Abstract
Diabetes mellitus (DM) and hypertension (HTN) are leading joint risk factors for both cardiovascular disease (CVD) and chronic kidney disease (CKD). In the nationwide KEEP (Kidney Early Evaluation Program) an estimated glomerular filtration rate less than 60 mL/min/1.73 m2 or a urine albumin:creatinine ratio ≥30 mg/g (3.4 mg/mmol) defines CKD. Overall in KEEP, the rates of identified CKD and self-reported CVD are 25.7% and 22.1%, respectively. The presence of CKD has been associated with younger ages of self-reported myocardial infarction and stroke. The combination of CVD and CKD in KEEP has been associated with shorter survival time. Finally, the presence of CVD or a prior history of coronary revascularization has been associated with modestly better rates of CVD risk factor control; however, the majority of patients with CKD have suboptimally controlled blood pressure, glucose, or lipids. These data suggest that patients with CKD are not only at higher risk for CVD and subsequent mortality, but are also ideal for targeted community—and practice-based interventions to improve risk factor control and, hopefully, reduce rates of subsequent cardiovacular events.
Keywords: Cardiovascular disease, Chronic kidney disease, Atherosclerosis, Myocardial infarction, Percutaneous coronary intervention, Microalbuminuria, Bypass surgery, Risk factors
Introduction
Excess adiposity, type 2 diabetes mellitus (DM), hypertension (HTN), and chronic kidney disease (CKD) are collectively a common constellation of health problems that are increasing globally with changes in lifestyle and advancing age [1]. CKD subsequently leads to additional systemic problems including uremia, volume retention, worsened HTN, coagulopathy, anemia, neuropathy, bone disease, and accelerated cardiovascular disease (CVD) [2]. The majority of persons with CKD do not die from kidney failure but rather succumb to CVD. Persons with CKD are at risk for CVD due to both traditional factors (eg, smoking, diabetes, dyslipidemia, HTN), as well as CKD-related factors (eg, reduced renal filtration, microalbuminuria, anemia, hyperparathyroidism, oxidative stress, etc.). CKD (defined as a urine albumin:creatinine ratio [ACR] ≥30 mg/g [3.4 mg/mmol] or an estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2) is now widely recognized as an independent CVD risk state [3]. Overall, there is support for the notion that the CKD state independently contributes to de novo and accelerated atherosclerotic disease in the coronary, cerebral, and peripheral circulations, and thus results in an earlier presentation of disease [4]. CKD is one of the most important predictors of complications after revascularization procedures [5]. Thus, CKD is known to complicate both the procedural results and the long-term management of cardiovascular risk factors. The National KEEP (Kidney Early Evaluation Program) has shed considerable light on the chronic disease epidemics of adiposity, DM, HTN, CKD, and CVD. This paper will review and summarize key findings published from KEEP concerning this issue and will identify key concepts for management and future investigation.
KEEP Methods
The National Kidney Foundation’s KEEP is a free, ongoing community-based screening program designed to identify individuals at increased risk for CKD and encourage them to seek follow-up care [6]. The KEEP program is the nation’s largest and only sustained chronic disease screening effort in the United States [7]. From 2000 through 2010, 47 National Kidney Foundation affiliates representing 49 states through thousands of screening events have recruited nearly 200,000 individuals. Eligible participants are adults ≥18 years old, with DM or HTN, or with a family history of DM, HTN, or CKD. Participants are prompted to attend screenings via announcements in the print media, Internet, and local community organization newsletters.
Measures
Screening data were collected on participant demographic characteristics and medical history including self-reported personal and family history of CVD. One-time systolic blood pressures (SBPs) and diastolic blood pressures (DBPs) were obtained, and blood and urine specimens were collected and processed for determination of blood glucose, creatinine, glycohemoglobin, lipids, and for urine ACR. A more detailed description of KEEP screening methods has been previously published [6]. In brief, standardized data collection forms were used at every site and scanned into a database program; the data are managed at the US Renal Data Systems Coordinating center in Minneapolis, Minnesota, where data integrity and consistency checks are performed.
Definitions and Outcomes
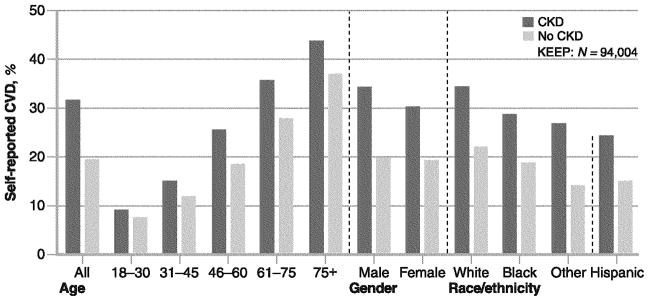
Participants who reported HTN or use of medications for HTN and those with SBP ≥140 mm Hg or DBP ≥90 mm Hg were categorized as having HTN. Participants who reported DM or use of antidiabetic medications and those with blood glucose values greater than 125 mg/dL (6.9 mmol/L), if reported as fasting, or greater than 200 mg/dL (11.1 mmol/L) otherwise were categorized as having DM. eGFRs were calculated using the formula (186.3*[serum Cr−1.154]*[age−.203]); calculated values were multiplied by 0.742 for women and by 1.21 for African Americans. Calculated eGFR values were categorized as less than 30, 30 to 59, 60 to 89, and ≥90 mL/min/1.73 m2 based on the Kidney Disease Outcomes Quality Initiative classification of kidney function; eGFR values less than 60 mL/min/1.73 m2 were considered abnormal and indicative of moderately reduced kidney function and referred to as prevalent CKD [4]. The urine ACR ≥30 mg/g (3.4 mg/mmol) was considered abnormal microalbuminuria. Coronary artery disease (CAD) has been defined in KEEP as a positive response to any of the questions: “heart attack,” “heart bypass surgery,” “heart angioplasty.” Myocardial infarction (MI) was defined as self-reported “heart attack”; percutaneous coronary intervention (PCI) was self-reported as “heart angioplasty”; and coronary artery bypass graft surgery (CABG) was self-reported as “heart bypass surgery” [8, 9]. Overall in KEEP, the rates of identified CKD and self-reported CVD are 25.7% and 22.1%, respectively. Among every age, gender, and ethnic group, rates of CVD are higher among those with CKD compared to those without (Fig. 1).
Fig. 1.
Rates of self-reported cardiovascular disease (CVD) in KEEP (Kidney Early Evaluation Program) by chronic kidney disease (CKD) status according to age group, gender, and race/ethnicity. CVD indicates any of the following cardiac events: heart attack, bypass surgery, heart angioplasty, stroke, heart failure, abnormal heart rhythm, or peripheral vascular disease. (Adapted from National Kidney Foundation [28])
Prematurity of MI, Stroke, and Mortality
In KEEP, premature CVD has been defined as self-reported MI or stroke before 55 and 65 years of age in men and women, respectively, as disclosed at the time of the community screening event. The composite prevalence of premature MI, stroke, or death for those with and without CKD has been reported to be 9.2% and 4.1%, respectively (P<0.0001) (Fig. 2) [4]. Multivariate analysis has demonstrated that CKD (OR, 1.44; 95% CI, 1.27–1.63), age (OR, 1.05 [per year]; 95% CI, 1.04–1.06), HTN (OR, 1.61; 95% CI, 1.40–1.84), diabetes (OR, 2.03; 95% CI, 1.79–2.29), smoking (OR, 1.91; 95% CI, 1.66–2.21), and less than high school education (OR, 1.59; 95% CI, 1.37–1.85) as the most significantly associated factors for premature CVD or death in KEEP (all P<0.0001). Although age at the time of first CVD event is not recorded in the KEEP database, Madala et al. [10•] have reported from the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the American College of Cardiology/American Heart Association Guidelines) database that excess adiposity is an additional and important determinant of premature first MI in addition to the factors listed above. Thus, it is reasonable to conclude that obese persons, with DM, HTN, and CKD, who also smoke and have a family history of premature CVD, could on average, anticipate first MI to occur in the fourth decade of life. Thus, this group during adolsence or early adulthood could be a prime target for multifactorial risk factor reduction and frequent surveillance. Conversely, an individual with none of these factors, if a CVD event is going to occur, is most likely to have its first presention beyond 70 years of age.
Fig. 2.
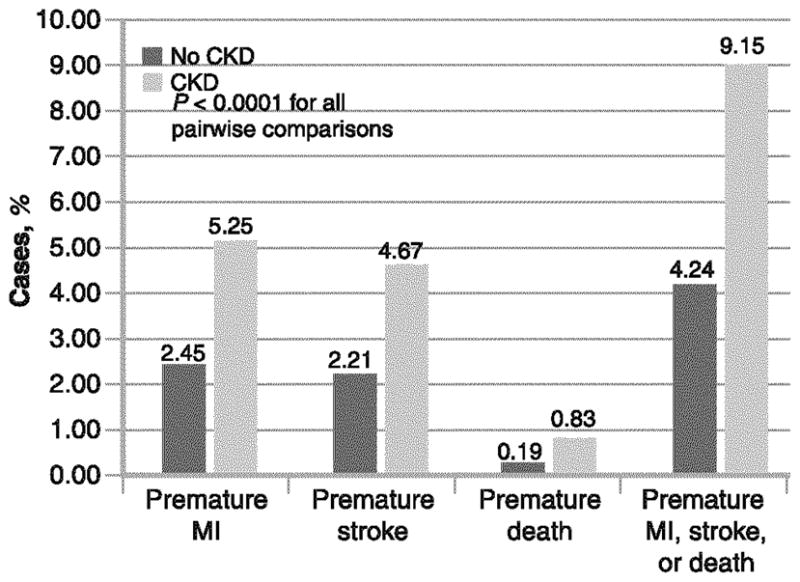
Rates of premature myocardial infarction (MI), stroke, or death in KEEP (Kidney Early Evaluation Program). CKD—chronic kidney disease; (From McCullough et al. [4]; with permission
Control of Coronary Heart Disease Risk Factors in Persons with CKD
In one analysis from 70,454 KEEP participants with a mean age of 53.5±15.7 years and 68.3% being female, 5410 (7.7%) had a self-reported history of CAD; 1295 (1.8%) had a history of prior PCI; and 1124 (1.6%) had a prior history of CABG (Table 1) [11•]. Among those with prior PCI or CABG, 415 had both PCI and CABG and the overall revascularization rates in KEEP were low (<5%). The rates of blood pressure control (defined as an SBP <130 mm Hg), blood glucose control (defined as a glucose <125 mg/dL [6.9 mmol/L]), total cholesterol (<200 mg/dL [5.2 mmol/L]), and current smoking among those with and without CKD are summarized in Table 2. However, the rates of achieving all three preventive cardiology goals (SBP <130 mm Hg, fasting glucose <125 mg/dL [6.9 mmol/L] if diabetic, and total cholesterol <200 mg/d [5.2 mmol/L]) or current smoking (composite cluster) occurred in 27.5%. Conversely, 72.5% of screenees in KEEP had at least one modifiable major risk factor that could be improved. Older age (OR, 1.04; 95% CI, 1.03–1.04; P<0.0001), male gender (OR, 1.40; 95% CI, 1.34–1.47; P<0.0001), ACR ≥30 mg/g (3.4 mg/mmol; OR, 1.66; 95% CI, 1.55–1.79; P<0.0001), one unit increase of body mass index (OR, 1.06; 95% CI, 1.05–1.06; P<0.0001), CAD without a history of revascularization (OR, 1.14; 95% CI, 1.02–1.28; P=0.02), and care received by a nephrologist (OR, 1.49; 95% CI, 1.22–1.83; P<0.0001) were associated with worse risk factor control. Importantly, having a prior history of PCI or CABG was not associated with improved risk factor control, suggesting that even manifest CVD does not prompt further intensification of risk factor management at any speciality level. KEEP does not record use of chronic medications, and thus we cannot draw conclusions regarding the rates of usage or effectiveness of cardioprotective medications or intensity of DM or HTN control. It is important to recognize that CKD does impact the efficacy of antihypertensive medications, so with respect to blood pressure, it makes this risk factor more difficult to control [12•].
Table 1.
Demographic characteristics of the KEEP population according to the presence of CAD
| No CAD (N=65,044) | CAD (no revascularization) (N=3406) | Any revascularization (PCI or CABG) (N=2004) | Total (N=70,454) | P valueb | |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | ||
| Mean age (years [range]) | 52.7±15.6 | 63.4±13.2 | 65.3±12.5 | 53.5±15.7 | <0.0001 |
| Malea | 19,977 (30.7) | 1411 (41.5) | 964 (48.4) | 22,352 (31.8) | <0.0001 |
| Racea | <0.0001 | ||||
| White | 28,953 (45.5) | 1774 (53.2) | 1285 (65.3) | 32,012 (46.4) | |
| African American | 22,192 (34.9) | 1019 (30.6) | 446 (22.7) | 23,657 (34.3) | |
| Other/unknown | 12,529 (19.7) | 539 (16.2) | 237 (12.0) | 13,305 (19.3) | |
| High school or better educationa | 54,690 (85.1) | 2500 (74.7) | 1594 (80.8) | 58,784 (84.5) | <0.0001 |
| Health insurance coveragea | 51,010 (81.5) | 2756 (84.9) | 1680 (89.4) | 55,446 (81.9) | <0.0001 |
| Family history of HTN, DM, or kidney disease | 59,602 (91.6) | 2999 (88.1) | 1661 (82.9) | 64,262 (91.2) | <0.0001 |
CABG coronary artery bypass graft surgery; CAD coronary artery disease; DM diabetes mellitus; HTN hypertension; KEEP Kidney Early Evaluation Program; PCI percutaneous coronary intervention
Missing values excluded from calculation
P value for comparing no CAD, CAD without revascularization, prior PCI, prior CABG, and both PCI and CABG
(Adapted from McCullough et al. [11•])
Table 2.
Health screening results according to CAD status
| No CAD (N=65,044) | CAD (no revascularization) (N=3406) | Any revascularization (PCI or CABG) (N=2004) | Total (N=70,454) | P valuea | |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | ||
| Body mass index (kg/m2) (missing=1180) | 30.1±6.8 | 30.8±6.8 | 30.1±6.2 | 30.2±6.8 | <0.0001 |
| Diabetic (reported DM or glucose >125 [6.9 mmol/L] if fasting, else >200 mg/dL [11.1 mmol/L]) (missing=18) | 17,005 (26.2) | 1653 (48.5) | 1019 (50.9) | 19,677 (27.9) | <0.0001 |
| Using insulin (KEEP question 7c) (missing=55,435) | 1058 (8.3) | 126 (14.1) | 270 (19.8) | 1454 (9.7) | <0.0001 |
| Plasma glucose (mean) for diabetics only in mg/dL [mmol/L] (n=19,243) | 158.9±81.1 [8.8±4.5] | 164.5±81.9 [9.2±4.6] | 154.2±71.6 [8.6±4.0] | 159.1±80.7 [8.8±4.5] | 0.0109 |
| N (%) glucose ≥125 mg/dL (6.9 mmol/L) (missing=1689) | 13,872 (21.9) | 1260 (37.9) | 722 (36.7) | 15,854 (23.1) | <0.0001 |
| Mean total cholesterol from central laboratory (n=26,293) | 201.1±41.0 | 197.9±45.0 | 182.6±43.6 | 199.6±41.6 | <0.0001 |
| N (%) with total cholesterol ≥200 mg/dL (5.2 mmol/L) (missing=44,161) | 11,219 (48.5) | 560 (45.3) | 589 (30.3) | 12,368 (47.0) | <0.0001 |
| Smoking (missing=4090) | <0.0001 | ||||
| Never smoked | 36,639 (59.8) | 1417 (44.3) | 894 (47.7) | 38,950 (58.7) | |
| Current smoker | 7464 (12.2) | 457 (14.3) | 193 (10.3) | 8114(12.2) | |
| Former smoker | 17,191 (28.1) | 1322 (41.4) | 787 (42.0) | 19,300 (29.1) | |
| Hypertensive (hypertension medications or SBP ≥140 or DBP ≥90 mm Hg) (missing=10) | 41,016(63.1) | 2874 (84.4) | 1721 (85.9) | 45,611 (64.8) | <0.0001 |
| N (%) with SBP ≥130 mm Hg (missing=938) | 35,727 (55.7) | 2194 (65.2) | 1263 (63.3) | 39,184 (56.4) | <0.0001 |
| Albumin:creatinine ≥30 mg/g (3.4 mg/mmol) (missing=9883) | 6257 (11.2) | 558 (19.2) | 359 (18.3) | 7174 (11.8) | <0.0001 |
| eGFR category (missing=3931) | <0.0001 | ||||
| eGFR <30 mL/min/1.73 m2 | 473 (0.8) | 84 (2.6) | 54 (2.8) | 611 (0.9) | |
| eGFR 30–59 mL/min/1.73 m2 | 9149 (14.9) | 932 (29.1) | 579 (30.5) | 10,660 (16.0) | |
| eGFR 60–89 mL/min/1.73 m2 | 31,421 (51.2) | 1553 (48.5) | 962 (50.7) | 33,936 (51.0) | |
| eGFR ≥90 mL/min/1.73 m2 | 20,382 (33.2) | 630 (19.7) | 304 (16.0) | 21,316 (32.0) | |
| eGFR <60 mL/min/1.73 m2 (missing=3931) | 9622 (15.7) | 1016 (31.8) | 633 (33.3) | 11,271 (16.9) | <0.0001 |
| Under care of cardiologist (KEEP question 24) | 1758 (2.7) | 293 (8.6) | 1103 (55.0) | 3154 (4.5) | <0.0001 |
| Under care of nephrologist (KEEP question 24) | 882 (1.4) | 83 (2.4) | 118 (5.9) | 1083 (1.5) | <0.0001 |
| Neither cardiologist nor nephrologist | 62,525 (96.1) | 3054 (89.7) | 875 (43.7) | 66,454 (94.3) | <0.0001 |
CABG coronary artery bypass graft surgery; CAD coronary artery disease; DBP diastolic blood pressure; DM diabetes mellitus; eGFR estimated glomerular filtration rate; HTN hypertension; KEEP Kidney Early Evaluation Program; PCI percutaneous coronary intervention; SBP systolic blood pressure
P value for comparing no CAD, CAD without revascularization, prior PCI, prior CABG, and both PCI and CABG
(Adapted from McCullough et al. [11•])
An analysis from the Cardiovascular Health Study has suggested that CKD alone should be considered a CVD risk equivalent [13]. However, compared with other conventional CVD risk factors, patient level awareness of CKD is low, less than 10% [14]. Whereas current society guidelines focus on the control of singular risk factors including blood pressure, blood glucose, and cholesterol, the totality of CVD risk has been difficult to summarize and measure in populations. It has been estimated that optimal CVD risk factor management should theoretically translate into an approximately 85% reduction in CVD events including MI and cardiovascular death [15]. Thus, the simple recognition of CKD based on the clinical evaluation and education of the patient that indeed CKD is present should consistently identify the very highest risk CVD subset who could benefit from frequent office visits, intensive surveillance, and preventive therapies that favorably influence the national history of both CKD and CVD before hard clinical end points develop [16–18].
We cannot infer from KEEP whether these results on CVD risk factors are based on lifestyle, medications, or both. On the positive side, younger individuals, women, having insurance, higher education, and more severe reductions in eGFR were all associated with better risk factor control. These data suggest efforts on patient and physician education and awareness will play an important future role in achieving stringent risk factor targets despite the presence of multiple comorbidities in patients with CKD [19–22].
Chronic Disease Awareness
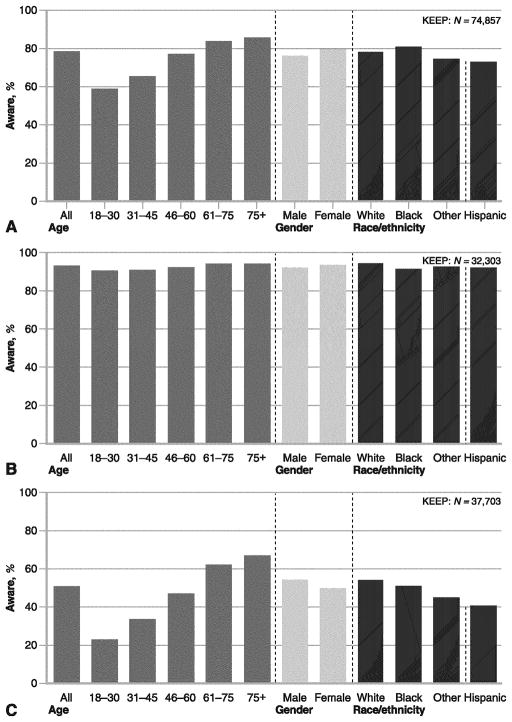
Prior work has demonstrated that KEEP screenees are generalizable to the NHANES (US National Health and Nutrition Examination Survey) in that the risk relationships between CKD, CVD, and outcomes hold, however, with respect to each risk factor, KEEP is an enriched population [23, 24]. Thus, KEEP has served as an excellent model to assess the issues of chronic disease awareness as they relate to both KD and CVD risk profiles. In a recent publication from KEEP, the definition of CVD was expanded to self-reported history of heart attack, heart angioplasty, bypass surgery, heart failure, abnormal heart rhythm, or stroke [25]. In this analysis, of 77,077 KEEP participants, 20,200 (26.25%) were identified with CKD and 23,082 (29.9%) with diabetes. Awareness of CKD among diabetic participants with CKD identified by laboratory measures was low at 9.4% (736 of 7853), with 6% in those with CKD stages 1 and 2 CKD (154 of 2548), and 10.9% in those with later-stage CKD (582 of 5305). There was a higher prevalence of CVD in KEEP participants with diabetes than without (39.5% vs 22.0%) and higher in stages 3 to 5 compared with stages 1 to 2 CKD (43.3% vs 34.4%). Among those aware of CKD, CVD prevalence was higher in participants with diabetes than without (53.7% vs 36.4%) and in participants with stages 3 to 5 compared with 1 to 2 (57% vs 41.4%). Awareness of HTN, DM, and elevated cholesterol is shown in Fig. 3. Participants with DM and CVD had a higher level of CKD awareness compared to those without DM and without CVD (8.2% vs 2.2%). Of participants with diabetic CVD, the presence of heart failure predicted CKD awareness (OR, 1.84; 95% CI, 1.40–2.43) moreso than presence of other cardiovascular conditions such as MI or stroke (1.35 [1.04–1.73] and 1.34 [1.04–1.72], respectively). These data suggest that patients often develop heart disease and then learn as a consequence that there is CKD present as well. In many cases, this is is too late for meaningful CKD risk factor intervention and slowing the progression of CKD becomes a secondary issue in the cardiovascular care model [26•]. In fact, CKD may be elevated to a clinical issue, particularly in heart failure, because it complicates care with respect to drugs that antagonize the renin-angiotensin system as well as diuretics and is associated with more severe heart failure symptoms [27].
Fig. 3.
Awareness of hypertension (a), diabetes (b), and elevated cholesterol (c) at the time of the KEEP (Kidney Early Evaluation Program) screening. (From National Kidney Foundation [28]; with permission)
Limitations of the Screening Program
The KEEP screening process does not recruit individuals using the terms “heart” or “CVD.” Thus, we believe that participants were enrolled based on the personal concern for DM, HTN, or CKD and that the self-report of CVD represents a measured variable disclosed by the individual. We acknowledge that self-reported CVD has inherent variance related both to over-reporting and under-reporting. However, the surveys were completed in an assisted manner by a health care professional trained in eliciting the most accurate and complete medical information possible. KEEP workers are unable to validate histories of revascularization and do not have access to discharge summaries or procedure reports because the screenings are held in communities and not in hospitals or research centers. Measurements are taken once (which is suboptimal), thus misclassification bias according groupings by measure (blood pressure, glucose, and cholesterol) works to bias hypothesis testing to the null. KEEP only recently has started testing specific measures including low-density lipoprotein cholesterol and glycohemoglobin, which will give more precise information about the treatment of CVD risk in the future. From the KEEP database, it is not possible to calculate the Framingham risk for each subject; thus, differing treatment goals according to baseline risk cannot be described. As noted above, KEEP does not have data collected on current and past medications (statins, oral antidiabetic agents, insulin, antihypertensives), drug intolerances, and number of medications used to treat each CVD risk factor. This information is critical for an indication of the clinical attempt to control conditions that may be difficult to manage in CKD. It is widely recognized that the eGFR variable may have worked to underestimate actual renal filtration function and thus misclassified patients with higher levels into those with eGFR less than 60 mL/min/1.73 m2, and thus diluted the biologic impact of CKD on the CVD risk factor control. All of these factors being considered, the overall rates of satisfactory control of four major modiafiable risk factors (for both CKD and CVD) are very low as shown in Fig. 4, indicating a considerable opportunity for improvement.
Fig. 4.
Rates of achieving one, two, or all three preventive cardiology goals according to chronic kidney disease (CKD) status for patients by coronary artery disease (CAD) status: no CAD, CAD but no revascularization, percutaneous coronary intervention (PCI), and coronary artery bypass graft surgery (CABG). (Preventive cardiology goals: systolic blood pressure <130 mm Hg, glucose <125 mg/dL [6.9 mmol/L] if diabetic, or total cholesterol <200 mg/dL [5.2 mmol/L]). (N=58,945. All paired P values between no CKD and CKD: no CAD, P<0.0001; CAD but no revascularization, P<0.0001; prior PCI, P= 0.033; prior CABG, P=0.0931; both PCI and CABG, P=0.2798; any revascularization [PCI or CABG], P=0.0057). (From McCullough et al. [11•]; with permission)
Conclusions
CKD as one of the most important cardiovascular risk states is associated with overall poor rates of optimal cardiovascular risk factor control. These data suggest there is considerable opportunity to raise awareness at the level of the patient, provider, and system for improved recognition and treatment of modifiable CVD risk in patients with CKD.
Footnotes
Disclosure No potential conflicts of interest relevant to this article were reported.
Contributor Information
Peter A. McCullough, Email: peteramccullough@gmail.com, Department of Medicine, Sections of Cardiology, Nephrology, and Endocrinology, St. John Providence Health System, Providence Park Hospital, Novi, MI, USA
Susan Steigerwalt, Department of Medicine, Sections of Cardiology, Nephrology, and Endocrinology, St. John Providence Health System, Providence Park Hospital, Novi, MI, USA.
Kirit Tolia, Department of Medicine, Sections of Cardiology, Nephrology, and Endocrinology, St. John Providence Health System, Providence Park Hospital, Novi, MI, USA.
Shu-Cheng Chen, Department of Medicine, U.S. Renal Data Systems Center, Minneapolis, MN, USA.
Suying Li, Department of Medicine, U.S. Renal Data Systems Center, Minneapolis, MN, USA.
Keith C. Norris, Charles R Drew University, Los Angeles, CA, USA
Adam Whaley-Connell, Division of Nephrology, University of Missouri School of Medicine, Columbia, MO, USA.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, Nahas ME, Jaber BL, Jadoul M, Levin A, Powe NR, Rossert J, Wheeler DC, Lameire N, Eknoyan G. Chronic kidney disease as a global public health problem: approaches and initiatives—a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007 Aug;72(3):247–59. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 2.Bakris G, Collins AJ. Executive summary: Kidney Early Evaluation Program (KEEP) 2007 Annual Data Report. Am J Kidney Dis. 2008 Apr;51(4 Suppl 2):Sl–2. doi: 10.1053/j.ajkd.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 3.McCullough PA, Jurkovitz CT, Pergola PE, McGill JB, Brown WW, Collins AJ, Chen SC, Li S, Singh A, Norris KC, Klag MJ, Bakris GL for the KEEP Investigators. Independent components of chronic kidney disease as a cardiovascular risk state: results from the Kidney Early Evaluation Program (KEEP) Arch Intern Med. 2007 Jun 11;167(11):1122–9. doi: 10.1001/archinte.167.11.1122. [DOI] [PubMed] [Google Scholar]
- 4.McCullough PA, Li S, Jurkovitz CT, Stevens L, Collins AJ, Chen SC, Norris KC, McFarlane S, Johnson B, Shlipak MG, Obialo CI, Brown WW, Vassalotti J, Whaley-Connell AT, Brenner RM, Bakris GL KEEP Investigators. Chronic kidney disease, prevalence of premature cardiovascular disease, and relationship to short-term mortality. Am Heart J. 2008 Aug;156(2):277–83. doi: 10.1016/j.ahj.2008.02.024. Epub 2008 Jun 4. Erratum in: Am Heart J. 2008 Dec; 156(6): 1132. [DOI] [PubMed] [Google Scholar]
- 5.McCullough PA. Why is chronic kidney disease the "spoiler" for cardiovascular outcomes? J Am Coll Cardiol. 2003 Mar 5;41( 5):725–8. doi: 10.1016/s0735-1097(02)02955-8. [DOI] [PubMed] [Google Scholar]
- 6.Brown WW, Peters RM, Ohmit SE, Keane WF, Collins A, Chen S-C, King K, Klag M, Molony DA, Flack JM. Early Detection of Kidney Disease in Community Settings: The Kidney Early Evaluation Program. Am J Kid Dis. 2003;42:22–35. doi: 10.1016/s0272-6386(03)00405-0. [DOI] [PubMed] [Google Scholar]
- 7.McCullough PA, Vassalotti JA, Collins AJ, Chen SC, Bakris GL. National Kidney Foundation’s Kidney Early Evaluation Program (KEEP) annual data report 2009: executive summary. Am J Kidney Dis. 2010 Mar;55(3 Suppl 2):Sl–3. doi: 10.1053/j.ajkd.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 8.Chobanian AV, Bakris GL, Black HR for the National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 9.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 10•.Madala MC, Franklin BA, Chen AY, Berman AD, Roe MT, Peterson ED, Ohman EM, Smith SC, Jr, Gibler WB, McCullough PA CRUSADE Investigators. Obesity and Age of First Non-ST-Segment Elevation Myocardial Infarction. J Am Coll Cardiol. 2008 Sep 16;52(12):979–985. doi: 10.1016/j.jacc.2008.04.067. Convincingly demonstrates that obesity is now the leading determinant of premature acute coronary syndrome, having a larger impact than smoking or family history. [DOI] [PubMed] [Google Scholar]
- 11•.McCullough PA, Whaley-Connell A, Brown WW, Collins AJ, Chen SC, Li S, Norris KC, Jurkovitz C, McFarlane S, Obialo C, Sowers J, Stevens L, Vassalotti JA, Bakris GL on Behalf of the KEEP Investigators. Cardiovascular risk modification in participants with coronary disease screened by the Kidney Early Evaluation Program. Intern Med J. 2010 Jan 4; doi: 10.1111/j.1445-5994.2009.02158.x. Evidence that the minority of CKD patients achieve satisfactory control of CVD risk factors. [DOI] [PubMed] [Google Scholar]
- 12•.Lubanski MS, McCullough PA. Kidney’s role in hypertension. Minerva Cardioangiol. 2009 Dec;57(6):743–59. Describes the mechanisms by which CKD elevates blood pressure and makes it more difficult to treat. [PubMed] [Google Scholar]
- 13.Rashidi A, Sehgal AR, Rahman M, O’Connor AS. The case for chronic kidney disease, diabetes mellitus, and myocardial infarction being equivalent risk factors for cardiovascular mortality in patients older than 65 years. Am J Cardiol. 2008 Dec 15;102( 12):1668–73. doi: 10.1016/j.amjcard.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 14.Saab G, Whaley-Connell AT, McCullough PA, Bakris GL. CKD awareness in the United States: the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2008 Aug;52(2):382–3. doi: 10.1053/j.ajkd.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 15.Lloyd-Jones DM, Dyer AR, Wang R, Daviglus ML, Greenland P. Risk factor burden in middle age and lifetime risks for cardiovascular and non-cardiovascular death (Chicago Heart Association Detection Project in Industry) Am J Cardiol. 2007 Feb 15;99(4):535–40. doi: 10.1016/j.amjcard.2006.09.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vassalotti JA, Li S, McCullough PA, Bakris GL. Kidney Early Evaluation Program: A Community-Based Screening Approach to Address Disparities in Chronic Kidney Disease. Semin Nephrol. 2010 Jan;30(1):66–73. doi: 10.1016/j.semnephrol.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Whaley-Connell AT, Sowers JR, McFarlane SI, Norris KC, Chen SC, Li S, Qiu Y, Wang C, Stevens LA, Vassalotti JA, Collins AJ Kidney Early Evaluation Program Investigators. Diabetes mellitus in CKD: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition and Examination Survey (NHANES) 1999–2004. Am J Kidney Dis. 2008 Apr;51(4 Suppl 2):S21–9. doi: 10.1053/j.ajkd.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Agrawal V, Kizilbash SH, McCullough PA. New therapeutic agents for diabetic kidney disease. Therapy. 2008 Jun;5(4):553–75. [Google Scholar]
- 19.Agrawal V, Ghosh AK, Barnes MA, McCullough PA. Awareness and Knowledge of Clinical Practice Guidelines for CKD among Internal Medicine Residents: A National Online Survey. Am J Kidney Dis. 2008 Oct 29; doi: 10.1053/j.ajkd.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 20.McCullough PA, Verrill TA. Cardiorenal interaction: appropriate treatment of cardiovascular risk factors to improve outcomes in chronic kidney disease. Postgrad Med. 2010 Mar;122(2):25–34. doi: 10.3810/pgm.2010.03.2119. [DOI] [PubMed] [Google Scholar]
- 21.Agrawal V, Shah A, Rice C, Franklin BA, McCullough PA. Impact of treating the metabolic syndrome on chronic kidney disease. Nat Rev Nephrol. 2009 Jul 28; doi: 10.1038/nrneph.2009.114. [DOI] [PubMed] [Google Scholar]
- 22.Stevens LA, Li S, Wang C, Huang C, Becker BN, Bomback AS, Brown WW, Burrows NR, Jurkovitz CT, McFarlane SI, Norris KC, Shlipak M, Whaley-Connell AT, Chen SC, Bakris GL, McCullough PA. Prevalence of CKD and comorbid illness in elderly patients in the United States: results from the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2010 Mar;55(3 Suppl 2):S23–33. doi: 10.1053/j.ajkd.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whaley-Connell AT, Sowers JR, Stevens LA, McFarlane SI, Shlipak MG, Norris KC, Chen SC, Qiu Y, Wang C, Li S, Vassalotti JA, Collins AJ Kidney Early Evaluation Program Investigators. CKD in the United States: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES) 1999–2004. Am J Kidney Dis. 2008 Apr;51(4 Suppl 2):S13–20. doi: 10.1053/j.ajkd.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 24.McCullough PA, Li S, Jurkovitz CT, Stevens LA, Wang C, Collins AJ, Chen SC, Norris KC, McFarlane SI, Johnson B, Shlipak MG, Obialo CI, Brown WW, Vassalotti JA, Whaley-Connell AT Kidney Early Evaluation Program Investigators. CKD and cardiovascular disease in screened high-risk volunteer and general populations: the Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES) 1999–2004. Am J Kidney Dis. 2008 Apr;51(4 Suppl 2):S38–45. doi: 10.1053/j.ajkd.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 25.Whaley-Connell A, Pavey BS, McCullough PA, Saab G, Li S, McFarlane SI, Chen SC, Vassalotti JA, Collins AJ, Bakris G, Sowers JR KEEP Investigators. Dysglycemia predicts cardiovascular and kidney disease in the Kidney Early Evaluation Program. J Clin Hypertens (Greenwich) 2010 Jan;12(1):51–8. doi: 10.1111/j.1751-7176.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26•.Agrawal V, Marinescu V, Agarwal M, McCullough PA. Cardiovascular implications of proteinuria: an indicator of chronic kidney disease. Nat Rev Cardiol. 2009 Apr;6(4):301–11. doi: 10.1038/nrcardio.2009.11. Best source of mechanistic figures demonstrating the relationship between proteinuria and atherosclerosis. [DOI] [PubMed] [Google Scholar]
- 27.McCullough PA, Franklin BA, Leifer E, Fonarow GC. Impact of Reduced Kidney Function on Cardiopulmonary Fitness in Patients with Systolic Heart Failure. Am J Nephrol. 2010 Jul 22;32( 3):226–233. doi: 10.1159/000317544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Kidney Foundation. KEEP Kidney Early Evaluation Program Annual Data Report 2009. Am J Kidney Dis. 2010;55(3 Suppl 2):S34–S154. doi: 10.1053/j.ajkd.2009.12.017. [DOI] [PubMed] [Google Scholar]