Abstract
Context
Anorexia nervosa and normal-weight hypothalamic amenorrhea are characterized by hypogonadism and hypercortisolemia. However, it is not known whether these endocrine abnormalities result in reductions in adrenal and/or ovarian androgens or androgen precursors in such women, nor is it known whether relative androgen deficiency contributes to abnormalities in bone density and body composition in this population.
Objective
Our objective was to determine whether endogenous androgen and dehydroepiandrosterone sulfate (DHEAS) levels: 1) are reduced in women with anorexia nervosa and normal-weight hypothalamic amenorrhea, 2) are reduced further by oral contraceptives in women with anorexia nervosa, and 3) are predictors of weight, body composition, or bone density in such women.
Design and Setting
We conducted a cross-sectional study at a general clinical research center.
Study Participants
A total of 217 women were studied: 137 women with anorexia nervosa not receiving oral contraceptives, 32 women with anorexia nervosa receiving oral contraceptives, 21 normal-weight women with hypothalamic amenorrhea, and 27 healthy eumenorrheic controls.
Main Outcome Measures
Testosterone, free testosterone, DHEAS, bone density, fat-free mass, and fat mass were assessed.
Results
Endogenous total and free testosterone, but not DHEAS, were lower in women with anorexia nervosa than in controls. More marked reductions in both free testosterone and DHEAS were observed in women with anorexia nervosa receiving oral contraceptives. In contrast, normal-weight women with hypothalamic amenorrhea had normal androgen and DHEAS levels. Lower free testosterone, total testosterone, and DHEAS levels predicted lower bone density at most skeletal sites measured, and free testosterone was positively associated with fat-free mass.
Conclusions
Androgen levels are low, appear to be even further reduced by oral contraceptive use, and are predictors of bone density and fat-free mass in women with anorexia nervosa. Interventional studies are needed to confirm these findings and determine whether oral contraceptive use, mediated by reductions in endogenous androgen levels, is deleterious to skeletal health in such women.
Anorexia nervosa primarily affects women of reproductive age and is complicated by severe bone loss, abnormalities in body composition, and endocrine dysfunction (1). Normal-weight women with hypothalamic amenorrhea are similarly, but less severely, affected (2). Endocrine abnormalities include hypogonadism, hypercortisolemia (3, 4), and IGF-I deficiency (5). The extent to which hypogonadism and/or adrenal dysfunction may lead to a reduction in adrenal and/or ovarian androgens and androgen precursors is not known. The potential contribution of androgen deficiency to abnormal bone density and body composition has not been investigated. Data derived from small numbers of subjects are contradictory, suggesting normal (6–10), low (11–19), or elevated (15, 20–23) androgens or dehydroepiandrosterone sulfate (DHEAS) levels in both anorexia nervosa and normal-weight women with hypothalamic amenorrhea. Limitations in testosterone and free testosterone assays at the low levels observed in women contribute to the controversy and the conflicting results. Whether hypoandrogenemia is present and contributes to reduced bone or lean body mass in women with anorexia nervosa and normal-weight women with hypothalamic amenorrhea is not known.
It is a common practice to prescribe estrogens for women with anorexia nervosa (24), despite the fact that two randomized trials have demonstrated a lack of efficacy to increase bone mineral density (BMD) in such women (25, 26). Hypothesized mechanisms underlying this reported lack of effectiveness of estrogen have focused on the impact of severe undernutrition and IGF-I deficiency. A known effect of oral contraceptives in healthy women of reproductive age is to decrease testosterone, free testosterone, and DHEAS (27–35). However, it is not known whether similar reductions occur in women with anorexia nervosa or whether estrogeninduced hypoandrogenemia is one of the mechanisms underlying the ineffectiveness of oral contraceptives to either prevent or reverse bone loss in women with anorexia nervosa.
We hypothesized that androgens and DHEAS, a prehormone that is converted endogenously to androgens and estrogens, would be reduced in women with anorexia nervosa and normal-weight women with hypothalamic amenorrhea and that oral contraceptive use would further reduce such levels in women with anorexia nervosa. We also investigated whether androgen and preandrogen levels were predictors of body composition and bone density. We therefore compared total testosterone, free testosterone, and DHEAS levels and examined the relationships between androgen levels and body composition in a total of 217 women in four groups: 1) women with anorexia nervosa not receiving oral contraceptives, 2) women with anorexia nervosa receiving oral contraceptives, 3) normal-weight women with hypothalamic amenorrhea, and 4) healthy controls.
Subjects and Methods
Subjects
We studied 217 women: 1) women with anorexia nervosa not receiving oral contraceptives (n = 137), 2) women with anorexia nervosa receiving oral contraceptives (n = 32), 3) normal-weight women with hypothalamic amenorrhea (n = 21), and 4) healthy controls of reproductive age (n = 27). All subjects were recruited from the community through advertisements and referrals from healthcare providers. Participants with anorexia nervosa were consecutive screens for our bone health studies from February 1997 through August 2004; all who did not meet Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) (37), criteria for anorexia nervosa, had discontinued or initiated oral contraceptives in the previous 3 months, or were receiving androgen or DHEA therapy were excluded from analysis. Normal-weight women with hypothalamic amenorrhea and healthy, eumenorrheic controls had been recruited for a previous cross-sectional comparison of bone density and leptin levels (2, 36).
Women with anorexia nervosa fulfilled all DSM-IV (37) diagnostic criteria for anorexia nervosa. These included intense fear of gaining weight, emphasis on body shape, weight less than 85% of ideal body weight (IBW) as determined by the 1983 Metropolitan Life tables (38), and lack of menses for at least three consecutive months. Women in the group receiving oral contraceptives had been receiving oral contraceptives for at least 3 months (range of 4–240 months, median of 36 months). Normal-weight participants with hypothalamic amenorrhea were 90–110% of IBW, amenorrheic for at least 3 months, and had normal, i.e. nonelevated, FSH, prolactin, testosterone, and free testosterone levels as well as an LH/FSH ratio less than 2.5 and the absence of hirsutism or other signs of hyperandrogenemia; history of an eating disorder was also an exclusion criterion. Twenty-eight healthy controls, 90–110% of IBW, with regular menses, were also studied. Exclusion criteria included history of amenorrhea, an eating disorder, or oral contraceptive use within the past 3 months. Current or past use of medications known to affect bone density, including glucocorticoids, bisphosphonates, and anticonvulsants, were exclusion criteria for all groups. Baseline characteristics, including BMD, of the normal-weight women with hypothalamic amenorrhea, healthy controls, and a subset of women with anorexia nervosa have been previously published (1, 2, 36, 39).
Methods
This study was approved by the Institutional Review Boards at Partners Health Care, Inc., and the Massachusetts Institute of Technology, and written informed consent was obtained from all subjects. All subjects were admitted to the General Clinical Research Centers at Massachusetts General Hospital and Massachusetts Institute of Technology for an outpatient visit, which included BMD determinations, nutritional evaluation, including weight in a gown, height, frame size, calculation of percent IBW and body mass index (BMI), performed by research dieticians. All healthy controls were studied during the early follicular phase, d 1–7, of the menstrual cycle. A study investigator interviewed study participants and obtained menstrual history, duration of anorexia nervosa, and medication use and confirmed the diagnosis of anorexia nervosa. BMD and body composition were measured using dual-energy x-ray absorptiometry with a Hologic 4500 densitometer (Hologic, Inc., Waltham, MA). This technique has a precision of 0.01 g/cm2 at the lumbar spine, 3% for fat mass, and 1.4% lean body mass (40, 41). Serum was collected and stored at −80 C for subsequent measurement of endocrine variables.
Assays
Serum testosterone was measured by RIA [Diagnostic Products Corp. (DPC), Los Angeles, CA] with a sensitivity of 2 ng/dl, an intraassay coefficient of variation (CV) of 4.1–10.5% and an interassay CV of 5.9–12%. SHBG was measured by immunoradiometric assay (DPC), with a sensitivity of 0.5 nmol/liter, an intraassay CV of 2.8 –5.3%, and an interassay CV of 7.9–8.5%. Free testosterone was calculated from total testosterone and SHBG using the laws of mass action. This calculated value has been validated to have a high degree of agreement with the free testosterone concentrations determined by equilibrium dialysis (42). DHEAS was measured by RIA (DPC), with a sensitivity of 2.5 mcg/dl, an intraassay CV of 3.8–5.3%, and an interassay CV of 6.3–11%.
Statistical analysis
Statistical analysis was performed using JMP Statistical Discoveries, version 4.0.2 (SAS Institute, Inc., Cary, NC). All variables were tested for normality by the Shapiro-Wilk test. All variables that were not normally distributed in all groups (total testosterone, free testosterone, DHEAS, and SHBG) were log-transformed. Clinical characteristics and bone densities were compared using ANOVA. Univariate regression analyses were performed to investigate androgens and preandrogens as potential predictors of bone density and body composition parameters. The term Z-score is defined as a sd score compared with an age-adjusted mean. Data are reported as mean ± sem
Results
Clinical characteristics of study subjects
Clinical characteristics of all four groups were compared in Table 1. The mean age did not differ among the four groups. As expected, mean BMI, percent IBW, total fat mass, percent fat, and fat-free mass were all lower in women with anorexia nervosa compared with healthy controls, but there was no difference between the normal-weight women with hypothalamic amenorrhea or healthy control groups. Mean fat-free mass was higher in the group of women with anorexia nervosa receiving oral contraceptives than in those not receiving oral contraceptives. BMD at the posteroanterior (PA) spine, lateral spine, hip, radius and total body were lower in women with anorexia nervosa compared with healthy controls and normal-weight women with hypothalamic amenorrhea. There was no difference in bone density at any skeletal site between women with anorexia nervosa not receiving oral contraceptives and those receiving oral contraceptives, after controlling for percent IBW. Bone density at the PA and lateral spine were lower in normal-weight women with hypothalamic amenorrhea compared with healthy controls.
TABLE 1.
Clinical characteristics
| AN − E (n = 137) | AN + E (n = 32) | HA (n = 21) | HC (n = 27) | |
|---|---|---|---|---|
| Age (yr) | 25.4 ± 0.6 | 24.6 ± 1.0 | 24.2 ± 1.1 | 24.2 ± 0.6 |
| BMI (kg/m2) | 16.7 ± 0.1a | 17.1 ± 0.2a | 20.7 ± 0.3 | 21.0 ± 0.3 |
| % BW | 74.8 ± 0.5a | 77.0 ± 1.2a | 93.0 ± 1.4 | 94.4 ± 1.1 |
| Total fat mass (kg) | 7.8 ± 0.3a | 8.9 ± 0.7a | 14.4 ± 0.8 | 15.5 ± 0.6 |
| % fat | 17.3 ± 0.5a | 18.2 ± 1.1a | 26.1 ± 1.2 | 27.2 ± 0.7 |
| Fat-free mass (kg) | 35.4 ± 0.4a | 37.2 ± 0.7b | 38.8 ± 1.1 | 39.0 ± 0.8 |
| PA spine BMD Z-score | −1.7 ± 0.1a | −1.4 ± 0.2a | −0.6 ± 0.2c | 0.3 ± 0.2 |
| Lateral spine BMD Z-score | −2.0 ± 0.1a | −1.5 ± 0.2b | −0.8 ± 0.2c | 0.0 ± 0.2 |
| Hip BMD Z-score | −1.5 ± 0.1a | −0.9 ± 0.2b | −0.3 ± 0.3 | 0.1 ± 0.2 |
| Radius BMD Z-score | −0.7 ± 0.1a | −1.0 ± 0.2a | 0.6 ± 0.2 | 0.7 ± 0.2 |
| Total-body BMD Z-score | −1.3 ± 0.1a | −0.9 ± 0.2a | 0.4 ± 0.2 | 0.7 ± 0.2 |
| Duration of amenorrhea (months) | 25.1 ± 3.1a | NA | 10.9 ± 2.1 | 0.0 ± 0.0 |
Data are reported as mean ± SEM. There was no difference between the AN − E and AN + E groups in BMD at any site after controlling for percent IBW. AN − E, Women with anorexia nervosa not receiving oral contraceptives; AN + E, women with anorexia nervosa receiving oral contraceptives; HA, normal-weight women with hypothalamic amenorrhea; HC, healthy controls of reproductive age; NA, not applicable.
P < 0.05 compared with HC and HA.
P < 0.05 compared with AN − E, HC, and HA.
P < 0.05 compared with HC only.
Hormone levels
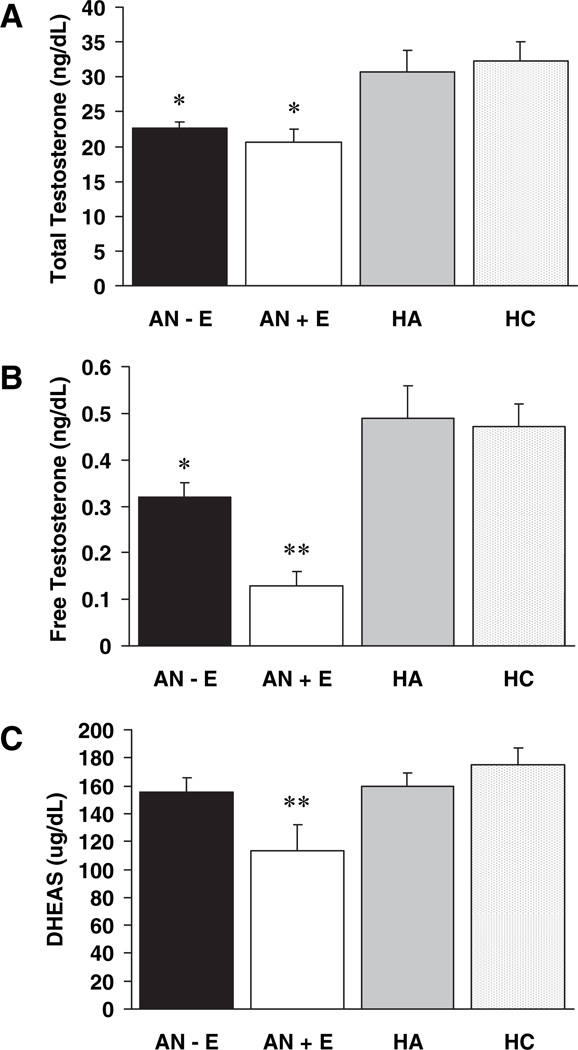
Mean androgen levels are shown in Table 2. Mean total testosterone levels were significantly lower in both groups of women with anorexia nervosa, those receiving and not receiving oral contraceptives, compared with both healthy controls and normal-weight women with hypothalamic amenorrhea. Mean free testosterone levels were significantly lower in both groups of women with anorexia nervosa compared with healthy controls and normal-weight women with hypothalamic amenorrhea. Mean free testosterone levels remained significantly lower in both groups of women with anorexia nervosa compared with healthy controls but not compared with normal-weight women with hypothalamic amenorrhea after controlling for percent IBW. In addition, mean free testosterone levels were lower in women with anorexia nervosa receiving oral contraceptives than in women with anorexia nervosa not receiving oral contraceptives. Mean total and free testosterone levels remained significantly lower in the women with anorexia nervosa not receiving oral contraceptives than in normal-weight women with hypothalamic amenorrhea after controlling for duration of amenorrhea. Mean DHEAS levels were lower in women with anorexia nervosa receiving oral contraceptives than in the other three groups. There were no significant differences in DHEAS levels among the other groups, including women with anorexia nervosa not receiving oral contraceptives and healthy controls. The differences in free testosterone and DHEAS among women with anorexia nervosa receiving and not receiving oral contraceptives remained significant after controlling for percent IBW or fat-free mass. Mean SHBG levels were higher in women with anorexia nervosa receiving oral contraceptives than the other three groups. Both total (r = 0.22; P = 0.02) and free testosterone (r = 0.73; P < 0.0001) were associated with SHBG levels. The comparisons of mean hormone levels among the groups are shown in Fig. 1.
TABLE 2.
Androgen, DHEAS, and SHBG levels
| AN − E (n = 137) | AN + E (n = 32) | HA (n = 21) | HC (n = 27) | |
|---|---|---|---|---|
| Total testosterone (ng/dl) | 22.6 ± 1.0a | 20.6 ± 1.9a | 30.7 ± 3.2 | 32.3 ± 2.7 |
| Free testosterone (ng/dl) | 0.32 ± 0.03a | 0.13 ± 0.03b | 0.49 ± 0.07 | 0.47 ± 0.05 |
| DHEAS (µg/dl) | 155.4 ± 9.9 | 113.4 ± 19.1b | 159.3 ± 9.4 | 175.5 ± 11.3 |
| SHBG (nmol/liter) | 61.8 ± 4.2 | 170.1 ± 19.2b | 46.5 ± 3.9 | 51.9 ± 3.2 |
Data are reported as mean ± SEM. AN − E, Women with anorexia nervosa not receiving oral contraceptives; AN + E, women with anorexia nervosa receiving oral contraceptives; HA, normal-weight women with hypothalamic amenorrhea; HC, healthy controls of reproductive age.
P < 0.05 compared with HC and HA.
P < 0.05 compared with AN − E, HC, and HA.
P < 0.05 compared with HC only.
Fig. 1.
Mean total and free testosterone levels are lower in women with anorexia nervosa not receiving oral contraceptives (AN − E) and women with anorexia nervosa receiving oral contraceptives (AN + E) than in normal-weight women with hypothalamic amenorrhea (HA) and healthy controls of reproductive age (HC); mean free testosterone and DHEAS levels are lower in AN + E than AN − E; DHEAS levels were not lower than normal in AN − E. *, P < 0.05 compared with HC and HA; **, P < 0.05 compared with AN − E, HC, and HA.
Testosterone, free testosterone, and DHEAS as predictors of bone density and body composition
Linear regression models were constructed for total testosterone, free testosterone, and DHEAS on bone density and body composition variables; the results are shown in Table 3. The analyses revealed significant positive associations between both androgens and preandrogens (testosterone, free testosterone, and DHEAS) and the following body composition variables: bone density at all sites, BMI, percent IBW, fat mass, and percent fat, except that total hip bone density was not significantly associated with DHEAS. Free testosterone was a positive predictor of fat-free mass, but total testosterone and DHEAS were not. All associations with DHEAS, except for with hip BMD and fat-free mass, and all associations with total and free testosterone remained significant or tended to be significant (P < 0.1) after controlling for age. After controlling for BMI, total testosterone, free testosterone, and DHEAS did not remain predictors of BMD or body composition variables.
TABLE 3.
Linear regression: hormones and body composition
| Total testosterone (ng/dl) | Free testosterone (ng/dl) | DHEAS (µg/dl) | ||||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| PA spine BMD (g/cm2) | 0.16 | 0.017 | 0.25 | 0.009 | 0.19 | 0.045 |
| Lateral spine BMD (g/cm2) | 0.23 | 0.001 | 0.28 | 0.005 | 0.19 | 0.053 |
| Hip BMD | 0.19 | 0.005 | 0.25 | 0.007 | 0.12 | 0.219 |
| Radius BMD (g/cm2) | 0.16 | 0.033 | 0.27 | 0.005 | 0.19 | 0.047 |
| Total body BMD (g/cm2) | 0.19 | 0.008 | 0.24 | 0.009 | 0.19 | 0.042 |
| BMI (kg/m2) | 0.27 | <0.001 | 0.44 | <0.001 | 0.19 | 0.041 |
| % IBW | 0.26 | <0.001 | 0.46 | <0.001 | 0.22 | 0.017 |
| Total fat mass (kg) | 0.29 | <0.001 | 0.35 | <0.001 | 0.18 | 0.057 |
| % fat | 0.27 | <0.001 | 0.32 | 0.001 | 0.19 | 0.039 |
| Fat-free mass (kg) | 0.08 | 0.293 | 0.19 | 0.039 | 0.03 | 0.771 |
Discussion
Our data demonstrate lower mean total testosterone and free testosterone, but not DHEAS, levels in women with anorexia nervosa compared with healthy controls and normal-weight women with hypothalamic amenorrhea. Women with anorexia nervosa who were receiving oral contraceptives had the lowest levels of free testosterone and DHEAS, much lower even than women with anorexia nervosa not receiving oral contraceptives. DHEAS levels were not reduced in women with anorexia nervosa, except for those who were receiving oral contraceptives. In addition, normal-weight women with hypothalamic amenorrhea did not have lower mean levels of total testosterone, free testosterone, or DHEAS than healthy controls. Of note, healthy, eumenorrheic controls were all studied during the early follicular phase of the menstrual cycle. Because testosterone and free testosterone levels increase 20–30% at midcycle, the differences between the women with anorexia nervosa and the controls would have been even greater if we had measured androgen levels at that time. Also of note, endogenous total testosterone, free testosterone, and DHEAS levels were all significant determinants of bone density in this cross-sectional study, raising the question of whether hypoandrogenemia may contribute to low bone density in women with anorexia nervosa and whether the lack of effectiveness of oral contraceptives as a therapy for bone loss may in part be attributed to the resultant reduction in free testosterone levels. Interventional studies would be necessary to investigate this possible mechanism further.
Previous reports in small numbers of women have shown variably low (11–18), normal (6 –10), or elevated (15, 23) androgens or DHEAS levels in women with anorexia nervosa. Some of these studies compared androgen or preandrogen levels with published normal ranges, instead of with those of recruited healthy control groups. Similarly, investigations of androgen levels in normal-weight women with hypothalamic amenorrhea have yielded conflicting results. There have been several reports of elevated testosterone levels in amenorrheic and oligoamenorrheic athletes (20–22), whereas other reports of normal-weight women with hypothalamic amenorrhea have demonstrated decreased levels (19). In contrast, the data regarding the effects of oral contraceptives on androgen levels in healthy women of reproductive age are congruent in that they clearly demonstrate decreases in free testosterone and DHEAS in healthy women of reproductive age (27–35). To our knowledge, ours is the first report of similar effects in women with anorexia nervosa, in whom the reduction of endogenous anabolic hormones could prove particularly relevant in that it may contribute to the known loss of bone mass and lean body mass in this disease.
Bone density is reduced in men with hypogonadism, and testosterone replacement results in reversal of the bone loss incurred. Moreover, we have recently shown that low-dose testosterone replacement therapy increases bone density at the hip and radius in women with severe androgen deficiency due to hypopituitarism (43). Three other randomized placebo-controlled studies have demonstrated increases in bone density with androgen administration in surgically or naturally menopausal women (44–46), whereas one randomized placebo-controlled study showed no difference with methyltestosterone plus esterified estrogens compared with esterified estrogens alone in postmenopausal women (47). In a study in which DHEA was administered to girls with anorexia nervosa for 1 yr, an increase in hip bone density compared with baseline was found in the initial analysis but was not detectable after controlling for weight gain; moreover, no increase in spine bone density was observed (13). Cross-sectional studies in healthy women have shown strong positive correlations between both free testosterone and percent free testosterone and bone density (48, 49). In a longitudinal study, Slemenda et al. (48) showed that lower androgen levels predicted subsequent bone loss in premenopausal, perimenopausal, and postmenopausal women. Our group has previously reported strong associations between change in free testosterone and change in surrogate markers of bone formation in adolescent girls with anorexia nervosa (50). However, to our knowledge, this is the first report of positive associations between bone mineral density and androgens in adults with anorexia nervosa. This finding could be clinically significant in a disease that is complicated by severe bone loss. The fact that associations did not remain significant after controlling for BMI reflects the relationship reported in this manuscript between undernutrition and hypoandrogenemia. Prospective, interventional studies will be necessary to determine whether there is an independent effect of circulating androgens on BMD in anorexia nervosa.
Limitations of this study include its cross-sectional design, assay limitations for androgen levels in women, and the fact that all samples were not drawn at 0800 h. The positive associations observed between both weight and body fat and androgen levels suggest that low weight and/or abnormalities in body composition are mechanisms underlying the hypoandrogenemia in women with anorexia nervosa. However, the reverse is also possible, and causality can be established only in prospective trials. Another limitation of the study is the assay for testosterone used. This is particularly true at the low levels observed in women (42, 51). Tandem mass spectrometry has been recently introduced and is increasingly accepted as the most accurate testosterone assay, but its use is limited by lack of wide availability and high cost. The direct RIA used in this study, along with one other assay, were shown to correlate most closely with tandem mass spectrometry of 11 manual and automated immunoassays examined in 122 men in a study by Wang et al. (51). In the current study, we calculated free testosterone levels using total testosterone and SHBG levels and the mass action equation. We have validated this method as yielding similar results to equilibrium dialysis (42), but it is dependent on the validity of the total testosterone and SHBG level used.
Our data demonstrate that total and free testosterone, but not DHEAS, are reduced in anorexia nervosa. Moreover, marked reductions in both free testosterone and DHEAS occur in women with anorexia nervosa who take oral contraceptives. In contrast, normal-weight women with hypothalamic amenorrhea appear to have normal androgen and DHEAS levels. Free testosterone, total testosterone, and DHEAS levels predict bone density at most skeletal sites tested in this cross-sectional study. It is not known whether the reduction in free testosterone and DHEAS levels in women with anorexia nervosa using oral contraceptives is harmful to skeletal health or has other deleterious effects. Interventional studies are needed to further investigate these issues.
Acknowledgments
We thank the nurses and bionutritionists at the Massachusetts General Hospital and Massachusetts Institute of Technology Clinical Research Centers and the study participants.
This work was supported by NIH-R01-DK52625 and MO1 RR01066.
Abbreviations
- BMD
Bone mineral density
- BMI
body mass index
- CV
coefficient of variation
- DHEAS
dehydroepiandrosterone sulfate
- IBW
ideal body weight
- PA
posteroanterior
Footnotes
Disclosure Summary: A.K. has received consulting fees from Proctor and Gamble. All other authors have nothing to disclose.
References
- 1.Miller KK, Grinspoon SK, Ciampa J, Hier J, Herzog D, Klibanski A. Medical findings in outpatients with anorexia nervosa. Arch Intern Med. 2005;165:561–566. doi: 10.1001/archinte.165.5.561. [DOI] [PubMed] [Google Scholar]
- 2.Grinspoon S, Miller K, Coyle C, Krempin J, Armstrong C, Pitts S, Herzog D, Klibanski A. Severity of osteopenia in estrogen-deficient women with anorexia nervosa and hypothalamic amenorrhea. J Clin Endocrinol Metab. 1999;84:2049–2055. doi: 10.1210/jcem.84.6.5792. [DOI] [PubMed] [Google Scholar]
- 3.Biller BM, Federoff HJ, Koenig JI, Klibanski A. Abnormal cortisol secretion and responses to corticotropin-releasing hormone in women with hypothalamic amenorrhea. J Clin Endocrinol Metab. 1990;70:311–317. doi: 10.1210/jcem-70-2-311. [DOI] [PubMed] [Google Scholar]
- 4.Biller BM, Saxe V, Herzog DB, Rosenthal DI, Holzman S, Klibanski A. Mechanisms of osteoporosis in adult and adolescent women with anorexia nervosa. J Clin Endocrinol Metab. 1989;68:548–554. doi: 10.1210/jcem-68-3-548. [DOI] [PubMed] [Google Scholar]
- 5.Grinspoon S, Baum H, Lee K, Anderson E, Herzog D, Klibanski A. Effects of short-term recombinant human insulin-like growth factor I administration on bone turnover in osteopenic women with anorexia nervosa. J Clin Endocrinol Metab. 1996;81:3864–3870. doi: 10.1210/jcem.81.11.8923830. [DOI] [PubMed] [Google Scholar]
- 6.Estour B, Pugeat M, Lang F, Dechaud H, Pellet J, Rousset H. Sex hormone binding globulin in women with anorexia nervosa. Clin Endocrinol (Oxf) 1986;24:571–576. doi: 10.1111/j.1365-2265.1986.tb03287.x. [DOI] [PubMed] [Google Scholar]
- 7.Sirinathsinghji DJ, Mills IH. Concentration patterns of plasma dehydroepiandrosterone, Δ5-androstenediol and their sulphates, testosterone and cortisol in normal healthy women and in women with anorexia nervosa. Acta Endocrinol (Copenh) 1985;108:255–260. doi: 10.1530/acta.0.1080255. [DOI] [PubMed] [Google Scholar]
- 8.Fisher EC, Nelson ME, Frontera WR, Turksoy RN, Evans WJ. Bone mineral content and levels of gonadotropins and estrogens in amenorrheic running women. J Clin Endocrinol Metab. 1986;62:1232–1236. doi: 10.1210/jcem-62-6-1232. [DOI] [PubMed] [Google Scholar]
- 9.Soyka LA, Grinspoon S, Levitsky LL, Herzog DB, Klibanski A. The effects of anorexia nervosa on bone metabolism in female adolescents. J Clin Endocrinol Metab. 1999;84:4489–4496. doi: 10.1210/jcem.84.12.6207. [DOI] [PubMed] [Google Scholar]
- 10.Skalba P, Zieba M, Olejek A. Testosterone and SHBG levels in blood serum in women with anorexia nervosa. Wiad Lek. 2001;54:532–536. (Polish) [PubMed] [Google Scholar]
- 11.Devesa J, Perez-Fernandez R, Bokser L, Gaudiero GJ, Lima L, Casanueva FF. Adrenal androgen secretion and dopaminergic activity in anorexia nervosa. Horm Metab Res. 1988;20:57–60. doi: 10.1055/s-2007-1010748. [DOI] [PubMed] [Google Scholar]
- 12.Brambilla F, Bellodi L, Arancio C, Limonta D, Ferrari E, Solerte B. Neurotransmitter and hormonal background of hostility in anorexia nervosa. Neuropsychobiology. 2001;43:225–232. doi: 10.1159/000054894. [DOI] [PubMed] [Google Scholar]
- 13.Gordon CM, Grace E, Emans SJ, Feldman HA, Goodman E, Becker KA, Rosen CJ, Gundberg CM, LeBoff MS. Effects of oral dehydroepiandrosterone on bone density in young women with anorexia nervosa: a randomized trial. J Clin Endocrinol Metab. 2002;87:4935–4941. doi: 10.1210/jc.2002-020545. [DOI] [PubMed] [Google Scholar]
- 14.Zumoff B, Walsh BT, Katz JL, Levin J, Rosenfeld RS, Kream J, Weiner H. Subnormal plasma dehydroisoandrosterone to cortisol ratio in anorexia nervosa: a second hormonal parameter of ontogenic regression. J Clin Endocrinol Metab. 1983;56:668–672. doi: 10.1210/jcem-56-4-668. [DOI] [PubMed] [Google Scholar]
- 15.Monteleone P, Luisi M, Colurcio B, Casarosa E, Ioime R, Genazzani AR, Maj M. Plasma levels of neuroactive steroids are increased in untreated women with anorexia nervosa or bulimia nervosa. Psychosom Med. 2001;63:62–68. doi: 10.1097/00006842-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Tomova A, Kumanov P, Kirilov G. Factors related to sex hormone binding globulin concentrations in women with anorexia nervosa. Horm Metab Res. 1995;27:508–510. doi: 10.1055/s-2007-980013. [DOI] [PubMed] [Google Scholar]
- 17.van Binsbergen CJ, Coelingh Bennink HJ, Odink J, Haspels AA, Koppeschaar HP. A comparative and longitudinal study on endocrine changes related to ovarian function in patients with anorexia nervosa. J Clin Endocrinol Metab. 1990;71:705–711. doi: 10.1210/jcem-71-3-705. [DOI] [PubMed] [Google Scholar]
- 18.Winterer J, Gwirtsman HE, George DT, Kaye WH, Loriaux DL, Cutler GB., Jr Adrenocorticotropin-stimulated adrenal androgen secretion in anorexia nervosa: impaired secretion at low weight with normalization after long-term weight recovery. J Clin Endocrinol Metab. 1985;61:693–697. doi: 10.1210/jcem-61-4-693. [DOI] [PubMed] [Google Scholar]
- 19.Tutten A, Laan E, Panhuysen G, Everaerd W, de Haan E, Koppeschaar H, Vroon P. Discrepancies between genital responses and subjective sexual function during testosterone substitution in women with hypothalamic amenorrhea. Psychosom Med. 1996;58:234–241. doi: 10.1097/00006842-199605000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Genazzani AD, Bersi C, Luisi S, Fruzzetti F, Malavasi B, Luisi M, Petraglia F, Genazzani AR. Increased adrenal steroid secretion in response to CRF in women with hypothalamic amenorrhea. J Steroid Biochem Mol Biol. 2001;78:247–252. doi: 10.1016/s0960-0760(01)00094-2. [DOI] [PubMed] [Google Scholar]
- 21.Rickenlund A, Carlstrom K, Ekblom B, Brismar TB, von Schoultz B, Hirschberg AL. Hyperandrogenicity is an alternative mechanism underlying oligomenorrhea or amenorrhea in female athletes and may improve physical performance. Fertil Steril. 2003;79:947–955. doi: 10.1016/s0015-0282(02)04850-1. [DOI] [PubMed] [Google Scholar]
- 22.Rickenlund A, Thoren M, Carlstrom K, von Schoultz B, Hirschberg AL. Diurnal profiles of testosterone and pituitary hormones suggest different mechanisms for menstrual disturbances in endurance athletes. J Clin Endocrinol Metab. 2004;89:702–707. doi: 10.1210/jc.2003-030306. [DOI] [PubMed] [Google Scholar]
- 23.de Rosa G, Corsello SM, de Rosa E, Della Casa S, Ruffilli MP, Grasso P, Pasargiklian E. Endocrine study of anorexia nervosa. Exp Clin Endocrinol. 1983;82:160–172. doi: 10.1055/s-0029-1210272. [DOI] [PubMed] [Google Scholar]
- 24.Robinson E, Bachrach LK, Katzman DK. Use of hormone replacement therapy to reduce the risk of osteopenia in adolescent girls with anorexia nervosa. J Adolesc Health. 2000;26:343–348. doi: 10.1016/s1054-139x(99)00121-4. [DOI] [PubMed] [Google Scholar]
- 25.Klibanski A, Biller BM, Schoenfeld DA, Herzog DB, Saxe VC. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab. 1995;80:898–904. doi: 10.1210/jcem.80.3.7883849. [DOI] [PubMed] [Google Scholar]
- 26.Grinspoon S, Thomas L, Miller K, Herzog D, Klibanski A. Effects of recombinant human IGF-I and oral contraceptive administration on bone density in anorexia nervosa. J Clin Endocrinol Metab. 2002;87:2883–2891. doi: 10.1210/jcem.87.6.8574. [DOI] [PubMed] [Google Scholar]
- 27.Coenen CM, Thomas CM, Borm GF, Hollanders JM, Rolland R. Changes in androgens during treatment with four low-dose contraceptives. Contraception. 1996;53:171–176. doi: 10.1016/0010-7824(96)00006-6. [DOI] [PubMed] [Google Scholar]
- 28.Coenen CM, Thomas CM, Borm GF, Rolland R. Comparative evaluation of the androgenicity of four low-dose, fixed-combination oral contraceptives. Int J Fertil Menopausal Stud. 1995;40 Suppl 2:92–97. [PubMed] [Google Scholar]
- 29.White T, Jain JK, Stanczyk FZ. Effect of oral versus transdermal steroidal contraceptives on androgenic markers. Am J Obstet Gynecol. 2005;192:2055–2059. doi: 10.1016/j.ajog.2005.02.067. [DOI] [PubMed] [Google Scholar]
- 30.Osmanagaoglu MA, Okumus B, Osmanagaoglu T, Bozkaya H. The relationship between serum dehydroepiandrosterone sulfate concentration and bone mineral density, lipids, and hormone replacement therapy in premenopausal and postmenopausal women. J Womens Health (Larchmt) 2004;13:993–999. doi: 10.1089/jwh.2004.13.993. [DOI] [PubMed] [Google Scholar]
- 31.Carlstrom K, Karlsson R, Von Schoultz B. Diurnal rhythm and effects of oral contraceptives on serum dehydroepiandrosterone sulfate (DHEAS) are related to alterations in serum albumin rather than to changes in adrenocortical steroid secretion. Scand J Clin Lab Invest. 2002;62:361–368. doi: 10.1080/00365510260296519. [DOI] [PubMed] [Google Scholar]
- 32.Murphy A, Cropp CS, Smith BS, Burkman RT, Zacur HA. Effect of low-dose oral contraceptive on gonadotropins, androgens, and sex hormone binding globulin in nonhirsute women. Fertil Steril. 1990;53:35–39. [PubMed] [Google Scholar]
- 33.Fern M, Rose DP, Fern EB. Effect of oral contraceptives on plasma androgenic steroids and their precursors. Obstet Gynecol. 1978;51:541–544. doi: 10.1097/00006250-197805000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Wiegratz I, Jung-Hoffmann C, Kuhl H. Effect of two oral contraceptives containing ethinylestradiol and gestodene or norgestimate upon androgen parameters and serum binding proteins. Contraception. 1995;51:341–346. doi: 10.1016/0010-7824(95)00098-u. [DOI] [PubMed] [Google Scholar]
- 35.Moutos D, Smith S, Zacur H. The effect of monophasic combinations of ethinyl estradiol and norethindrone on gonadotropins, androgens and sex hormone binding globulin: a randomized trial. Contraception. 1995;52:105–109. doi: 10.1016/s0010-7824(95)00137-9. [DOI] [PubMed] [Google Scholar]
- 36.Miller KK, Parulekar MS, Schoenfeld E, Anderson E, Hubbard J, Klibanski A, Grinspoon SK. Decreased leptin levels in normal weight women with hypothalamic amenorrhea: the effects of body composition and nutritional intake. J Clin Endocrinol Metab. 1998;83:2309–2312. doi: 10.1210/jcem.83.7.4975. [DOI] [PubMed] [Google Scholar]
- 37.American Psychiatric Association. Diagnostic and statistical manual of mental disorder. 4th ed. (text revision) Arlington, VA: American Psychiatric Publishing, Inc.; 2000. [Google Scholar]
- 38.Metropolitan Life Insurance Co. Metropolitan height and weight tables. Stat Bull. 1983;64:2–9. [PubMed] [Google Scholar]
- 39.Miller KK, Grinspoon S, Gleysteen S, Grieco KA, Ciampa J, Breu J, Herzog DB, Klibanski A. Preservation of neuroendocrine control of reproductive function despite severe undernutrition. J Clin Endocrinol Metab. 2004;89:4434–4438. doi: 10.1210/jc.2004-0720. [DOI] [PubMed] [Google Scholar]
- 40.Finkelstein JS, Klibanski A, Schaefer EH, Hornstein MD, Schiff I, Neer RM. Parathyroid hormone for the prevention of bone loss induced by estrogen deficiency. N Engl J Med. 1994;331 doi: 10.1056/NEJM199412153312404. 1618–16123. [DOI] [PubMed] [Google Scholar]
- 41.Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr. 1990;51:1106–1112. doi: 10.1093/ajcn/51.6.1106. [DOI] [PubMed] [Google Scholar]
- 42.Miller KK, Rosner W, Lee H, Hier J, Sesmilo G, Schoenfeld D, Neubauer G, Klibanski A. Measurement of free testosterone in normal women and women with androgen deficiency: comparison of methods. J Clin Endocrinol Metab. 2004;89:525–533. doi: 10.1210/jc.2003-030680. [DOI] [PubMed] [Google Scholar]
- 43.Miller KK, Biller BM, Beauregard C, Lipman JG, Jones J, Schoenfeld D, Sherman JC, Swearingen B, Loeffler J, Klibanski A. Effects of testosterone replacement in androgen-deficient women with hypopituitarism: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2006;91:1683–1690. doi: 10.1210/jc.2005-2596. [DOI] [PubMed] [Google Scholar]
- 44.Davis SR, McCloud P, Strauss BJ, Burger H. Testosterone enhances estradiol’s effects on postmenopausal bone density and sexuality. Maturitas. 1995;21:227–236. doi: 10.1016/0378-5122(94)00898-h. [DOI] [PubMed] [Google Scholar]
- 45.Barrett-Connor E, Young R, Notelovitz M, Sullivan J, Wiita B, Yang HM, Nolan J. A two-year, double-blind comparison of estrogen-androgen and conjugated estrogens in surgically menopausal women. Effects on bone mineral density, symptoms and lipid profiles. J Reprod Med. 1999;44:1012–1020. [PubMed] [Google Scholar]
- 46.Miller BE, De Souza MJ, Slade K, Luciano AA. Sublingual administration of micronized estradiol and progesterone, with and without micronized testosterone: effect on biochemical markers of bone metabolism and bone mineral density. Menopause. 2000;7:318–326. doi: 10.1097/00042192-200007050-00006. [DOI] [PubMed] [Google Scholar]
- 47.Watts NB, Notelovitz M, Timmons MC, Addison WA, Wiita B, Downey LJ. Comparison of oral estrogens and estrogens plus androgen on bone mineral density, menopausal symptoms, and lipid-lipoprotein profiles in surgical menopause. Obstet Gynecol. 1995;85:529–537. doi: 10.1016/0029-7844(94)00448-m. [DOI] [PubMed] [Google Scholar]
- 48.Slemenda C, Longcope C, Peacock M, Hui S, Johnston CC. Sex steroids, bone mass, and bone loss. A prospective study of pre-, peri-, and postmenopausal women. J Clin Invest. 1996;97:14–21. doi: 10.1172/JCI118382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steinberg KK, Freni-Titulaer LW, DePuey EG, Miller DT, Sgoutas DS, Coralli CH, Phillips DL, Rogers TN, Clark RV. Sex steroids and bone density in premenopausal and perimenopausal women. J Clin Endocrinol Metab. 1989;69:533–539. doi: 10.1210/jcem-69-3-533. [DOI] [PubMed] [Google Scholar]
- 50.Soyka LA, Misra M, Frenchman A, Miller KK, Grinspoon S, Schoenfeld DA, Klibanski A. Abnormal bone mineral accrual in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2002;87:4177–4185. doi: 10.1210/jc.2001-011889. [DOI] [PubMed] [Google Scholar]
- 51.Wang C, Catlin DH, Demers LM, Starcevic B, Swerdloff RS. Measurement of total serum testosterone in adult men: comparison of current laboratory methods versus liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab. 2004;89:534–543. doi: 10.1210/jc.2003-031287. [DOI] [PubMed] [Google Scholar]