Abstract
Background
To compare the treatment of heterotopic ossification (HO) within traumatic brain and spinal cord injured populations.
Methods
MEDLINE/Pubmed, CINAHL, EMBASE, and PsycINFO databases were searched for articles addressing treatment of HO post-injury. Articles were constrained to: English language and human subjects. Studies were included if: n≥50% of the subjects had a SCI or TBI, n≥3 SCI or TBI subjects, and study subjects participated in a treatment or intervention. Study quality, for randomized control trials (RCTs), were assessed using the PEDro assessment scale, while non-RCTs was assessed using the Downs and Black evaluation tool. A modified Sackett scale was used to apply levels of evidence for each intervention.
Results
In total 26 studies (NTBI=12; NSCI=14) met inclusion criteria. The majority of studies (10/12) conducted in the TBI population were surgical interventions. Studies conducted with the SCI population investigated diverse pharmacological treatments including: bisphosphonates, non-steroidal anti-inflammatory drugs (NSAIDs) and Warfarin. Non-pharmacological studies investigated the benefits of pulse low-intensity electromagnetic field therapy, surgical excision, and radiotherapy in the treatment of HO.
Conclusions
Within the SCI literature, NSAIDs showed the greatest efficacy in the prevention of HO when administered early after a SCI, and biphosphonates were found to be the most effective treatment strategy. In the TBI population, surgical excision was the most effective treatment.
Keywords: spinal cord injury, brain injury, therapeutic interventions, heterotopic ossification
1. Introduction
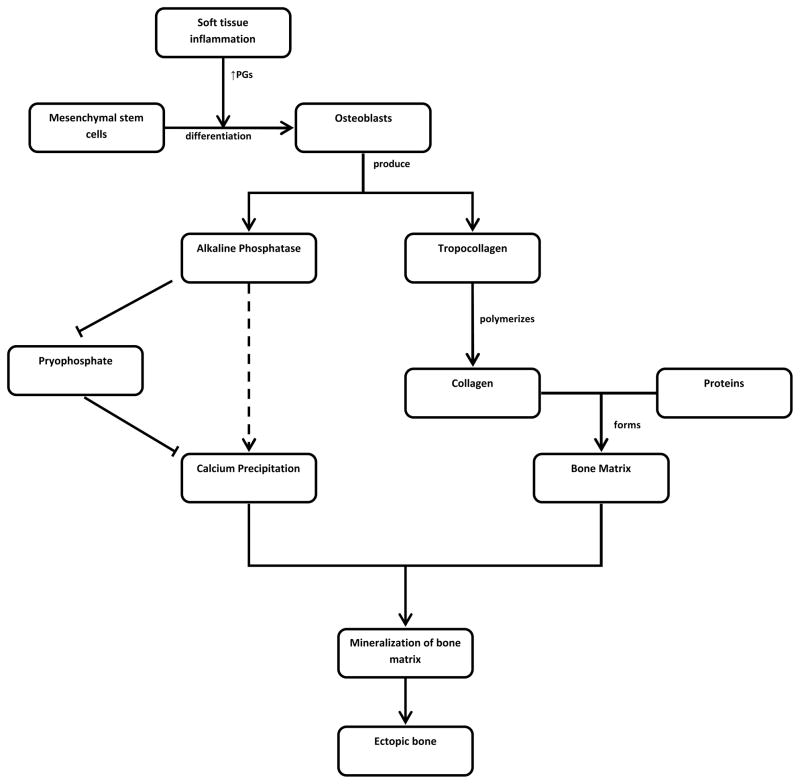
The formation of neurogenic heterotopic ossification (HO) appears to be similar following both traumatic brain injury and traumatic spinal cord injury. HO is thought to be associated with local inflammation [1] which affects mesenchymal stem cells present in soft tissues [2]. These mesenchymal cells transform into osteoblasts which are regulated by prostaglandins (PGs) [3–5]. Inflammation releases prostaglandins, particularly prostaglandin E2, which has been found to lead to lamellar heterotopic bone formation in experimental animal studies [5–7].
Osteoblasts are integral to the formation of heterotopic bone through production of tropocollagen and alkaline phosphatase (AP). Tropocollagen polymerizes to form collagen fibers which are involved in the formation of the bone matrix. AP inactivates pyrophosphate which allows calcium deposition and mineralization of bone matrix [1]. Histological and radiological examination of mature heterotopic bone resembles that of normal bone [1].
For those who sustain a TBI, the incidence of HO has been reported as ranging from 11 to 73.3% [8–10] thus making it a relatively common occurrence. For those who sustain a brain injury, soft tissue areas around the hips, elbows and knees are more commonly involved in the development of HO [11]. Reported incidence of HO varies greatly in the SCI population, ranging from 10–78% [1;12] is more likely to occur if spasticity is present, there is a prolonged loss of consciousness, or if there are long bone fractures, or where there is decreased range of motion. For individuals who remain in coma for an extended period of time (greater than 2 weeks) the risk of developing HO increases significantly [13]. Many who develop HO, experience pain (the most common symptom), warmth, and swelling in the areas affected [14]. Individuals who sustain a spinal cord injury may not report feeling pain in the affected area [9].
Previous systematic reviews of HO following brain injury [15] and spinal cord injury [16] by our group have examined the effectiveness of interventions for HO. The purpose of this systematic review was to examine and compare the effectiveness of treatments used to prevent and treat neurogenic HO in both the TBI and SCI populations. This review was conducted as part of the SCIRE project (http://www.scireproject.com) [17], an evidence-based review of the literature assessing rehabilitation interventions in SCI patients and the ERABI project (http://www.abiebr.com) [18] an evidence-based review of the literature assessing rehabilitation interventions in brain injury patients.
2. Methods
2.1 Literature Search Strategy
A systematic review of the literature, published from 1980 to May 2010, of studies investigating interventions to prevent or treat HO occurring in those who had sustained a spinal cord injury or a brain injury was undertaken. Several databases were searched, including MEDLINE, CINAHL, EMBASE and PsycINFO. Key words included: heterotopic ossification, HO, ectopic ossification, brain injury, spinal cord injury, treatment, intervention, excision, radiation. All retrieved references were scanned for relevant citations.
2.2 Study Selection
Studies were selected based on the previously established Spinal Cord Injury Rehabilitation Evidence (SCIRE) [19] and the Evidence Based Review of Acquired Brain Injury (ERABI) [20] methodologies. Studies were only included for review and analysis if: at least 50% of the study population had either an ABI or a SCI; if the study included 3 or more individuals who had an ABI or SCI; and there was a definable intervention for the prevention or treatment of HO. Literature and secondary hand searching resulted in 194 studies in the SCI population and 55 studies in the ABI population. Further evaluation of the studies resulted in 26 meeting inclusion criteria.
2.3 Study Appraisal
Once selected, studies were carefully reviewed and scored using both the Physiotherapy Evidence Database (PEDro)[21] and Downs and Black (D&B)[22] quality assessment tools. The PEDro assessment tool consists of 11 questions with a maximum score of 10, while the D&B tool, which was modified for this study, consists of 27 questions with a maximum score of 28.
2.4 Data Synthesis
Investigations involving similar interventions were grouped and tabulated. The tables included, study author(s) and year, the PEDro or Downs and Black score, the type of study, treatment administered, dosage and frequency and time post injury. A modified Sackett scale, was used to assign a Level of Evidence for each treatment [23].
3. Results
Results from this systematic review revealed 3 primary types of treatments: pharmacological (see Table 2), non pharmacological (see Table 3) and combined (pharmacological and non pharmacological) (see Table 4). All treatments were used with both the ABI and SCI populations
Table 2.
HO Pharmacological Treatments
| Study Characteristics: Author/Yr N Design-Quality Score | Population | Treatment | ||
|---|---|---|---|---|
| Treatment | Dosage/Frequency | Time post Injury | ||
| NSAIDS | ||||
| Banovac et al., 2004 N=76 RCT-PEDro=10 |
SCI | Rofecoxib | 25mg 1×daily for 4 weeks | 25±7days |
|
Banovac et al., 2001 N=33 RCT-PEDro=9 |
SCI | Indomethacin | 75mg 1 daily for 3 weeks | Not stated |
| Bisphosphonates | ||||
|
Spielman et al., 1983 N=20 Non-RCT D&B=14 |
BI | Etidronate | 20mg/kg body weight/day for 1st 3 months 10 mg/kg body weight/day for last 3 months |
2–7 days post injury |
|
Banovac et al., 1993 N=38 PCT-D&B=12 |
SCI | Etidronate | 300mg (IV) daily for 3–5 days and 20 mg orally for 6 months. | Not stated |
|
Garland 1983 N=14 Case series-D&B=9 |
SCI | Etidronate | 20mg/kg body weight/day First two weeks 10mg/kg/day for 2 years |
Not stated |
| Banavoc et al. 1997 N=46 PCT-D&B=7 |
SCI | Etidronate | IV for 3/day for 3 days Oral dose for 6 months |
Not stated |
|
Banavoc et al. 2000 N=40 Case series-D&B=7 |
SCI | Etidronate | IV plus 20mg/kg body weight/day for 6 months | Not stated |
Table 3.
HO Non-Pharmacological Treatments
| Study Characteristics: Author/Yr N Design-Quality Score | Population | Treatment | ||
|---|---|---|---|---|
| Treatment | Dosage/Frequency | Time post Injury | ||
|
Durovic et al 2009 N=29 RCT-PEDro=6 |
SCI | Pulse Low-Intensity Electromagnetic Field Therapy |
Induction of 10mT, frequency of 25Hz for 30 mins for 4 weeks | Mean of 7 weeks |
| Garland & Orwin 1987 N=19 Case series-D&B=14 |
SCI | Excision | N/A | Not stated |
|
Melamed et al. 2002 N=9 Pre-Post-D&B=11 |
BI | Excision | N/A | Not stated |
|
Sautter-Bihl 2001 N=52 Case series-D&B=12 |
SCI | Radiotherapy | A linear accelerator at 6 to 8 MV photons with single doses between 2 to 10 Gy | Not stated |
|
Sautter Bihl et al. 2000 N=36 Case series D&B=9 |
SCI | Radiotherapy | 10 Gy in increments of 2–2.5Gy | Not stated |
|
Garland et al. 1982 N=16 Case series-D&B=10 |
BI | Physiotherapy and ROM Exercises | Not stated | 3.6 months |
| Meiners et al. 1997 N=29 Case series-D&B=12 |
SCI | Excision/irradiation/passive movement | N/A | Not stated |
|
Ippolito et al. 1999b N=14 Case series-D&B=9 |
BI | Excision/passive movement | Not stated | 16–120 days |
|
Ippolito et al. 1999c N=5 Case series-D&B=8 |
BI | Excision/passive motion | At least twice a day for1 month | Not stated |
Table 4.
Combined Treatments
| Study Characteristics: Author/Yr N Design-Quality Score | Population | Treatment | ||
|---|---|---|---|---|
| Treatment | Dosage/Frequency | Time post Injury | ||
|
Kolessar et al. 1996 N=17 Case series-D&B=15 |
BI | Excision/Indomethacin/Etidronate | Indomethacin (75 mg/day) + Etidronate (20 mg/kg/day) | Mean of 30 months |
|
Fuller et al. 2005 N=17 Case series-D&B=14 |
BI | Excision/Etidronate | 20 mg/kg body weight for 2 months | Not stated |
|
Lazarus et al. 1999 N=24 Pre-Post-D&B=13 |
BI | Excision/Indomethacin | Indomethacin 25–50mg | Average of 35.4 months |
|
Ippolito et al. 1999a N=12 Pre-Post-D&B=12 |
BI | Excision | N/A | Not stated |
|
Moore 1993 N=17 Case series-D&B=9 |
BI | Excision/Etidronate | 10mg/kg body weight per day for 3 months | Not stated |
|
Schuetz et al. 2005 N=5 Case studies-D&B=9 |
SCI | Excision/Pamidronate | 120 mg for 12 hrs post surgery and then increase over 6–14 days. | Not stated |
|
Subbarao et al. 1987 N=5 Case series-D&B=8 |
SCI | Excision/Etidronate | 20 mg/kg body weight preoperatively for 10–14 days 10 mg/kg body weight post-operatively for at least 3 months |
Not stated |
|
Kolessar et al. 1996 N=17 Case series-D&B=15 |
BI | Excision/Indomethacin/Etidronate | Indomethacin (75 mg/day) + Etidronate (20 mg/kg/day) | Mean of 30 months |
|
Charnley et al. 1996 N=5 Case series-D&B=9 |
BI | Excision/Indomethacin | Not stated | Over 18 months |
|
De Palma et al. 2002 N=10 Case series-D&B=8 |
BI | Excision/Indomethacin/active motion therapy | 25 mg 3 times a day for 6 weeks | 18–20 months |
3.1 Pharmacological Interventions
Banovac et al. [24;25] examined the use of NSAIDs in treating HO post SCI. In their first RCT, Banovac et al. [24] compared the prophylactic effect of 3 weeks of indomethacin or placebo treatment in SCI patients (n=33) and then followed the patients to determine who developed HO. HO was diagnosed through clinical presentation, nuclear bone scans or radiographs. Banovac et al. [24] found a significantly lower incidence of HO in the treatment group (25.0%) compared to the placebo group (64.7%) (p<0.001). Furthermore, patients in the treatment group experiencing HO symptoms presented significantly later than those in the placebo group (31.7 days vs. 19.2 days; p<0.048). In the second RCT, Banovac et al [24] again found SCI individuals (n=76) were significantly less likely to develop clinical and radiographic evidence of HO if they prophylactically received 25 mg of Rofecoxib daily for 4 weeks when compared to those in a non-treatment control group. Overall there was Level 1 evidence that NSAIDS (robecoxib and indomethacin) reduced the incidence of HO post SCI.
Six studies examined the use of bisphosphonates, specifically etidronate, to treat HO. Of these, there was only one study which looked at administering etidronate to a group of ABI patients [26]. In this cohort study, patients (n=10) were administered etidronate prophylactically within a week of injury for 6 months. Those receiving etidronate were compared to a retrospective control group (n=10) who did not receive treatment. Radiographic and clinical evidence found those receiving etidronate had a significantly (p<0.025) lower incidence of developing HO when compared to those without treatment. Study results indicate there is Level 2 evidence that etridonate reduces the development of heterotopic ossification in severe head injury patients.
In the SCI population, etidronate treatment was examined in 5 studies. In a prospective controlled trial (PCT), Banavoc et al.[25] studied SCI patients diagnosed with HO based on radiographs and three-phase nuclear bone scans. In the first group, patients were administered intravenous etidronate for 3–5 days followed by oral etidronate treatment for 6 months. The second group received only the oral editronate treatment for 6 months. The authors found no significant difference between the two groups in the development of HO. However, intravenous etidronate treatment significantly reduced swelling from baseline (p<0.01). There is Level 2 evidence that etidronate treatment is effective in the treatment HO post SCI.
In a second PCT study, Banavoc et al. [27] studied treatment with intravenous etidronate treatment followed by oral etidrontate treatment for 6 months in two groups of individuals post SCI. The first group (n=33) presented as positive for HO on bone scintigraphy with negative radiographic findings; the second group (n=13) presented as positive for both bone scintigraphy and radiographs. The study found 78% of participants in the first group that completed treatment showed no radiographic evidence of HO. However, only 46% of patients in the second group showed no further progression of HO. Overall there is Level 2 evidence that etidronate can halt post-SCI HO progression if initiated before radiographic evidence is present.
In a case series, Banavoc et al. [28] followed SCI patients (n=40) who had positive bone scans but negative radiographs for HO treated with intravenous etidronate for 3 days and oral etidronate for 6 months over a 6 year period. The study found only 27.5% of patients developed radiographic evidence for HO. In another case series, Garland et al. [29] found no evidence of improvement in SCI patients (n=14) with clinical signs of HO following long term (2 years) etidronate treatment. Results of these studies indicate there is Level 4 evidence that etidronate is not effective in treating HO post SCI once there is radiographic or clinical evidence of HO.
3.2 Non-Pharmacological Interventions
In an RCT reported by Durovic et al.[30], individuals post SCI were divided into two groups. The treatment group received prophylactic pulse low intensity electromagnetic field therapy with range of motion and exercise therapy, while the control group received only range of motion and exercise therapy [29]. The study found a significantly higher incidence of HO (measured by Brooker grades and radiographs) in the control group when compared to the treatment group (p=0.04). Progression of HO was seen in 33% of the individuals in the control group while none of the individuals in the treatment group developed HO. Based on this one RCT, there is Level 1 evidence supporting prophylactic treatment of HO using pulse low intensity electromagnetic field therapy post SCI.
Only one study [31] examined the effectiveness of surgical excision alone on HO. This SCI study found large functional gains directly following excision. At the 6th year follow up, range of motion in 3 of 24 hips (12.5%) returned to preoperative levels or worse whereas 21 of 24 hips, (87.5%) improved compared to preoperative levels. Preoperative range of motion (ROM) was approximately 11.5° and post operatively, during the final assessment period, ROM was approximately 35°. Study results indicate there was a total recurrence rate for HO in 22 of 24 hips (92%). There is Level 4 evidence that surgical excision alone does not significantly improve HO post SCI.
The use of radiotherapy to improve the success of surgical excision of HO post injury was studied in two case series, both of which included only individuals with SCI [32;33]. The studies found neither progression nor recurrence of the excised bone in 71% [32] and 90.9% [33] of patients based on the Brooker scale. Furthermore, adverse effects were associated with irradiation. Results from these two case studies indicate there is Level 4 evidence radiotherapy halts the progression of HO post SCI.
Garland et al.[34] conducted a retrospective chart review of 16 TBI patients with HO who underwent range of motion therapy. The study found initial improvement in ROM for 82% of patients; furthermore, 64% of these patients continued to maintain their ROM or gained further range through rehabilitation. Based on the results of this one study, there is Level 4 evidence excision improves range of motion in TBI individuals.
Multicomponent non-pharmacological treatments were common, most of which included surgical excision as the primary treatment intervention. Meiners et al. [35], in a case series, examined the effects of surgical excision, irradiation and passive range of motion therapy of the hip joints in individuals with SCI. Study results indicate ROM increased from 21.95 degrees preoperatively to 94.51 degrees intra-operatively and 82.68 degrees at 4 year follow-up in all of the 29 study participants. There is Level 4 evidence multicomponent treatment using surgical excision, radiotherapy and passive range of motion therapy improves range of motion post SCI.
In a prospective case series, preoperative examination of 9 patients found HO in 12 joints (hips, knees and elbows): 9 joints with Brooker class IV ossification and three joints with class III based on radiographic review [36]. Irradiation (750 cGy) was provided to 7 of the 9 patients within the first 24 hours post surgery. Joint ROM and ambulatory levels were assessed at one year post surgery. Bone scans showed no recurrence of HO in any patients at 1 year follow up and ROM improved in 7 patients.
Two other case series followed TBI patients who underwent excision of HO from 16 elbows [37] and 7 knees[38] followed by passive range of motion therapy. Both studies found large improvements in the ROM of affected joints and no recurrence of HO in any of the joints. Results of these studies indicate there is Level 4 evidence surgical excision followed by passive movement therapy improves ROM in individuals post TBI.
3.3 Combined Treatments
The use of pharmacological treatment in combination with non-pharmacological treatment was seen in 10 studies (see Table 4); all of these involved surgical excision as the primary treatment. In six studies, bisphosphonate treatment was supplemented with the surgical excision. Fuller et al. [39] reported on a case series of 17 brain injury patients who underwent surgical excision of knee HO followed by etidronate treatment. A significant increase in arc of motion by an average of 65° was seen (p<0.0001). No recurrence of HO was seen on clinical and radiographic examination. Moore [40] followed 17 brain injured patients with surgical excision of HO at hips and elbows along with etidronate treatment for the prevention of secondary HO. Range of motion gained immediately following surgical excision was maintained on average 23 months post surgery in 17 of the 20 joints. A recurrence rate of 15% was reported.
Kolessar et al. [41] retrospectively reviewed the charts of 17 patients who had sustained an ABI and underwent HO resection. All patients reviewed were also administered indomethacin and etidronate postoperatively. The radiographic review at about 13 months follow-up found a recurrence rate of 23.8% according to Brooker classification; however only 1 case had definite motion restriction.
Lazarus et al. [42] examined effectiveness of surgical excision followed by indomecthacin treatment in 24 patients with traumatic brain injury and elbow HO. Maximum flexion and extension increased significantly at 2 year follow-up (p=0.0003, 0.0005 respectively). The study found patients receiving continuous passive motion (CPM) had significantly higher motion gain than those that did not receive CPM (p=0.04).
Ippolito et al. [43] reviewed 21 TBI patients with HO receiving indomethacin post excision. Minute ossification was still seen in radiographs of all patients post surgery; however, the ossification had no apparent clinical effect. At followup (average of 38 months), 10 patients were able to ambulate and 2 were able to sit in a wheelchair. Excision was combined with either paminodrate [44] or etidronate [45] post SCI in two case series. Both studies found no evidence of HO recurrence.
In two small case series, Charnley et al. [46] and de Palma et al. [47] excised heterotopic bone which developed post brain injury followed by indomethacin treatment. After an average of 18 months, Charnley et al. [46] found no recurrence of HO. De Palma et al. found improvement in range of motion was greatest in patients with the largest restriction preoperatively. Overall there is Level 4 evidence supporting the use of combined therapies to treat HO post SCI or ABI.
Discussion
This systematic review found interventions used to treat HO in individuals post SCI were predominately pharmacological while the majority of interventions used post ABI were non-pharmacological, specifically surgical excision. Furthermore, interventions for treating HO post ABI commonly involved muticomponent treatments, while those post SCI primarily involved a single drug treatment. The spinal cord injury literature presented with stronger levels of evidence with a few well done Level 1 and Level 2 studies, while studies presented in the ABI literature were predominantly Level 4 evidence.
Diagnosis of HO was primarily established using either radiographic or clinical evidence. Furthermore, location of HO varied in the brain injury population between the hip, knee and elbow while HO in SCI patients was predominantly seen in the hip.
Only two studies examined the use of NSAIDs on the development of HO, both of which included post-SCI individuals. Both studies found a significant reduction in development of HO in individuals receiving either indomethacin or rofecoxib treatment. Interestingly, Banovac et al. found patients receiving 3 weeks (21 days) of indomethacin presented with HO symptoms an average of 31.7 days, while the placebo group presented symptoms an average of 19.2 days. The late development of HO in the treatment group may be correlated with the halting of indomethacin treatment. Although the use of NSAIDS is an intriguing prophylacitic with a generally acceptable side effect profile, more study is needed. Studies assessing administration of indomethacin over a longer term may establish a more prolonged and potentially more effective prophylactic effect.
Literature on the use of bisphosphonates to treat HO (once diagnosed by radiographs) in the spinal cord injury population found etidronate treatment was not effective in halting its progression; however, beginning treatment before radiographs became positive has been shown to result in lowering the incidence of HO post injury. Similarly, Spielman et al., in a study of individuals who had sustained a TBI showed earlier treatment with eidronate using a similar regimen as in the spinal cord studies resulted in a significantly lower incidence of HO. Since, there is a relatively low incidence of HO post ABI it is important to evaluate if the positive results found were due to the low probability of developing HO post injury or due to the treatment itself; hence, further evaluation on the prophylactic effectiveness of etidronate is needed.
Surgical excision was a common mode of treatment in many multicomponent interventions which included both pharmacological and nonpharmacological interventions in both populations. In most studies, the average time of surgical excision was about 30 months post injury. Secondary treatments including pharmacological, range of motion therapy and radiotherapy were found to be effective following excision. Of the pharmacological treatments etidronate and indomethacin administration post surgery greatly reduced recurrence of HO and increased ROM in both TBI and SCI populations.
Conclusion
Pharmacological interventions were found to be effective prophylactic treatments for HO; however, once HO had developed surgical excision was the most effective mode of treatment. There was an abundant, albeit not high quality, body of research literature on surgical excision in the TBI population when compared to the SCI population. Pharmacological research on the prevention and treatment of HO in traumatic brain injury is lacking, while research in SCI offers strong support for the use of NSAIDs and bisphosphonates to prevent the development of HO or slow its progression. New emerging technologies such as pulse low intensity electromagnetic field therapy show great promise; however, due to difficulties in administrating this therapy and the lack of follow up data, its use at this point may be limited. At present prophylactic multi-component treatments which include NSAID or bisphosphonates and range of motion therapy show strong evidence of effectiveness. Surgical excision of heterotopic bone in combination with pharmacological treatment post excision should be considered once HO has formed.
Figure 1.
The Development of Heterotopic Ossification Post SCI or ABI.
Table 1.
Levels of Evidence Modified from the Sackett Scale.
| Level 1 | RCTs with a PEDro score of >/=6 |
| Level 2 | RCTs with a PEDro score of <6, Cohort, and Prospective Controlled Trails |
| Level 3 | Case-Control studies |
| Level 4 | Pre-Post, Case Series, and Post Interventions |
| Level 5 | Case Reports, Clinical Consensus or Observational Studies |
| Conflicting | Disagreement between the findings of at least 2 RCTs or when not available between 2 non-RCTs. |
Table 5.
Effectiveness of the Various Treatments Used within the ABI and SCI populations
| Treatment | SCI | ABI |
|---|---|---|
| NSAIDS | + | |
| Bisphosphonates | + | + |
| Excision | − | + |
| Radiotherapy | + | |
| Excision/Etidronate | + | |
| Excision/Indomethacin | + | |
| Excision/Indomethacin/Etidronate | + | |
| Excision/Pamidronate | + | |
| Excision/Indomethacin | + | |
| Pulse Low-Intensity Electromagnetic Field Therapy | + | |
| Radiotherapy | − | |
| Physiotherapy and ROM Exercises | + | |
| Excision/irradiation/passive movement | + | |
| Excision/passive movement | + |
Acknowledgments
We would like to acknowledge the Ontario Neurotrauma Fund, SCI Solutions Network and ICORD/the Rick Hansen Man in Motion Foundation for their support of the project.
Footnotes
Reprints available from author.
Reference List
- 1.van Kuijk AA, Geurts AC, van Kuppevelt HJ. Neurogenic heterotopic ossification in spinal cord injury.[see comment]. [Review] [180 refs] Spinal Cord. 2002;40:313–326. doi: 10.1038/sj.sc.3101309. [DOI] [PubMed] [Google Scholar]
- 2.Bone R. Systemic inflammatory response syndrome: A unifying concept of systemic inflammation. In: Fein A, Abraham E, Balk R, Bernard G, Bone R, Dantzker D, Fink M, editors. Sepsis and Multiorgan Failure. Williams & Wilkins; Baltimore: 1997. pp. 3–10. [Google Scholar]
- 3.Buring K. On the origin of cells in heterotopic bone formation. Clin Orthop Relat Res. 1975:293–301. doi: 10.1097/00003086-197507000-00040. [DOI] [PubMed] [Google Scholar]
- 4.Jensen LL, Halar E, Little JW, Brooke MM. Neurogenic heterotopic ossification. Am J Phys Med. 1987;66:351–363. [PubMed] [Google Scholar]
- 5.High WB. Effects of orally administered prostaglandin E-2 on cortical bone turnover in adult dogs: a histomorphometric study. Bone. 1987;8:363–373. doi: 10.1016/8756-3282(87)90068-8. [DOI] [PubMed] [Google Scholar]
- 6.Jee WS, Ueno K, Kimmel DB, Woodbury DM, Price P, Woodbury LA. The role of bone cells in increasing metaphyseal hard tissue in rapidly growing rats treated with prostaglandin E2. Bone. 1987;8:171–178. doi: 10.1016/8756-3282(87)90017-2. [DOI] [PubMed] [Google Scholar]
- 7.Schurch B, Capaul M, Vallotton MB, Rossier AB. Prostaglandin E2 measurements: Their value in the early diagnosis of heterotopic ossification in spinal cord injury patients. Archives of Physical Medicine & Rehabilitation. 1997;78:687–691. doi: 10.1016/s0003-9993(97)90074-5. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe TK, Sant MO. Common medical complications of traumatic brain injury. Physical Medicine and Rehabilitation: state of the arts reviews. 2001;15:283–299. [Google Scholar]
- 9.Black K, DeSantis N. Medical Complications Common to Spinal-Cord-Injured and Brain-Injured Patients. Topics in Spinal Cord Injury Rehabilitation. 1999;5:47–75. [Google Scholar]
- 10.Simonsen LL, Sonne-Holm S, Krasheninnikoff M, Engberg AW. Symptomatic heterotopic ossification after very severe traumatic brain injury in 114 patients: incidence and risk factors. Injury. 2007;38:1146–1150. doi: 10.1016/j.injury.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Cullen N, Bayley M, Bayona N, Hilditch M, Aubut J. Management of heterotopic ossification and venous thromboembolism following acquired brain injury. Brain Inj. 2007;21:215–230. doi: 10.1080/02699050701202027. [DOI] [PubMed] [Google Scholar]
- 12.Banovac K, Williams JM, Patrick LD, Haniff YM. Prevention of heterotopic ossification after spinal cord injury with indomethacin. Spinal Cord. 2001;39:370–374. doi: 10.1038/sj.sc.3101166. [DOI] [PubMed] [Google Scholar]
- 13.Anderson MC, Lais RL. Excision of heterotopic ossification of the popliteal space following traumatic brain injury. J Orthop Trauma. 2004;18:190–192. doi: 10.1097/00005131-200403000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Garland DE, Blum CE, Waters RL. Periarticular heterotopic ossification in head-injured adults. Incidence and location. J Bone Joint Surg Am. 1980;62:1143–1146. [PubMed] [Google Scholar]
- 15.Teasell R, Hilditch M, Marshall S, Cullen N, Bayonna N. Heterotopic Ossification and Venous Thromboembolism. In: Teasell R, Marshall S, Bayley M, Cullen N, editors. The Evidence Based Review of Acquired Brain Injury. Vol. 5. 2009. pp. 1–36. Ref Type: Electronic Citation. [Google Scholar]
- 16.Teasell R, Mehta S, Aubut JL, Ashe MC, Sequeira K, Macaluso S, Tu L for the SCIRE Research Team. A systematic reveiw of therapeutic interventions for heterotopic ossification following spinal cord injury. Spinal Cord. 2010 doi: 10.1038/sc.2009.175. (in Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eng JJ, Teasell R, Miller W, Wolfe D, Townsen A. Spinal Cord Injury Rehabilitation Evidence. Lulu; Vancouver: 2008. [Google Scholar]
- 18.Teasell RW, Marshall S, Cullen N, Rees L, Bayley M, Weiser M, Lippert C, Colantonio A, McCabe P, Welch-West P. Evidence Based Review of Moderate to Severe Acquired Brain Injury. London: 2009. pp. 1–790. [Google Scholar]
- 19.Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Aubut JL, Abramson C, Hsieh J, Connolly S, Konnyu K. Spinal Cord Injury Rehabilitation Evidence: Methods of the SCIRE systematic review. TOP SPINAL CORD INJ REHABIL. 2007;13:1–10. doi: 10.1310/sci1301-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teasell R, Bayona N, Marshall S, Cullen N, Bayley M, Chundamala J, Villamere J, Mackie D, Rees L, Hartridge C, Lippert C, Hilditch M, Welch-West P, Weiser M, Ferri C, McCabe P, McCormick A, Aubut JA, Comper P, Salter K, Van RR, Collins D, Foley N, Nowak J, Jutai J, Speechley M, Hellings C, Tu L. A systematic review of the rehabilitation of moderate to severe acquired brain injuries. Brain Inj. 2007;21:107–112. doi: 10.1080/02699050701201524. [DOI] [PubMed] [Google Scholar]
- 21.Moseley AM, Herbert RD, Sherrington C, Maher CG. Evidence for physiotherapy practice: a survey of the Physiotherapy Evidence Database (PEDro) Aust J Physiother. 2002;48:43–49. doi: 10.1016/s0004-9514(14)60281-6. [DOI] [PubMed] [Google Scholar]
- 22.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Straus SE, Richardson WS, Glasziou P, Haynes RB. Evidence-Based Medicine: How to practice and teach EBM. Elsevier Churchill Livingstone; Toronto: 2005. [Google Scholar]
- 24.Banovac K, Williams JM, Patrick LD, Haniff YM. Prevention of heterotopic ossification after spinal cord injury with indomethacin. Spinal Cord. 2001;39:370–374. doi: 10.1038/sj.sc.3101166. [DOI] [PubMed] [Google Scholar]
- 25.Banovac K, Gonzalez F, Wade N, Bowker JJ. Intravenous disodium etidronate therapy in spinal cord injury patients with heterotopic ossification. Paraplegia. 1993;31:660–666. doi: 10.1038/sc.1993.106. [DOI] [PubMed] [Google Scholar]
- 26.Spielman G, Gennarelli TA, Rogers CR. Disodium etidronate: its role in preventing heterotopic ossification in severe head injury. Arch Phys Med Rehabil. 1983;64:539–542. [PubMed] [Google Scholar]
- 27.Banovac K, Gonzalez F. Evaluation and management of heterotopic ossification in patients with spinal cord injury. Spinal Cord. 1997;35:158–162. doi: 10.1038/sj.sc.3100380. [DOI] [PubMed] [Google Scholar]
- 28.Banovac K. The effect of etidronate on late development of heterotopic ossification after spinal cord injury. J SPINAL CORD MED. 2000;23:40–44. doi: 10.1080/10790268.2000.11753507. [DOI] [PubMed] [Google Scholar]
- 29.Garland DE, Alday B, Venos KG, Vogt JC. Diphosphonate treatment for heterotopic ossification in spinal cord injury patients. Clin Orthop Relat Res. 1983:197–200. [PubMed] [Google Scholar]
- 30.Durovic A, Miljkovic D, Brdareski Z, Plavsic A, Jevtic M. Pulse low-intensity electromagnetic field as prophylaxis of heterotopic ossification in patients with traumatic spinal cord injury. Vojnosanit Pregl. 2009;66:22–28. doi: 10.2298/vsp0901022d. [DOI] [PubMed] [Google Scholar]
- 31.Garland DE, Orwin JF. Resection of heterotopic ossification in patients with spinal cord injuries. Clin Orthop Relat Res. 1989:169–176. [PubMed] [Google Scholar]
- 32.Sautter-Bihl ML, Hultenschmidt B, Liebermeister E, Nanassy A. Fractionated and single-dose radiotherapy for heterotopic bone formation in patients with spinal cord injury. A phase-I/II study. Strahlenther Onkol. 2001;177:200–205. doi: 10.1007/pl00002399. [DOI] [PubMed] [Google Scholar]
- 33.Sautter-Bihl ML, Liebermeister E, Nanassy A. Radiotherapy as a local treatment option for heterotopic ossifications in patients with spinal cord injury. Spinal Cord. 2000;38:33–36. doi: 10.1038/sj.sc.3100847. [DOI] [PubMed] [Google Scholar]
- 34.Garland DE, Razza BE, Waters RL. Forceful joint manipulation in head-injured adults with heterotopic ossification. Clin Orthop Relat Res. 1982:133–138. [PubMed] [Google Scholar]
- 35.Meiners T, Abel R, Lindel K, Mesecke U. Improvements in activities of daily living following functional hand surgery for treatment of lesions to the cervical spinal cord: self-assessment by patients. Spinal Cord. 2002;40:574–580. doi: 10.1038/sj.sc.3101384. [DOI] [PubMed] [Google Scholar]
- 36.Melamed E, Robinson D, Halperin N, Wallach N, Keren O, Groswasser Z. Brain injury-related heterotopic bone formation: treatment strategy and results. Am J Phys Med Rehabil. 2002;81:670–674. doi: 10.1097/00002060-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Ippolito E, Formisano R, Caterini R, Farsetti P, Penta F. Resection of elbow ossification and continuous passive motion in postcomatose patients. J Hand Surg [Am ] 1999;24:546–553. doi: 10.1053/jhsu.1999.0546. [DOI] [PubMed] [Google Scholar]
- 38.Ippolito E, Formisano R, Farsetti P, Caterini R, Penta F. Excision for the treatment of periarticular ossification of the knee in patients who have a traumatic brain injury. J Bone Joint Surg Am. 1999;81:783–789. doi: 10.2106/00004623-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Fuller DA, Mark A, Keenan MA. Excision of heterotopic ossification from the knee: a functional outcome study. Clin Orthop Relat Res. 2005;438:197–203. doi: 10.1097/00003086-200509000-00033. [DOI] [PubMed] [Google Scholar]
- 40.Moore TJ. Functional outcome following surgical excision of heterotopic ossification in patients with traumatic brain injury. J Orthop Trauma. 1993;7:11–14. doi: 10.1097/00005131-199302000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Kolessar DJ, Katz SD, Keenan MA. Functional outcome following surgical resection of heterotopic ossification in patients with brain injury. J Head Trauma Rehabil. 1996;11:78–87. [Google Scholar]
- 42.Lazarus MD, Guttmann D, Rich CE, Keenan MAE. Heterotopic ossification resection about the elbow. NeuroRehabilitation. 1999;12:145–153. [Google Scholar]
- 43.Ippolito E, Formisano R, Caterini R, Farsetti P, Penta F. Operative treatment of heterotopic hip ossification in patients with coma after brain injury. Clin Orthop Relat Res. 1999:130–138. doi: 10.1097/00003086-199908000-00018. [DOI] [PubMed] [Google Scholar]
- 44.Schuetz P, Mueller B, Christ-Crain M, Dick W, Haas H. Amino-bisphosphonates in heterotopic ossification: first experience in five consecutive cases. Spinal Cord. 2005;43:604–610. doi: 10.1038/sj.sc.3101761. [DOI] [PubMed] [Google Scholar]
- 45.Subbarao JV, Nemchausky BA, Gratzer M. Resection of heterotopic ossification and Didronel therapy--regaining wheelchair independence in the spinal cord injured patient. J Am Paraplegia Soc. 1987;10:3–7. doi: 10.1080/01952307.1987.11719626. [DOI] [PubMed] [Google Scholar]
- 46.Charnley G, Judet T, Garreau dL, Mollaret O. Excision of heterotopic ossification around the knee following brain injury. Injury. 1996;27:125–128. doi: 10.1016/0020-1383(95)00180-8. [DOI] [PubMed] [Google Scholar]
- 47.de Palma L, Rapali S, Paladini P, Ventura A. Elbow heterotopic ossification in head-trauma patients: diagnosis and treatment. Orthopedics. 2002;25:665–668. doi: 10.3928/0147-7447-20020601-17. [DOI] [PubMed] [Google Scholar]