This article highlights the important scientific contributions of Dr Pawan Singal in the area of cardiomyocyte necrosis such as the importance of intracellular Ca2+ overloading in mediating cell injury and the pathogenic role of oxidative stress. Parallel to Dr Singal’s findings, the authors also present their own research on a mitochondriocentric signal-transducer-effector pathway to cardiomyocyte necrosis. Understanding these pathogenic origins and pathophysiological expressions of congestive heart failure is paramount to developing medical management strategies.
Keywords: Antioxidant defenses, Calcium overloading, Cardiomyocyte necrosis, Catecholamines, Mitochondria, Oxidative stress, Parathyroid hormone
Abstract
Congestive heart failure (CHF), a common clinical syndrome, has reached epidemic proportions. Its disabling symptoms account for frequent hospitalizations and readmissions. Pathophysiological mechanisms that lead to CHF and account for its progressive nature are of considerable interest. Important scientific observations obtained from Dr Pawan K Singal’s laboratory in Winnipeg, Manitoba, have provided crucial insights to our understanding of the pathophysiological factors that contribute to cardiomyocyte necrosis (the heart is a postmitotic organ incapable of tolerating an ongoing loss of these cells without adverse functional consequences). This increment in knowledge and the mechanistic insights afforded by Dr Singal and his colleagues have highlighted the role of excessive intracellular calcium accumulation and the appearance of oxidative stress in CHF, in which the rate of reactive oxygen species generation overwhelms their rate of detoxification by antioxidant defenses. They have shown that this common pathophysiological scenario applies to diverse entities such as ischemia/reperfusion and hypoxia/reoxygenation forms of injury, myocardial infarction and the cardiomyopathies that accompany diabetes and excess levels of catecholamines and adriamycin. The authors are honoured to be invited to contribute to the present focus issue of Experimental & Clinical Cardiology in recognizing Dr Singal’s numerous scholarly accomplishments. The present article reviews the authors’ recent work on a mitochondriocentric signal-transducer-effector pathway to cardiomyocyte necrosis found in rats with either an acute stressor state that accompanies isoproterenol administration or a chronic stressor state manifested after four weeks of aldosterone/salt treatment.
Congestive heart failure (CHF) has reached epidemic proportions due, in part, to the reduced mortality rate observed with acute coronary events. The disabling symptoms that constitute the clinical syndrome of CHF now account for the leading cause of hospitalizations in the United States. Understanding pathogenic origins and pathophysiological expressions of CHF is paramount to developing its optimal medical management.
In this context, the present focus issue of Experimental & Clinical Cardiology collectively highlights the important scientific contributions of Dr Pawan K Singal, Professor of Physiology at the University of Manitoba, and Director at the Institute of Cardiovascular Sciences of the St Boniface General Hospital Research Centre in Winnipeg, Manitoba. His laboratory has contributed substantively to our understanding of the cellular-molecular mechanisms leading to cardiomyocyte necrosis, a pathological event accounting for the progressive nature of the failing heart in what is arguably a postmitotic organ with a fixed number of adult cardiomyocytes. Over the past 30 years, his insightful research has expanded our knowledge of the importance of intracellular Ca2+ [Ca2+]i overloading in mediating cell injury. Singal and colleagues reported on the excessive [Ca2+]i accumulation (EICA) that evolves from diverse pathophysiological origins. These include catecholamine-mediated [Ca2+]i accumulation that occurs due to a hyperadrenergic state (1); and ischemia/reperfusion injury, in which the rise in [Ca2+]i occurs during reperfusion when extracellular Ca2+ levels remain normal (2). Second, they reported on the pathogenic role of oxidative stress, in which the rate of injurious reactive oxygen species (ROS) generation overwhelms their rate of detoxification through endogenous antioxidant defenses in diverse entities such as myocardial infarction and the cardiomyopathies associated with either catecholamines, diabetes or adriamycin treatment. In these entities, with either acute or chronic oxidative stress, endogenous antioxidant reserves become inadequate while the addition of exogenous antioxidants (eg, probucol and propranolol) provide cardioprotection (3–11).
Parallel to Dr Singal’s findings, we present our work on a mitochondriocentric signal-transducer-effector (MSTE) pathway to cardiomyocyte necrosis. Its three major components, representing signal, transducer and effector, respectively, includes EICA, especially Ca2+ overloading of the subsarcolemmal population of mitochondria; the generation of ROS by these organelles; and the terminal effector, which involves the opening of the inner membrane-bound mitochondrial permeability transition pore (mPTP). It is our privilege to contribute to the present focus issue of Experimental and Clinical Cardiology, which is dedicated to Professor Singal’s career and his numerous scholarly contributions to the field.
MYOCARDIAL FIBROSIS: A FOOTPRINT OF CARDIOMYOCYTE NECROSIS
Foci of microscopic scarring are scattered throughout the myocardium of the explanted failing human heart (12). The loss of cardiomyocytes to necrosis and their subsequent replacement with fibrillar stiff collagen each contribute to the pathological remodelling of myocardium and the progressive nature of heart failure. Fibrosis is considered to be the major component of the progressive pathological structural remodelling found in the failing heart (12). Its presence underscores the importance of cardiomyocyte necrosis to the failing heart and implicates it as an ongoing event. Apoptosis, which may also be ongoing and important, is not accompanied by tissue repair and the appearance of scarring. Necrotic cells, on the other hand, spill their contents, including troponins, which serve as ‘danger’ signals to invoke inflammatory cell and fibroblast responses that lead to wound healing. In patients hospitalized because of CHF, elevations in serum troponin levels are found on admission and recur with subsequent admissions. Accordingly, elevated troponin levels are associated with an increased risk of morbidity and mortality due to cardiovascular events (13–22). Factors other than myocardial infarction, wherein a critical reduction in coronary blood flow leads to the loss of a segment of myocardium, can account for cardiomyocyte necrosis and raises the importance of neurohormonal activation.
PATHOPHYSIOLOGICAL MECHANISMS IN CARDIOMYOCYTE NECROSIS
Acute stressor states
Acute stressor states are inextricably linked to neurohormonal activation. This includes the hypothalamic-pituitary-adrenal axis, the adrenergic nervous system and the renin-angiotensin-aldosterone system (RAAS) whose effector hormones can be cytotoxic (23–27). The hyperadrenergic state, which accompanies bodily injury (eg, subarachnoid hemorrhage, acute myocardial infarction, burns and trauma), leads to catecholamine-mediated EICA, which importantly includes the accumulation of Ca2+ in subsarcolemmal mitochondria. The ensuing dysfunction of these Ca2+-overloaded mitochondria, coupled with diminished synthesis of high-energy phosphate and their structural degeneration, leads to cardiomyocyte necrosis. The adverse consequences of elevated plasma adrenaline levels (eg, 5000 pg/mL) have been well described (27–31). Excess catecholamine levels also accompany marked emotional and/or physical stress and lead to stress-related apical ballooning or Takotsubo cardiomyopathy (32).
Isoproterenol was used to address the cytotoxicity associated with hyperadrenergic states. Myosin labelling of cells was demonstrated within 2 h of isoproterenol treatment (27) using a monoclonal antibody to cardiac myosin, which enters cardiomyocytes through their hyperpermeable membrane when cell death is imminent. Cells residing within the endomyocardium of the left ventricular (LV) apex were particularly vulnerable to necrosis. More recently, a mitochondriocentric pathway was identified, leading to cardiomyocyte necrosis following isoproterenol treatment (33), in which EICA and oxidative stress were self-evident in cardiomyocytes harvested from the LV apex (vis-à-vis the equator or base). These findings were interpreted to be in keeping with the increased density of β1 receptors reported at this site and the known apical to basal activation of the LV where blood is propelled from the apex toward the base and out into the aorta in a peristaltic-like manner (34–36).
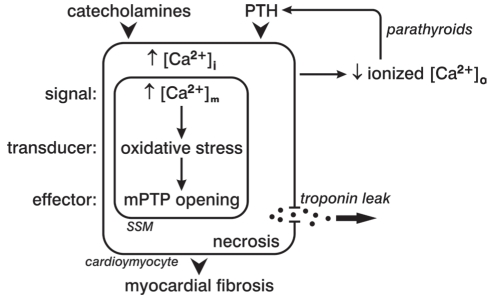
[Ca2+]i overloading involving subsarcolemmal mitochondria is the signal that drives the MSTE pathway to cardiomyocyte necrosis during the acute hyperadrenergic state (Figure 1). The transducer induces oxidative stress, which is invoked in response to EICA, in which the rate of ROS and reactive nitrogen species generation overwhelms their rate of elimination by endogenous antioxidant defenses. Finally, the effector to this pathway is represented by mPTP opening, with consequent solute entry, osmotic swelling and organellar dysfunction with structural degeneration and cell death.
Figure 1).
A subsarcolemmal (SSM) mitochondriocentric signal-transducer-effector pathway to cardiomyocyte necrosis evoked by either catecholamines or parathyroid hormone (PTH). [Ca2+]i Intracellular calcium; [Ca2+]m Mitochondrial-free calcium concentration; [Ca2+]o Extracellular calcium; mPTP Mitochondrial permeability transition pore. Adapted from reference 118
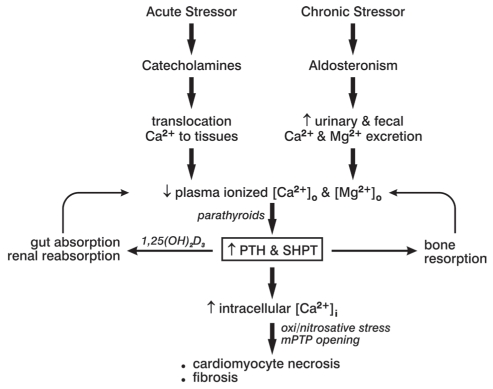
Other pathophysiological responses that accompany catecholamine excess have also proven to be cytotoxic. These include a contemporaneous dyshomeostasis of essential cations manifested as hypokalemia, ionized hypocalcemia and hypomagnesemia (37). The shift in electrolytes from blood to soft tissues accounts for these aberrations and, in turn, invoke secondary hyperparathyroidism (SHPT). The parathyroid glands’ elaboration of the calcitropic parathyroid hormone (PTH) seeks to restore Ca2+ homeostasis through bone mineral resorption (Figure 2). Acute stressor states are accompanied by plasmaionized hypocalcemia with elevations in plasma PTH, in which the severity of injury and extent of the catecholamine response directly correlate with the fall in ionized hypocalcemia and corresponding risk of adverse cardiovascular events (38–48). Elevated PTH levels, however, promote [Ca2+]i overloading, especially in the presence of acute or chronic stressor states. Intracellular cationic shifts, particularly during catecholamine- and PTH-mediated EICA, converge on mitochondria to induce oxidative stress and increase the opening potential of their inner membrane-bound mPTP (Figure 1). The ensuing loss of intracellular cationic homeostasis and cardiomyocyte necrosis is followed by the spillage of cell contents including the leakage of troponins, which ultimately appear in the circulation as a biomarker confirming cardiomyonecrosis.
Figure 2).
Acute and chronic stressor states with excess catecholamines or aldosteronism, respectively, can lead to plasma-ionized hypocalcemia with secondary hyperparathyroidism (SHPT), in which elevations in plasma parathyroid hormone (PTH) levels seek to restore extracellular Ca2+ [Ca2+]o homeostasis via bone resorption, and increase Ca2+ absorption and reabsorption from the colon and the kidneys, respectively. Paradoxically, PTH raises intracellular Ca2+ [Ca2+]i to induce oxidative stress. [Mg2+]o Extracellular magnesium; mPTP Mitochondrial permeability transition pore. Adapted from reference 119
Chronic stressor states
The secondary aldosteronism of CHF, a chronic stressor state, leads to increased fecal and urinary Ca2+ excretion and consequent ionized hypocalcemia with elevated plasma PTH levels (Figure 2) (49–53). A dyshomeostasis of divalent cations is found in patients hospitalized with decompensated biventricular failure with a dilated cardiomyopathy of ischemic or nonischemic origin. This cation-hormone profile is also found in patients with primary aldosteronism (54–57), in whom aberrations in serum ionized and total Ca2+, together with elevated PTH levels, are normalized by either a spironolactone – an aldosterone receptor antagonist – or adrenal surgery (56,57). Furthermore, elevated PTH levels serve as an endogenous stimulus to adrenal aldosterone production, and can further account for contemporaneous elevated plasma aldosterone levels. In patients with primary hyper-parathyroidism, preoperative PTH levels in excess of 100 ng/mL are independent predictors of abnormal elevations in plasma aldosterone levels (58). However, the relative importance of PTH-mediated [Ca2+]i overloading and induction of oxidative stress as major pathogenic events accounting for cardiomyocyte necrosis as contrasted with elevations in circulating aldosterone, per se, remain unclear (59–61).
Abnormal elevations in serum PTH levels (>65 pg/mL) serve as a potent mediator of EICA in cardiomyocytes and mitochondria (59,62,63). They are found in patients hospitalized with decompensated heart failure and those awaiting cardiac transplantation (49,53,64,65), and serve as an independent predictor of CHF and the need for hospitalization (66–68). Moreover, they have been shown to be an independent risk factor for mortality and cardiovascular events in community-dwelling individuals (69–71). SHPT is especially prevalent in the African-American (AA) population with protracted (>4 weeks) decompensated biventricular failure, in which chronic elevations in plasma aldosterone levels contribute to CHF symptoms (53). SHPT is also related to the prevalence of hypovitaminosis D in the AA population; the increased melanin content in dark-skinned individuals serves as a natural sunscreen (53). Accordingly, the presence of hypovitaminosis D, often of marked severity (<20 ng/mL), compromises Ca2+ homeostasis, predisposing the AA population to ionized hypocalcemia and consequent SHPT (53,72,73). Vitamin D deficiency is also common in Caucasian and Asian people with heart failure whose effort intolerance promotes an indoor lifestyle (66,74–76).
Other factors that may be associated with compromised Ca2+ stores and contribute to the appearance of SHPT, especially in AA individuals with CHF, have been reviewed elsewhere (77). In brief, these include a high-salt diet and consequential hypercalciuria, which predisposes to ionized hypocalcemia and SHPT with bone resorption. Osteopenia and osteoporosis are the adverse outcomes of chronic SHPT; they predispose to atraumatic bone fractures (78,79). Patients with heart failure have reduced bone density, which is related to SHPT and vitamin D deficiency, coupled with effort intolerance due to symptomatic failure and consequent reduced physical activity (49,64,80–84). The risk of such fractures is further increased in elderly patients with heart failure receiving a loop diuretic, in which hypercalciuria is also contributory, but preventable, when given in combination with spironolactone (85–87).
Summary
Elevations in serum troponin levels – biomarkers of cardiomyocyte necrosis – not due to ischemia-mediated myocardial infarction are found in patients hospitalized with acute or chronic stressor states and are associated with increased in-hospital and overall cardiac mortality (13–22). The role of EICA and oxidative stress induced by neurohormonal activation, which includes the calcitropic hormones, catecholamines and PTH, in promoting necrosis is now evident. An ongoing loss of cardiomyocytes undoubtedly contributes to the progressive nature of heart failure (the heart is a postmitotic organ with a fixed number of these cells).
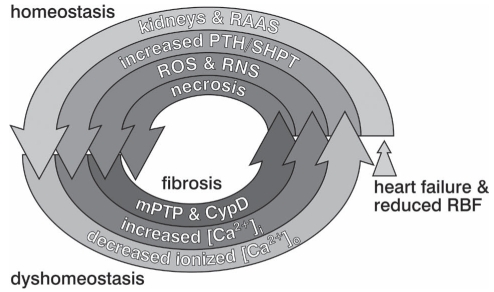
A progressive downward spiral, in which homeostasis begets dyshomeostasis at the organ, cellular and subcellular levels leading to cardiomyocyte necrosis is depicted in Figure 3. The cycle begins with heart failure and reduced renal blood flow leading to the homeostatic activation of the RAAS. Ionized extracellular hypocalcemia appears to result from an accompanying increased excretory loss of Ca2+. In turn, this dyshomeostatic reaction accounts for the subsequent homeostatic response, which is initiated by the appearance of SHPT with increased level of circulating PTH. The dyshomeostatic response to SHPT is PTH-mediated [Ca2+]i overloading, wherein induction of oxidative stress follows with the generation of ROS and reactive nitrogen species. Together, [Ca2+]i overloading and oxidative stress contribute to the pathological opening of the mPTP and activation of cyclophilin D with ensuing osmotic injury to mitochondria and, ultimately, necrotic cell death.
Figure 3).
Homeostasis gone awry begets dyshomeostasis leading to cardiomyocyte necrosis and myocardial fibrosis. [Ca2+]i Intracellular calcium; [Ca2+]o Extracellular calcium; CypD Cyclophilin D; mPTP Mitochondrial permeability transition pore; PTH Parathyroid hormone; RAAS Renin-angiotensin-aldosterone system; RBF Renal blood flow; RNS Reactive nitrogen species; ROS Reactive oxygen species; SHPT Secondary hyperparathyroidism. Reproduced with permission from reference 119
DEFICIENT ANTIOXIDANT RESERVES
Singal and Kirshenbaum (88), Dhaliwal et al (89), and Kirshenbaum and Singal (90) emphasized the importance of a deficiency in anti-oxidant reserves as being contributory to the imbalance in the prooxidant to antioxidant ratio leading to cardiomyocyte necrosis, which accompanies neurohormonal activation. In aldosteronism with CHF, together with increased urinary and fecal losses of K+, Ca2+ and Mg2+, there is a simultaneous cellular and subcellular dyshomeostasis of Zn2+ with resultant hypozincemia (91,92). Accompanying Zn2+ deficiency compromises the activity of Cu/Zn superoxide dismutase – an important metalloenzyme that serves as an antioxidant. Urinary Zn2+ excretion is also increased in response to an angiotensin-converting enzyme inhibitor or an angiotensin receptor antagonist, commonly used in the management of CHF; hypozincemia can be associated with abnormalities in taste (or dysgeusia) (93,94). Furthermore, serum Zn2+ and Se2+ levels are reduced in AA patients (51,52) including those with decompensated failure and compensated failure, as well as those with heart disease without heart failure. Intricate interactions between anti-oxidants, Zn2+ and Se2+, and Zn2+ with prooxidant Ca2+, have also been noted (63,95). Underlying causes for the simultaneous deficiencies of these divalent cations in AA patients, including inadequate dietary intake, remain to be investigated.
Zn2+ dyshomeostasis
The prooxidant effect representing [Ca2+]i overloading that accompanies elevations in either plasma catecholamines or PTH levels is intrinsically coupled to Zn2+ entry, which acts as an antioxidant (62,63,96,97). Although less robust, Zn2+ entry is known to occur via L-type Ca2+ channels, whereas more substantive amounts enter via Zn2+ transporters activated by oxidative stress. Increased cytosolic-free intracellular zinc [Zn2+]i also occur via release of inactive Zn2+ bound to metallothionein-1 induced by nitric oxide derived from nitric oxide synthase. Elevations in [Zn2+]i can also be achieved via a ZnSO4 supplement (3,62,97–102). Increased cytosolic-free [Zn2+]i activates its sensor, metal-responsive transcription factor 1 which, on its translocation to the nucleus, upregulates the expression of antioxidant defense genes. These observations raise the therapeutic prospect that cation-modulating nutriceuticals capable of favourably influencing the [Ca2+]o, [Ca2+]i and Zn2+ equilibrium, enhancing overall antioxidant capacity, could prove pivotal to combating oxidative injury and cardiomyocyte necrosis while promoting Zn2+-based cardioprotective potential.
Se2+ dyshomeostasis
Se2+ is a cofactor of metalloenzyme-based antioxidants, such as glutathione-peroxidase and thioredoxin reductase, each of which promote optimal antioxidant/oxidant balance at the cellular and sub-cellular levels (103). Monitoring serum Se2+ and Se2+-dependent enzyme activities could be useful in addressing optimal Se2+ status and the need for Se2+ supplementation (104,105). Appearance of a dilated cardiomyopathy has been reported in populations in whom dietary Se2+ deficiencies are found, such as in the Se2+-poor soil of the Keshan Province of China, or when parenteral nutrition is deficient in Se2+ (106–108). The Se2+-deficiency-induced cardiomyopathy is often reversible with Se2+ supplementation (109).
MITOCHONDRIA-TARGETED ANTIOXIDANTS
Quercetin and cyclosporine A
Our previous studies indicated that the MSTE pathway leading to necrotic cell death during either isoproterenol administration or chronic aldosterone/salt treatment (ALDOST) includes intramitochondrial Ca2+ overloading, together with induction of oxidative stress and opening of the mPTP. To further validate this concept, we hypothesized that the mitochondria-targeted interventions would be cardioprotective. Accordingly, eight-week-old male Sprague-Dawley rats receiving four weeks of ALDOST were cotreated with either quercetin, a flavonoid with mitochondrial antioxidant properties, or cyclosporine A, an mPTP inhibitor, and compared with ALDOST alone or untreated age/sex-matched controls. Compared with controls, the following results were obtained in the rats treated with ALDOST: a marked increase in mitochondrial H2O2 production and 8-isoprostane levels, an increased propensity for mPTP opening, and greater concentrations of mitochondrial-free calcium [Ca2+]m and total tissue Ca2+, coupled with a five-fold rise in collagen volume fraction without any terminal deoxynucleotidyl-transferase-mediated dUTP nick-end labelling (TUNEL)-based evidence of cardiomyocyte apoptosis. Each of these pathophysiological responses to ALDOST were prevented by quercetin or cyclosporine A cotreatment (110). Thus, mitochondria play a central role in initiating the cellular-subcellular pathway that leads to necrotic cell death and myocardial scarring. This destructive cycle can be interrupted, and myocardium salvaged with its structure and function preserved by mitochondria-targeted cardioprotective strategies.
Carvedilol and nebivolol
Using cardiomyocytes and subsarcolemmal mitochondria (SSM) harvested from rats receiving four weeks of ALDOST, major components of the MSTE pathway to necrosis were identified. Mitochondria-targeted pharmaceutical interventions were used as cardioprotective strategies using four weeks cotreatment with either carvedilol (Carv) or nebivolol (Nebiv). Compared with controls, the following results were obtained in the rats treated with ALDOST: elevated levels of cardiomyocyte-free [Ca2+]i and SSM-free [Ca2+]m; increased H2O2 production and 8-isoprostanes in SSM, increased cardiac tissue and plasma levels; and enhanced opening of mPTP and myocardial scarring. Overall, antioxidant capacity was augmented by increased levels of cytosolic-free [Zn2+]i. Cotreatment with either Carv or Nebiv attenuated [Ca2+]i and [Ca2+]m overloading, prevented oxidative stress and reduced mPTP opening, while further enhancing [Zn2+]i and conferring cardioprotection. Thus, major components of the MSTE pathway to cardiomyocyte necrosis seen with ALDOST include [Ca2+]i overloading coupled with oxidative stress and mPTP opening. This subcellular pathway can be favourably regulated by Carv or Nebiv cotreatment to salvage cardiomyocytes and prevent fibrosis (111).
Summary
The prooxidant pathophysiological scenario anticipates assertions to whether the ensuing adverse consequences are the result of excessive generation of prooxidants, compromised endogenous antioxidant defenses, or both. Clearly, deficiencies of Zn2+ and Se2+ can be counterproductive to metal-dependent antioxidant enzymes. Zn2+ supplementation, as an antioxidant, has shown promise in enhancing antioxidant defenses and serving as a cardioprotective strategy in rodents receiving ALDOST or having streptozocin-induced diabetes (62,97,98,112). A polynutrient supplement, which includes Ca2+, Mg2+, Zn2+ and Se2+, together with a vitamin D supplement, however, will likely be necessary to address the contemporaneous dyshomeostasis of these cations. Promising results with a polynutrient supplement have been reported in critically ill patients including those with heart failure (113–117).
SUMMARY AND CONCLUSIONS
Acute and chronic stressor states are each accompanied by neurohormonal activation that include the adrenergic nervous system and RAAS. The ensuing hyperadrenergic state and SHPT provoke cardiomyocyte Ca2+ overloading, including [Ca2+]i and [Ca2+]m of SSM, with mitochondria-based induction of oxidative stress and opening of their inner membrane-bound mPTP representing the major components of the MSTE pathway to organellar degeneration and cardiomyocyte necrosis. The release of troponins from nonischemic necrotic cardiomyocytes causes elevated serum troponin levels and a wound healing response leading to foci of microscopic scarring. The ongoing nature of the necrosis accounts for scarring to be scattered throughout the endomyocardium of the LV, especially its apex. The loss of cardiomyocytes and their replacement by fibrous tissue contributes to the progressive nature of failure. Fibrosis is a major component of the adverse structural remodelling of the failing myocardium.
Further adverse events, orchestrated by neurohormonal activation, are the coordinated translocation of cations to injured tissues. This facilitates the concordant appearance of hypokalemia, ionized hypocalcemia and hypomagnesemia, hypozincemia and hyposelenemia. Intracellular cationic shifts adaptively regulate the equilibrium between prooxidants and antioxidants – a critical determinant of myocardial cell survival. The intrinsically coupled dyshomeostasis of Ca2+ and Zn2+, representing prooxidant and antioxidant, respectively, can be pharmacologically uncoupled in favour of increased [Zn2+]i and enhanced antioxidant defenses. In doing so, cardiomyocytes susceptible to necrotic cell death can be rescued. The use of nutriceuticals to achieve these goals should be considered as complementary strategies to the current standard of care based on pharmaceuticals.
Footnotes
NOTE: This work was supported, in part, by National Institutes of Health grants R01-HL73043 and R01 HL90867 (KTW). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. The authors have no conflicts of interest to declare.
REFERENCES
- 1.Singal PK, Matsukubo MP, Dhalla NS. Calcium-related changes in the ultrastructure of mammalian myocardium. Br J Exp Pathol. 1979;60:96–106. [PMC free article] [PubMed] [Google Scholar]
- 2.Singh RP, Schellenberg D, Weinberg L, Singal PK. Effects of ouabain on calcium paradox in rat hearts. Can J Physiol Pharmacol. 1986;64:235–9. doi: 10.1139/y86-037. [DOI] [PubMed] [Google Scholar]
- 3.Singal PK, Kapur N, Dhillon KS, Beamish RE, Dhalla NS. Role of free radicals in catecholamine-induced cardiomyopathy. Can J Physiol Pharmacol. 1982;60:1390–7. doi: 10.1139/y82-207. [DOI] [PubMed] [Google Scholar]
- 4.Singal PK, Beamish RE, Dhalla NS. Potential oxidative pathways of catecholamines in the formation of lipid peroxides and genesis of heart disease. Adv Exp Med Biol. 1983;161:391–401. doi: 10.1007/978-1-4684-4472-8_22. [DOI] [PubMed] [Google Scholar]
- 5.Kirshenbaum LA, Thomas TP, Randhawa AK, Singal PK. Time-course of cardiac myocyte injury due to oxidative stress. Mol Cell Biochem. 1992;111:25–31. doi: 10.1007/BF00229570. [DOI] [PubMed] [Google Scholar]
- 6.Kirshenbaum LA, Singal PK. Increase in endogenous antioxidant enzymes protects hearts against reperfusion injury. Am J Physiol. 1993;265:H484–H93. doi: 10.1152/ajpheart.1993.265.2.H484. [DOI] [PubMed] [Google Scholar]
- 7.Siveski-Iliskovic N, Kaul N, Singal PK. Probucol promotes endogenous antioxidants and provides protection against adriamycin-induced cardiomyopathy in rats. Circulation. 1994;89:2829–35. doi: 10.1161/01.cir.89.6.2829. [DOI] [PubMed] [Google Scholar]
- 8.Khaper N, Rigatto C, Seneviratne C, Li T, Singal PK. Chronic treatment with propranolol induces antioxidant changes and protects against ischemia-reperfusion injury. J Mol Cell Cardiol. 1997;29:3335–44. doi: 10.1006/jmcc.1997.0558. [DOI] [PubMed] [Google Scholar]
- 9.Li T, Singal PK. Adriamycin-induced early changes in myocardial antioxidant enzymes and their modulation by probucol. Circulation. 2000;102:2105–10. doi: 10.1161/01.cir.102.17.2105. [DOI] [PubMed] [Google Scholar]
- 10.Li T, Danelisen I, Bello-Klein A, Singal PK. Effects of probucol on changes of antioxidant enzymes in adriamycin-induced cardiomyopathy in rats. Cardiovasc Res. 2000;46:523–30. doi: 10.1016/s0008-6363(00)00039-0. [DOI] [PubMed] [Google Scholar]
- 11.Khullar M, Al-Shudiefat AA, Ludke A, Binepal G, Singal PK. Oxidative stress: A key contributor to diabetic cardiomyopathy. Can J Physiol Pharmacol. 2010;88:233–40. doi: 10.1139/Y10-016. [DOI] [PubMed] [Google Scholar]
- 12.Beltrami CA, Finato N, Rocco M, et al. Structural basis of end-stage failure in ischemic cardiomyopathy in humans. Circulation. 1994;89:151–63. doi: 10.1161/01.cir.89.1.151. [DOI] [PubMed] [Google Scholar]
- 13.Ishii J, Nomura M, Nakamura Y, et al. Risk stratification using a combination of cardiac troponin T and brain natriuretic peptide in patients hospitalized for worsening chronic heart failure. Am J Cardiol. 2002;89:691–5. doi: 10.1016/s0002-9149(01)02341-4. [DOI] [PubMed] [Google Scholar]
- 14.Kuwabara Y, Sato Y, Miyamoto T, et al. Persistently increased serum concentrations of cardiac troponin in patients with acutely decompensated heart failure are predictive of adverse outcomes. Circ J. 2007;71:1047–51. doi: 10.1253/circj.71.1047. [DOI] [PubMed] [Google Scholar]
- 15.Peacock WF, IV, De Marco T, Fonarow GC, et al. Cardiac troponin and outcome in acute heart failure. N Engl J Med. 2008;358:2117–26. doi: 10.1056/NEJMoa0706824. [DOI] [PubMed] [Google Scholar]
- 16.Zairis MN, Tsiaousis GZ, Georgilas AT, et al. Multimarker strategy for the prediction of 31 days cardiac death in patients with acutely decompensated chronic heart failure. Int J Cardiol. 2009;141:284–90. doi: 10.1016/j.ijcard.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 17.Löwbeer C, Gustafsson SA, Seeberger A, Bouvier F, Hulting J. Serum cardiac troponin T in patients hospitalized with heart failure is associated with left ventricular hypertrophy and systolic dysfunction. Scand J Clin Lab Invest. 2004;64:667–76. doi: 10.1080/00365510410003002. [DOI] [PubMed] [Google Scholar]
- 18.Horwich TB, Patel J, MacLellan WR, Fonarow GC. Cardiac troponin I is associated with impaired hemodynamics, progressive left ventricular dysfunction, and increased mortality rates in advanced heart failure. Circulation. 2003;108:833–8. doi: 10.1161/01.CIR.0000084543.79097.34. [DOI] [PubMed] [Google Scholar]
- 19.Sukova J, Ostadal P, Widimsky P. Profile of patients with acute heart failure and elevated troponin I levels. Exp Clin Cardiol. 2007;12:153–6. [PMC free article] [PubMed] [Google Scholar]
- 20.Ilva T, Lassus J, Siirilä-Waris K, et al. Clinical significance of cardiac troponins I and T in acute heart failure. Eur J Heart Fail. 2008;10:772–9. doi: 10.1016/j.ejheart.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Sato Y, Nishi K, Taniguchi R, et al. In patients with heart failure and non-ischemic heart disease, cardiac troponin T is a reliable predictor of long-term echocardiographic changes and adverse cardiac events. J Cardiol. 2009;54:221–30. doi: 10.1016/j.jjcc.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Miller WL, Hartman KA, Burritt MF, Grill DE, Jaffe AS. Profiles of serial changes in cardiac troponin T concentrations and outcome in ambulatory patients with chronic heart failure. J Am Coll Cardiol. 2009;54:1715–21. doi: 10.1016/j.jacc.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 23.Fleckenstein A, Kanke J, Döring HJ, Leder O. Key role of Ca in the production of noncoronarogenic myocardial necroses. Recent Adv Stud Cardiac Struct Metab. 1975;6:21–32. [PubMed] [Google Scholar]
- 24.Lossnitzer K, Janke J, Hein B, Stauch M, Fleckenstein A. Disturbed myocardial calcium metabolism: A possible pathogenetic factor in the hereditary cardiomyopathy of the Syrian hamster. Recent Adv Stud Cardiac Struct Metab. 1975;6:207–17. [PubMed] [Google Scholar]
- 25.Tan LB, Burniston JG, Clark WA, Ng Y, Goldspink DF. Characterization of adrenoceptor involvement in skeletal and cardiac myotoxicity Induced by sympathomimetic agents: Toward a new bioassay for beta-blockers. J Cardiovasc Pharmacol. 2003;41:518–25. doi: 10.1097/00005344-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Goldspink DF, Burniston JG, Ellison GM, Clark WA, Tan LB. Catecholamine-induced apoptosis and necrosis in cardiac and skeletal myocytes of the rat in vivo: The same or separate death pathways? Exp Physiol. 2004;89:407–16. doi: 10.1113/expphysiol.2004.027482. [DOI] [PubMed] [Google Scholar]
- 27.Benjamin IJ, Jalil JE, Tan LB, Cho K, Weber KT, Clark WA. Isoproterenol-induced myocardial fibrosis in relation to myocyte necrosis. Circ Res. 1989;65:657–70. doi: 10.1161/01.res.65.3.657. [DOI] [PubMed] [Google Scholar]
- 28.Bier CB, Rona G. Mineralocorticoid potentiation of isoproterenol-induced myocardial injury: Ultrastructural equivalent. J Mol Cell Cardiol. 1979;11:961–6. doi: 10.1016/0022-2828(79)90387-0. [DOI] [PubMed] [Google Scholar]
- 29.Rona G, Boutet M, Huttner I. Reperfusion injury. A possible link between catecholamine-induced and ischemic myocardial alterations. Adv Myocardiol. 1983;4:427–39. [PubMed] [Google Scholar]
- 30.Yates JC, Taam GM, Singal PK, Beamish RE, Dhalla NS. Modification of adrenochrome-induced cardiac contractile failure and cell damage by changes in cation concentrations. Lab Invest. 1980;43:316–26. [PubMed] [Google Scholar]
- 31.Singal PK, Forbes MS, Sperelakis N. Occurrence of intramitochondrial Ca2+ granules in a hypertrophied heart exposed to adriamycin. Can J Physiol Pharmacol. 1984;62:1239–44. doi: 10.1139/y84-207. [DOI] [PubMed] [Google Scholar]
- 32.Bybee KA, Prasad A. Stress-related cardiomyopathy syndromes. Circulation. 2008;118:397–409. doi: 10.1161/CIRCULATIONAHA.106.677625. [DOI] [PubMed] [Google Scholar]
- 33.Shahbaz AU, Zhao T, Zhao W, et al. Calcium and zinc dyshomeostasis during isoproterenol-induced acute stressor state. Am J Physiol Heart Circ Physiol. 2011;300:H636–H44. doi: 10.1152/ajpheart.00900.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rushmer RF, Thal N. The mechanics of ventricular contraction; a cinefluorographic study. Circulation. 1951;4:219–28. doi: 10.1161/01.cir.4.2.219. [DOI] [PubMed] [Google Scholar]
- 35.Sedmera D, Reckova M, Bigelow MR, et al. Developmental transitions in electrical activation patterns in chick embryonic heart. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:1001–9. doi: 10.1002/ar.a.20107. [DOI] [PubMed] [Google Scholar]
- 36.Buchalter MB, Rademakers FE, Weiss JL, Rogers WJ, Weisfeldt ML, Shapiro EP. Rotational deformation of the canine left ventricle measured by magnetic resonance tagging: Effects of catecholamines, ischaemia, and pacing. Cardiovasc Res. 1994;28:629–35. doi: 10.1093/cvr/28.5.629. [DOI] [PubMed] [Google Scholar]
- 37.Whitted AD, Stanifer JW, Dube P, et al. A dyshomeostasis of electrolytes and trace elements in acute stressor states: Impact on the heart. Am J Med Sci. 2010;340:48–53. doi: 10.1097/MAJ.0b013e3181e5945b. [DOI] [PubMed] [Google Scholar]
- 38.Carlstedt F, Lind L, Joachimsson PO, Rastad J, Wide L, Ljunghall S. Circulating ionized calcium and parathyroid hormone levels following coronary artery by-pass surgery. Scand J Clin Lab Invest. 1999;59:47–53. doi: 10.1080/00365519950185995. [DOI] [PubMed] [Google Scholar]
- 39.Carlstedt F, Lind L, Rastad J, Stjernstrom H, Wide L, Ljunghall S. Parathyroid hormone and ionized calcium levels are related to the severity of illness and survival in critically ill patients. Eur J Clin Invest. 1998;28:898–903. doi: 10.1046/j.1365-2362.1998.00391.x. [DOI] [PubMed] [Google Scholar]
- 40.Carlstedt F, Lind L, Wide L, et al. Serum levels of parathyroid hormone are related to the mortality and severity of illness in patients in the emergency department. Eur J Clin Invest. 1997;27:977–81. doi: 10.1046/j.1365-2362.1997.2310778.x. [DOI] [PubMed] [Google Scholar]
- 41.Hästbacka J, Pettilä V. Prevalence and predictive value of ionized hypocalcemia among critically ill patients. Acta Anaesthesiol Scand. 2003;47:1264–9. doi: 10.1046/j.1399-6576.2003.00236.x. [DOI] [PubMed] [Google Scholar]
- 42.Choi YC, Hwang SY. The value of initial ionized calcium as a predictor of mortality and triage tool in adult trauma patients. J Korean Med Sci. 2008;23:700–5. doi: 10.3346/jkms.2008.23.4.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cherry RA, Bradburn E, Carney DE, Shaffer ML, Gabbay RA, Cooney RN. Do early ionized calcium levels really matter in trauma patients? J Trauma. 2006;61:774–9. doi: 10.1097/01.ta.0000239516.49799.63. [DOI] [PubMed] [Google Scholar]
- 44.Dickerson RN, Henry NY, Miller PL, Minard G, Brown RO. Low serum total calcium concentration as a marker of low serum ionized calcium concentration in critically ill patients receiving specialized nutrition support. Nutr Clin Pract. 2007;22:323–8. doi: 10.1177/0115426507022003323. [DOI] [PubMed] [Google Scholar]
- 45.Burchard KW, Simms HH, Robinson A, DiAmico R, Gann DS. Hypocalcemia during sepsis. Relationship to resuscitation and hemodynamics. Arch Surg. 1992;127:265–72. doi: 10.1001/archsurg.1992.01420030027005. [DOI] [PubMed] [Google Scholar]
- 46.Joborn H, Hjemdahl P, Larsson PT, et al. Platelet and plasma catecholamines in relation to plasma minerals and parathyroid hormone following acute myocardial infarction. Chest. 1990;97:1098–105. doi: 10.1378/chest.97.5.1098. [DOI] [PubMed] [Google Scholar]
- 47.Karlsberg RP, Cryer PE, Roberts R. Serial plasma catecholamine response early in the course of clinical acute myocardial infarction: Relationship to infarct extent and mortality. Am Heart J. 1981;102:24–9. doi: 10.1016/0002-8703(81)90408-7. [DOI] [PubMed] [Google Scholar]
- 48.Magnotti LJ, Bradburn EH, Webb DL, et al. Admission ionized calcium levels predict the need for multiple transfusions: A prospective study of 591 critically ill trauma patients. J Trauma. 2011;70:391–7. doi: 10.1097/TA.0b013e31820b5d98. [DOI] [PubMed] [Google Scholar]
- 49.Shane E, Mancini D, Aaronson K, et al. Bone mass, vitamin D deficiency, and hyperparathyroidism in congestive heart failure. Am J Med. 1997;103:197–207. doi: 10.1016/s0002-9343(97)00142-3. [DOI] [PubMed] [Google Scholar]
- 50.Khouzam RN, Dishmon DA, Farah V, Flax SD, Carbone LD, Weber KT. Secondary hyperparathyroidism in patients with untreated and treated congestive heart failure. Am J Med Sci. 2006;331:30–4. doi: 10.1097/00000441-200601000-00009. [DOI] [PubMed] [Google Scholar]
- 51.LaGuardia SP, Dockery BK, Bhattacharya SK, Nelson MD, Carbone LD, Weber KT. Secondary hyperparathyroidism and hypovitaminosis D in African-Americans with decompensated heart failure. Am J Med Sci. 2006;332:112–8. doi: 10.1097/00000441-200609000-00003. [DOI] [PubMed] [Google Scholar]
- 52.Arroyo M, LaGuardia SP, Bhattacharya SK, et al. Micronutrients in African-Americans with decompensated and compensated heart failure. Transl Res. 2006;148:301–8. doi: 10.1016/j.trsl.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 53.Alsafwah S, LaGuardia SP, Nelson MD, et al. Hypovitaminosis D in African Americans residing in Memphis, Tennessee with and without heart failure. Am J Med Sci. 2008;335:292–7. doi: 10.1097/MAJ.0b013e318167b0bd. [DOI] [PubMed] [Google Scholar]
- 54.Fertig A, Webley M, Lynn JA. Primary hyperparathyroidism in a patient with Conn’s syndrome. Postgrad Med J. 1980;56:45–7. doi: 10.1136/pgmj.56.651.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hellman DE, Kartchner M, Komar N, Mayes D, Pitt M. Hyperaldosteronism, hyperparathyroidism, medullary sponge kidneys, and hypertension. JAMA. 1980;244:1351–3. [PubMed] [Google Scholar]
- 56.Resnick LM, Laragh JH. Calcium metabolism and parathyroid function in primary aldosteronism. Am J Med. 1985;78:385–90. doi: 10.1016/0002-9343(85)90328-6. [DOI] [PubMed] [Google Scholar]
- 57.Rossi E, Sani C, Perazzoli F, Casoli MC, Negro A, Dotti C. Alterations of calcium metabolism and of parathyroid function in primary aldosteronism, and their reversal by spironolactone or by surgical removal of aldosterone-producing adenomas. Am J Hypertens. 1995;8:884–93. doi: 10.1016/0895-7061(95)00182-O. [DOI] [PubMed] [Google Scholar]
- 58.Brunaud L, Germain A, Zarnegar R, et al. Serum aldosterone is correlated positively to parathyroid hormone (PTH) levels in patients with primary hyperparathyroidism. Surgery. 2009;146:1035–41. doi: 10.1016/j.surg.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 59.Chhokar VS, Sun Y, Bhattacharya SK, et al. Hyperparathyroidism and the calcium paradox of aldosteronism. Circulation. 2005;111:871–8. doi: 10.1161/01.CIR.0000155621.10213.06. [DOI] [PubMed] [Google Scholar]
- 60.Vidal A, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC, Weber KT. Calcium paradox of aldosteronism and the role of the parathyroid glands. Am J Physiol Heart Circ Physiol. 2006;290:H286–H94. doi: 10.1152/ajpheart.00535.2005. [DOI] [PubMed] [Google Scholar]
- 61.Selektor Y, Ahokas RA, Bhattacharya SK, Sun Y, Gerling IC, Weber KT. Cinacalcet and the prevention of secondary hyperparathyroidism in rats with aldosteronism. Am J Med Sci. 2008;335:105–10. doi: 10.1097/MAJ.0b013e318134f013. [DOI] [PubMed] [Google Scholar]
- 62.Gandhi MS, Deshmukh PA, Kamalov G, et al. Causes and consequences of zinc dyshomeostasis in rats with chronic aldosteronism. J Cardiovasc Pharmacol. 2008;52:245–52. doi: 10.1097/FJC.0b013e3181833eb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kamalov G, Deshmukh PA, Baburyan NY, et al. Coupled calcium and zinc dyshomeostasis and oxidative stress in cardiac myocytes and mitochondria of rats with chronic aldosteronism. J Cardiovasc Pharmacol. 2009;53:414–23. doi: 10.1097/FJC.0b013e3181a15e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee AH, Mull RL, Keenan GF, et al. Osteoporosis and bone morbidity in cardiac transplant recipients. Am J Med. 1994;96:35–41. doi: 10.1016/0002-9343(94)90113-9. [DOI] [PubMed] [Google Scholar]
- 65.Schmid C, Kiowski W. Hyperparathyroidism in congestive heart failure. Am J Med. 1998;104:508–9. [PubMed] [Google Scholar]
- 66.Ogino K, Ogura K, Kinugasa Y, et al. Parathyroid hormone-related protein is produced in the myocardium and increased in patients with congestive heart failure. J Clin Endocrinol Metab. 2002;87:4722–7. doi: 10.1210/jc.2002-020314. [DOI] [PubMed] [Google Scholar]
- 67.Zittermann A, Schleithoff SS, Tenderich G, Berthold HK, Korfer R, Stehle P. Low vitamin D status: A contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol. 2003;41:105–12. doi: 10.1016/s0735-1097(02)02624-4. [DOI] [PubMed] [Google Scholar]
- 68.Sugimoto T, Tanigawa T, Onishi K, et al. Serum intact parathyroid hormone levels predict hospitalisation for heart failure. Heart. 2009;95:395–8. doi: 10.1136/hrt.2008.147652. [DOI] [PubMed] [Google Scholar]
- 69.Pilz S, Tomaschitz A, Drechsler C, et al. Parathyroid hormone level is associated with mortality and cardiovascular events in patients undergoing coronary angiography. Eur Heart J. 2010;31:1591–8. doi: 10.1093/eurheartj/ehq109. [DOI] [PubMed] [Google Scholar]
- 70.Hagström E, Hellman P, Larsson TE, et al. Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation. 2009;119:2765–71. doi: 10.1161/CIRCULATIONAHA.108.808733. [DOI] [PubMed] [Google Scholar]
- 71.Hagström E, Ingelsson E, Sundström J, et al. Plasma parathyroid hormone and risk of congestive heart failure in the community. Eur J Heart Fail. 2010;12:1186–92. doi: 10.1093/eurjhf/hfq134. [DOI] [PubMed] [Google Scholar]
- 72.Bell NH, Greene A, Epstein S, Oexmann MJ, Shaw S, Shary J. Evidence for alteration of the vitamin D-endocrine system in blacks. J Clin Invest. 1985;76:470–3. doi: 10.1172/JCI111995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sawaya BP, Monier-Faugere MC, Ratanapanichkich P, Butros R, Wedlund PJ, Fanti P. Racial differences in parathyroid hormone levels in patients with secondary hyperparathyroidism. Clin Nephrol. 2002;57:51–5. doi: 10.5414/cnp57051. [DOI] [PubMed] [Google Scholar]
- 74.Zittermann A, Fischer J, Schleithoff SS, Tenderich G, Fuchs U, Koerfer R. Patients with congestive heart failure and healthy controls differ in vitamin D-associated lifestyle factors. Int J Vitam Nutr Res. 2007;77:280–8. doi: 10.1024/0300-9831.77.4.280. [DOI] [PubMed] [Google Scholar]
- 75.Zittermann A, Schleithoff SS, Gotting C, et al. Poor outcome in end-stage heart failure patients with low circulating calcitriol levels. Eur J Heart Fail. 2008;10:321–7. doi: 10.1016/j.ejheart.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 76.Zittermann A, Schleithoff SS, Koerfer R. Vitamin D and vascular calcification. Curr Opin Lipidol. 2007;18:41–6. doi: 10.1097/MOL.0b013e328011c6fc. [DOI] [PubMed] [Google Scholar]
- 77.Borkowski BJ, Cheema Y, Shahbaz AU, Bhattacharya SK, Weber KT. Cation dyshomeostasis and cardiomyocyte necrosis. The Fleckenstein hypothesis revisited. Eur Heart J. 2011 doi: 10.1093/eurheartj/ehr063. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cohen AJ, Roe FJ. Review of risk factors for osteoporosis with particular reference to a possible aetiological role of dietary salt. Food Chem Toxicol. 2000;38:237–53. doi: 10.1016/s0278-6915(99)00145-3. [DOI] [PubMed] [Google Scholar]
- 79.Teucher B, Dainty JR, Spinks CA, et al. Sodium and bone health: Impact of moderately high and low salt intakes on calcium metabolism in postmenopausal women. J Bone Miner Res. 2008;23:1477–85. doi: 10.1359/jbmr.080408. [DOI] [PubMed] [Google Scholar]
- 80.Kerschan-Schindl K, Strametz-Juranek J, Heinze G, et al. Pathogenesis of bone loss in heart transplant candidates and recipients. J Heart Lung Transplant. 2003;22:843–50. doi: 10.1016/s1053-2498(02)00806-9. [DOI] [PubMed] [Google Scholar]
- 81.Nishio K, Mukae S, Aoki S, et al. Congestive heart failure is associated with the rate of bone loss. J Intern Med. 2003;253:439–46. doi: 10.1046/j.1365-2796.2003.01130.x. [DOI] [PubMed] [Google Scholar]
- 82.Kenny AM, Boxer R, Walsh S, Hager WD, Raisz LG. Femoral bone mineral density in patients with heart failure. Osteoporos Int. 2006;17:1420–7. doi: 10.1007/s00198-006-0148-4. [DOI] [PubMed] [Google Scholar]
- 83.Frost RJ, Sonne C, Wehr U, Stempfle HU. Effects of calcium supplementation on bone loss and fractures in congestive heart failure. Eur J Endocrinol. 2007;156:309–14. doi: 10.1530/EJE-06-0614. [DOI] [PubMed] [Google Scholar]
- 84.Abou-Raya S, Abou-Raya A. Osteoporosis and congestive heart failure (CHF) in the elderly patient: Double disease burden. Arch Gerontol Geriatr. 2009;49:250–4. doi: 10.1016/j.archger.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 85.van Diepen S, Majumdar SR, Bakal JA, McAlister FA, Ezekowitz JA. Heart failure is a risk factor for orthopedic fracture: A population-based analysis of 16,294 patients. Circulation. 2008;118:1946–52. doi: 10.1161/CIRCULATIONAHA.108.784009. [DOI] [PubMed] [Google Scholar]
- 86.Carbone LD, Cross JD, Raza SH, et al. Fracture risk in men with congestive heart failure. Risk reduction with spironolactone. J Am Coll Cardiol. 2008;52:135–8. doi: 10.1016/j.jacc.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 87.Law PH, Sun Y, Bhattacharya SK, Chhokar VS, Weber KT. Diuretics and bone loss in rats with aldosteronism. J Am Coll Cardiol. 2005;46:142–6. doi: 10.1016/j.jacc.2005.03.055. [DOI] [PubMed] [Google Scholar]
- 88.Singal PK, Kirshenbaum LA. A relative deficit in antioxidant reserve may contribute in cardiac failure. Can J Cardiol. 1990;6:47–9. [PubMed] [Google Scholar]
- 89.Dhaliwal H, Kirshenbaum LA, Randhawa AK, Singal PK. Correlation between antioxidant changes during hypoxia and recovery on reoxygenation. Am J Physiol. 1991;261:H632–H8. doi: 10.1152/ajpheart.1991.261.3.H632. [DOI] [PubMed] [Google Scholar]
- 90.Kirshenbaum LA, Singal PK. Antioxidant changes in heart hypertrophy: Significance during hypoxia-reoxygenation injury. Can J Physiol Pharmacol. 1992;70:1330–5. doi: 10.1139/y92-186. [DOI] [PubMed] [Google Scholar]
- 91.Garcia Zozaya JL, Padilla Viloria M. [Alterations of calcium, magnesium, and zinc in essential hypertension: Their relation to the renin-angiotensin-aldosterone system.] Invest Clin. 1997;38(Suppl 2):27–40. [PubMed] [Google Scholar]
- 92.Tubek S. Zinc content in lymphocytes and the activity of zinc ion efflux from lymphocytes in primary arterial hypertension. Biol Trace Elem Res. 2005;107:89–99. doi: 10.1385/BTER:107:2:089. [DOI] [PubMed] [Google Scholar]
- 93.Golik A, Modai D, Averbukh Z, et al. Zinc metabolism in patients treated with captopril versus enalapril. Metabolism. 1990;39:665–7. doi: 10.1016/0026-0495(90)90098-w. [DOI] [PubMed] [Google Scholar]
- 94.Golik A, Zaidenstein R, Dishi V, et al. Effects of captopril and enalapril on zinc metabolism in hypertensive patients. J Am Coll Nutr. 1998;17:75–8. doi: 10.1080/07315724.1998.10720459. [DOI] [PubMed] [Google Scholar]
- 95.Chmielnicka J, Zareba G, Witasik M, Brzeznicka E. Zinc-selenium interaction in the rat. Biol Trace Elem Res. 1988;15:267–76. doi: 10.1007/BF02990143. [DOI] [PubMed] [Google Scholar]
- 96.Kamalov G, Ahokas RA, Zhao W, et al. Temporal responses to intrinsically coupled calcium and zinc dyshomeostasis in cardiac myocytes and mitochondria during aldosteronism. Am J Physiol Heart Circ Physiol. 2010;298:H385–H94. doi: 10.1152/ajpheart.00593.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kamalov G, Ahokas RA, Zhao W, et al. Uncoupling the coupled calcium and zinc dyshomeostasis in cardiac myocytes and mitochondria seen in aldosteronism. J Cardiovasc Pharmacol. 2010;55:248–54. doi: 10.1097/FJC.0b013e3181cf0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang J, Song Y, Elsherif L, et al. Cardiac metallothionein induction plays the major role in the prevention of diabetic cardiomyopathy by zinc supplementation. Circulation. 2006;113:544–54. doi: 10.1161/CIRCULATIONAHA.105.537894. [DOI] [PubMed] [Google Scholar]
- 99.Chung MJ, Hogstrand C, Lee SJ. Cytotoxicity of nitric oxide is alleviated by zinc-mediated expression of antioxidant genes. Exp Biol Med (Maywood) 2006;231:1555–63. doi: 10.1177/153537020623100916. [DOI] [PubMed] [Google Scholar]
- 100.Chung MJ, Walker PA, Brown RW, Hogstrand C. Zinc-mediated gene expression offers protection against H2O2-induced cytotoxicity. Toxicol Appl Pharmacol. 2005;205:225–36. doi: 10.1016/j.taap.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 101.Singal PK, Dhillon KS, Beamish RE, Dhalla NS. Protective effect of zinc against catecholamine-induced myocardial changes electrocardiographic and ultrastructural studies. Lab Invest. 1981;44:426–33. [PubMed] [Google Scholar]
- 102.Chvapil M, Owen JA. Effect of zinc on acute and chronic isoproterenol induced heart injury. J Mol Cell Cardiol. 1977;9:151–9. doi: 10.1016/0022-2828(77)90046-3. [DOI] [PubMed] [Google Scholar]
- 103.Prabhu KS, Zamamiri-Davis F, Stewart JB, Thompson JT, Sordillo LM, Reddy CC. Selenium deficiency increases the expression of inducible nitric oxide synthase in RAW 264.7 macrophages: Role of nuclear factor-kB in up-regulation. Biochem J. 2002;366(Pt 1):203–9. doi: 10.1042/BJ20020256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hatanaka N, Nakaden H, Yamamoto Y, Matsuo S, Fujikawa T, Matsusue S. Selenium kinetics and changes in glutathione peroxidase activities in patients receiving long-term parenteral nutrition and effects of supplementation with selenite. Nutrition. 2000;16:22–6. doi: 10.1016/s0899-9007(99)00183-5. [DOI] [PubMed] [Google Scholar]
- 105.Weiss SL, Evenson JK, Thompson KM, Sunde RA. The selenium requirement for glutathione peroxidase mRNA level is half of the selenium requirement for glutathione peroxidase activity in female rats. J Nutr. 1996;126:2260–7. doi: 10.1093/jn/126.9.2260. [DOI] [PubMed] [Google Scholar]
- 106.Xia YM, Hill KE, Burk RF. Biochemical studies of a selenium-deficient population in China: Measurement of selenium, glutathione peroxidase and other oxidant defense indices in blood. J Nutr. 1989;119:1318–26. doi: 10.1093/jn/119.9.1318. [DOI] [PubMed] [Google Scholar]
- 107.van Rij AM, Thomson CD, McKenzie JM, Robinson MF. Selenium deficiency in total parenteral nutrition. Am J Clin Nutr. 1979;32:2076–85. doi: 10.1093/ajcn/32.10.2076. [DOI] [PubMed] [Google Scholar]
- 108.Reeves WC, Marcuard SP, Willis SE, Movahed A. Reversible cardiomyopathy due to selenium deficiency. JPEN J Parenter Enteral Nutr. 1989;13:663–5. doi: 10.1177/0148607189013006663. [DOI] [PubMed] [Google Scholar]
- 109.Boldery R, Fielding G, Rafter T, Pascoe AL, Scalia GM. Nutritional deficiency of selenium secondary to weight loss (bariatric) surgery associated with life-threatening cardiomyopathy. Heart Lung Circ. 2007;16:123–6. doi: 10.1016/j.hlc.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 110.Shahbaz AU, Kamalov G, Zhao W, et al. Mitochondria-targeted cardioprotection in aldosteronism. J Cardiovasc Pharmacol. 2011;57:37–43. doi: 10.1097/FJC.0b013e3181fe1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cheema Y, Sherrod JN, Shahbaz AU, et al. Mitochondriocentric pathway to cardiomyocyte necrosis in aldosteronism: Cardioprotective responses to carvedilol and nebivolol. J Cardiovasc Pharmacol. 2011;58:80–6. doi: 10.1097/FJC.0b013e31821cd83c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Song Y, Wang J, Li XK, Cai L. Zinc and the diabetic heart. Biometals. 2005;18:325–32. doi: 10.1007/s10534-005-3689-7. [DOI] [PubMed] [Google Scholar]
- 113.Demling RH, DeBiasse MA. Micronutrients in critical illness. Crit Care Clin. 1995;11:651–73. [PubMed] [Google Scholar]
- 114.Sriram K, Lonchyna VA. Micronutrient supplementation in adult nutrition therapy: Practical considerations. JPEN J Parenter Enteral Nutr. 2009;33:548–62. doi: 10.1177/0148607108328470. [DOI] [PubMed] [Google Scholar]
- 115.Witte KK, Clark AL, Cleland JG. Chronic heart failure and micronutrients. J Am Coll Cardiol. 2001;37:1765–74. doi: 10.1016/s0735-1097(01)01227-x. [DOI] [PubMed] [Google Scholar]
- 116.Soukoulis V, Dihu JB, Sole M, et al. Micronutrient deficiencies an unmet need in heart failure. J Am Coll Cardiol. 2009;54:1660–73. doi: 10.1016/j.jacc.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 117.Bhattacharya SK, Ahokas RA, Carbone LD, et al. Macro- and micronutrients in African-Americans with heart failure. Heart Fail Rev. 2006;11:45–55. doi: 10.1007/s10741-006-9192-6. [DOI] [PubMed] [Google Scholar]
- 118.Robinson AD, Ramanathan KB, McGee JE, Newman KP, Weber KT. Oxidative stress and cardiomyocyte necrosis with elevated serum troponins: Pathophysiologic mechanisms. Am J Med Sci. 2011 doi: 10.1097/MAJ.0b013e3182231ee3. (In press) [DOI] [PubMed] [Google Scholar]
- 119.Kamalov G, Bhattacharya SK, Weber KT. Congestive heart failure: Where homeostasis begets dyshomeostasis. J Cardiovasc Pharmacol. 2010;56:320–8. doi: 10.1097/FJC.0b013e3181ed064f. [DOI] [PMC free article] [PubMed] [Google Scholar]