0.94) and postmenopausal patients (HR 0.78, 95% CI 0.68–0.90) after pooling data from 6 trials [20]–[21], [23], [31], [35], [37]. Efficacy data of adjuvant chemotherapy according to tumer size (<2 cm and  2 cm) was available in 4 trials [20], [24], [27], [35] and 5 trials [20], [24], [27], [35], [38], respectively. The pooled HRs for DFS favoured the taxane-based treatment arm when compared with the taxane-free treatment arm both in the tumor size <2 cm subgroup (HR 0.84, 95% CI 0.72–0.99) and in the tumor size
2 cm) was available in 4 trials [20], [24], [27], [35] and 5 trials [20], [24], [27], [35], [38], respectively. The pooled HRs for DFS favoured the taxane-based treatment arm when compared with the taxane-free treatment arm both in the tumor size <2 cm subgroup (HR 0.84, 95% CI 0.72–0.99) and in the tumor size  2 cm subgroup (HR 0.87, 95% CI 0.75–0.99) (Figure 5). Eleven trials [20]–[21], [23]–[24], [27], [29], [31], [35], [37], [40]–[41] reported subgroup results of ER status. For ER-positive subgroup the pooled HR of 0.83 (95% CI 0.76–0.90) for DFS indicated a 17% reduction in the risk of recurrence presented in the taxanes-based treatment arm, and for the ER-negative subgroup the pooled HR of 0.80 (95% CI 0.73–0.88) for DFS indicated a 20% reduction in the risk of recurrence presented among patients received taxanes. However, subgroup analysis of HER-2 (human epidermal growth factor receptor-2) status (5 trials [23]–[25], [29], [37] included) showed no significantly statistical difference in efficacy of taxanes when comparing the taxane-based treatment arm with the taxane-free treatment arm in either HER-2-positive group (HR 0.84, 95% CI 0.68–1.03) or HER-2-negative group (HR 0.87, 95% CI 0.73–1.03; Figure 6). Although statistical significance was not attained, when examining the data by HER-2 status, there were similar trends favouring taxanes.
2 cm subgroup (HR 0.87, 95% CI 0.75–0.99) (Figure 5). Eleven trials [20]–[21], [23]–[24], [27], [29], [31], [35], [37], [40]–[41] reported subgroup results of ER status. For ER-positive subgroup the pooled HR of 0.83 (95% CI 0.76–0.90) for DFS indicated a 17% reduction in the risk of recurrence presented in the taxanes-based treatment arm, and for the ER-negative subgroup the pooled HR of 0.80 (95% CI 0.73–0.88) for DFS indicated a 20% reduction in the risk of recurrence presented among patients received taxanes. However, subgroup analysis of HER-2 (human epidermal growth factor receptor-2) status (5 trials [23]–[25], [29], [37] included) showed no significantly statistical difference in efficacy of taxanes when comparing the taxane-based treatment arm with the taxane-free treatment arm in either HER-2-positive group (HR 0.84, 95% CI 0.68–1.03) or HER-2-negative group (HR 0.87, 95% CI 0.73–1.03; Figure 6). Although statistical significance was not attained, when examining the data by HER-2 status, there were similar trends favouring taxanes.
Figure 5. Efficacy of taxanes in subgroups of tumor size <2 cm, tumor size  2 cm: meta-analysis of DFS.
2 cm: meta-analysis of DFS.
Figure 6. Efficacy of taxanes in subgroups of HER-2 status: meta-analysis of DFS.
Toxicities
Data concerning AEs were extracted from 15 trials [20]–[21], [23]–[25], [27], [29], [32], [34]–[35], [37], [39]–[42]. A summary of drug-related toxicities ( grade 3) was shown in figure 7. The pooled ORs of each group, stratified according to grade 3 or greater toxicities, indicated that a significant increase in toxicity associated with taxane treatment was observed for neutropenia (OR = 1.54, 95%CI 1.10–2.15), febrile neutropenia (OR = 2.28, 95% CI 1.25–4.16), fatigue (OR = 2.10, 95% CI 1.37–3.22), diarrhea (OR = 2.16, 95% CI 1.32–3.53), stomatitis (OR = 1.68, 95% CI 1.04–2.71), and oedema (OR = 6.61, 95%CI 2.14–20.49). However, heterogeneity among trials was found in these analyses, possibly due to the use of different agents at various dosage and the use of different control arms. Moreover, subgroup analyses were performed based on stratification with the 2 types of taxanes. The results suggested that paclitaxel was associated with statistically fewer toxicity events when compared with taxane-free therapy in some toxicities, such as neutropenia (OR = 0.72, 95%CI 0.53–0.98), and febrile neutropenia (OR = 0.51, 95%CI 0.32–0.79) but not in other toxicities. However, figure 7 showed that docetaxel was associated with a significant increase in neutropenia, febrile neutropenia, leukopenia, stomatitis, oedema, fatigue and/or asthenia, and diarrhea (Figure 7).
grade 3) was shown in figure 7. The pooled ORs of each group, stratified according to grade 3 or greater toxicities, indicated that a significant increase in toxicity associated with taxane treatment was observed for neutropenia (OR = 1.54, 95%CI 1.10–2.15), febrile neutropenia (OR = 2.28, 95% CI 1.25–4.16), fatigue (OR = 2.10, 95% CI 1.37–3.22), diarrhea (OR = 2.16, 95% CI 1.32–3.53), stomatitis (OR = 1.68, 95% CI 1.04–2.71), and oedema (OR = 6.61, 95%CI 2.14–20.49). However, heterogeneity among trials was found in these analyses, possibly due to the use of different agents at various dosage and the use of different control arms. Moreover, subgroup analyses were performed based on stratification with the 2 types of taxanes. The results suggested that paclitaxel was associated with statistically fewer toxicity events when compared with taxane-free therapy in some toxicities, such as neutropenia (OR = 0.72, 95%CI 0.53–0.98), and febrile neutropenia (OR = 0.51, 95%CI 0.32–0.79) but not in other toxicities. However, figure 7 showed that docetaxel was associated with a significant increase in neutropenia, febrile neutropenia, leukopenia, stomatitis, oedema, fatigue and/or asthenia, and diarrhea (Figure 7).
Figure 7. Summary of drug-related toxicities grade 3 or greater.
Discussion
Nineteen randomized, open-label, phase III trials of 30698 women (with 8426 recurrence events and 3803 deaths) were included to examine the role of taxanes added in adjuvant chemotherapy for patients with early or operable breast cancer. The pooled HRs for DFS and OS for all available trials showed that taxane-based therapy was associated with significant reduction in the risk of recurrence and death, and the similar results were observed in the sensitivity analysis. There was no significant evidence of statistical heterogeneity among individual trials. This meta-analysis also indicated that taxane-based adjuvant chemotherapy was more efficacious in improving DFS and OS when compared with taxane-free therapy. This result was consistent with results reported in 2 previous reviews [6]–[7].
The study conducted by Sparano et al reported that there were no significant differences in DFS between the paclitaxel-treated groups and docetaxel-treated groups [43]. This finding was similar to the results of our study (test for interaction between docetaxel and paclitaxel, p = 0.824) as well as the meta-analysis conducted by De Laurentiis et al (test for interaction between docetaxel and paclitaxel, p = 0.16) [6]. However, Sparano et al also reported that greater benefits in improving DFS were observed in the group receiving paclitaxel weekly and the group receiving docetaxel every 3 weeks when compared with the group receiving paclitaxel every 3 weeks. The results of our subgroup analysis according to the paclitaxel schedule showed that patients receiving paclitaxel weekly and every 3 weeks, but not those receiving paclitaxel every 2 weeks, demonstrated superior efficacy to patients in the control arm of the study. We recognize that comparisons between 2 types of taxanes can be confounded by drug schedule, as shown in the Sparano trial [43]. Because of insufficient data, we were unable to make firm conclusion about the efficacy of various drug schedule (only 4 trials [23], [38], [41]–[42] included in paclitaxel every 3 weeks group and 1 trial [29] included in paclitaxel weekly group).
The results of subgroup analysis also indicated that there were significantly gains in DFS in the taxane-based treatment arm, except for patients with HER-2 status. The pooled HRs of analysis of among women with either HER-2 positive or HER-2 negative status showed a favorable trend but no statistical difference between the 2 treatment arms. However, De Laurentiis et al reported that the HR for DFS in the HER-2 positive subgroup was 0.51 (95% CI 0.29–0.87) and in the HER-2 negative subgroup was 0.70 (95% CI 0.55–0.91) [6]. Only 2 trials [29], [37] were included in their research and the estimated HR may be less reliable. Nowadays, the predictive value of hormone receptor (particularly HER-2) in determining taxane responsiveness remains controversial [44]–[45]. Additional 3 trials [23]–[25] were included in our analysis, and the pooled HR did not confirm the predictive value of HER-2, however, the point estimate of HRs of most trials favoured taxanes. Therefore, the presence of HER-2 as a predictor of taxane responsiveness needs to be further investigated.
A different toxicity profile was confirmed between taxane-based and taxane-free treatment arms. Drug-related toxicities, such as neutropenia, febrile neutropenia, and oedema, were reported in both this study and previous meta-analyses [7]. In our study, the subgroup analyses of toxicity showed paclitaxel may be associated with fewer toxicities than docetaxel. However, this conclusion could not be definitively confirmed because of less available data on paclitaxel. We need more data to support our result in the future. Unfortunately, only 3 of these trials provided information about quality of life (QoL) [20], [37], [40]. These trials showed no significant difference in QoL scores between the 2 treatment arms. Although GEICAM 9805 trial and BCIRG 001 trial [20], [37] found that taxane was associated with a transient reduction in QoL scores, these scores returned to baseline values afterwards.
The efficacy of taxanes for patients with node-negative breast cancer, longer observation periods, and varying tumor size was not reported in the 2 previous meta-analyses. To resolve these uncertainties, we investigated the efficacy by subgroup analysis of available trials.
There was insufficient evidence to define the efficacy of taxanes among the patients with high-risk, node-negative BC, although the efficacy for node-positive, early-stage breast cancer had been confirmed. The benefits of adjuvant chemotherapy (cyclophosphamide combined with methotrexate and 5-fluorouracil) for node-negative disease was confirmed in 3 trials (NSABP B-13, B-19, B-23) [46]. The GEICAM 9805 trial [20] randomly assigned 1060 patients with high-risk, axillary-nod-negative BC to TAC(docetaxel, doxorubicin, and cyclophosphamide) arm or FAC (fluorouracil, doxorubicin, and cyclophosphamide) arm, and the trial reported that the hazard ratio for DFS significantly favoured the TAC arm (HR 0.68, 95% CI 0.49–0.93, p = 0.01). However, 4 trials [24], [26]–[27], [36] reported the efficacy of taxane (docetaxel) for patients with node-negative BC in subgroup analysis. The results of these 4 trials did not show a significant difference between the docetaxel and control arms (the NSABP B-27 trial [36] did not report exact data of HR for DFS in subgroup analysis). Patients in the GEICAM 9805 trial received docetaxel with 6 cycles, and patients in the other 4 trials received docetaxel with 4 cycles. More therapy cycles of docetaxel may be much more beneficial for node-negative BC. Nevertheless, our study did not compare different therapy cycles because of the limited availability of trials. Data concerning DFS from 4 available trials [20], [24], [26]–[27] were pooled (excluded NSABP B-27), and the result (HR 0.83 95% CI 0.71–0.97) significant favored the docetaxel regimen. Therefore, this subgroup analysis provided evidence that docetaxel was useful in improving DFS among patients with high-risk, node-negative BC, which was consistent with the result of the GEICAM 9805 trial.
Trials included in this study were observed with various median follow-up periods. Our aim was to determine whether taxane-based therapy could be efficacious against BC during longer observation periods. The results demonstrated that the benefits of taxanes were still observed during longer follow-up period (HR 0.86, 95% CI 0.82–0.90). However, the results in 2 two trials [22]–[23] (Anglo-Celtic trial and the Boccardo et al. trial with median follow-up of 99 and 102 months, respectively) did not show significant efficacy of taxanes in improving DFS (HR 0.79, 95% CI 0.56–1.12; HR 1.16, 95% CI 0.79–1.75; for these two trials respectively). These results differed from results of our meta-analysis, possibly due to the small sample size of these 2 trials (only 363 patients recruited in Anglo-Celtic trial and 244 patients in Boccardo et al trial). Our study provided stronger evidence demonstrating the efficacy of taxanes for early or operable BC under longer observation periods. Moreover, RCTs which recruit larger population with longer follow-up time will be required to confirm the efficacy of the agent.
The patients included in the ECTO trial [38] all had tumor size greater than 2 cm, with efficacy results showing that the paclitaxel produced significant benefit for this group of patients. For the remaining 4 trials [20], [24], [27], [35], the results did not show any significantly statistical difference between 2 arms in subgroup analysis of tumor size (either tumor size <2 cm or  2 cm). However, the estimated HRs using the data of these 5 trials showed taxane-based therapy was statistically superior in reducing the risk of cancer recurrence among patients with both tumor size <2 cm and
2 cm). However, the estimated HRs using the data of these 5 trials showed taxane-based therapy was statistically superior in reducing the risk of cancer recurrence among patients with both tumor size <2 cm and  2 cm compared with taxane-free therapy (Figure 5). Consequently, the pooled analysis confirmed the efficacy of taxanes and was consistent with the results of the overall analysis.
2 cm compared with taxane-free therapy (Figure 5). Consequently, the pooled analysis confirmed the efficacy of taxanes and was consistent with the results of the overall analysis.
Our meta-analysis also has several potential limitations. First, our study was based on abstracted data and not on individual patient data (IPD), which may not provide robust estimation for the association. Second, the characteristics of the included trials were varied in the follow-up observation period, therapy regimen, agents and dosage. Third, there may be several trials with available data that were ongoing or unpublished at the time of the writing of this manuscript that were not included in this meta-analysis, in addition to the 19 trials included in this study. Consequently, publication bias may be unavoidable in this meta-analysis. However, the results form the funnel plots and the Begg-Mazumdar test did not indicate significant publication bias.
Despite the limitations of our research, the results strongly suggest that adjuvant chemotherapy that includs taxanes provides a significant advantage in improving both DFS and OS among patients with early or operable BC compared with therapy without taxanes. Moreover, the subgroup analysis concerning node status also demonstrated that docetaxel-based therapy is superior to docetaxel-free therapy, for high risk node-negative BC, in reducing the risk of cancer recurrence. Additional well-designed RCTs with varying drug schedules for both operable and node-negative BC are warranted to further evaluate these conclusions. The benefit of taxanes should be balanced against their toxicity, and additional data on QoL should be provided in further analysis. Physicians should take these adverse drug events into consideration and interpret the results carefully and comprehensively in clinical practice.
Supporting Information
PRISMA Checklist.
(DOC)
PRISMA Flowchart.
(DOC)
Baseline characteristics for included trials.
(DOC)
Acknowledgments
We all thank Zhi-Chao Jin for his generous assistance with this study. Editorial support in the final preparation of the manuscript was provided by Editage Company.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: National Nature Science Foundation of China (30872186, 81072388), a grant from the leading talents of science in Shanghai 2010 (022) and a grant sponsored by Program of Shanghai Subject Chief Scientist (09XD1405500). The funders had no role in the study design, data collection, analysis, decision to publish or preparation of the manuscript. The corresponding author had full access to all the data in the study and had the final responsibility for the decision to submit for publication.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Verma S, Lavasani S, Mackey J, Pritchard K, Clemons M, et al. Optimizing the management of her2-positive early breast cancer: the clinical reality. Curr Oncol. 2010;17:20–33. doi: 10.3747/co.v17i4.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.EBCTCG. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 5.EBCTCG. Polychemotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists' Collaborative Group. Lancet. 1998;352:930–942. [PubMed] [Google Scholar]
- 6.De Laurentiis M, Cancello G, D'Agostino D, Giuliano M, Giordano A, et al. Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol. 2008;26:44–53. doi: 10.1200/JCO.2007.11.3787. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson T, Wilcken N, Vagg R, Ghersi D, Nowak AK. Taxanes for adjuvant treatment of early breast cancer. Cochrane Database Syst Rev. 2007:CD004421. doi: 10.1002/14651858.CD004421.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Plos Medicine. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 9.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 10.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326:219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deeks JJ, Higgins JPT, Altman DG. Analyzing data and undertaking meta-analyses. In: Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.0.1. Oxford, UK: The Cochrane Collaboration: chap 9; 2008. [Google Scholar]
- 13.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making. 2005;25:646–654. doi: 10.1177/0272989X05282643. [DOI] [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 17.Bonnefoi H, Potti A, Delorenzi M, Mauriac L, Campone M, et al. Validation of gene signatures that predict the response of breast cancer to neoadjuvant chemotherapy: a substudy of the EORTC 10994/BIG 00-01 clinical trial. Lancet Oncology. 2007;8:1071–1078. doi: 10.1016/S1470-2045(07)70345-5. [DOI] [PubMed] [Google Scholar]
- 18.Buzdar AU, Singletary SE, Valero V, Booser DJ, Ibrahim NK, et al. Evaluation of paclitaxel in adjuvant chemotherapy for patients with operable breast cancer: Preliminary data of a prospective randomized trial. Clinical Cancer Research. 2002;8:1073–1079. [PubMed] [Google Scholar]
- 19.Lee KS, Ro J, Nam BH, Lee ES, Kwon Y, et al. A randomized phase-III trial of docetaxel/capecitabine versus doxorubicin/cyclophosphamide as primary chemotherapy for patients with stage II/III breast cancer. Breast Cancer Research and Treatment. 2008;109:481–489. doi: 10.1007/s10549-007-9672-y. [DOI] [PubMed] [Google Scholar]
- 20.Martin M, Segui MA, Anton A, Ruiz A, Ramos M, et al. Adjuvant docetaxel for high-risk, node-negative breast cancer. N Engl J Med. 2010;363:2200–2210. doi: 10.1056/NEJMoa0910320. [DOI] [PubMed] [Google Scholar]
- 21.Polyzos A, Malamos N, Boukovinas I, Adamou A, Ziras N, et al. FEC versus sequential docetaxel followed by epirubicin/cyclophosphamide as adjuvant chemotherapy in women with axillary node-positive early breast cancer: A randomized study of the Hellenic Oncology Research Group (HORG). Breast Cancer Research and Treatment. 2010;119:95–104. doi: 10.1007/s10549-009-0468-0. [DOI] [PubMed] [Google Scholar]
- 22.Mansi JL, Yellowlees A, Lipscombe J, Earl HM, Cameron DA, et al. Five-year outcome for women randomised in a phase III trial comparing doxorubicin and cyclophosphamide with doxorubicin and docetaxel as primary medical therapy in early breast cancer: an Anglo-Celtic Cooperative Oncology Group study. Breast Cancer Res Treat. 2010;122:787–794. doi: 10.1007/s10549-010-0989-6. [DOI] [PubMed] [Google Scholar]
- 23.Boccardo F, Amadori D, Guglielmini P, Sismondi P, Farris A, et al. Epirubicin followed by cyclophosphamide, methotrexate and 5-fluorouracil versus paclitaxel followed by epirubicin and vinorelbine in patients with high-risk operable breast cancer. Oncology. 2010;78:274–281. doi: 10.1159/000315735. [DOI] [PubMed] [Google Scholar]
- 24.Ellis P, Barrett-Lee P, Johnson L, Cameron D, Wardley A, et al. Sequential docetaxel as adjuvant chemotherapy for early breast cancer (TACT): an open-label, phase III, randomised controlled trial. The Lancet. 2009;373:1681–1692. doi: 10.1016/S0140-6736(09)60740-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones S, Holmes FA, O'Shaughnessy J, Blum JL, Vukelja SJ, et al. Docetaxel With Cyclophosphamide Is Associated With an Overall Survival Benefit Compared With Doxorubicin and Cyclophosphamide: 7-Year Follow-Up of US Oncology Research Trial 9735. J Clin Oncol. 2009;27:1177–1183. doi: 10.1200/JCO.2008.18.4028. [DOI] [PubMed] [Google Scholar]
- 26.Jones SE, Savin MA, Holmes FA, O'Shaughnessy JA, Blum JL, et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. Journal of Clinical Oncology. 2006;24:5381–5387. doi: 10.1200/JCO.2006.06.5391. [DOI] [PubMed] [Google Scholar]
- 27.Goldstein LJ, O'Neill A, Sparano JA, Perez EA, Shulman LN, et al. Concurrent doxorubicin plus docetaxel is not more effective than concurrent doxorubicin plus cyclophosphamide in operable breast cancer with 0 to 3 positive axillary nodes: North American breast cancer intergroup trial E 2197. Journal of Clinical Oncology. 2008;26:4092–4099. doi: 10.1200/JCO.2008.16.7841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldstein L, O'Neill A, Sparano J, Perez E, Shulman L, et al. E2197: Phase III AT (doxorubicin/docetaxel) vs. AC (doxorubicin/cyclophosphamide) in the adjuvant treatment of node positive and high risk node negative breast cancer.(abstract). J Clin Oncol ASCO. 2005;(suppl 16):abstr 512. [Google Scholar]
- 29.Martin M, Rodriguez-Lescure A, Ruiz A, Alba E, Calvo L, et al. Randomized phase 3 trial of fluorouracil, epirubicin, and cyclophosphamide alone or followed by paclitaxel for early breast cancer. Journal of the National Cancer Institute. 2008;100:805–814. doi: 10.1093/jnci/djn151. [DOI] [PubMed] [Google Scholar]
- 30.Cognetti F, De Laurentiis M. Sequential epirubicin–docetaxel–CMF as adjuvant therapy for node-positive early stage breast cancer: updated results of the TAXit216 randomized trial. Ann Oncol. 2008;19(Suppl):a1820. [Google Scholar]
- 31.Bianco AR, De Laurentiis M, De Placido S, De Matteis A, Manzione L, et al. Sequential epirubicin-docetaxel-CMF as adjuvant therapy for node-positive early-stage breast cancer: Subgroup analysis of the TAXit216 randomized trial. Breast Cancer Symposium. 2008;(suppl):abstr 187. [Google Scholar]
- 32.Moore HC, Green SJ, Gralow JR, Bearman SI, Lew D, et al. Intensive dose-dense compared with high-dose adjuvant chemotherapy for high-risk operable breast cancer: Southwest Oncology Group/Intergroup study 9623. J Clin Oncol. 2007;25:1677–1682. doi: 10.1200/JCO.2006.08.9383. [DOI] [PubMed] [Google Scholar]
- 33.Crown JP, Francis P, Di Leo A, Buyse M, Balil A, et al. Docetaxel (T) given concurrently with or sequentially to anthracycline-based (A) adjuvant therapy (adjRx) for patients (pts) with node-positive (N+) breast cancer (BrCa), in comparison with non-T adjRx: First results of the BIG 2–98 Trial at 5 years median follow-up (MFU). J Clin Oncol ASCO. 2006;(suppl 18):abstr LBA519. [Google Scholar]
- 34.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, Alanko T, Kataja V, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. New England Journal of Medicine. 2006;354:809–820. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 35.Roche H, Fumoleau P, Spielmann M, Canon JL, Delozier T, et al. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: The FNCLCC PACS 01 trial. Journal of Clinical Oncology. 2006;24:5664–5671. doi: 10.1200/JCO.2006.07.3916. [DOI] [PubMed] [Google Scholar]
- 36.Bear HD, Anderson S, Smith RE, Geyer CE, Jr, Mamounas EP, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006;24:2019–2027. doi: 10.1200/JCO.2005.04.1665. [DOI] [PubMed] [Google Scholar]
- 37.Martin M, Pienkowski T, Mackey J, Pawlicki M, Guastalla JP, et al. Adjuvant docetaxel for node-positive breast cancer. New England Journal of Medicine. 2005;352:2302–2313. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 38.Gianni L, Baselga J, Eiermann W, Guillem Porta V, Semiglazov V, et al. European Cooperative Trial in Operable Breast Cancer (ECTO): Improved freedom from progression (FFP) from adding paclitaxel (T) to doxorubicin (A) followed by cyclophosphamide methotrexate and fluorouracil (CMF). J Clin Oncol ASCO. 2005;(suppl 16):abstr 513. doi: 10.1200/JCO.2008.19.2567. [DOI] [PubMed] [Google Scholar]
- 39.Gianni L, Baselga J, Eiermann W, Guillem Porta V, Semiglazov V, et al. Feasibility and tolerability of sequential doxorubicin/paclitaxel followed by cyclophosphamide, methotrexate, and fluorouracil and its effects on tumor response as preoperative therapy. Clin Cancer Res. 2005;11:8715–8721. doi: 10.1158/1078-0432.CCR-05-0539. [DOI] [PubMed] [Google Scholar]
- 40.Fountzilas G, Skarlos D, Dafni U, Gogas H, Briasoulis E, et al. Postoperative dose-dense sequential chemotherapy with epirubicin, followed by CMF with or without paclitaxel, in patients with high-risk operable breast cancer: A randomized phase III study conducted by the Hellenic Cooperative Oncology Group. Annals of Oncology. 2005;16:1762–1771. doi: 10.1093/annonc/mdi366. [DOI] [PubMed] [Google Scholar]
- 41.Mamounas EP, Bryant J, Lembersky B, Fehrenbacher L, Sedlacek SM, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: Results from NSABP B-28. Journal of Clinical Oncology. 2005;23:3686–3696. doi: 10.1200/JCO.2005.10.517. [DOI] [PubMed] [Google Scholar]
- 42.Henderson IC, Berry DA, Demetri GD, Cirrincione CT, Goldstein LJ, et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. Journal of Clinical Oncology. 2003;21:976–983. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 43.Sparano JA, Wang M, Martino S, Jones V, Perez EA, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–1671. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andre F, Broglio K, Roche H, Martin M, Mackey JR, et al. Estrogen receptor expression and efficacy of docetaxel-containing adjuvant chemotherapy in patients with node-positive breast cancer: results from a pooled analysis. J Clin Oncol. 2008;26:2636–2643. doi: 10.1200/JCO.2007.14.9146. [DOI] [PubMed] [Google Scholar]
- 45.Martin M, Mackey J, Vogel C. Benefit from adjuvant taxanes and endocrine responsiveness in breast cancer. Breast. 2007;16(Suppl 2):S127–131. doi: 10.1016/j.breast.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 46.Fisher B, Jeong JH, Anderson S, Wolmark N. Treatment of axillary lymph node-negative, estrogen receptor-negative breast cancer: updated findings from National Surgical Adjuvant Breast and Bowel Project clinical trials. J Natl Cancer Inst. 2004;96:1823–1831. doi: 10.1093/jnci/djh338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist.
(DOC)
PRISMA Flowchart.
(DOC)
Baseline characteristics for included trials.
(DOC)