Abstract
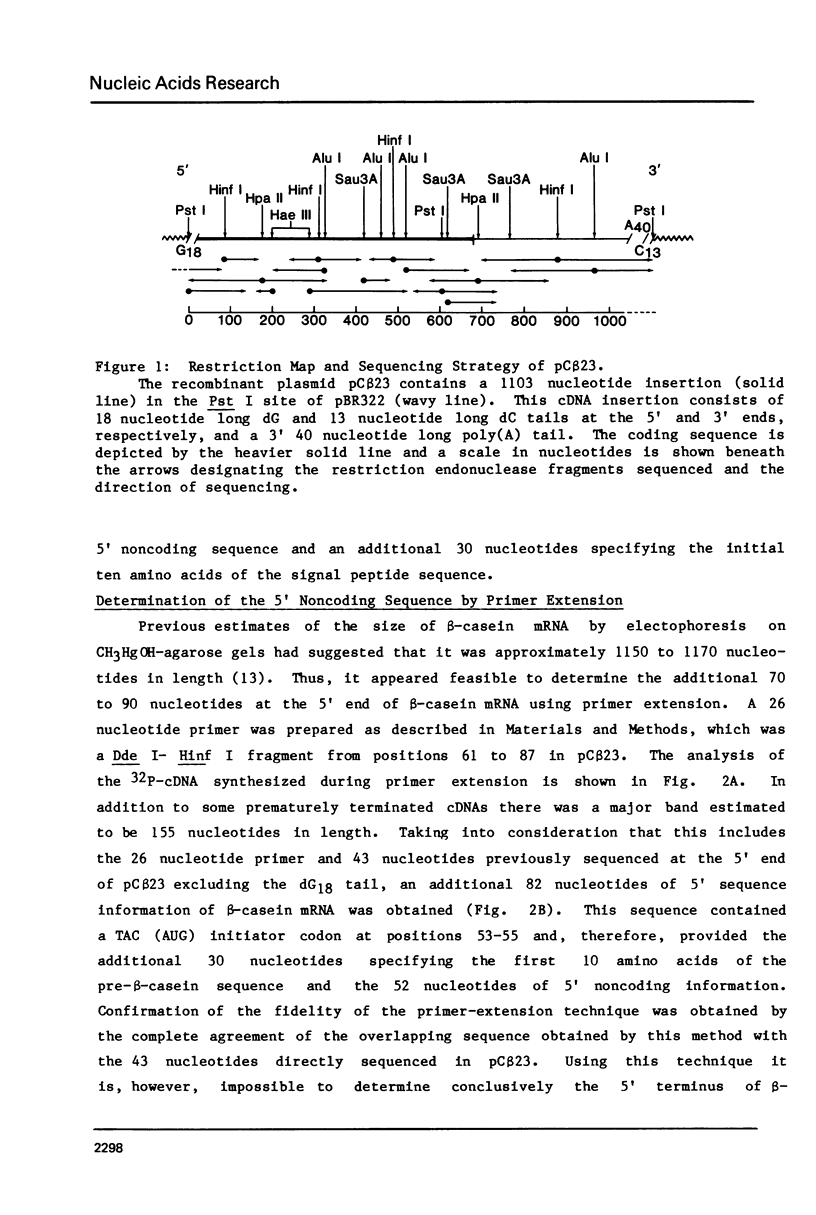
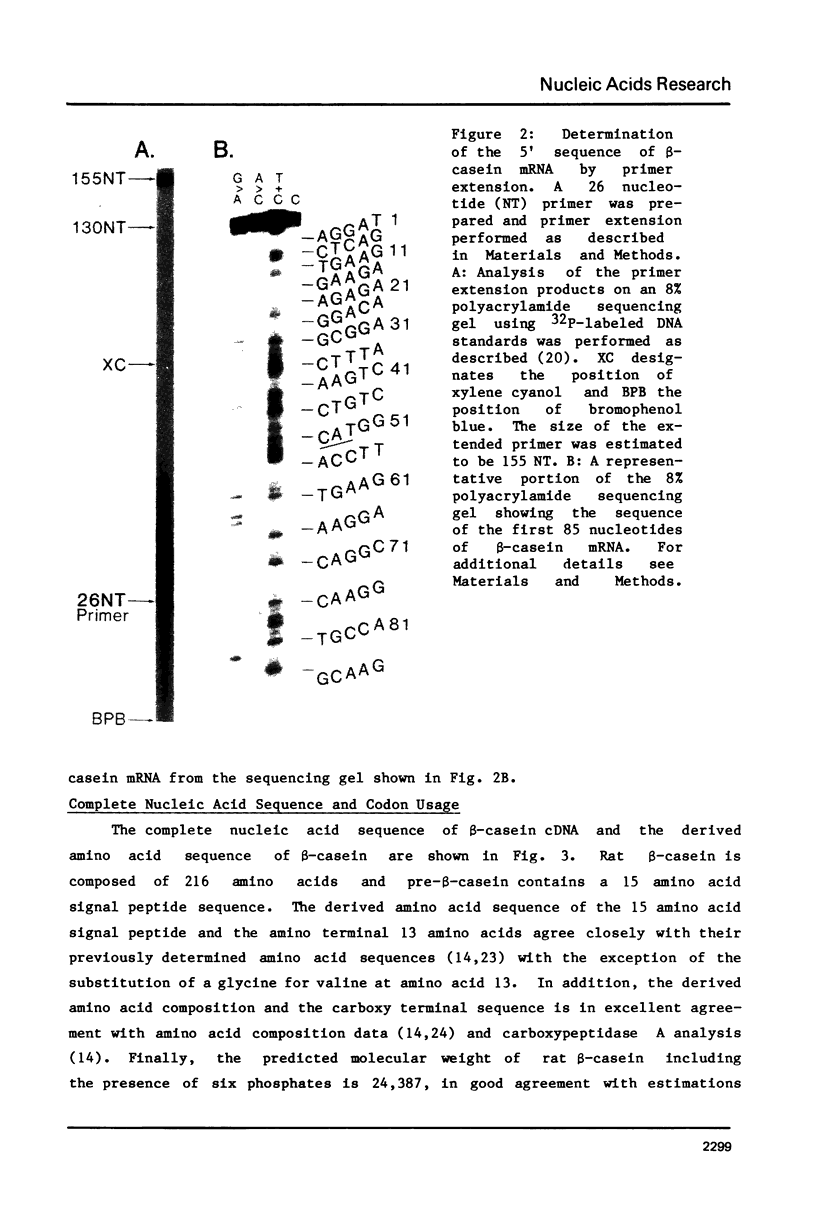
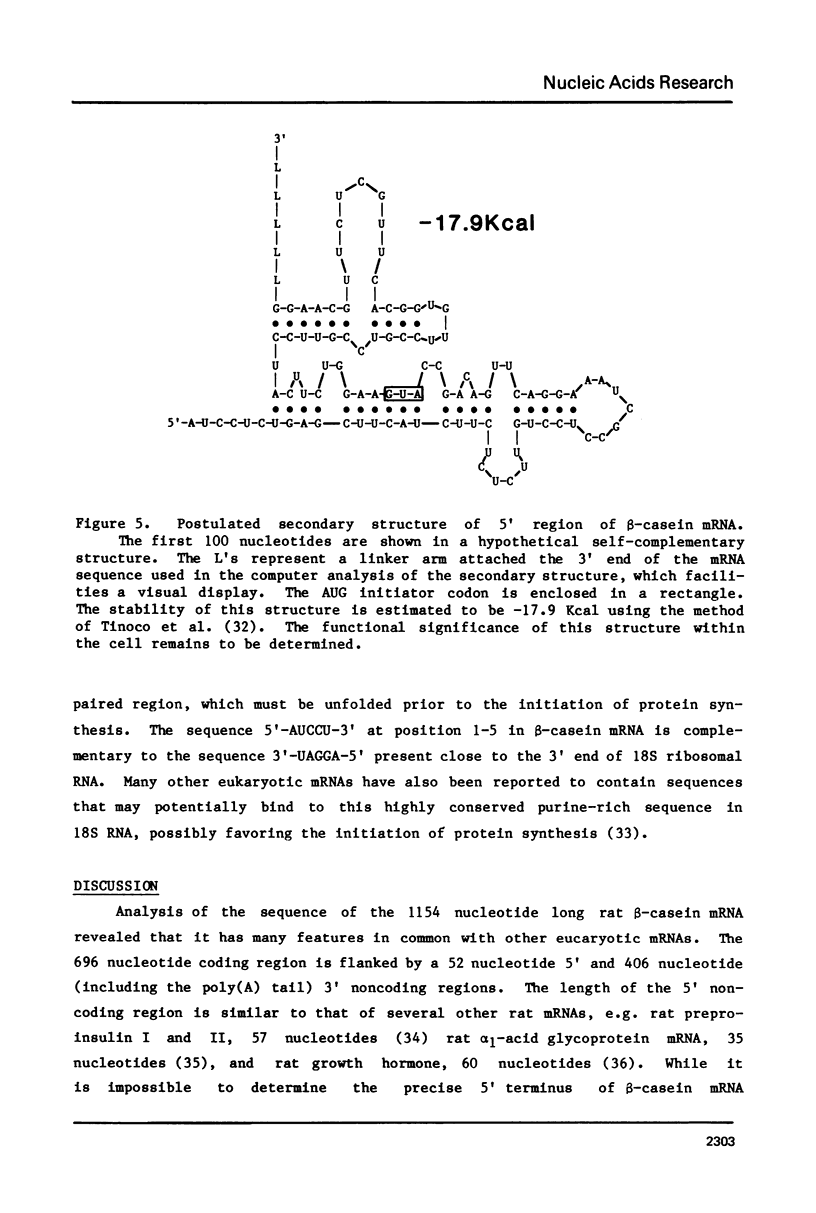
The complete sequence of a 1072 nucleotide rat beta-casein cDNA insertion in the hybrid plasmid pC beta 23 has been determined. Primer extension was employed to determine the sequence of an additional 82 5'-terminal nucleotides in beta-casein mRNA. Rat beta-casein mRNA consists of a 696 nucleotide coding region, flanked by 52 nucleotide 5' and 406 nucleotide 3' noncoding regions, including a 40 nucleotide poly(A) tail. The derived 216 amino acid sequence of rat beta-casein was compared to the previously determined sequences of beta-caseins from several other species. Approximately 38% of the amino acids have been conserved among the rat, ovine, bovine and human sequences and these conserved amino acids occurred in clusters throughout the protein. One such cluster containing the majority of the potential casein phosphorylation sites was located near the amino terminus. Contrary to the considerable divergence observed for the processed beta-casein, 14 of 15 amino acids in the signal peptide sequence of the precasein were identical between the rat and ovine caseins.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Walter P., Chang C. N., Goldman B. M., Erickson A. H., Lingappa V. R. Translocation of proteins across membranes: the signal hypothesis and beyond. Symp Soc Exp Biol. 1979;33:9–36. [PubMed] [Google Scholar]
- Brignon G., Ribadeau Dumas B., Mercier J. C. Premiers elements de structure primaire des caseines alphas2 bovines. FEBS Lett. 1976 Nov 15;72(1):111–116. doi: 10.1016/0014-5793(76)80910-6. [DOI] [PubMed] [Google Scholar]
- Dandekar A. M., Qasba P. K. Rat alpha-lactalbumin has a 17-residue-long COOH-terminal hydrophobic extension as judged by sequence analysis of the cDNA clones. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4853–4857. doi: 10.1073/pnas.78.8.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Kafatos F. C., Maniatis T. The primary structure of rabbit beta-globin mRNA as determined from cloned DNA. Cell. 1977 Apr;10(4):571–585. doi: 10.1016/0092-8674(77)90090-3. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M., Shenk T. The sequence 5'-AAUAAA-3'forms parts of the recognition site for polyadenylation of late SV40 mRNAs. Cell. 1981 Apr;24(1):251–260. doi: 10.1016/0092-8674(81)90521-3. [DOI] [PubMed] [Google Scholar]
- Greenberg R., Groves M. L. Human beta-casein. J Dairy Res. 1979 Apr;46(2):235–239. doi: 10.1017/s0022029900017118. [DOI] [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Hagenbüchle O., Santer M., Steitz J. A., Mans R. J. Conservation of the primary structure at the 3' end of 18S rRNA from eucaryotic cells. Cell. 1978 Mar;13(3):551–563. doi: 10.1016/0092-8674(78)90328-8. [DOI] [PubMed] [Google Scholar]
- Jenness R. Proceedings: Biosynthesis and composition of milk. J Invest Dermatol. 1974 Jul;63(1):109–118. doi: 10.1111/1523-1747.ep12678111. [DOI] [PubMed] [Google Scholar]
- Jollès J., Fiat A. M., Schoentgen F., Alais C., Jollès P. The amino acid sequence of sheep kappa A-casein. II. Sequence studies concerning the kappa A-caseinoglycopeptide and establishment of the complete primary structure of the protein. Biochim Biophys Acta. 1974 Oct 9;365(2):335–343. [PubMed] [Google Scholar]
- Kozak M. How do eucaryotic ribosomes select initiation regions in messenger RNA? Cell. 1978 Dec;15(4):1109–1123. doi: 10.1016/0092-8674(78)90039-9. [DOI] [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg H. M., McDevitt B. E., Majzoub J. A., Nathans J., Sharp P. A., Potts J. T., Jr, Rich A. Cloning and nucleotide sequence of DNA coding for bovine preproparathyroid hormone. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4981–4985. doi: 10.1073/pnas.76.10.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land H., Grez M., Hauser H., Lindenmaier W., Schütz G. 5'-Terminal sequences of eucaryotic mRNA can be cloned with high efficiency. Nucleic Acids Res. 1981 May 25;9(10):2251–2266. doi: 10.1093/nar/9.10.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebhaber S. A., Goossens M., Kan Y. W. Homology and concerted evolution at the alpha 1 and alpha 2 loci of human alpha-globin. Nature. 1981 Mar 5;290(5801):26–29. doi: 10.1038/290026a0. [DOI] [PubMed] [Google Scholar]
- Lomedico P., Rosenthal N., Efstratidadis A., Gilbert W., Kolodner R., Tizard R. The structure and evolution of the two nonallelic rat preproinsulin genes. Cell. 1979 Oct;18(2):545–558. doi: 10.1016/0092-8674(79)90071-0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McReynolds L., O'Malley B. W., Nisbet A. D., Fothergill J. E., Givol D., Fields S., Robertson M., Brownlee G. G. Sequence of chicken ovalbumin mRNA. Nature. 1978 Jun 29;273(5665):723–728. doi: 10.1038/273723a0. [DOI] [PubMed] [Google Scholar]
- Mercier J. C., Gaye P. Study of secretory lactoproteins: primary structures of the signals and enzymatic processing. Ann N Y Acad Sci. 1980;343:232–251. doi: 10.1111/j.1749-6632.1980.tb47255.x. [DOI] [PubMed] [Google Scholar]
- Mercier J. C., Grosclaude F., Ribadeau-Dumas B. Structure primaire de la caséine s1 -bovine. Séquence complète. Eur J Biochem. 1971 Nov 11;23(1):41–51. doi: 10.1111/j.1432-1033.1971.tb01590.x. [DOI] [PubMed] [Google Scholar]
- Mercier J. C. Phosphorylation of caseins, present evidence for an amino acid triplet code posttranslationally recognized by specific kinases. Biochimie. 1981 Jan;63(1):1–17. doi: 10.1016/s0300-9084(81)80141-1. [DOI] [PubMed] [Google Scholar]
- Miller W. L., Coit D., Baxter J. D., Martial J. A. Cloning of bovine prolactin cDNA and evolutionary implications of its sequence. DNA. 1981;1(1):37–50. doi: 10.1089/dna.1.1981.1.37. [DOI] [PubMed] [Google Scholar]
- Norgard M. V., Keem K., Monahan J. J. Factors affecting the transformation of Escherichia coli strain chi1776 by pBR322 plasmid DNA. Gene. 1978 Jul;3(4):279–292. doi: 10.1016/0378-1119(78)90038-0. [DOI] [PubMed] [Google Scholar]
- Page G. S., Smith S., Goodman H. M. DNA sequence of the rat growth hormone gene: location of the 5' terminus of the growth hormone mRNA and identification of an internal transposon-like element. Nucleic Acids Res. 1981 May 11;9(9):2087–2104. doi: 10.1093/nar/9.9.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricca G. A., Taylor J. M. Nucleotide sequence of rat alpha 1-acid glycoprotein messenger RNA. J Biol Chem. 1981 Nov 10;256(21):11199–11202. [PubMed] [Google Scholar]
- Richards D. A., Blackburn D. E., Rosen J. M. Restriction enzyme mapping and heteroduplex analysis of the rat milk protein cDNA clones. J Biol Chem. 1981 Jan 10;256(1):533–538. [PubMed] [Google Scholar]
- Richards D. A., Rodgers J. R., Supowit S. C., Rosen J. M. Construction and preliminary characterization of the rat casein and alpha-lactalbumin cDNA clones. J Biol Chem. 1981 Jan 10;256(1):526–532. [PubMed] [Google Scholar]
- Richardson B. C., Mercier J. C. The primary structure of the ovine beta-caseins. Eur J Biochem. 1979 Sep;99(2):285–297. doi: 10.1111/j.1432-1033.1979.tb13255.x. [DOI] [PubMed] [Google Scholar]
- Rosen J. M. Isolation and characterization of purified rat casein messenger ribonucleic acids. Biochemistry. 1976 Nov 30;15(24):5263–5271. doi: 10.1021/bi00669a011. [DOI] [PubMed] [Google Scholar]
- Smith A. J. DNA sequence analysis by primed synthesis. Methods Enzymol. 1980;65(1):560–580. doi: 10.1016/s0076-6879(80)65060-5. [DOI] [PubMed] [Google Scholar]
- Staden R. Further procedures for sequence analysis by computer. Nucleic Acids Res. 1978 Mar;5(3):1013–1016. doi: 10.1093/nar/5.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Sequence data handling by computer. Nucleic Acids Res. 1977 Nov;4(11):4037–4051. doi: 10.1093/nar/4.11.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Topper Y. J. Multiple hormone interactions in the development of mammary gland in vitro. Recent Prog Horm Res. 1970;26:287–308. doi: 10.1016/b978-0-12-571126-5.50011-x. [DOI] [PubMed] [Google Scholar]
- Weaver C. A., Gordon D. F., Kemper B. Introduction by molecular cloning of artifactual inverted sequences at the 5' terminus of the sense strand of bovine parathyroid hormone cDNA. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4073–4077. doi: 10.1073/pnas.78.7.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]