Phosphodiesterase type-5 (PDE-5) inhibitors – sildenafil, vardenafil and tadalafil – have been successfully used to treat male erectile dysfunction. In 2006, sildenafil and tadalafil were among the 75 most popular prescription drugs in the United States. Recent studies suggest that these PDE-5 inhibitors could be used as novel drug therapies for cardiac hypertrophy, cardiomyopathy, heart failure, stroke, neurodegenerative diseases and other circulatory disorders. This reviews discusses these suggested applications of PDE-5 inhibitors in the areas mentioned above.
Keywords: Cardiovascular disease, Ischemia/reperfusion injury, Phosphodiesterase-5 inhibitors
Abstract
Phosphodiesterase type-5 (PDE-5) is an enzyme that catalyzes the hydrolytic degradation of cyclic GMP – an essential intracellular second messenger that modulates diverse biological processes in living cells. Three selective inhibitors of PDE-5 – sildenafil, vardenafil and tadalafil – have been successfully used by millions of men worldwide for the treatment of erectile dysfunction. Also, sildenafil and tadalafil are currently approved for the treatment of pulmonary hypertension. Recent powerful basic science data and clinical studies suggest potential nonurological applications of PDE-5 inhibitors, including ischemia/reperfusion injury, myocardial infarction, cardiac hypertrophy, cardiomyopathy, heart failure, stroke, neurodegenerative diseases and other circulatory disorders including Raynaud’s phenomenon. Future carefully controlled clinical trials would hopefully expedite their expanding therapeutic use in patients with cardiovascular disease.
Phosphodiesterase (PDE) is an enzyme that catalyzes the hydrolytic degradation of cyclic AMP (cAMP) and/or cyclic GMP (cGMP) – the two essential intracellular second messengers that modulate diverse biological processes in living cells (1,2). A total of 11 PDE families, along with several splice variants, have been identified (2–4). The distribution of the PDE isozymes varies across different tissues and cell types, and most likely the different subcellular compartments (2,5). Another important difference among the PDE isoforms is their substrate specificity for cAMP versus cGMP. Such a diversified tissue distribution and selectivity in hydrolyzing cAMP/cGMP may provide a molecular basis for PDE in participating in the complex functional processes in the body. PDE-5 was originally identified and purified from rat platelets (6) and lungs (7) in 1980. Subsequent studies showed that PDE-5 is widely present in vascular and bronchial smooth muscles, platelets, renal tubules and lung tissues. Recent studies from our laboratory and others have confirmed PDE-5A expression in isolated cardiomyocytes from mice (8) and dogs (9). PDE-5 messenger RNA was also detected in human heart samples (10).
For the majority of medical professionals, PDE-5 may be best known as the molecular target of three well-advertised PDE-5 inhibitors – sildenafil (Viagra, Pfizer Canada Inc), vardenafil (Levitra, Bayer Inc, Canada) and tadalafil (Cialis, Eli Lilly Canada Inc) – that have been prescribed by urologists worldwide to treat erectile dysfunction (ED). In fact, sildenafil and tadalafil were among the 75 most popular prescription drugs dispensed in the United States in 2006. Sildenafil citrate was developed by Pfizer Inc, and in March 1998, was the first PDE-5 inhibitor approved by the United States Food and Drug Administration (FDA) for the treatment of ED. Vardenafil, co-developed by Bayer and GlaxoSmithKline, has a duration of action that is similar to sildenafil, but has higher biochemical potency and selectivity (11). Clinical trials have shown that vardenafil has a high efficacy and low adverse event profile in a population with mixed ED etiologies (12). Tadalafil was co-developed by ICOS Corporation and Eli Lilly, and its long-acting inhibitory effect on PDE-5 may last for up to 36 h (13).
The mechanism of action for the three PDE-5 inhibitors involves increased tissue levels of cGMP, which causes smooth muscle relaxation and vasodilation. Because erection is largely a hemodynamic event, which is regulated by vascular tone and blood flow balance in the penis (14), and because cGMP modulates vascular tone, PDE-5 inhibitors could enhance erectile function by blocking the enzymatic hydrolysis of cGMP. The recent advancing basic and clinical studies suggest some very promising new applications of PDE-5 inhibitors, far beyond their urological scope, which are reviewed as follows.
ISCHEMIA/REPERFUSION INJURY AND MYOCARDIAL INFARCTION
Myocardial ischemia/reperfusion injury occurs in a wide spectrum of patients – ranging from survivors of out-of-hospital cardiac arrest to acute myocardial infarction (MI) victims and patients undergoing cardiac surgery – and represents a major public health burden. The infarct size needs to be limited because for patients with MI who do not die from out-of-hospital arrhythmias, the prognosis is dependent on the amount of heart muscle that is lost as a result of ischemia/reperfusion injury. Thus, there is a compelling need to protect the heart muscle in patients experiencing a heart attack. The first study showing a powerful preconditioning-like effect of sildenafil against myocardial ischemia/reperfusion injury in an in vivo rabbit model was published by our research group in 2002 (15). This discovery of the infarct-limiting effect of sildenafil has been duplicated in several models including mouse hearts (16–18), infant rabbit hearts (19) and rat hearts (20–22). A similar cardioprotective effect has been observed with vardenafil in rabbits (23) and tadalafil in rats (24), which reinforces the idea that PDE-5 inhibitors as a class had cardioprotective effects against ischemia/reperfusion injury. The anti-ischemic effects of sildenafil were also observed against ischemia/reperfusion-triggered ventricular arrhythmias (20,25) as well as improvement of postischemic ventricular contractile function (19–21). In addition to the preconditioning-like effects of these drugs, two recent studies showed the infarct-limiting effect of sildenafil or vardenafil when administered just before reperfusion (26,27). Likewise, tadalafil reduced infarct size when given 30 min to 120 min before coronary occlusion through protein kinase G (PKG)-dependent generation of H2S in the myocardium (28). The duration of cardioprotection from tadalafil remained until 36 h to 40 h after a single dose, and repeat administration at 36 h and 72 h extended protection until 108 h (29).
The protective effect of sildenafil against necrosis and apoptosis was also observed in isolated cardiomyocytes subjected to simulated ischemia/reoxygenation (8), suggesting that the vascular effects of this drug or participation of other cell types, eg, endothelial cells or fibroblasts, were not necessarily required to protect against cell death. Sildenafil combined with low-dose atorvastatin – another widely used drug for lowering cholesterol – has been shown to have a potent protective effect against MI in rats (22). These studies suggest that PDE-5 inhibitors, either alone or possibly in combination with statins, may be useful as adjunct therapy in patients undergoing coronary artery bypass grafting, coronary angioplasty or heart transplantation. Based on our animal data, we believe that timely administration of clinically relevant doses of ED drugs can significantly reduce heart muscle damage in patients after a major heart attack and improve chances of survival. In addition, there is a potential application of these studies in preventing multiple organ damage that occurs following cardiac arrest/resuscitation or shock. Clinically, there was an interesting observational cohort study using prescription-event monitoring in the United Kingdom, which showed no evidence for an increased risk of MI or ischemic heart disease in patients taking sildenafil during the first months of treatment. Also, the mortality in sildenafil takers was lower than in the general population (30).
The mechanism by which PDE-5 inhibitors exert cytoprotective effects following ischemia/reperfusion is not completely understood. We first hypothesized that the vasodilatory action of PDE-5 inhibitors, particularly in vivo, could release endogenous mediators of preconditioning such as adenosine and bradykinin from endothelial cells, which may trigger a signalling cascade with an activation of kinases resulting in phosphorylation of endothelial nitric oxide synthase (eNOS) (22) and synthesis of eNOS and inducible nitric oxide synthase (8,16,22). Nitric oxide (NO) generated from these enzymes may subsequently activate guanylate cyclase and enhance the formation of cGMP (20,21). cGMP could activate PKG, which can subsequently open mitochondrial (15,21,22,29) and sarcolemmal (21) ATP-sensitive K+ (KATP) channels, resulting in acute and delayed cardioprotective effects. The role of PKG in sildenafil-induced protection against necrosis and apoptosis was abolished by the PKG inhibitors, KT5823, guanosine 3′,5′-cyclic monophosphorothioate, 8-(4-chlorophenylthio)(Rp-8-pCPT-cGMPs) or DT-2 in cardiomyocytes. Moreover, the selective knockdown of PKG in cardiomyocytes with adenoviral vector containing short hairpin RNA of PKG also blocked sildenafil-induced protection. We further showed that sildenafil-induced phosphorylation of ERK1/2 and glycogen synthase kinase 3β, and sildenafil-enhanced Bcl-2-Bax ratio are blocked by KT5823 and short hairpin RNA of PKG (31). We, therefore, suggest that sildenafil (and, potentially, other PDE-5 inhibitors) may induce survival by NO synthase-dependent cGMP accumulation and subsequent activation of PKG that leads to phosphorylation of ERK and inactivation of glycogen synthase kinase 3β.
Mitochondria are known to play an essential role in cell survival by ATP synthesis and maintenance of Ca2+ homeostasis. Opening the mitochondrial KATP channel partially compensates the membrane potential, which enables additional protons to be pumped out to form an H+ electrochemical gradient for both ATP synthesis and Ca2+ transport. The mitochondrial stabilizing effect of sildenafil was further confirmed in our isolated cardiomyocyte study (8), which showed an increase in the Bcl-2/Bax ratio and preservation of mitochondrial membrane potential in the sildenafil-pretreated myocytes. Nevertheless, the protective mechanisms for PDE-5 inhibitors given only at reperfusion appear to be independent of eNOS, inducible NO synthase and cGMP (26), but certainly require opening of the mitochondrial KATP channels (27). Calcium-activated potassium channels are K+-selective, high-conductance channels that are critically dependent on intracellular Ca2+ flux and concentration. These channels are key mediators in cellular processes and are critical in maintaining Ca2+ homeostasis, mainly via their ability to sense transmembrane voltage and intracellular Ca2+ concentration (32). Recent studies have shown localization of these channels in the inner membrane of cardiomyocyte mitochondria (33). We recently showed that opening of mitochondrial calcium-activated potassium channels as well as mitochondrial KATP serve both as triggers and as mediators of sildenafil-elicited delayed cardioprotection (17).
Madhani et al (34) provided an interesting mechanism of protection: they showed that sildenafil-induced PKG-mediated phosphorylation of phospholemman at position 69 in cardiomyocytes during reperfusion is associated with the stimulation of the Na+/K+-ATPase. The limitation of Na+ and Ca2+ overload as a result of increased Na+/K+-ATPase could contribute to infarct limitation by attenuation of intracellular Na+ concentration accumulation during ischemia and/or reperfusion.
VASCULAR ENDOTHELIAL DYSFUNCTION
Endothelial dysfunction, defined as a reduction in the bioavailability of NO, is associated with many of the common risk factors for cardiovascular disease and, for instance, plays a major role in the development of atherosclerosis and acute coronary syndromes (35). Drugs, which are capable of improving endothelial function, usually provide benefits in reducing morbidity and mortality in patients suffering from ischemic heart disease. The effects of PDE-5 inhibitors on endothelial function have been extensively investigated in recent years. For example, in patients with chronic heart failure, acute administration of sildenafil increased endothelium-dependent, flow-mediated vasodilation in the brachial artery (36). It was also shown that sildenafil dilated the epicardial coronary arteries, improved endothelial dysfunction in the brachial artery, reduced exercise-induced ischemia and inhibited platelet activation in patients with coronary artery disease (37). Similarly, oral treatment with sildenafil in dogs caused vasodilation of coronary resistance vessels with an increase of blood flow into an ischemic myocardial region during exercise (38). A recent double-blinded, placebo-controlled crossover study of healthy male volunteers (39) showed that sildenafil significantly reduced the impairment of endothelium-dependent vasodilation caused by pneumatic cuff-induced radial artery ischemia and reperfusion. Interestingly, the vasoprotective effect of sildenafil was abrogated by previous administration of sulphonylurea glibenclamide, a KATP channel blocker, suggesting that opening of these channels played a crucial role, similar to protection against infarction in the intact heart. In contrast, a recent laboratory investigation (40) showed no change in coronary flow or myocardial oxygen consumption following sildenafil treatment in dogs with congestive heart failure at rest or during exercise. In addition, myocardial expression of PDE-5 protein was found to be downregulated in failing hearts, suggesting that this enzyme plays a minor role in the regulation of coronary hemodynamics in congestive heart failure (9,40).
PULMONARY HYPERTENSION
Pulmonary hypertension (PHT) is a rapidly progressive disease of the pulmonary vasculature, which subsequently leads to right heart failure. PHT is provoked by prolonged exposure to hypoxia, which leads to structural remodelling of pulmonary vessels, comprising increased thickness of the adventitial and medial layers, and muscularization of precapillary vessels (41). The combination of vasoconstriction and vascular remodelling, coupled with an increase in hematocrit triggered by hypoxia, results in PHT. Other illnesses, such as congenital heart disease, collagen vascular disease and HIV infection, are frequently associated with PHT. The lung is an organ with abundant PDE-5 expression (7). It has been shown that sildenafil attenuated the rise in pulmonary artery pressure and vascular remodelling when it was given before chronic exposure to hypoxia and during ongoing hypoxia-induced PHT in rats (42). Clinical investigations in patients with PHT also indicated that sildenafil therapy helps improve patients’ cardiac function and exercise capacity (43). Another study involving PHT patients showed that sildenafil significantly increased cardiac output and decreased pulmonary artery systolic pressure, mean pulmonary artery pressure, pulmonary vascular resistance and mean arterial pressure at peak measurements (44). In children with congenital heart disease, intravenous sildenafil was shown to be a pulmonary vasodilator that is as effective as inhaled NO (45). Sildenafil increases exercise capacity during severe hypoxia both at sea level and at high altitudes (46). In a randomized, double-blinded, placebo-controlled crossover study of 14 healthy mountaineers and trekkers, an oral dose of 50 mg sildenafil significantly increased arterial oxygen saturation, maximum workload and cardiac output, and reduced systolic pulmonary artery pressure during exercise under normobaric hypoxia (10% inspired O2). During a 14-day sojourn at high altitude (5400 m above sea level), sildenafil significantly reduced systolic pulmonary artery pressure at rest and during exercise, and also increased maximum workload and cardiac output (46).
Based on the overwhelmingly reproducible positive results, in June 2005, the FDA approved the use of sildenafil for the treatment of PHT patients with New York Heart Association class II to IV symptoms. The 20 mg pill of sildenafil, marketed as Revatio (Pfizer Canada Inc), is the only form approved by the FDA to treat PHT (taken orally three times a day). Tadalafil, which has a longer half-life, has also been recently licensed for the treatment of PHT (47). Vardenafil is currently undergoing phase III evaluation for the same indication.
CARDIAC HYPERTROPHY AND HEART FAILURE
It has been reported that blocking PDE-5 with sildenafil suppresses both chamber and myocyte hypertrophy, and improves in vivo heart function in mice exposed to chronic transverse aortic constriction (48). Sildenafil also reversed pre-established hypertrophy induced by pressure load while restoring chamber function. These authors showed that sildenafil deactivated multiple signalling pathways, including calcineurin/NFAT, PI3K/Akt and ERK1/2, which are triggered by the pressure load. Because ventricular hypertrophy and remodelling are a prelude to heart failure, the antihypertrophic and antinecrotic/apoptotic effects of sildenafil (8) could be translated into a rationale for the use of PDE-5 inhibitors for slowing the pathological progression of heart failure. In fact, chronic treatment with sildenafil immediately following MI also attenuated ischemic cardiomyopathy (49) as indicated by an improvement in cardiac function, an increased survival rate and a reduction in apoptosis in the border zone of the infarcted myocardium. More recently, we showed that sildenafil treatment beginning at three days post-MI also reduced the progression of heart failure, suggesting that PDE-5 inhibition is a promising therapeutic in patients with advanced heart failure. Interestingly, PKG activation with sildenafil was associated with the inhibition of Rho kinase (50), which is known to suppress left ventricular remodelling following MI in mice (51).
Clinically, an inhibitory effect of sildenafil on cardiac sympathetic activity was recently reported in 10 heart failure patients (52). A mean (± SEM) intravenous bolus dose of 2.4±0.2 mg and a mean maintenance dose of 0.7±0.1 mg of sildenafil (approximately 100 ng/mL plasma concentration) led to a 20% reduction in cardiac noradrenaline spillover – an index of cardiac sympathetic activity that has been considered as an independent predictor of mortality in heart failure patients (53). Sildenafil may also improve clinical outcomes in patients with existing heart failure. In 13 patients with New York Heart Association class III heart failure, oral administration of 50 mg sildenafil improved peak oxygen consumption (an indicator of exercise capacity) and right heart hemodynamics during exercise performed 60 min after the drug was administered (54). The observed benefits were evident only in heart failure patients with secondary PHT. It appears that a reduction of pulmonary artery pressure and right ventricle afterload caused by sildenafil could lead to an improvement in exercise capacity.
CARDIOMYOPATHY
Anticancer drugs (such as doxorubicin) may cause severe cardiotoxicity that ultimately leads to cardiomyopathy. The cardiotoxic effects of doxorubicin continue to be the major limitation in the current cancer chemotherapy. We demonstrated that in vivo treatment of mice with sildenafil before administration of doxorubicin conferred protective effects against doxorubicin-induced cardiotoxicity (55). Sildenafil pretreatment attenuated myocyte apoptosis, maintained mitochondrial membrane potential, preserved myofibrillar integrity, and alleviated left ventricular dysfunction and ST segment prolongation. Similarly, in the isolated cardiomyocytes, doxorubicin treatment caused a significant increase in apoptosis, caspase-3 activation and disruption of mitochondrial membrane potential, all of which were attenuated by sildenafil. In addition, the protective effects were abolished by either L-NAME (an inhibitor of NO synthase) or 5-HD (a blocker of mitochondrial KATP), indicating the participation of NO and mitochondrial KATP in mediating the protective effects of sildenafil against doxorubicin-induced cardiomyopathy (55). More recently, we showed that tadalafil also attenuated doxorubicin-induced cardiomyopathy in mice and prevented the depletion of prosurvival proteins including Bcl-2 and GATA-4 (56,57). In these studies, tadalafil given in combination with doxorubicin attenuated oxidative stress and improved antioxidant capacity via upregulation of mitochondrial superoxide dismutase. Interestingly, tadalafil did not interfere with the efficacy of DOX in killing human osteosarcoma cells in vitro or its antitumour effect in vivo in a tumour xenograft model.
STROKE AND NEURODEGENERATIVE DISEASES
PDE-5 inhibitors may also protect the brain against stroke and other neurodegenerative diseases. Oral treatment with sildenafil for seven consecutive days starting 2 h or 24 h after embolic middle cerebral artery occlusion significantly enhanced neurological recovery without any effect on infarct volume (58). The authors proposed that an increase in the cortical levels of cGMP after sildenafil treatment may have evoked neurogenesis and reduced neurological deficits. Sildenafil has also been shown to influence cerebral hemodynamics during acute exposure to high altitudes and after acclimatization (59). Cerebral oxygenation was significantly enhanced with sildenafil in subjects after rapid ascent to 3480 m as demonstrated by an improvement in several physiological parameters such as arterial oxygen saturation, regional cerebral oxygen saturation and middle cerebral artery velocity. Improvement in cerebral oxygenation with sildenafil was also observed in children with elevated pulmonary vascular resistance due to congenital heart defects after cardiac surgery (60). There was a significant increase in cerebral oxygenated hemoglobin and total hemoglobin, and a decrease in deoxygenated hemoglobin following sildenafil administration. These changes in hemoglobin elevated the cerebral tissue oxygenation index, probably due to general endothelial dysfunction after cardiopulmonary bypass. These findings may be clinically important because they indicate that sildenafil may increase cerebral blood flow through amelioration of general endothelial dysfunction after cardiopulmonary bypass surgery (60). Similar cerebral vascular-protective effects of sildenafil were recently reported in 11 patients suffering from severe PHT and 22 healthy volunteers (61). Both groups inhaled iloprost (a commonly used drug to treat PHT) and oral sildenafil, which led to a significant reduction in pulmonary arterial pressure and vascular resistance, accompanied by minor changes in systemic vascular resistance. The cerebral vascular tone and micro-vascular reactivity were significantly improved by sildenafil, but slightly worsened by iloprost. Taken together, these studies suggest a potential use of PDE-5 inhibitors to improve cerebral circulation, particularly under hypoxic conditions.
OTHER NONUROLOGICAL USES
Sildenafil may also offer a potential therapeutic strategy to improve uteroplacental blood flow in fetal growth restriction (FGR). FGR affects up to 8% of all pregnancies and has massive health implications including morbidity, mortality and increased incidence of cardiovascular disease in adulthood. Sildenafil treatment reduced vasoconstriction and improved relaxation of small vessels in women whose pregnancies were complicated by FGR (62). Patients suffering from liver cirrhosis may also benefit from sildenafil therapy in the future. Hyperammonemia (elevated ammonia levels in the blood) appears to contribute to mental deficits and other neurological abnormalities in cirrhosis patients. The underlying molecular mechanism appears to be related to the impaired function of the glutamate-NO-cGMP pathway in the brain. It has been shown that chronic oral administration of sildenafil normalized the glutamate-NO-cGMP pathway and extracellular cGMP in the brain of rats with portacaval anastomosis or hyperammonemia. Moreover, sildenafil restored the ability of rats with hyperammonemia or with portacaval shunts to learn a conditional discrimination task (63).
It has been shown that human coronary arteriolar endothelial cells exposed to sildenafil (1 μM to 20 μM) demonstrated significantly accelerated tubular morphogenesis with the induction of thioredoxin-1, hemeoxygenase-1 and vascular endothelial growth factor (VEGF). Sildenafil induced VEGF and angiopoietin-specific receptors such as KDR, Tie-1 and Tie-2 (64). These authors also showed that sildenafil protected the heart against ischemia/reperfusion injury by upregulating VEGF and the Ang-1 system (65). Other studies have shown that both sildenafil and vardenafil enhance ischemia-induced angiogenesis as measured by vascular perfusion, tissue blood flow and vascular density in a mouse model of unilateral hindlimb ischemia (66,67). Thus, PDE-5 inhibitors may have therapeutic potential in treating ischemic cardiovascular diseases such as peripheral artery disease and critical limb ischemia.
Because of the potent systemic vasodilatory effects of PDE-5 inhibitors, these drugs may be beneficial in patients with Raynaud’s phenomenon. It has been shown that vardenafil (10 mg by mouth, twice daily), when given to patients with Raynaud’s disease, improved digital blood flow in 70% of patients and improved clinical symptoms in 68% of patients (68). Sildenafil also reduced the frequency and duration of Raynaud attacks, and markedly increased capillary blood flow velocity with visible healing of chronic digital ulcerations in patients (69). However, similar benefits were not observed with tadalafil (70,71). Thus, sildenafil may be a promising agent for the treatment of Raynaud patients in the future.
FUTURE PERSPECTIVE
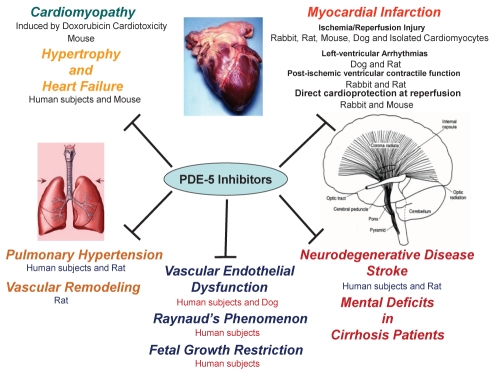
The studies reviewed above suggest that PDE-5 inhibitors have great promise for further development as novel drug therapies for MI, cardiac hypertrophy, cardiomyopathy, heart failure, stroke, neurodegenerative diseases and, potentially, other circulatory disorders (Figure 1).
Figure 1).
Illustrative summary of nonurological applications of phosphodiesterase (PDE)-5 inhibitors
In fact, there is an ongoing National Institutes of Health multicentre trial (RELAX: Evaluating the Effectiveness of Sildenafil at Improving Health Outcomes and Exercise Ability in People with Diastolic Heart Failure; NCT00763867) of patients with heart failure. In addition, PDE-5 inhibitors have been proposed to benefit preeclampsia patients (72) and patients undergoing cardiac surgery (73). Therefore, the time is ripe for a series of carefully designed clinical trials that, hopefully, could expedite the expansion of nonurological therapeutic applications of PDE-5 inhibitors.
Acknowledgments
This study was supported by grants from the National Institutes of Health (HL51045, HL79424 and HL93685) to Rakesh C Kukreja and a National Scientist Development Grant from the American Heart Association (10SDG3770011) to Fadi N Salloum. Saisudha Koka is supported by a postdoctoral fellowship from the American Heart Association (11POST7400028).
REFERENCES
- 1.Beavo JA. Cyclic nucleotide phosphodiesterases: Functional implications of multiple isoforms. Physiol Rev. 1995;75:725–48. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- 2.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: Molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- 3.Corbin JD, Francis SH, Webb DJ. Phosphodiesterase type 5 as a pharmacologic target in erectile dysfunction. Urology. 2002;60:4–11. doi: 10.1016/s0090-4295(02)01686-2. [DOI] [PubMed] [Google Scholar]
- 4.Corbin JD, Francis SH. Pharmacology of phosphodiesterase-5 inhibitors. Int J Clin Pract. 2002;56:453–9. [PubMed] [Google Scholar]
- 5.Wallis RM, Corbin JD, Francis SH, Ellis P. Tissue distribution of phosphodiesterase families and the effects of sildenafil on tissue cyclic nucleotides, platelet function, and the contractile responses of trabeculae carneae and aortic rings in vitro. Am J Cardiol. 1999;83:3C–12C. doi: 10.1016/s0002-9149(99)00042-9. [DOI] [PubMed] [Google Scholar]
- 6.Coquil JF, Franks DJ, Wells JN, Dupuis M, Hamet P. Characteristics of a new binding protein distinct from the kinase for guanosine 3′:5′-monophosphate in rat platelets. Biochim Biophys Acta. 1980;631:148–65. doi: 10.1016/0304-4165(80)90063-x. [DOI] [PubMed] [Google Scholar]
- 7.Francis SH, Lincoln TM, Corbin JD. Characterization of a novel cGMP binding protein from rat lung. J Biol Chem. 1980;255:620–6. [PubMed] [Google Scholar]
- 8.Das A, Xi L, Kukreja RC. Phosphodiesterase-5 inhibitor sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis. Essential role of nitric oxide signaling. J Biol Chem. 2005;280:12944–55. doi: 10.1074/jbc.M404706200. [DOI] [PubMed] [Google Scholar]
- 9.Senzaki H, Smith CJ, Juang GJ, et al. Cardiac phosphodiesterase 5 (cGMP-specific) modulates beta-adrenergic signaling in vivo and is down-regulated in heart failure. FASEB J. 2001;15:1718–26. doi: 10.1096/fj.00-0538com. [DOI] [PubMed] [Google Scholar]
- 10.Loughney K, Hill TR, Florio VA, et al. Isolation and characterization of cDNAs encoding PDE5A, a human cGMP-binding, cGMP-specific 3′,5′-cyclic nucleotide phosphodiesterase. Gene. 1998;216:139–47. doi: 10.1016/s0378-1119(98)00303-5. [DOI] [PubMed] [Google Scholar]
- 11.Saenz de Tejada I, Angulo J, Cuevas P, et al. The phosphodiesterase inhibitory selectivity and the in vitro and in vivo potency of the new PDE5 inhibitor vardenafil. Int J Impot Res. 2001;13:282–90. doi: 10.1038/sj.ijir.3900726. [DOI] [PubMed] [Google Scholar]
- 12.Porst H, Rosen R, Padma-Nathan H, et al. The efficacy and tolerability of vardenafil, a new, oral, selective phosphodiesterase type 5 inhibitor, in patients with erectile dysfunction: The first at-home clinical trial. Int J Impot Res. 2001;13:192–9. doi: 10.1038/sj.ijir.3900713. [DOI] [PubMed] [Google Scholar]
- 13.Eardley I, Cartledge J. Tadalafil (Cialis) for men with erectile dysfunction. Int J Clin Pract. 2002;56:300–4. [PubMed] [Google Scholar]
- 14.Rotella DP. Phosphodiesterase 5 inhibitors: Current status and potential applications. Nat Rev Drug Discov. 2002;1:674–82. doi: 10.1038/nrd893. [DOI] [PubMed] [Google Scholar]
- 15.Ockaili R, Salloum F, Hawkins J, Kukreja RC. Sildenafil (Viagra) induces powerful cardioprotective effect via opening of mitochondrial KATP channels in rabbits. Am J Physiol Heart Circ Physiol. 2002;283:H1263–9. doi: 10.1152/ajpheart.00324.2002. [DOI] [PubMed] [Google Scholar]
- 16.Salloum F, Yin C, Xi L, Kukreja RC. Sildenafil induces delayed preconditioning through inducible nitric oxide synthase-dependent pathway in mouse heart. Circ Res. 2003;92:595–7. doi: 10.1161/01.RES.0000066853.09821.98. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Fisher P, Xi L, Kukreja RC. Activation of mitochondrial calcium-activated and ATP-sensitive potassium channels is essential for sildenafil-induced cardioprotection. J Mol Cell Cardiol. 2008;44:105–13. doi: 10.1016/j.yjmcc.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Das A, Salloum FN, Xi L, Rao YJ, Kukreja RC. ERK phosphorylation mediates sildenafil-induced myocardial protection against ischemia-reperfusion injury in mice. Am J Physiol. 2009;296:H1236–43. doi: 10.1152/ajpheart.00100.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bremer YA, Salloum F, Ockaili R, Chou E, Moskowitz WB, Kukreja RC. Sildenafil citrate (Viagra) induces cardioprotective effects after ischemia/reperfusion injury in infant rabbits. Pediatr Res. 2005;57:22–7. doi: 10.1203/01.PDR.0000147736.27672.15. [DOI] [PubMed] [Google Scholar]
- 20.Das S, Maulik N, Das DK, Kadowitz PJ, Bivalacqua TJ. Cardioprotection with sildenafil, a selective inhibitor of cyclic 3′,5′-monophosphate-specific phosphodiesterase 5. Drugs Exp Clin Res. 2002;28:213–9. [PubMed] [Google Scholar]
- 21.du Toit EF, Rossouw E, Salie R, Opie LH, Lochner A. Effect of sildenafil on reperfusion function, infarct size, and cyclic nucleotide levels in the isolated rat heart model. Cardiovasc Drugs Ther. 2005;19:23–31. doi: 10.1007/s10557-005-6894-2. [DOI] [PubMed] [Google Scholar]
- 22.Rosanio S, Ye Y, Atar S, et al. Enhanced cardioprotection against ischemia-reperfusion injury with combining sildenafil with low-dose atorvastatin. Cardiovasc Drugs Ther. 2006;20:27–36. doi: 10.1007/s10557-005-5203-4. [DOI] [PubMed] [Google Scholar]
- 23.Salloum FN, Ockaili RA, Wittkamp M, Marwaha VR, Kukreja RC. Vardenafil: A novel type 5 phosphodiesterase inhibitor reduces myocardial infarct size following ischemia/reperfusion injury via opening of mitochondrial K(ATP) channels in rabbits. J Mol Cell Cardiol. 2006;40:405–11. doi: 10.1016/j.yjmcc.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Sesti C, Florio V, Johnson EG, Kloner RA. The phosphodiesterase-5 inhibitor tadalafil reduces myocardial infarct size. Int J Impot Res. 2007;19:55–61. doi: 10.1038/sj.ijir.3901497. [DOI] [PubMed] [Google Scholar]
- 25.Nagy O, Hajnal A, Parratt JR, Vegh A. Sildenafil (Viagra) reduces arrhythmia severity during ischaemia 24 h after oral administration in dogs. Br J Pharmacol. 2004;141:549–51. doi: 10.1038/sj.bjp.0705658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elrod JW, Greer JJ, Lefer DJ. Sildenafil-mediated acute cardioprotection is independent of the NO/cGMP pathway. Am J Physiol Heart Circ Physiol. 2007;292:H342–7. doi: 10.1152/ajpheart.00306.2006. [DOI] [PubMed] [Google Scholar]
- 27.Salloum FN, Takenoshita Y, Ockaili RA, et al. Sildenafil and vardenafil but not nitroglycerin limit myocardial infarction through opening of mitochondrial K(ATP) channels when administered at reperfusion following ischemia in rabbits. J Mol Cell Cardiol. 2007;42:453–8. doi: 10.1016/j.yjmcc.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salloum FN, Chau VQ, Hoke NN, et al. Phosphodiesterase-5 inhibitor, tadalafil, protects against myocardial ischemia/reperfusion through protein-kinase G dependent generation of hydrogen sulfide. Circulation (Suppl) 2009;120:S31–6. doi: 10.1161/CIRCULATIONAHA.108.843979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmad N, Wang Y, Ali AK, Ashraf M. Long-acting phosphodiesterase-5 inhibitor, tadalafil, induces sustained cardioprotection against lethal ischemic injury. Am J Physiol Heart Circ Physiol. 2009;297:H387–91. doi: 10.1152/ajpheart.00169.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shakir SA, Wilton LV, Boshier A, Layton D, Heeley E. Cardiovascular events in users of sildenafil: Results from first phase of prescription event monitoring in England. BMJ. 2001;322:651–2. doi: 10.1136/bmj.322.7287.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Das A, Xi L, Kukreja RC. Protein kinase G-dependent cardioprotective mechanism of phosphodiesterase-5 inhibition involves phosphorylation of ERK and GSK3beta. J Biol Chem. 2008;283:29572–85. doi: 10.1074/jbc.M801547200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shieh CC, Coghlan M, Sullivan JP, Gopalakrishnan M. Potassium channels: Molecular defects, diseases, and therapeutic opportunities, Pharmacol Rev. 2000;52:557–94. [PubMed] [Google Scholar]
- 33.Xu W, Liu Y, Wang S, et al. Cytoprotective role of Ca2+-activated K+ channels in the cardiac inner mitochondrial membrane. Science. 2002;298:1029–33. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- 34.Madhani M, Hall AR, Cuello F, et al. Phospholemman Ser69 phosphorylation contributes to sildenafil-induced cardioprotection against reperfusion injury. Am J Physiol Heart Circ Physiol. 2010;299:H827–36. doi: 10.1152/ajpheart.00129.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cayatte AJ, Palacino JJ, Horten K, Cohen RA. Chronic inhibition of nitric oxide production accelerates neointima formation and impairs endothelial function in hypercholesterolemic rabbits. Arterioscler Thromb. 1994;14:753–9. doi: 10.1161/01.atv.14.5.753. [DOI] [PubMed] [Google Scholar]
- 36.Katz SD, Balidemaj K, Homma S, Wu H, Wang J, Maybaum S. Acute type 5 phosphodiesterase inhibition with sildenafil enhances flow-mediated vasodilation in patients with chronic heart failure. J Am Coll Cardiol. 2000;36:845–51. doi: 10.1016/s0735-1097(00)00790-7. [DOI] [PubMed] [Google Scholar]
- 37.Halcox JP, Nour KR, Zalos G, et al. The effect of sildenafil on human vascular function, platelet activation, and myocardial ischemia. J Am Coll Cardiol. 2002;40:1232–40. doi: 10.1016/s0735-1097(02)02139-3. [DOI] [PubMed] [Google Scholar]
- 38.Traverse JH, Chen YJ, Du R, Bache RJ. Cyclic nucleotide phosphodiesterase type 5 activity limits blood flow to hypoperfused myocardium during exercise. Circulation. 2000;102:2997–3002. doi: 10.1161/01.cir.102.24.2997. [DOI] [PubMed] [Google Scholar]
- 39.Gori T, Sicuro S, Dragoni S, Donati G, Forconi S, Parker JD. Sildenafil prevents endothelial dysfunction induced by ischemia and reperfusion via opening of adenosine triphosphate-sensitive potassium channels: A human in vivo study. Circulation. 2005;111:742–6. doi: 10.1161/01.CIR.0000155252.23933.2D. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y, Traverse JH, Hou M, Li Y, Du R, Bache RJ. Effect of PDE5 inhibition on coronary hemodynamics in pacing-induced heart failure. Am J Physiol Heart Circ Physiol. 2003;284:H1513–20. doi: 10.1152/ajpheart.00529.2001. [DOI] [PubMed] [Google Scholar]
- 41.Hislop A, Reid L. New findings in pulmonary arteries of rats with hypoxia-induced pulmonary hypertension. Br J Exp Pathol. 1976;57:542–54. [PMC free article] [PubMed] [Google Scholar]
- 42.Sebkhi A, Strange JW, Phillips SC, Wharton J, Wilkins MR. Phosphodiesterase type 5 as a target for the treatment of hypoxia-induced pulmonary hypertension. Circulation. 2003;107:3230–5. doi: 10.1161/01.CIR.0000074226.20466.B1. [DOI] [PubMed] [Google Scholar]
- 43.Wilkins MR, Paul GA, Strange JW, et al. Sildenafil versus Endothelin Receptor Antagonist for Pulmonary Hypertension (SERAPH) study. Am J Respir Crit Care Med. 2005;171:1292–7. doi: 10.1164/rccm.200410-1411OC. [DOI] [PubMed] [Google Scholar]
- 44.Bhatia S, Frantz RP, Severson CJ, Durst LA, McGoon MD. Immediate and long-term hemodynamic and clinical effects of sildenafil in patients with pulmonary arterial hypertension receiving vasodilator therapy. Mayo Clin Proc. 2003;78:1207–13. doi: 10.4065/78.10.1207. [DOI] [PubMed] [Google Scholar]
- 45.Schulze-Neick I, Hartenstein P, Li J, et al. Intravenous sildenafil is a potent pulmonary vasodilator in children with congenital heart disease. Circulation. 2003;108:II167–73. doi: 10.1161/01.cir.0000087384.76615.60. [DOI] [PubMed] [Google Scholar]
- 46.Ghofrani HA, Reichenberger F, Kohstall MG, et al. Sildenafil increased exercise capacity during hypoxia at low altitudes and at Mount Everest base camp: A randomized, double-blind, placebo-controlled crossover trial. Ann Intern Med. 2004;141:169–77. doi: 10.7326/0003-4819-141-3-200408030-00005. [DOI] [PubMed] [Google Scholar]
- 47.Galiè N, Brundage BH, Ghofrani HA, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119:2894–903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 48.Takimoto E, Champion HC, Li M, et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214–22. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 49.Salloum FN, Abbate A, Das A, et al. Sildenafil (Viagra) attenuates ischemic cardiomyopathy and improves left ventricular function in mice. Am J Physiol Heart Circ Physiol. 2008;294:H1398–406. doi: 10.1152/ajpheart.91438.2007. [DOI] [PubMed] [Google Scholar]
- 50.Chau VQ, Salloum FN, Hoke NN, Abbate A, Kukreja RC. Mitigation of the progression of heart failure with sildenafil involves inhibition of RhoA/Rho-kinase pathway. Am J Physiol Heart Circ Physiol. 2011;300:H2272–9. doi: 10.1152/ajpheart.00654.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hattori T, Shimokawa H, Higashi M, et al. Long-term inhibition of Rho-kinase suppresses left ventricular remodeling after myocardial infarction in mice. Circulation. 2004;109:2234–9. doi: 10.1161/01.CIR.0000127939.16111.58. [DOI] [PubMed] [Google Scholar]
- 52.Al Hesayen A, Floras JS, Parker JD. The effects of intravenous sildenafil on hemodynamics and cardiac sympathetic activity in chronic human heart failure. Eur J Heart Fail. 2006;8:864–8. doi: 10.1016/j.ejheart.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 53.Kaye DM, Lefkovits J, Jennings GL, Bergin P, Broughton A, Esler MD. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol. 1995;26:1257–63. doi: 10.1016/0735-1097(95)00332-0. [DOI] [PubMed] [Google Scholar]
- 54.Lewis GD, Lachmann J, Camuso J, et al. Sildenafil improves exercise hemodynamics and oxygen uptake in patients with systolic heart failure. Circulation. 2007;115:59–66. doi: 10.1161/CIRCULATIONAHA.106.626226. [DOI] [PubMed] [Google Scholar]
- 55.Fisher PW, Salloum F, Das A, Hyder H, Kukreja RC. Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation. 2005;111:1601–10. doi: 10.1161/01.CIR.0000160359.49478.C2. [DOI] [PubMed] [Google Scholar]
- 56.Koka S, Das A, Zhu SG, Durrant D, Xi L, Kukreja RC. Long-acting phosphodiesterase-5 inhibitor tadalafil attenuates doxorubicin-induced cardiomyopathy without interfering with chemotherapeutic effect. J Pharmacol Exp Ther. 2010;334:1023–30. doi: 10.1124/jpet.110.170191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koka S, Kukreja RC. Attenuation of doxorubicin-induced cardiotoxicity by tadalafil: A long acting phosphodiesterase-5 inhibitor. Mol Cell Pharmacol. 2010;2:173–8. [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang R, Wang Y, Zhang L, et al. Sildenafil (Viagra) induces neurogenesis and promotes functional recovery after stroke in rats. Stroke. 2002;33:2675–80. doi: 10.1161/01.str.0000034399.95249.59. [DOI] [PubMed] [Google Scholar]
- 59.Chan CW, Hoar H, Pattinson K, Bradwell AR, Wright AD, Imray CH. Effect of sildenafil and acclimatization on cerebral oxygenation at altitude. Clin Sci (Lond) 2005;109:319–24. doi: 10.1042/CS20050036. [DOI] [PubMed] [Google Scholar]
- 60.Nagdyman N, Fleck T, Bitterling B, et al. Influence of intravenous sildenafil on cerebral oxygenation measured by near-infrared spectroscopy in infants after cardiac surgery. Pediatr Res. 2006;59:462–5. doi: 10.1203/01.pdr.0000198772.26417.66. [DOI] [PubMed] [Google Scholar]
- 61.Rosengarten B, Schermuly RT, Voswinckel R, et al. Sildenafil improves dynamic vascular function in the brain: Studies in patients with pulmonary hypertension. Cerebrovasc Dis. 2006;21:194–200. doi: 10.1159/000090555. [DOI] [PubMed] [Google Scholar]
- 62.Wareing M, Myers JE, O’Hara M, Baker PN. Sildenafil citrate (Viagra) enhances vasodilatation in fetal growth restriction. J Clin Endocrinol Metab. 2005;90:2550–5. doi: 10.1210/jc.2004-1831. [DOI] [PubMed] [Google Scholar]
- 63.Erceg S, Monfort P, Hernandez-Viadel M, Rodrigo R, Montoliu C, Felipo V. Oral administration of sildenafil restores learning ability in rats with hyperammonemia and with portacaval shunts. Hepatology. 2005;41:299–306. doi: 10.1002/hep.20565. [DOI] [PubMed] [Google Scholar]
- 64.Vidavalur R, Penumathsa SV, Zhan L, Thirunavukkarasu M, Maulik N. Sildenafil induces angiogenic response in human coronary arteriolar endothelial cells through the expression of thioredoxin, hemeoxygenase and vascular endothelial growth factor. Vascul Pharmacol. 2006;45:91–5. doi: 10.1016/j.vph.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 65.Koneru S, Varma Penumathsa S, Thirunavukkarasu M, et al. Sildenafil-mediated neovascularization and protection against myocardial ischaemia reperfusion injury in rats: Role of VEGF/angiopoietin-1. J Cell Mol Med. 2008;12:2651–64. doi: 10.1111/j.1582-4934.2008.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Senthilkumar A, Smith RD, Khitha J, et al. Sildenafil promotes ischemia-induced angiogenesis through a PKG-dependent pathway. Arterioscler Thromb Vasc Biol. 2007;27:1947–54. doi: 10.1161/ATVBAHA.107.147421. [DOI] [PubMed] [Google Scholar]
- 67.Sahara M, Sata M, Morita T, Nakajima T, Hirata Y, Nagai R. A phosphodiesterase-5 inhibitor vardenafil enhances angiogenesis through a protein kinase G-dependent hypoxia-inducible factor-1/vascular endothelial growth factor pathway. Arterioscler Thromb Vasc Biol. 2010;30:1315–24. doi: 10.1161/ATVBAHA.109.201327. [DOI] [PubMed] [Google Scholar]
- 68.Caglayan E, Huntgeburth M, Karasch T, et al. Phosphodiesterase type 5 inhibition is a novel therapeutic option in Raynaud disease. Arch Intern Med. 2006;166:231–3. doi: 10.1001/archinte.166.2.231. [DOI] [PubMed] [Google Scholar]
- 69.Fries R, Shariat K, von WH, Bohm M. Sildenafil in the treatment of Raynaud’s phenomenon resistant to vasodilatory therapy. Circulation. 2005;112:2980–5. doi: 10.1161/CIRCULATIONAHA.104.523324. [DOI] [PubMed] [Google Scholar]
- 70.Friedman EA, Harris PA, Wood AJ, Stein CM, Kurnik D. The effects of tadalafil on cold-induced vasoconstriction in patients with Raynaud’s phenomenon. Clin Pharmacol Ther. 2007;81:503–9. doi: 10.1038/sj.clpt.6100103. [DOI] [PubMed] [Google Scholar]
- 71.Schiopu E, Hsu VM, Impens AJ, et al. Randomized placebo-controlled crossover trial of tadalafil in Raynaud’s phenomenon secondary to systemic sclerosis. J Rheumatol. 2009;36:2264–8. doi: 10.3899/jrheum.090270. [DOI] [PubMed] [Google Scholar]
- 72.Downing JW, Ramasubramanian R, Johnson RF, et al. Hypothesis: Selective phosphodiesterase-5 inhibition improves outcome in preeclampsia. Med Hypotheses. 2004;63:1057–64. doi: 10.1016/j.mehy.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 73.Fung E, Fiscus RR, Yim AP, Angelini GD, Arifi AA. The potential use of type-5 phosphodiesterase inhibitors in coronary artery bypass graft surgery. Chest. 2005;128:3065–73. doi: 10.1378/chest.128.4.3065. [DOI] [PubMed] [Google Scholar]