Abstract
Background
In earlier studies we found that a high-fat, high-energy diet (HFED) attenuates proteinuria, azotemia and lipid accumulation in the remnant kidney of rats subjected to 5/6 nephrectomy. This study was conducted to explore the mechanism of the salutary effect of HFED in association with moderate protein restriction in this model.
Methods
The 5/6 nephrectomized male rats were randomized to receive regular rat chow (CRF group, n = 6) or HFED diet (CRF + HFED, n = 7) for 12 weeks. Sham-operated rats served as controls (n = 6).
Results
The CRF group exhibited azotemia, hypertension, proteinuria, diminished body weight, oxidative stress, glomerulosclerosis, tubulo-interstitial inflammation and upregulation of pro-oxidant [NAD(P)H oxidase], pro-inflammatory (NF-κB activation, increased MCP-1, lipoxygenase, ICAM-1, VCAM-1), pro-fibrotic (TGF-β, CTGF) and pro-apoptotic pathways (Bax, caspase-3) in the remnant kidney. Consumption of the HFED resulted in a 66% increment in lipid intake, 8% increment in carbohydrate intake and a 24% reduction in protein intake. The CRF + HFED group gained weight normally, had increments in leptin and adiponectin levels, and despite increments in plasma cholesterol and fatty acids, showed significant attenuation of oxidative stress, proteinuria and inflammation, and partial reversal of the remnant kidney upregulation of pro-oxidant, pro-inflammatory, pro-fibrotic and pro-apoptotic pathways.
Conclusion
Consumption of high-energy diet in association with mild protein restriction results in suppression of upregulated pathways that drive progression of renal injury in the remnant kidney model. These findings may have relevance in the management of chronic kidney disease in humans.
Keywords: Dyslipidemia, Malnutrition, High-calorie diet, Chronic renal disease progression, Inflammation, Uremic cachexia
Introduction
Large epidemiological studies have demonstrated inverse relationships between mortality and body mass index [1–8], overweight and obesity [4, 9], and a U-shaped mortality curve for blood pressure [10], serum cholesterol [11], homocysteine [12] and creatinine [13] in dialysis-dependent end-stage renal disease (ESRD) patients. While the mechanisms responsible for the survival advantage conferred by overweight in patients maintained on hemodialysis, so-called “reverse epidemiology,” has been extensively debated [14], the effect of high-calorie diets with a high fat content on the diseased/remnant kidney itself has received little attention. This relative disregard is due to the assumption that a high lipid intake could worsen the oxidative stress and inflammation that are hallmarks of chronic renal injury. In fact, the genetically obese Zucker rats develop renal injury that can be arrested or ameliorated with immunosuppressive/anti-inflammatory therapy [15], indicating that oxidative stress and inflammation, driven by the local effects of oxidized lipoprotein, play a pivotal role in the hyperlipidemia-induced nephropathy. Furthermore, oxidized low-density lipoproteins are known to accumulate in the renal interstitium in human renal allograft patients and in experimental animals [16, 17], and induce macrophage influx [18], inflammation and collagen synthesis [16, 17]. In this process, scavenger receptors of oxidized lipoproteins play a significant role in the modulation of the resulting renal interstitial injury [19].
In a recent study, some of us examined the effects of a high-fat, high-energy diet (HFED) in the remnant kidney model assuming that it might accelerate progression of renal disease, and contrary to our expectations, we observed improvements in proteinuria and diminished lipid accumulation in the remnant kidney [20]. The present study was designed to discern the potential mechanism(s) of the salutary effects of HFED in this model. To this end, the effects of chronic consumption of a high-fat, high-energy diet on pro-oxidant, pro-inflammatory, pro-apoptotic and pro-fibrotic pathways and renal histology were examined in rats subjected to 5/6 nephrectomy, an experimental model characterized by development of unrelenting glomerulosclerosis, tubulointerstitial injury, proteinuria and chronic renal failure (CRF). This resembles the progression of chronic renal disease in humans.
Materials and methods
The experimental protocol was approved by the Institutional Animal Care and Use Committee of the University of California (Irvine, CA). Male Sprague-Dawley rats with an average body weight of 250–300 g (Harlan Sprague-Dawley Inc., Indianapolis, IL) housed in a climate-controlled vivarium with 12-h day and night cycles were used in this study.
The animals were randomly assigned to the CRF and sham-operated control groups. The CRF group underwent 5/6 nephrectomy by surgical resection of the upper and lower thirds of the left kidney, followed by right nephrectomy 7 days later. The control group had a similar surgical procedure, and kidneys were manipulated but left intact. The procedures were carried out under general anesthesia (sodium pentobarbital, 50 mg/kg, IP) using strict hemostasis and aseptic techniques. The 5/6 nephrectomized animals were randomized to a group fed with regular laboratory diet (Prolab® RMH 2500) (CRF group, n = 6) and a group that received a high-fat, high-energy diet (HFED) diet, resembling the Western diet (CRF + HFED group, n = 6). Sham-operated rats (n = 6) were fed regular laboratory diet (Prolab, RMH 2500). The regular diet (RD) provided 59.1% of the energy as carbohydrate, 12.1% as fat and 28.8% as protein; the HFED diet provided 42.7% of the energy as carbohydrate, 42.0% as fat and 15.2% as protein. The composition of the nutrients (g/100 g) in the HFED-fed CRF and RD-fed CRF groups was: carbohydrate, 49.1 and 49.3; protein, 19.5 and 24.0; fat, 21.0 and 10.5; fiber, 5.0 and 5.3; digestible energy (kcal/g), 4.5 and 3.0, respectively. The fat content of the diets consisted of anhydrous milk fat. Food consumption was evaluated every 4 weeks.
Animals were observed for 12 weeks and then were euthanized by exsanguination using cardiac puncture under general anesthesia (sodium pentobarbital, 50 mg/kg IP). Visceral abdominal fat and epididymal fat pads were removed and weighed. Kidneys were removed and weighed. A section of the harvested kidney was separated and fixed in 10% formalin, and the remainder was cleaned with phosphate-buffered saline, snap-frozen in liquid nitrogen and stored at −70°C until processed.
Blood pressure was determined by tail cuff plethysmography (CODA2, Kent Scientific Corporation, Torrington, CT) at 4, 8 and 12 weeks. Conscious rats were placed in a restrainer on a warming pad and allowed to rest inside the cage for 15 min before blood pressure measurements. Rat tails were placed inside a tail cuff, and the cuff was inflated and released several times to allow the animal to be conditioned to the procedure.
Biochemical determinations
Proteinuria (Chondrex Inc., Redmond, WA) and creatinine clearance were determined by placing the animals in individual metabolic cages for a timed urine collection. Plasma total cholesterol (Stanbio Laboratory, Boerne, TX), triglyceride (Stanbio Laboratory, Boerne, TX), free fatty acid, HDL cholesterol and blood glucose (Wako Chemicals, Richmond, VA), urea, albumin and creatinine (Bioassay Systems, Hayward, CA, based in the Jaffé alkaline picrate assay) were measured using the specified reagents. Rat insulin, leptin and adiponectin were determined with ELISA kits purchased from Linco Research Inc, (St. Charles, MO). All laboratory determinations were made in samples taken between 9 and 11 a.m. without overnight food restriction. Thiobarbituric acid reactive substance (TBARS) assay was performed in the plasma and kidney tissue homogenates as described previously [21].
Histology and immunohistology
Light microscopy studies were done in formalin-fixed renal sections stained with periodic acid-Schiff and hematoxylin-eosin stains as described in previous investigations [22–24]. Briefly, glomerulosclerosis was graded from 0 (normal) to 4+ (sclerosis in more than 75% of the glomerular tuft) and calculated the score with the following formula: [(1 × n glomeruli with 1+) + (2× n glomeruli with 2+) + (3 × n glomeruli with 3+) + (4 × n glomeruli with 4+)] × 100/total number of glomeruli examined. Tubulointerstitial damage was graded according the extension (%) of tubular damage (infiltration, fibrosis, tubular dilatation/atrophy) in successively evaluated fields in the renal cortex. Immunohistology was used to identify infiltration of lymphocytes (CD5-positive cells) and macrophages (ED1-positive cells) by avidin-biotin-peroxidase methodology) and collagen IV (by alkaline phosphatase methodology). Positive cells were separately evaluated within the glomeruli (per glomerular cross section) and in tubulointestitial areas of the cortex (positive cells/mm2). Computer-assisted image analysis was used to evaluate the extent of the lesions in tubulointerstitial areas using an Olympus BX51 system and DP70 microscopic digital camera and Sigma Pro (Leesburg, VA) image analysis software as in previous work [22–26]. All histological and immunohistological determinations were done by an observer who was blinded with respect to the identity of the tissue under scrutiny.
Primary antibodies used were mouse anti-ED1 (monocytes and macrophages, Biosource, Camarillo, CA); mouse anti-rat thymocytes and T lymphocytes CD5 (Biosource, Camarillo, CA) and rabbit anti-human collagen IV (Accurate Chemical Scientific Corporation, Westbury, NY). Secondary antibodies were rat anti-mouse IgG biotin-conjugated (Accurate Chemical Scientific Corporation, Westbury, NJ) and donkey anti-rabbit IgG biotin-conjugated (Accurate Chemical Scientific Corporation, West-bury, NJ). Other immunostaining reagents were Dako Fast Red Substrate System for Immunochemistry (Dako catalog K-0597), extravidin alkaline phosphatase conjugate and extravidin peroxidase (Sigma); levamisol solution (Vector Laboratories SP-5000) and diamino benzidine (Sigma).
Preparation of kidney homogenates and nuclear extracts
All solutions, tubes and centrifuges were maintained at 0–4°C. The nuclear extract was prepared as described previously [20, 21]. Briefly, 100 mg of kidney cortex was homogenized in 0.5 ml buffer A containing 10 mM HEPES, pH 7.8, 10 mM KCl, 2 mM MgCl2, 1 mM dithiothreitol (DTT), 0.1 mM EDTA, 0.1 mM PMSF, 1 μM pepstatin and 1 mM P-aminobenzamidine using a tissue homogenizer for 20 s. Homogenates were kept on ice for 15 min, and 125 μl of a 10% Nonidet p40 (NP 40) solution was added and mixed for 15 s. The mixture was centrifuged for 2 min at 12,000 rpm. The supernatant containing cytosolic proteins was collected. The pelleted nuclei were washed once with 200 μl of buffer A plus 25 μl of 10% NP 40, centrifuged, then suspended in 50 μl of buffer B [50 mM HEPES, pH 7.8, 50 mM KCl, 300 mM NaCl, 0.1 mM EDTA, 1 mM DTT, 0.1 mM PMSF, 10% (v/v) glycerol], mixed for 20 min and centrifuged for 5 min at 12,000 rpm. The supernatant containing nuclear proteins was stored at −80°C. The protein concentration in tissue homogenates and nuclear extracts was determined by the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA).
Western blot analyses
Target proteins in the homogenates of the kidney tissue were measured by Western blot analysis as described previously [21, 22] using the antibodies against nNOS, eNOS, Rac-1, gp91phox, p47phox (BD Bioscience, San Jose, CA), 12-lipoxygenase (12-LO) (Cayman chemical, Ann Arbor, MI), ICAM-1 (Siekagaku Corp., Tokyo, Japan), VCAM-1 (Amac, Inc., Westbrook, ME), MCP-1 (Biosource, Int, Camarillo, CA), phosphorylated I kappa B (p-IκB), pERK and ERK, Bax, caspase-3 (Cell Signaling Technology, Inc., Denver, CO) and Histone H1, NF-κB p65, NF-κB p50, NOX4, p22phox, TGF-β, CTGF (Santa Cruz Biotechnology Inc., Santa Cruz, CA). β-Actin (Assay Designs, Ann Arbor, MI) and Histone H1 served as housekeeping control for cytosolic and nuclear target proteins. Briefly, aliquots containing 50 μg proteins were fractionated on 4–20% Tris-glycine gel (Novex Inc., San Diego, CA) at 120 V for 2 h and transferred to a Hybond-ECL membrane (Amersham Life Science Inc., Arlington Heights, IL). The membrane was incubated for 1 h in blocking buffer (1 × TBS, 0.05% Tween-20 and 5% nonfat milk) and then overnight in the same buffer containing the given antibodies. The membrane was washed three times for 5 min in 1 × TBS, 0.05% Tween-20 prior to 2-h incubation in a buffer (1 × TBS, 0.05% Tween-20 and 3% nonfat milk) containing horseradish peroxidase-linked anti-rabbit IgG and anti-mouse IgG (Amersham Life Science Inc.) at 1:1,000 dilution. The membrane was washed four times and developed by autoluminography using ECL chemiluminescent agents (Amersham Life Science Inc.)
Statistical analysis
Statistical comparisons were done with multi-group ANOVA analysis and Tukey post-tests with the help of a commercial statistical program (InstatR, Graph Pad, San Diego, CA). Two-tailed p values of p < 0.05 were considered statistically significant. Data are given as mean and SD.
Results
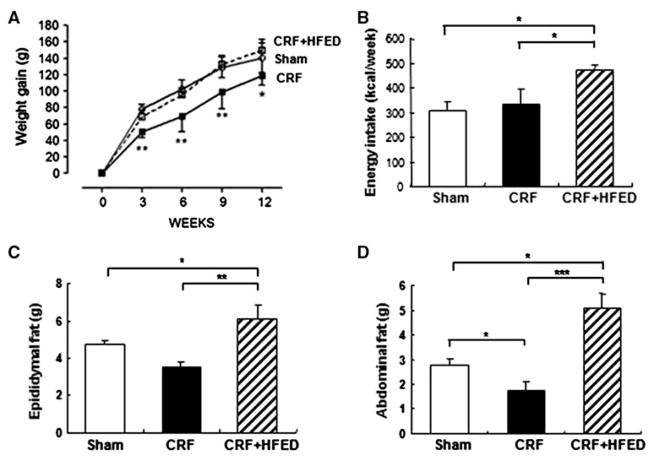
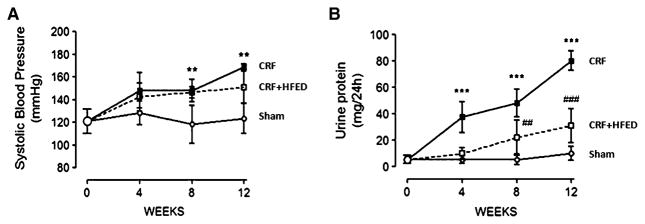
Table 1 shows the weekly food consumption in the study groups. The caloric intake and the fat intake were significantly higher in the CRF group fed the test HFED diet. The carbohydrate and protein intakes were not significantly different in the study groups, but, nevertheless, the mean protein intake was 24% lower in the CRF + HFED group than in the CRF group. The mean body weights at the onset and conclusion of the study period were 319.2 ± 9.1 and 459.8 ± 21.5 g in the control; 310.8 ± 16.7 and 435.6 ± 17.3 g in the CRF and 307.3 ± 13.9 and 457.1 ± 24.3 g in the CRF + HFED groups, respectively. The longitudinal measurements of body weight, energy intake, epididymal and abdominal fat mass are shown in Fig. 1. Compared to the control group the CRF group consuming regular diet gained significantly less weight during the study period. Consumption of the HFED diet restored body growth to that found in the sham-operated control rats. Longitudinal measurements of arterial blood pressure and urinary protein excretion are shown in Fig. 2. The CRF animals exhibited significant hypertension and proteinuria. Consumption of HFED diet resulted in a significant reduction in proteinuria, but did not modify the severity of hypertension. Remnant kidney weight was 2.38 ± 0.38 g in the CRF group and 1.65 ± 0.13 g in the CRF + HFED group (p < 0.01). As expected 5/6 nephrectomy resulted in a significant reduction in creatinine clearance and the corresponding rise in plasma urea and creatinine concentrations. Consumption of the HFED resulted in a significant reduction of the plasma urea concentration. While mean plasma creatinine was lower and creatinine clearance was higher in the HFED-fed CRF animals, the differences did not reach statistical significance. Compared with the control group the CRF group had a significant reduction of hematocrit, reflecting the anemia of chronic kidney disease. Consumption of the HFED resulted in partial correction of the CRF-induced anemia (Table 2). Likewise, the plasma albumin concentration was significantly reduced in the CRF animals fed regular diet and normalized by HFED. Plasma triglycerides, total cholesterol and LDL cholesterol were increased in the CRF groups compared to the sham-operated group. Consumption of the HFED resulted in a further increase in triglyceride concentration, but a significant reduction of total and LDL cholesterol values. The plasma insulin concentration was elevated, and the HOMA index was increased in the CRF groups pointing to insulin resistance. Consumption of HFED resulted in partial improvement of these parameters. Data are summarized in Table 2.
Table 1.
Weekly protein, fat and carbohydrate intakes in the sham-operated control and 5/6 nephrectomized rats fed regular (CRF) and high-fat energy (CRF + HFED) diets
| Sham | CRF | CRF + HFED | |
|---|---|---|---|
| Protein intake (g/day) | 0.50 ± 0.09 | 0.54 ± 0.17 | 0.41 ± 0.04 |
| Fat intake (g/day) | 1.18 ± 0.12 | 1.90 ± 0.65 | 3.15 ± 0.31*** |
| Carbohydrate intake (g/day) | 4.9 ± 0.54 | 6.84 ± 2.14 | 7.38 ± 0.74 |
Values are given as mean ± SD (n = 6–7)
p < 0.001 versus the rest
Fig. 1.
Weight gain (a), energy intake (b), epididymal fat mass (c) and abdominal fat (d) in the sham-operated rats, 5/6 nephrectomized rats fed regular diet (CRF group) and CRF rats fed high-energy diet (CRF + HFED group). Data are mean ± SD *p < 0.05, **p < 0.01, ***p < 0.001
Fig. 2.
Systolic blood pressure (a) and urine protein excretion (b) in the sham-operated rats and 5/6 nephrectomized rats fed regular diet (CRF group) and CRF rats fed high-energy diet (CRF + HFED group). Data are mean ± SD. **p < 0.01; ***p < 0.001 versus sham. ##p < 0.01; ###p < 0.001 versus CRF groups
Table 2.
Blood chemistries at the end of experiment (12 weeks post nephrectomy)
| Sham | CRF | CRF + HFED | |
|---|---|---|---|
| Urea (mg/dl) | 54.3 ± 1.8 | 114.7 ± 4.8** | 77.2 ± 14.8# |
| Scr (mg/dl) | 0.5 ± 0.1 | 1.6 ± 0.5* | 1.4 ± 0.3* |
| Ccr (ml/min/kg BW) | 5.7 ± 1.3 | 1.7 ± 0.4* | 1.5 ± 0.4* |
| Hematocrit (%) | 50.2 ± 3.1 | 38.1 ± 9.6* | 45.3 ± 6.3 |
| Blood sugar (mg/dl) | 189.3 ± 10.0 | 179.3 ± 32.6 | 172.2 ± 24.3 |
| HOMA-IR index | 0.42 ± 0.17 | 2.87 ± 0.92** | 1.21 ± 0.39 |
| Plasma albumin (g/l) | 27.6 ± 2.1 | 23.4 ± 2.4** | 28.6 ± 1.6## |
| Tryglycerides (mg/dl) | 62.2 ± 48.7 | 121.8 ± 44.3 | 176.2 ± 64.4** |
| Total cholesterol (mg/dl) | 71.2 ± 7.7 | 221.2 ± 20.5*** | 164.5 ± 45.0***,# |
| LDL cholesterol (mg/dl) | 15.4 ± 8.9 | 95.9 ± 14.1*** | 58.6 ± 18.5***,### |
| HDL cholesterol (mg/dl) | 43.3 ± 6.8 | 100.9 ± 10.4*** | 70.7 ± 30.7# |
| HDL/LDL cholesterol | 2.75 ± 1.2 | 1.07 ± 0.2** | 1.19 ± 0.2** |
| Free fatty acids (mg/dl) | 0.17 ± 0.1 | 0.17 ± 0.01 | 0.40 ± 0.1***,### |
Values are given as mean ± SD (n = 6–7)
Scr serum creatinine, Ccr creatinine clearance, HOMA-IR index homeostasis model assessment insulin resistance index
p <0.05,
p < 0.01,
p < 0.001 versus sham groups
p < 0.05
p < 0.01,
p < 0.001 versus CRF groups
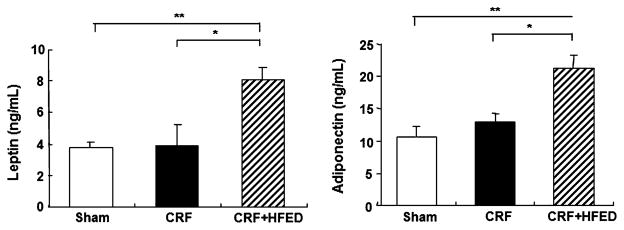
Plasma concentrations of leptin and adiponectin were slightly elevated (p > 0.5) in the CRF rats compared to those found in the control group. Consumption of the HFED resulted in a significant increase in plasma adiponectin and leptin levels paralleling the increase in the body weight and fat content in the study animals (Fig. 3).
Fig. 3.
Plasma leptin and adiponectin levels in the sham-operated rats and 5/6 nephrectomized rats fed regular diet (CRF group) and CRF rats fed high-energy diet (CRF + HFED group). Data are mean ± SD. *p < 0.05, **p < 0.01
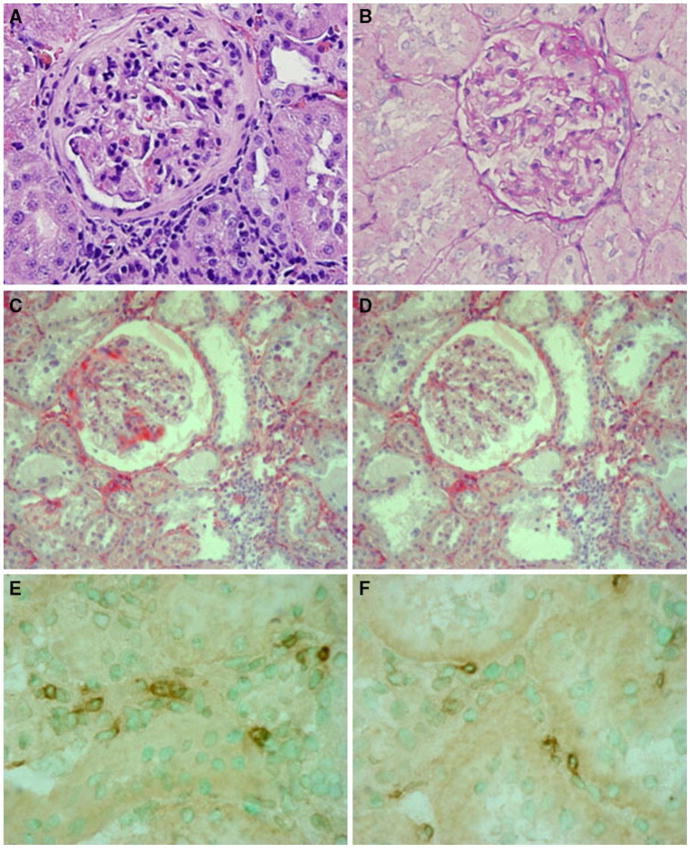
As expected, 5/6 nephrectomy was associated with glomerulosclerosis (sham = 1.5 ± 1.64 index score, CRF = 80.0 ± 24.9; CRF + HFED = 60.6 ± 19.0, both CRF groups p < 0.01 vs. sham), tubulointerstitial damage (sham = 1.13 ± 0.65 damage score; CRF = 44.5 ± 16.8; CRF + HFED = 35.6 ± 17.2, both CRF groups p < 0.01 vs. sham), and these changes were partially ameliorated by the HFED diet. However, the improvement did not reach statistical significance. Collagen deposition in the remnant kidney was significantly increased (p < 0.01) in the both CRF groups compared with the control group, and while partially lower in the CRF + HFED group, the difference between the CRF and CRF + HFED groups did not reach statistical significance (sham = 1.13 ± 0.65% positive area of total area; CRF = 3.17 ± 1.07; CRF + HFED = 2.30 ± 0.24). Glomerular immune cell infiltration was scarce (0–3 cells/glomerular cross section), and tubulointerstitial infiltration of lymphocytes (sham = 12.5 ± 2.77 CD5+ cells/mm2; CRF = 48.7 ± 2.09; CRF + HFED = 22.4 ± 11.4) and macrophages (sham = 10.5 ± 1.00 ED1+ cells/mm2; CRF = 30.0 ± 5.03; CRF + HFED = 19.1 ± 6.16) was increased in the CRF group (p < 0.01) and decreased by the HFED (p < 0.05 vs. CRF). Microphotographs are shown in Fig. 4.
Fig. 4.
Histology and immunohistology of representative areas in the CRF group (a, c, e) and the CRF + HFED group (b, d, f). Light microscopy in a and b (PAS staining); collagen IV (alkaline phosphatase staining) in c and d and ED1 positive cells (macrophages) with immunoperoxidase staining in e and f. Structural changes in the CRF + HFED group had a wide range of variability, and differences did not reach statistical significance. Microphotographs shown in b, d and f correspond to biopsies with relatively minor damage
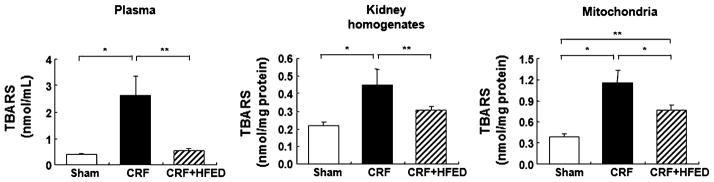
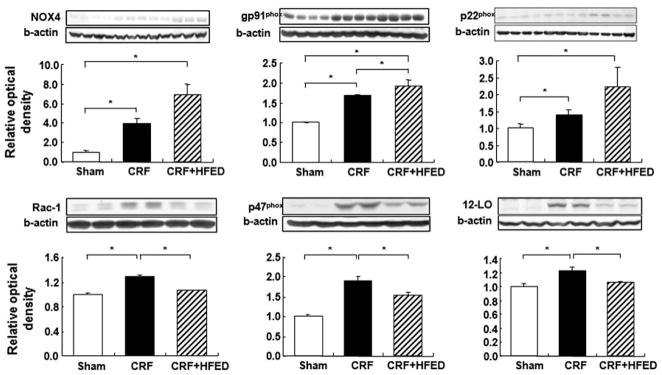
The concentrations of thiobarbituric acid reactive substances (TBARS) in the plasma, renal tissue homogenate and isolated renal mitochondria were significantly elevated in the CRF rats compared to the sham-operated group, signifying heightened peroxidation of the polyunsaturated fatty acids in the plasma and cellular membranes. Consumption of HFED significantly reduced the TBARS concentration in the plasma and renal tissue homogenate and mitochondria of the study animals (Fig. 5). Compared to the sham-operated group, the CRF group exhibited significant upregulation of gp91phox, NOX-4, P22phox, p47phox and Rac1 subunits of the NAD(P)H oxidase and of 12-lipoxygenase in the remnant kidney. Consumption of HFED significantly reduced p47phox, Rac1 and 12-LO protein abundance, but had either no effect on or further increased gp91phox, NOX-4 and P22phox abundance in the remnant kidney (Fig. 6).
Fig. 5.
Plasma, renal homogenates and mitochondrial thiobarbituric acid reactive substance (TBARS) levels in the study groups. Data are mean ± SD. *p < 0.05, **p < 0.01
Fig. 6.
Representative Western blots and group data depicting NOX4, gp91phox, p22phox, p47phox, Rac-1 and 12-LO abundance in the remnant kidney tissues of the study groups. Data are mean ± SD. *p < 0.05
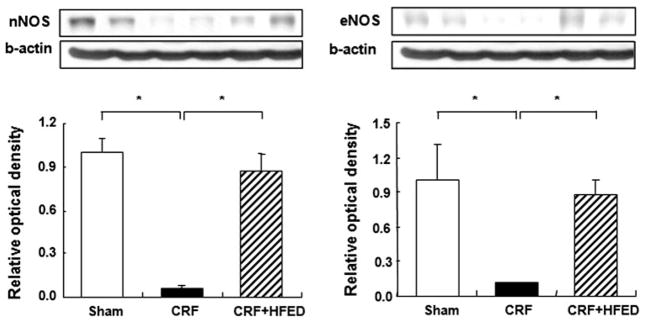
The eNOS and nNOS protein abundance was significantly lower in the remnant kidneys of the CRF groups when compared with those found in the control group. Consumption of HFED resulted in partial restoration of eNOS and nNOS expressions in the study animals (Fig. 7).
Fig. 7.
Representative Western blots and group data depicting eNOS and nNOS abundance in the remnant kidney tissues of the study groups. Data are mean ± SD. *p < 0.05
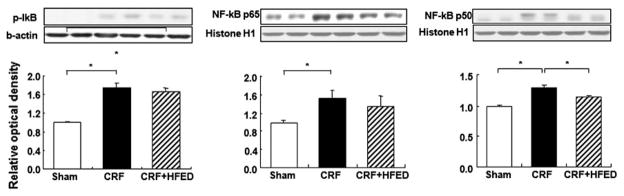
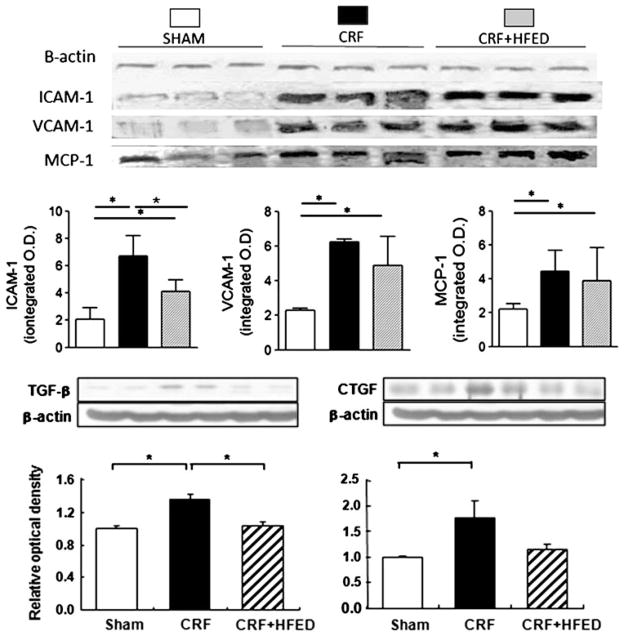
Compared to the control group, the CRF group showed a significant increase in p-IκB and nuclear translocation of P65 and P50 active subunits of NF-κB signifying activation of this pro-inflammatory nuclear factor in the remnant kidney. Consumption of HFED reduced p-IκB and nuclear P65 and P50 values. However, the HFED-induced reduction in NF-κB activation did not reach statistical significance (Fig. 8). The CRF animals exhibited increased TGF-β, CTGF, MCP-1, ICAM-1 and VCAM-1 expression in the remnant kidneys. Consumption of HFED significantly reduced (p < 0.05) TGF-β and ICAM-1 abundance in the remnant kidney (Fig. 9).
Fig. 8.
Representative Western blots and group data depicting p-IκB and NF-κB p50 and p65 abundance in the remnant kidney tissues of the study groups. Data are mean ± SD. *p <0.05
Fig. 9.
Representative Western blots and group data depicting TGF-β, CTGF, MCP-1, ICAM-1 and VCAM-1 abundance in the remnant kidney tissues of the study groups. Data are mean ± SD. *p < 0.05
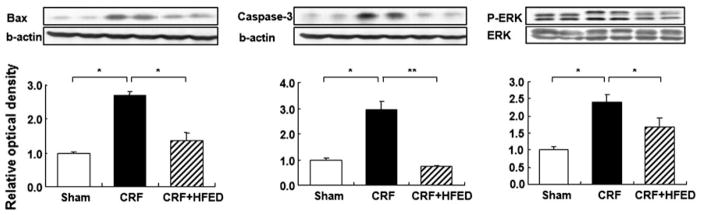
Renal tissue Bax and cleaved caspase-3 protein abundance was significantly increased in CRF rats. Consumption of HFED significantly (p < 0.05) attenuated the CRF-associated elevation of Bax and cleaved caspase-3 in the remnant kidney. Compared with the control group the CRF groups showed a significant increase in phosphorylated ERK1/2 in the remnant kidney. Consumption of HFED significantly (p < 0.05) reduced phosphorylated ERK1/2 abundance in study animals (Fig. 10).
Fig. 10.
Representative Western blots and group data depicting Bax, caspase-3 and activated ERK1/2 abundance in the remnant kidney tissues of the study groups. Data are mean ± SD. *p < 0.05, **p < 0.01
Discussion
Oxidative stress and inflammation are common features of chronic kidney disease and are major mediators of progressive renal injury, atherosclerosis, catabolic state and wasting syndrome. The CRF animals employed in this study exhibited oxidative stress as evidence by marked elevation of lipid peroxidation products (TBARS) in the plasma and remnant kidney tissue. This was associated with marked upregulations of NAD(P)H oxidase and 12-lipoxygenase, which are a major source of reactive oxygen species. In addition to oxidative stress, the remnant kidney tissue showed significant inflammation as evidenced by activation of NF-κB (master regulator of pro-inflammatory cytokines and chemokines), upregulation of MCP-1, ICAM-1, VCAM-1 and LOX-1, and monocyte/lymphocyte infiltration. Chronic oxidative stress and inflammation contribute to tissue damage and dysfunction by promoting fibrosis and cellular apoptosis. In fact, the remnant kidney tissues in our CRF animals showed a marked increase in TGF-β and mediators of apoptosis.
The improvement observed in the remnant kidney of rats with renal ablation is reminiscent of the survival benefit associated with cholesterol levels and body weight in uremic patients [9, 11], and it may be related to avoidance of uremic cachexia. Is well recognized that reduction in protein intake retards the progression of chronic renal disease [27], and a moderate reduction in protein intake, equivalent to approximately 1.74 g/kg/day in the CRF group and 1.33 g/kg/day in the CRF + HFED group, may be in part responsible for the beneficial effects observed in the remnant kidney of the rats fed the test diet. Nevertheless, a critical finding was that a high-fat diet that induced increments in blood lipid concentrations had no deleterious effects on the remnant kidney. On the contrary, the CRF + HFED group gained weight normally, maintained normal plasma albumin concentration, had reduced proteinuria, attenuated oxidative stress and tubulointerstitial inflammation, and showed a partial reversal of the upregulation of oxidative and inflammatory pathways in the remnant kidney. The observed improvement in proteinuria and renal function with HFED was independent of hypertension since blood pressure was equally elevated in both CRF groups.
The beneficial effect of high fat (calorie) intake on kidney injury may be related to a reduction in oxidative and inflammatory stress shown in this paper in association with the improvement in the intra-renal lipid metabolism that has been recently reported [20]. As expected, the CRF animals exhibited a pro-atherogenic serum lipid profile. The mechanism by which HFED attenuated hypercholesterolemia in the study animals is not known. However, it may be related to the reduction of proteinuria, which is known to cause of hypercholesterolemia [28].
Adipokines are increased in chronic renal failure. Circulating adiponectin is increased in ESRD [29], but its prognostic significance in chronic renal failure is controversial [30, 31]. The levels of adiponectin are inversely related to body mass index [32], and therefore it is likely that the increased circulating levels of this adipokine in the CRF + HFED group reflect the higher body weight and adipose tissue mass in these rats. Since adiponectin has anti-atherogenic and anti-inflammatory properties [33], it is likely that the increased adiponectin levels could be a factor in the nephroprotection afforded by the high-calorie diet in this model.
The blood leptin level is commonly elevated in renal failure due to its reduced urinary clearance [34] and increased release by adipose tissue [35]. The HFED-fed CRF group had significantly higher leptin levels than the CRF group consuming regular diet; this finding likely reflects the higher adipose tissue mass in the former group. Interestingly, increased circulating leptin levels in ESRD patients are associated with improved response to erythropoietin [36], which may account for the reported inverse correlation between body mass index and the erythropoietin requirement and for the observation that hemodialysis patients with a high body mass index are less likely to experience severe anemia [14]. In the present study, the higher leptin level in CRF rats consuming HFED was accompanied by a higher hematocrit when compared to those consuming regular diet. This is most likely the result of the improved nutritional status and reduction in severity of oxidative stress and inflammation in the HFED group.
The remnant kidney weight was significantly less in the CRF rats receiving a high-calorie diet than in those fed regular diet. Since the amount of kidney left after the 5/6 nephrectomy was approximately the same in the CRF rats consuming HFED and regular diet, the lower remnant kidney weight in the HFED-fed suggests a reduction of hypertrophy in the HFED-fed animals. The nephrectomy-induced renal hypertrophy is an incompletely understood phenomenon that takes place to a large measure in the first 2 weeks after surgery and is temporally associated with upregulation of TGF-β and involves the cycline kinase pathways [37]. The reduction in the TGF-β abundance in the remnant kidney in CRF rats fed a HFED diet provides a plausible explanation for the lower post-nephrectomy hypertrophy in these animals.
The unexpected beneficial effects of the HFED with a high fat content were associated with significant reductions of LOX-1 reported in reference [20] and ICAM-1 expressions in the remnant kidney. LOX-1 is a type II membrane glycoprotein with a C-terminal extracellular lectin-like domain that is essential for binding to oxidized LDL [38]. In addition to macrophages, the microvascular endothelial cells express LOX-1 where it modulates nitric oxide production and facilitates leukocyte recruitment into the tissues by inducing production of MCP-1, and expression of adhesion molecules ICAM-1 and VCAM-1 [38, 39]. Since LOX-1 expression is increased by hyperlipidemia, reactive oxidant species and pro-inflammatory cytokines [40], it is not surprising that the CRF group would have higher levels of this scavenger receptor. The reduction the tubulointerstitial inflammation and oxidative stress in the rats receiving the HFED is likely the result of the reduction in the production of MCP-1 and adhesion molecules by the test diet.
As noted above, protein restriction retards progression of renal disease, and high protein intake accelerates the progression of renal disease. The adverse effect of high protein intake is mediated by glomerular hyperfiltration and the increased burden of pro-oxidant and pro-inflammatory byproducts of protein metabolism, including but not limited to indoxyl sulfate and polyamines polyamines such as putrescine, spermidine and spermine, among others [41–45]. The CKD animals employed in the present study exhibited attenuation of proteinuria, azotemia and oxidative stress and inflammation in the remnant kidney with long-term consumption of a high-calorie diet and only a minimal reduction of dietary protein intake. This was associated with prevention of CKD-induced body mass growth retardation and a markedly blunted rise in serum urea concentration. These observations point to attenuation and/or prevention of the CKD-induced catabolic state that usually leads to body mass reduction, muscle wasting and growth retardation. Thus, by preventing catabolism of tissue proteins, a high-calorie diet appears to exert a protein-sparing effect resulting in significant reduction of total body protein catabolism and hence reduced generation of pro-inflammatory byproducts of protein metabolism.
In summary, rats with extensive renal ablation consuming a high-fat, high-sucrose diet gained weight normally, had reduced proteinuria and suppressed oxidative stress and inflammation in the remnant kidney. The downregulation of pro-inflammatory, pro-oxidant, pro-fibrotic and pro-apoptotic pathways may be, in part, due to moderate reduction in protein intake and diminished catabolism of endogenous proteins. These findings provide an experimental basis for the positive relationship between survival and body mass index in patients with ESRD.
Acknowledgments
This study was in part supported by the NIH Division of Research Resources Grant 5 U54 RR-0119234 and by FONACYT Grant 2005000283.
Footnotes
Conflict of interest None
Contributor Information
Hyun Ju Kim, Division of Nephrology and Hypertension, Department of Medicine, University of California, Irvine, CA, USA.
Nosratola D. Vaziri, Division of Nephrology and Hypertension, Department of Medicine, University of California, Irvine, CA, USA
Keith Norris, Charles Drew University, Los Angeles, CA, USA.
Won Suk An, Division of Nephrology and Hypertension, Department of Medicine, University of California, Irvine, CA, USA.
Yasmir Quiroz, Centro de Investigaciones Biomédicas, Instituto Venezolano de Investigaciones Científicas (IVIC), Zulia, Hospital Universitario and Universidad del Zulia, Maracaibo, Venezuela.
Bernardo Rodriguez-Iturbe, Email: bernardori@telcel.net.ve, Centro de Investigaciones Biomédicas, Instituto Venezolano de Investigaciones Científicas (IVIC), Zulia, Hospital Universitario and Universidad del Zulia, Maracaibo, Venezuela. Servicio de Nefrología, 9°Piso, Hospital Universitario, Ave Goajira s/n, Maracaibo, Zulia, Venezuela.
References
- 1.Degoulet P, Legrain M, Réach I, Aimé F, Devriés C, Rojas P, et al. Mortality risk factors in patients treated by chronic hemodialysis. Report of the Diaphane collaborative study. Nephron. 1982;31:103–10. doi: 10.1159/000182627. [DOI] [PubMed] [Google Scholar]
- 2.Leavy SD, Strawderman RL, Jones CA, Port FK, Held PJ. Simple nutritional indicators as independent predictors of mortality in hemodialysis patients. Am J Kidney Dis. 1998;31:997–1006. doi: 10.1053/ajkd.1998.v31.pm9631845. [DOI] [PubMed] [Google Scholar]
- 3.Kopple JD, Zhu X, Lew NL, Lowrie EG. Body weight-for-height relationships predict mortality in hemodialysis patients. Kidney Int. 1999;56:1136–48. doi: 10.1046/j.1523-1755.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 4.Fleishmann E, Teal N, Dudley J, May W, Bower JD, Salahudeen AK. Influence of excess weight on mortality and hospital stay in 1346 hemodialysis patients. Kidney Int. 1999;55:1560–7. doi: 10.1046/j.1523-1755.1999.00389.x. [DOI] [PubMed] [Google Scholar]
- 5.Leavy SF, McCullough K, Hecking E, Goodkin D, Port FK, Young EW. Body mass index and mortality in “healthier” as compared with “sicker” hemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2001;16:2386–94. doi: 10.1093/ndt/16.12.2386. [DOI] [PubMed] [Google Scholar]
- 6.Glanton CW, Hypolite IO, Hshieh PB, Agoada LY, Yuan CM, Abott KC. Factors associated with short term survival in obese end stage renal disease patients. Ann Epidemiol. 2003;13:136–43. doi: 10.1016/s1047-2797(02)00251-x. [DOI] [PubMed] [Google Scholar]
- 7.Kalantar-Zadeh K, McAllister CJ, Lehn RS, Lee GH, Nissenson AR, Kopple JD. Effect of malnutrition-inflammation complex syndrome on EPO hyporesponsiveness in maintenance hemodialysis patients. Am J Kidney Dis. 2003;42:761–73. doi: 10.1016/s0272-6386(03)00915-6. [DOI] [PubMed] [Google Scholar]
- 8.Abbott KC, Glanton CW, Trespalacios FC, Oliver DK, Ortiz MI, Agodoa LY, et al. Body mass index, dialysis modality and survival: analysis of the United States Data System Dialysis Morbidity and Mortality Wave II Study. Kidney Int. 2004;65:597–605. doi: 10.1111/j.1523-1755.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- 9.Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9:S16–23. [PubMed] [Google Scholar]
- 10.Zager PG, Nikolic J, Brown RH, Campbell MA, Hunt WC, Peterson D, et al. U” curve association of blood pressure and mortality in hemodialysis patients. Medical Directors of Dialysis Clinic, Inc. Kidney Int. 1998;54:561–9. doi: 10.1046/j.1523-1755.1998.00005.x. [DOI] [PubMed] [Google Scholar]
- 11.Nishizawa Y, Shoji T, Ishimura E, Inaba M, Morii H. Paradox of risk factors for cardiovascular mortality in uremia: is a higher cholesterol level better for atherosclerosis in uremia? Am J Kidney Dis. 2001;38:S4–7. doi: 10.1053/ajkd.2001.27380. [DOI] [PubMed] [Google Scholar]
- 12.Kalantar-Zadeh K, Block G, Humphreys MH, McAllister CJ, Kopple JD. A low, rather than a high, total plasma homocysteine is an indicator of poor outcome in hemodialysis patients. J Am Soc Nephrol. 2004;15:442–52. doi: 10.1097/01.asn.0000107564.60018.51. [DOI] [PubMed] [Google Scholar]
- 13.Lowrie EJ, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an elevation of death rate differences between facilities. Am J Kidney Dis. 1990;15:458–82. doi: 10.1016/s0272-6386(12)70364-5. [DOI] [PubMed] [Google Scholar]
- 14.Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular disease risk factors in maintenance dialysis. Kidney Int. 2003;63:793–808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez-Iturbe B, Quiroz Y, Shahkarami A, Li Z, Vaziri ND. Mycophenolate mofetil ameliorates nephropathy in the obese Zucker rat. Kidney Int. 2005;68:1041–7. doi: 10.1111/j.1523-1755.2005.00496.x. [DOI] [PubMed] [Google Scholar]
- 16.Reddy S, Santanam N, Reddy PP, Rock JA, Murphy AA, Parthasarathy S. Interaction of interceed oxidized regenerated cellulose with macrophages: a potential mechanism by which interceed may prevent adhesions. Am J Obstet Gynecol. 1997;177:1315–20. doi: 10.1016/s0002-9378(97)70070-x. [DOI] [PubMed] [Google Scholar]
- 17.Chade AR, Mushin OP, Zhu X, Rodriguez-Porcel M, Grande JP, Textor SC, et al. Pathways of renal fibrosis and modulation of matrix turnover in experimental hypercholesterolemia. Hypertension. 2005;46:772–9. doi: 10.1161/01.HYP.0000184250.37607.da. [DOI] [PubMed] [Google Scholar]
- 18.Ruan XZ, Varghese Z, Powis SH, Moorhead JF. Human mesangial cells express inducible macrophage scavenger receptor. Kidney Int. 1999;56:440–51. doi: 10.1046/j.1523-1755.1999.00587.x. [DOI] [PubMed] [Google Scholar]
- 19.Okamura DM, Lopez-Guisa JM, Koelsch K, Collins S, Eddy AA. Atherogenic scavenger receptor modulation in the tubulointerstitium in response to chronic renal injury. Am J Physiol Renal Physiol. 2007;293:F575–85. doi: 10.1152/ajprenal.00063.2007. [DOI] [PubMed] [Google Scholar]
- 20.Kim H-J, Yuan J, Norris K, Vaziri ND. High calorie diet partially ameliorates dysregulation of intra-renal lipid metabolism in the remnant kidney. J Nutr Biochem. 2009 doi: 10.1016/j.jnutbio.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim HJ, Vaziri ND. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am J Physiol Renal Physiol. 2010;298:F662–71. doi: 10.1152/ajprenal.00421.2009. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez V, Quiroz Y, Nava M, Pons H, Rodriguez-Iturbe B. Overload proteinuria is followed by salt-sensitive hypertension caused by renal infiltration of immune cells. Am J Physiol Renal Physiol. 2002;283:F1132–41. doi: 10.1152/ajprenal.00199.2002. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Iturbe B, Ferrebuz A, Vanegas V, Quiroz Y, Mezzano S, Vaziri ND. Early and sustained inhibition of Nuclear Factor kappa B prevents hypertension in spontaneously hypertensive rats. J Pharmacol Exp Ther. 2005;315:51–7. doi: 10.1124/jpet.105.088062. [DOI] [PubMed] [Google Scholar]
- 24.Quiroz Y, Ferrebuz A, Romero F, Vaziri ND, Rodriguez-Iturbe B. Melatonin ameliorates oxidative stress, inflammation, proteinuria and progression of renal damage in rats with renal mass reduction. Am J Physiol Renal Physiol. 2008;294:F336–44. doi: 10.1152/ajprenal.00500.2007. [DOI] [PubMed] [Google Scholar]
- 25.Cho K-H, Kim H-J, Rodriguez-Iturbe B, Vaziri ND. Niacin ameliorates oxidative stress, inflammation, proteinuria, and hypertension in rats with chronic renal failure. Am J Physiol Renal Physiol. 2009;297:F106–13. doi: 10.1152/ajprenal.00126.2009. [DOI] [PubMed] [Google Scholar]
- 26.Quiroz Y, Ferrebuz A, Vaziri ND, Rodriguez-Iturbe B. Effect of chronic antioxidant therapy with superoxide dismutase-mimetic drug, tempol, on progression of renal disease in rats with renal mass reduction. Nephron Exp Nephrol. 2009;112:e31–42. doi: 10.1159/000210577. [DOI] [PubMed] [Google Scholar]
- 27.Fouque D, Aparicio M. Eleven reasons to control the protein intake of patients with chronic kidney disease. Nat Clin Pract Nephrol. 2007;3:383–92. doi: 10.1038/ncpneph0524. [DOI] [PubMed] [Google Scholar]
- 28.Vaziri ND. Molecular mechanisms of lipid dysregulation in nephrotic syndrome. Kidney Int. 2003;63:1964–76. doi: 10.1046/j.1523-1755.2003.00941.x. [DOI] [PubMed] [Google Scholar]
- 29.Zoccali C, Mallamaci F, Tripepi G, Benedetto FA, Cutrupi S, Parlongo S, et al. Adiponectin, metabolic risk factors, and cardiovascular events among patients with endstage renal disease. J Am Soc Nephrol. 2002;13:134–41. doi: 10.1681/ASN.V131134. [DOI] [PubMed] [Google Scholar]
- 30.Zoccali C, Mallamaci F. Adiponectin and renal disease progression: another epidemiologic conundrum. Kidney Int. 2007;71:1195–7. doi: 10.1038/sj.ki.5002319. [DOI] [PubMed] [Google Scholar]
- 31.Pecoits-Filho R, Lindholm B, Stenvinkel P. The malnutrition, inflammation, and atherosclerosis (MIA) syndrome––the heart of the matter. Nephrol Dial Transplant. 2002;17(Suppl 11):28–31. doi: 10.1093/ndt/17.suppl_11.28. [DOI] [PubMed] [Google Scholar]
- 32.Díez JJ, Iglesias P, Fernández-Reyes MJ, Aguilera A, Bajo MA, Alvarez-Fidalgo P, et al. Serum concentrations of leptin, adiponectin and resistin, and their relationship with cardiovascular disease in patients with end-stage renal disease. Clin Endocrinol. 2005;62:242–9. doi: 10.1111/j.1365-2265.2005.02207.x. [DOI] [PubMed] [Google Scholar]
- 33.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptor. Endocr Rev. 2005;26:439–45. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 34.Johansen KL, Mulligan K, Tai V, Schambelan M. Leptin, body composition and indices of malnutrition in patients in dialysis. J Amer Soc Nephrol. 1998;9:1080–4. doi: 10.1681/ASN.V961080. [DOI] [PubMed] [Google Scholar]
- 35.Aminzadeh MA, Pahl MV, Barton CH, Vaziri ND. Human uremic plasma stimulates release of leptin and uptake of tumor necrosis factor-α in visceral adipocytes. Nephrol Dial Transplant. 2009;24:3626–31. doi: 10.1093/ndt/gfp405. [DOI] [PubMed] [Google Scholar]
- 36.Hung S-C, Tung T-Y, Yang C-S, Tarng D-C. High-calorie supplementation increases serum leptin levels and improves response to rHuEPO in long-term hemodialysis patients. Am J Kidney Dis. 2005;45:1073–83. doi: 10.1053/j.ajkd.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 37.Takeda A, Toda T, Shinohara S, Mogi Y, Matsui N. Factors contributing to higher hematocrit levels in hemodialysis patients not receiving recombinant human erythropoietin. Am J Kidney Dis. 2002;40:104–9. doi: 10.1053/ajkd.2002.33918. [DOI] [PubMed] [Google Scholar]
- 38.Sinuani I, Weissgarten J, Beberashvili I, Rapoport MJ, Sandbank J, Feldman L, et al. The cyclin kinase inhibitor p57kip2 regulates TGF-β-induced compensatory tubular hypertrophy: effect of the immunomodulator AS101. Nephrol Dial Transplant. 2009;24:2328–38. doi: 10.1093/ndt/gfn742. [DOI] [PubMed] [Google Scholar]
- 39.Chen M, Narumiya S, Masaki T, Sawamura T. Conserved C-terminal residues within the lectin-like domain of LOX-1 are essential for oxidized low-density-lipoprotein binding. Biochem J. 2001;355:289–96. doi: 10.1042/0264-6021:3550289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mita S, Kobayashi N, Yoshida K, Nakano S, Matsuoka H. Cardioprotective mechanisms of Rho-kinase inhibition associated with NOS and oxidative stress-LOX-1 pathway in Dahl salt-sensitive hypertensive rats. J Hypertens. 2005;23:87–96. doi: 10.1097/00004872-200501000-00017. [DOI] [PubMed] [Google Scholar]
- 41.Metha JL, Chen J, Hermonat PL, Romeo F, Novelli G. Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1): a critical player in the development of atherosclerosis and related disorders. Cardiovasc Res. 2006;69:36–45. doi: 10.1016/j.cardiores.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Kim HJ, Moradi H, Yuan J, Norris K, Vaziri ND. Renal mass reduction results in accumulation of lipids and dysregulation of lipid regulatory proteins in the remnant kidney. Am J Physiol Renal Physiol. 2009;296:F1297–306. doi: 10.1152/ajprenal.90761.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tumur Z, Shimizu H, Enomoto A, Miyazaki H, Niwa T. Indoxyl sulfate upregulates expression of ICAM-1 and MCP-1 by oxidative stress-induced NF-kappaB activation. Am J Nephrol. 2010;31:435–41. doi: 10.1159/000299798. [DOI] [PubMed] [Google Scholar]
- 44.Sinha-Hikim I, Shen R, Paul Lee WN, Crum A, Vaziri ND, Norris KC. Effects of a novel cystine based glutathione precursor on oxidative stress in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2010 doi: 10.1152/ajpcell.00434.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Igarashi K, Ueda S, Yoshida K, Kashiwagi K. Polyamines in renal failure. Amino Acids. 2006;31:477–83. doi: 10.1007/s00726-006-0264-7. [DOI] [PubMed] [Google Scholar]