Taurine is a beta-amino acid that is found in excitable tissues; however, its deficiency leads to reduced ATP generation and oxidative stress, and contributes to cell shrinkage during apoptosis. In addition, taurine has been found to modulate calcium movement and superoxide generation, which are important to cell survival. Accordingly, this study examined the involvement of reactive oxygen species and calcium movement in the initiation of cellular apoptosis by testing the taurine content of neonatal cardiomyocytes (isolated from two- to three-day-old Wistar rats) after 48 h incubation in medium containing the taurine transport inhibitor beta-alanine.
Keywords: Apoptosis, Mitochondrial permeability transition, Oxidative stress, Taurine deficiency
Abstract
It has recently been shown that taurine deficiency leads to impaired respiratory chain function, resulting in reduced ATP generation and enhanced oxidative stress. Because cardiomyopathy develops in taurine-deficient animals, the hypothesis that mitochondrial oxidative stress may contribute to the development of cardiomyocyte dysfunction and cell death was tested. Isolated neonatal cardiomyocytes incubated in medium containing the taurine transport inhibitor, beta-alanine, lost nearly one-half of their cellular taurine content after 48 h. Accompanying the loss of taurine was a time-dependent increase in apoptosis, which was prevented by the mitochondrial permeability transition inhibitor, cyclosporin A. Two taurine-dependent factors, oxidative stress and calcium overload, serve as important regulators of the mitochondrial permeability transition. Although taurine deficiency slowed the removal of calcium from the cytosol, it had no effect on diastolic calcium content and only modestly reduced systolic calcium content, suggesting that calcium overload is not the trigger for mitochondrial permeability transition pore formation. On the other hand, the glutathione redox ratio was significantly altered in the taurine-deficient cardiomyocyte, suggesting that oxidative stress is the primary initiator of mitochondrial permeability transition and apoptosis in the taurine-deficient cardiomyocyte.
Taurine is a beta-amino acid found in very high concentrations in excitable tissues (1). Although taurine was discovered more than 100 years ago, only recently have studies explored its potential physiological functions, which include osmoregulation, immunoregulation, modulation of ion transport, determination of energy metabolism and regulation of cholesterol metabolism (1–5). Many of these taurine functions are mediated by taurine conjugation products (2,3,5). The most recently discovered conjugation product, 5-taurinomethyluridine-tRNALeu(UUR), which is formed in the wobble position of tRNALeu(UUR), stabilizes U-G pairing in the anticodon loop of the transfer RNA (tRNA), resulting in efficient decoding of UUG (5,6). Thus, by diminishing the formation of 5-taurinomethyluridine-tRNALeu(UUR), taurine deficiency decreases the rate of UUG-dependent mitochondria-encoded protein synthesis, leading to impaired assembly of respiratory chain complexes. Through this series of events, taurine is a key determinant of oxidative phosphorylation and the rate of mitochondrial superoxide generation; the latter arises from the diversion of electrons from the respiratory chain to the acceptor oxygen (4,7).
The evidence supporting taurine as a cytoprotective agent has been accumulating (8). According to Lang et al (9), preloading Jurkat T lymphocytes with taurine significantly inhibits Fas-induced DNA fragmentation and apoptotic cell shrinkage. Because taurine loss contributes to cell shrinkage during apoptosis, Lang et al (10) suggested that taurine preloading prevents the apoptotic cascade from proceeding beyond the cell shrinkage step, implying that the cytoprotective actions of taurine are linked to its osmoregulatory function. However, taurine also modulates calcium movement and superoxide generation, which are important determinants of cell survival (7,11,12). Therefore, the present study examined the involvement of reactive oxygen species and calcium movement in the initiation of cellular apoptosis of taurine-deficient cardiomyocytes.
METHODS
Cardiomyocyte preparation
Neonatal cardiomyocytes were obtained from two- to three-day-old Wistar rats, as previously described by Grishko et al (13). Briefly after enzymatic homogenization, cells were preplated for nonmyocyte attachment. The unattached cells were then suspended in minimum essential medium, and supplemented with 10% newborn calf serum and 0.1 mM 5-bromo-2-deoxyuridine, before being plated and incubated overnight. The serum-containing medium was replaced the next day with serum-substituted medium. The neonatal cardiomyocyte culture was maintained for two days at 37°C before treatment at various time intervals with medium containing either 0 mM (control) or 5 mM beta-alanine, which is a taurine transport inhibitor.
Cellular Ca2+ content
Control and beta-alanine-treated cardiomyocytes were loaded with 2 μM Indo-1 for 20 min at 37°C. After rinsing, calcium transients were measured by confocal microscopy. Values for peak systolic intracellular calcium concentration [Ca2+]i, diastolic [Ca2+]i and the time required to achieve 90% relaxation are expressed as percentages of control. Data are expressed as the ratio of two wavelengths (F410/F490), which is proportional to [Ca2+]i.
Cellular taurine content and reduced/oxidized glutathione level analysis
Cells were homogenized in ice-cold perchloric acid (1 M) and 2 mM EDTA before being subjected to centrifugation, as described by Schaffer et al (14). The resulting supernatant was used to measure intracellular taurine content according to the Shaffer and Kocsis (15) method. Oxidized glutathione (GSSG) and reduced glutathione (GSH) levels were determined according to the glutathione assay kit (Cayman Chemical, USA).
Western blot analysis
Cytochrome c levels were determined by Western blot analysis. Following the method described by Grishko et al (13), cells were lysed and homogenized in ice-cold isolation buffer (pH 7.25 at 4°C). The homogenate was centrifuged twice at 800 g and then at 16,000 g. The resulting supernatant was defined as the cytosolic fraction and used to determine cytochrome c content. Protein concentration was determined by the bicinchoninic acid method, using bovine serum albumin as the standard. Samples were mixed with an equal volume of 5× electrophoresis sample buffer, and were then subjected to one-dimensional electrophoresis on a 12% sodium dodecyl sulfate polyacrylamide gel. The proteins were then transferred to nitrocellulose membranes and blocked before being incubated with the appropriate antibody. The Western blots were analyzed by the enhanced chemiluminescence reaction.
Annexin V and propidium iodide procedures
Cells were treated with either 0 mM (control) or 5 mM beta-alanine for 48 h in the presence and absence of 200 nM cyclosporin A (CsA) – an inhibitor of the mitochondrial permeability transition (MPT) pore. The number of apoptotic cells were assessed using the TACS Annexin V-FITC kit (Trevigen, USA), as described by Ricci et al (16). Annexin V and propidium iodide staining were observed by fluorescence microscopy using an Olympus IX70 (Olympus America Inc, USA) inverted microscope. Five separate fields under the microscope were examined for bright green (apoptotic) and red (necrotic) nuclei.
Statistical analysis
All results were reported as mean ± SEM. Statistical significance of the data was determined using Student’s t test for comparison within groups, and ANOVA combined with Tukey’s post hoc test for comparison between groups. P<0.05 was considered to be statistically significant.
RESULTS
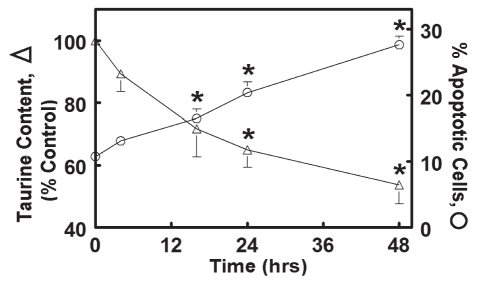
Incubation of isolated neonatal rat cardiomyocytes for 48 h with medium containing the taurine transport inhibitor, beta-alanine (5 mM), resulted in a 45% decline in cellular taurine levels. A time-dependent increase in the number of apoptotic cells was associated with the loss of taurine (Figure 1).
Figure 1).
Relationship between cellular taurine content and apoptosis. Rat neonatal cardiomyocytes were incubated for various time intervals, in a medium containing the taurine transport inhibitor beta-alanine (5 mM). At each time interval, some cells were harvested and examined for taurine content. The number of apoptotic cells was also examined in other cell preparations. Cells incubated for 48 h with the normal medium contained identical taurine levels to cells forgoing the 48 h incubation (the zero-point control). Values shown represent the mean ± SEM of four to five different cell preparations. *Significant difference between the control (zero time point) and the beta-alanine-treated group (P<0.05)
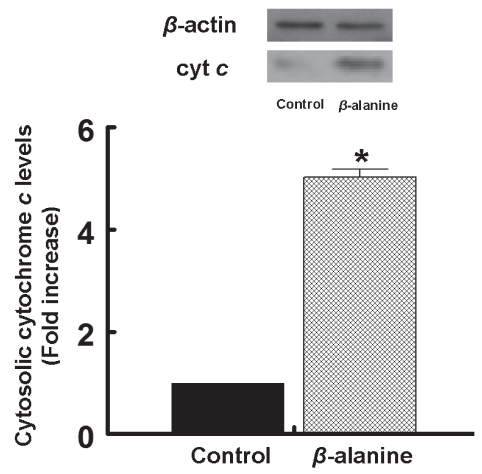
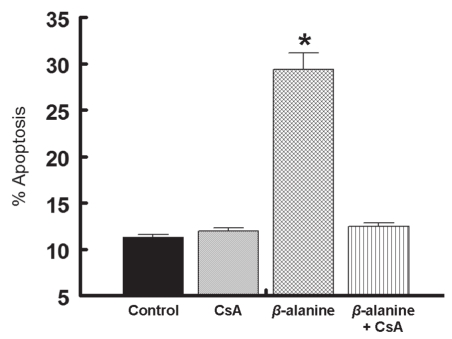
Several pathways are believed to initiate the apoptotic cascade including receptor signalling coupled to caspase activation (17), endoplasmic reticular disruption (18) and two types of mitochondrial permeability changes (both of which release proapoptotic factors, such as cytochrome c, from the mitochondria) (19,20). Thus, to determine whether mitochondrial permeabilization is involved in beta-alanine-mediated apoptosis, the release of cytochrome c from the mitochondria was examined. As shown in Figure 2, taurine deficiency was associated with a fivefold increase in the amount of cytochrome c found in the cytosol of beta-alanine-treated cardiomyocytes. To determine which type of mitochondrial permeability event contributed to beta-alanine-mediated apoptosis, the two types of mitochondrial permeabilization were distinguished on the basis of their mechanism of permeabilization. In one type of permeabilization, referred to as MPT, a pore forms that spans the two mitochondrial membranes (20). In another mechanism, the outer mitochondrial membrane is permeabilized by the oligomers Bax and Bak (19). To evaluate the importance of MPT in beta-alanine-mediated apoptosis, the effect of an MPT inhibitor, CsA, was examined. As shown in Figure 3, CsA completely prevented beta-alanine-mediated apoptosis, while having no effect on apoptosis in the control cells. Although cytochrome c also leaks from outer membrane permeabilized cells, Bax-mediated per-meabilization does not appear to contribute to beta-alanine-mediated apoptosis, because the levels of phosphorylated Bad increase despite no alteration in the Bcl-2/Bax ratio (21). Cumulatively, these data suggest that activation of the MPT is a major initiating event in beta-alanine-mediated apoptosis.
Figure 2).
Cytosolic levels of cytochrome c (cyt c) in taurine-deficient cells. Cardiomyocytes were incubated for 48 h in medium containing or lacking (control) 5 mM beta (β)-alanine. The cells were then harvested and the cytosolic fraction was isolated. Cyt c content of the cytosol was determined by Western blot analysis. Upper panel: A representative gel of cytosolic cyt c and beta-actin content of control and beta-alanine-treated cells. Lower panel: Average levels of cyt c in the cytosol of control and beta-alanine-treated cells. The cyt c data, which were normalized relative to beta-actin content, represent the mean ± SEM of three different cell preparations. *Significant difference between the control and beta-alanine-treated cells (P<0.05)
Figure 3).
Initiation of mitochondrial permeability transition in beta (β)-alanine-treated cells. Cardiomyocytes were incubated for 48 h with either no addition (control), 200 nM cyclosporin A (CsA), 5 mM β-alanine, or 200 nM CsA and 5 mM β-alanine (β-alanine plus CsA). The percentage of apoptotic cells was determined by the annexin V and propidium iodide method. Values shown represent the mean ± SEM of five different cell preparations. *Significant difference between the β-alanine-treated group and the other three groups (P<0.05)
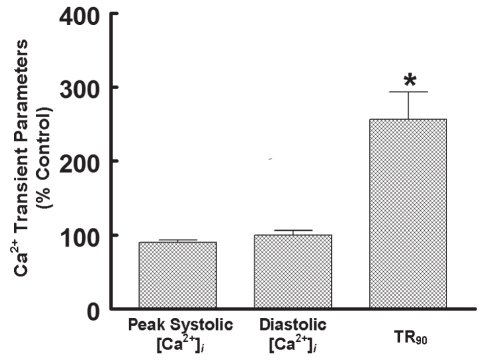
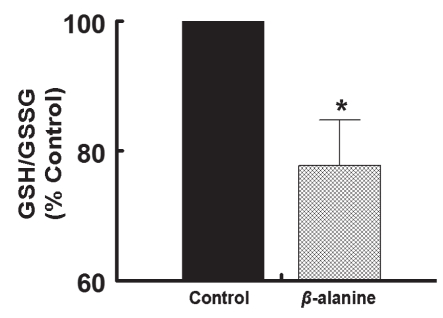
MPT is highly regulated, with elevated [Ca2+]i and oxidative stress being key initiators of the process (22–24). The Ca2+ binding sites that serve as triggers of MPT face the mitochondrial matrix. Calcium-mediated damage to the mitochondria is generally associated with the accumulation of large amounts of Ca2+ in both the mitochondria and the cytosol. Because [Ca2+]i fluctuations in the mitochondria and cytosol of the neonatal cardiomyocyte are broadly similar (24), insight into the effect of taurine deficiency on mitochondrial-free Ca2+ can be obtained by examining Ca2+ transients of taurine-deficient and taurine-replete cardiomyocytes. Figure 4 shows that the major effect of taurine depletion on the Ca2+ cycle is prolongation of the relaxation phase, as reflected by the 260% increase in the time required to achieve 90% relaxation. While [Ca2+]i remained elevated throughout much of the relaxation phase of the taurine-deficient cell, diastolic Ca2+ content was unchanged, while systolic Ca2+ content was actually decreased by 14% relative to the control cell. Because some cells underwent apoptosis despite minor changes in diastolic and systolic [Ca2+]i, Ca2+ overload is unlikely to represent the major cause of MPT in the taurine-deficient cell. Therefore, it was examined whether mitochondrial oxidative stress might predispose the mitochondria to MPT pore formation (23). As shown in Figure 5, taurine deficiency was associated with a 45% decrease in the GSH/GSSG state. Because the MPT pore forms at very low GSH/GSSG ratios (25), it is likely that oxidative stress is the major trigger for the onset of apoptosis in the taurine-deficient cardiomyocyte.
Figure 4).
Effect of taurine deficiency on Ca2+ transient. Cells were incubated for 48 h with medium containing or lacking 5 mM beta-alanine. The cells were then loaded with the fluorescent Ca2+ probe, Indo-1. Each Ca2+ transient parameter is expressed as a percentage of control. Values shown represent mean ± SEM of four to five different cell preparations. *Significant difference from the control value, which was fixed at 100%. [Ca2+]i Intracellular calcium concentration; TR90 Time required to achieve 90% relaxation
Figure 5).
Reduction in reduced glutathione (GSH) and oxidized glutathione (GSSG) ratio in taurine-deficient cells. Cardiomyocytes were incubated for 48 h in medium containing or lacking 5 mM beta (β)-alanine. After harvesting the cells, GSH and GSSG glutathione content was determined. The GSH/GSSG ratio was expressed as a percentage of control. Values shown represent the mean ± SEM of three to four cell preparations. *Significant difference between the control and β-alanine-treated cells (P<0.05)
DISCUSSION
Cardiomyopathy develops in cats fed a taurine-free diet (26,27) and in taurine-deficient rats lacking the taurine transporter (28). The mechanism underlying the development of cardiomyopathy is poorly understood. Based on results observed in the present study, it appears that apoptosis contributes to the development of myocardial dysfunction in taurine deficiency (29).
Apoptosis is also a common feature of the mitochondrial disease – MELAS (mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes) (30). In most MELAS cases, a mutation in the D-loop of tRNALeu(UUR) reduces the post-translational modification of a uridine moiety located in the wobble position of the tRNA. Because the post-translational modification of tRNALeu(UUR) stabilizes U-G pairing in the anticodon loop of tRNALeu(UUR), MELAS is associated with inefficient decoding of UUG, impaired synthesis of UUG-dependent mitochondria-encoded proteins and reduced respiratory chain activity (31). Although impaired ATP generation is a widely recognized consequence of reduced respiratory chain complex activity, oxidative stress also increases because electrons are diverted from the electron transport chain to the acceptor oxygen, forming a superoxide anion in the process. Thus, MELAS and taurine deficiency are both associated with enhanced superoxide generation. However, in the case of taurine deficiency, the availability of the substrate (taurine) for the post-translational modification of tRNALeu(UUR) to 5-taurinomethyluridine-tRNALeu(UUR) is reduced; while in MELAS, a mutation in tRNALeu(UUR) alters the structure of the tRNA, resulting in a defect in the post-translational modification of the wobble position uridine by taurine.
Mitochondria are highly sensitive to oxidative damage (16,25). Among the key targets of oxidants in the mitochondria is mitochondrial DNA (mtDNA). Although a DNA repair system is present in the mitochondria to restore mtDNA, the repair system can be overwhelmed, causing severe mtDNA oxidative damage that triggers mutations or impairs transcription of mitochondria-encoded proteins. Because reduced expression of mitochondria-encoded proteins can further reduce electron transport flux, a vicious cycle of increased oxidative stress and impaired mitochondrial protein synthesis can ensue. Invariably, this sequence of events leads to the initiation of the MPT and apoptosis (16). Also, the oxidation of key proteins promotes MPT and apoptosis in oxidatively stressed cells. Key proteins include adenine nucleotide translocase, which appears to regulate the formation of the MPT pore (32) and respiratory chain components that promote mitochondrial superoxide generation (33,34).
In the present study, the glutathione redox state served as a measure of oxidative stress in taurine-deficient cardiomyocytes. However, recent studies reveal that the glutathione redox ratio (GSH/GSSG) is not only a measure of oxidative stress, but also a determinant of oxidative stress (25). According to Aon et al (25), the GSH/GSSG ratio is directly coupled to both cellular membrane potential and the mitochondrial NADH/NAD ratio. At very low GSH/GSSG ratios, the cellular membrane potential collapses and MPT is initiated. In this regard, it is significant that overexpression of glutathione peroxide 1 in the mitochondria is cytoprotective (35). A key element of the cytoprotective activity of GSH and glutathione peroxidase is the scavenging of toxic oxidants. These data are consistent with the present finding that reductions in the GSH/GSSG ratio are associated with the onset of the MPT, the release of cytochrome c from the mitochondria and the onset of the apoptotic cascade.
REFERENCES
- 1.Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101–63. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- 2.Yokogoshi H, Mochizuki H, Nanami K, Hida Y, Miyachi F, Oda H. Dietary taurine enhances cholesterol degradation and reduces serum and liver cholesterol concentrations in rats fed a high-cholesterol diet. J Nutr. 1999;129:1705–12. doi: 10.1093/jn/129.9.1705. [DOI] [PubMed] [Google Scholar]
- 3.Schuller-Levis GB, Park E. Taurine: New implications for an old amino acid. FEMS Microbiol Lett. 2003;226:195–202. doi: 10.1016/S0378-1097(03)00611-6. [DOI] [PubMed] [Google Scholar]
- 4.Jong CJ, Azuma J, Schaffer S. Mechanism underlying the antioxidant activity of taurine: Prevention of mitochondrial oxidant production. Amino Acids. 2011. (In press) [DOI] [PubMed]
- 5.Suzuki T, Suzuki T, Wada T, Saigo K, Watanabe K. Taurine as a constituent of mitochondrial tRNAs: New insights into the functions of taurine and human mitochondrial diseases. Embo J. 2002;21:6581–9. doi: 10.1093/emboj/cdf656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurata S, Weixlbaumer A, Ohtsuki T, et al. Modified uridines with C5-methylene substituents at the first position of the tRNA anticodon stabilize U-G wobble pairing during decoding. J Biol Chem. 2008;283:18801–11. doi: 10.1074/jbc.M800233200. [DOI] [PubMed] [Google Scholar]
- 7.Schaffer SW, Azuma J, Mozaffari M. Role of antioxidant activity of taurine in diabetes. Can J Physiol Pharmacol. 2009;87:91–9. doi: 10.1139/Y08-110. [DOI] [PubMed] [Google Scholar]
- 8.Schaffer S, Azuma J, Takahashi K, Mozaffari M. Why is taurine cytoprotective? Adv Exp Med Biol. 2003;526:307–21. doi: 10.1007/978-1-4615-0077-3_39. [DOI] [PubMed] [Google Scholar]
- 9.Lang F, Madlung J, Siemen D, Ellory C, Lepple-Wienhues A, Gulbins E. The involvement of caspases in the CD95 (Fas/Apo-1)-but not swelling-induced cellular taurine release from Jurkat T-lymphocytes. Pflugers Arch. 2000;440:93–9. doi: 10.1007/s004240000247. [DOI] [PubMed] [Google Scholar]
- 10.Lang F, Busch GL, Ritter M, et al. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- 11.Kramer JH, Chovan JP, Schaffer SW. Effect of taurine on calcium paradox and ischemia heart failure. Am J Physiol. 1981;240:H238–H246. doi: 10.1152/ajpheart.1981.240.2.H238. [DOI] [PubMed] [Google Scholar]
- 12.Nahashima T, Seto Y, Nakajima T, et al. Calcium-associated cytoprotective effect of taurine on the calcium and oxygen paradoxes in isolated hepatocytes. Liver. 1990;10:167–72. doi: 10.1111/j.1600-0676.1990.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 13.Grishko V, Pastukh V, Solodushko V, Gillespie M, Azuma J, Schaffer SW. Apoptotic cascade initiated by angiotensin II in neonatal cardiomyocytes: Role of DNA damage. Am J Physiol. 2003;285:H2364–H2372. doi: 10.1152/ajpheart.00408.2003. [DOI] [PubMed] [Google Scholar]
- 14.Schaffer SW, Solodushko V, Kakhniashvili D. Beneficial effect of taurine depletion on osmotic sodium and calcium loading during chemical hypoxia. Am J Physiol. 2002;282:C1113–C1120. doi: 10.1152/ajpcell.00485.2001. [DOI] [PubMed] [Google Scholar]
- 15.Shaffer JE, Kocsis JJ. Methods of reducing tissue taurine levels. In: Schaffer SW, Baskin SI, Kocsis JJ, editors. The effects of taurine on excitable tissues. New York: Spectrum; 1981. pp. 219–29. [Google Scholar]
- 16.Ricci C, Pastukh V, Schaffer SW. Involvement of the mitochondrial permeability transition pore in angiotensin II-mediated apoptosis. Exp Clin Cardiol. 2005;10:160–4. [PMC free article] [PubMed] [Google Scholar]
- 17.Ashkenazi A, Dixit VM. Death receptors: Signaling and modulation. Science. 1998;281:1305–8. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa T, Zhu H, Horishima N, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 19.Hacker G, Weber A. BH3-only proteins trigger cytochrome c release, but how? Arch Biochem Biophys. 2007;462:150–5. doi: 10.1016/j.abb.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 20.Gogvadze V, Robertson JD, Zhivotovsky B, Orrenius S. Cytochrome c release occurs via Ca2+-dependent and Ca2+-independent mechanisms that are regulated by Bax. J Biol Chem. 2001;276:19066–71. doi: 10.1074/jbc.M100614200. [DOI] [PubMed] [Google Scholar]
- 21.Schaffer S, Solodushko V, Pastukh V, Ricci C, Azuma J. Possible cause of taurine deficient cardiomyopathy: Potentiation of angiotensin II action. J Cardiovasc Pharmacol. 2003;41:751–9. doi: 10.1097/00005344-200305000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol. 2009;46:821–31. doi: 10.1016/j.yjmcc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 23.Halestrap AP, Woodfield KY, Connern CP. Oxidative stress, thiol reagents and membrane potential modulate the mitochondrial permeability transition by affecting nucleotide binding to the adenine nucleotide translocase. J Biol Chem. 1997;272:3346–54. doi: 10.1074/jbc.272.6.3346. [DOI] [PubMed] [Google Scholar]
- 24.Griffiths EJ, Bell CJ, Balaska D, Rutter GA. Mitochondrial calcium: Role in the normal and ischaemic/reperfused myocardium. In: Schaffer SW, Suleiman MS, editors. Mitochondria: The dynamic organelle. New York: Springer Science and Business Media, LLC; 2007. pp. 197–220. [Google Scholar]
- 25.Aon MA, Cortassa S, Maack C, O’Rouke B. Sequential opening of mitochondrial ion channels as a function of glutathione redox thiol status. J Biol Chem. 2007;282:21889–900. doi: 10.1074/jbc.M702841200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Novotny MJ, Hogan PM, Flannigan G. Echocardiographic evidence for myocardial failure induced by taurine deficiency in domestic cats. Can J Vet Res. 1994;58:6–12. [PMC free article] [PubMed] [Google Scholar]
- 27.Pion PD, Kittleson MD, Rogers QR, Morris JG. Myocardial failure in cats associated with low plasma taurine: A reversible cardiomyopathy. Science. 1987;237:764–8. doi: 10.1126/science.3616607. [DOI] [PubMed] [Google Scholar]
- 28.Ito T, Kimura Y, Uozumi Y, et al. Taurine deletion caused by knocking out the taurine transporter gene leads to a cardiomyopathy with cardiac atrophy. J Mol Cell Cardiol. 2008;44:927–37. doi: 10.1016/j.yjmcc.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Kang PM, Izumo S. Apoptosis and heart failure. Circ Res. 2000;86:1107–13. doi: 10.1161/01.res.86.11.1107. [DOI] [PubMed] [Google Scholar]
- 30.van Eijsden RGE, Eijssen LMT, Lindsey PJ, et al. Termination of damaged protein repair defines the occurrence of symptoms in carriers of the m.3243A>G tRNALeu mutation. J Med Genet. 2008;45:525–34. doi: 10.1136/jmg.2008.057497. [DOI] [PubMed] [Google Scholar]
- 31.Yasukawa T, Suzuki T, Suzuki T, Ueda T, Ohta S, Watanabe K. Modification defect at anticodon wobble nucleotide of mitochondrial tRNAsLeu(UUR) with pathogenic mutations of mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes. J Biol Chem. 2000;275:4251–7. doi: 10.1074/jbc.275.6.4251. [DOI] [PubMed] [Google Scholar]
- 32.Halestrap AP, Brenner C. The adenine nucleotide translocase: A central component of the mitochondrial permeability transition pore and key player in cell death. Curr Med Chem. 2003;10:1507–25. doi: 10.2174/0929867033457278. [DOI] [PubMed] [Google Scholar]
- 33.Ide T, Tsutsui H, Kinugawa S, et al. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing heart. Circ Res. 1999;85:357–63. doi: 10.1161/01.res.85.4.357. [DOI] [PubMed] [Google Scholar]
- 34.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: Central role of complex III. J Biol Chem. 2003;278:36027–31. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 35.Thu VT, Kim HK, Ha SH, et al. Glutathione peroxidase I protects mitochondria against hypoxia/reoxygenation damage in mouse hearts. Pflugers Arch. 2010;460:55–68. doi: 10.1007/s00424-010-0811-7. [DOI] [PubMed] [Google Scholar]