Abnormal functioning of the cystathionine gamma-lyase (CSE)/hydrogen sulphide pathway has been found to lead to cardiovascular diseases such as hypertension. Hydrogen sulphide has been recognized as a regulator of vascular integrity; however, its role in smooth muscle cells during hypoxia has not been explored in a CSE deficiency model. This study sought to evaluate the response of smooth muscle cells from CSE-wildtype mice and CSE-knockout mice to hypoxic stress.
Keywords: Apoptosis, Cystathionine gamma-lyase, Hydrogen sulphide, Oxidative stress, Vascular smooth muscle cell
Abstract
BACKGROUND:
Hydrogen sulphide (H2S) has recently emerged as a novel and important gasotransmitter in the cardiovascular system, where it is generated mainly by cystathionine gamma-lyase (CSE). Abnormal metabolism and functions of the CSE/H2S pathway have been linked to various cardiovascular diseases including atherosclerosis and hypertension. An important role for H2S in regulating the balance between cellular growth and death has been demonstrated whereby inhibition of the endogenous CSE/H2S pathway results in greater apoptosis of vascular smooth muscle cells (SMCs). H2S is increasingly recognized as a critical regulator of vascular integrity, but its role in SMCs during hypoxia has not been explored in a model of CSE deficiency.
METHODS:
Cell viability, apoptosis, redox status and mitochondrial activity in hypoxia-exposed (12 h at 1% O2) SMCs derived from the mesenteric artery of CSE-knockout (CSE-KO) mice were analyzed. These were compared with those from CSE-wild-type (CSE-WT) mice.
RESULTS:
CSE-KO cells exhibited redox imbalance and aberrant mitochondrial activity versus CSE-WT cells, indicating an essential regulatory role for the endogenous CSE/H2S pathway on SMC function. CSE-KO cells were also more susceptible to hypoxia-induced cell death, indicating a critical contribution of endogenous CSE/H2S pathway to the protective hypoxia stress response.
CONCLUSION:
These findings support the concept that H2S is a crucial regulator of vascular homeostasis, the deficiency of which is associated with various pathologies, and provide further evidence that H2S is a potent vasculoprotectant.
Hydrogen sulphide (H2S) has recently emerged as a novel and important endogenous gaseous signalling transmitter, or gasotransmitter, in the cardiovascular system (1–4). Cystathionine γ-lyase (CSE), a pyridoxal-5′-phosphate-dependent enzyme that acts on L-cysteine in the trans-sulfuration pathway, is the principal H2S-generating enzyme in vascular tissues. Abnormal metabolism and functions of the CSE/H2S pathway have been linked to various cardiovascular diseases including atherosclerosis (5,6) and hypertension (2). Vascular CSE expression and H2S production were shown to be significantly decreased in hypertensive rats, wherein exogenous H2S was found to attenuate increased blood pressure and structural remodelling during the development of hypertension (7,8). CSE-deficient mice exhibited age-dependent hypertension and decreased endog enous H2S levels, and provided the first direct evidence that H2S is a physiological vasodilator and regulator of blood pressure (2).
Vascular remodelling contributes to increased peripheral resistance in hypertension, and involves smooth muscle cell (SMC) hypertrophy/hyperplasia (9,10), wherein dysregulation of SMC proliferation is a causative factor (11). More recently, investigators have demonstrated that overexpression of CSE resulted in increased endogenous H2S production that stimulated SMC apoptosis (12); exogenous H2S has been shown to produce a similar effect (13,14). We recently reported that SMCs isolated from CSE-deficient mice featured increased proliferation both in vitro and in vivo versus their CSE-wild-type (CSE-WT) counterparts (15), thereby elucidating a contributing mechanism to the observed hypertension (2). These studies indicate that decreased endogenous H2S due to suppression of CSE expression could be pathogenic in diverse cardiovascular diseases (16) – a concept that further investigation and characterization of the cellular effects of CSE deficiency promises to enlighten.
Elevated oxidative stress is a well-recognized factor in the development of many cardiovascular diseases including hypertension and atherosclerosis (17,18). H2S is, itself, known to have antioxidant effects in the neuronal (19,20) and cardiovascular systems (21–26). Indeed, exogenous H2S has been shown to reduce homocysteine-induced levels of hydrogen peroxide, peroxynitrite and superoxide (23), and to decrease superoxide via inhibition of NADPH oxidase in vascular SMCs (25). Furthermore, H2S delayed the accumulation of lipid per-oxidation products in hemin-mediated oxidation in human umbilical vein endothelial cells (24). Such antioxidant effects of H2S may prove significant for vascular remodelling and the development of atherosclerosis (5,26), particularly given the recent observations that a CSE/H2S pathway deficiency was associated with atherosclerotic progression, which was reversible via exogenous H2S (27).
Hypoxia is a hallmark of many cardiovascular pathologies including hypertension, atherosclerosis and myocardial infarction (28). Many studies have shown that H2S can mimic the cardioprotective effects of hypoxic pre- and postconditioning (29), and new interest in the relationship of H2S with O2 homeostasis was recently spurred by the demonstration that the consumption of exogenous H2S by mitochondria was O2 dependent (30). In the present study, we analyzed cell viability and apoptosis, redox status, and mitochondrial activity in hypoxia-exposed SMCs derived from the mesenteric artery of CSE-knockout (CSE-KO) mice. We then compared them with those from CSE-WT mice. Our findings suggest a significant role for the endogenous CSE/H2S pathway in the homeostatic regulation of SMC proliferation, redox balance and mitochondrial function, as well as in protecting SMCs against hypoxia-induced cell death.
METHODS
Cell culture and hypoxia
Single SMCs from the mesenteric artery of CSE-WT and CSE-KO mice were isolated and identifed as previously described (15). Cells were cultured in Dulbecco’s modifed Eagle’s medium containing 10% supplemented fetal bovine serum, 100 U/mL penicillin and 100 mg/mL streptomycin. Cell counts and viability were assessed via automated Trypan blue exclusion assay using a Vi-Cell XR Cell Viability Analyzer (Beckman Coulter, Canada). Doubling time analyses were completed as per convention, and cells were maintained at a passage density of 1:3 to 1:6. All experiments were completed when the cells reached 70% to 80% confluence with matched (ie, same passage number for both WT and KO) cultures between passages 6 and 12. A humidified hypoxia glove box chamber with automatic controller (Coy, USA) was used for the 12 h hypoxia exposure regimen, set at 37°C, 5% CO2 and 1% O2.
Assessment of cell proliferation/viability
Cell proliferation/viability was assessed via spectrophotometry using the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay. Cells were seeded onto sterile flat-bottom 96-well plates (Corning, USA) and incubated overnight to achieve the desired confluence. With 4 h of treatment time remaining, MTT reagent (Thiazolyl Blue Tetrazolium Bromide; Sigma-Aldrich, USA) was added to plate wells to achieve a final concentration of 10% (v/v), and cells were incubated at 37°C for an additional 4 h, during which time the MTT reagent was converted to purple formazan crystals in living cells according to their metabolic activity. Following this, the incubation medium was aspirated and 50 μL of dimethylsulfoxide per well was added to solubilize the formazan crystals. Following 10 min of agitation on a Belly Dancer shaker (Stovall Life Science, USA) at its highest setting, absorbance was measured spectrophotometrically at a wavelength of 490 nm (650 nm correction wavelength) using a PowerWave XS Microplate Spectrophotometer (BioTek, USA).
Apoptosis assay
Active caspase-3/7 activity was assessed via flow cytometry using the CaspaTag Caspase-3/7 In Situ Assay kit (Chemicon International, USA). Cells were seeded onto sterile flat-bottom 25 cm2 culture flasks (Corning) and grown overnight to achieve the desired confluence. Following treatment, cells were washed with buffer and suspended via trypsinization in phosphate buffered saline (PBS) to achieve 1×107 cells/mL. Freshly prepared fluorochrome inhibitors of caspases reagent (10 μL) was added to 290 μL of cell suspension, mixed gently and incubated at 37°C for 1 h in the absence of light (gently mixing twice during this incubation). Following several wash and count steps, samples were immediately analyzed using the FL1-H channel of a BD FACSCalibur Flow Cytometer (BD Biosciences, USA) supported by BD CellQuest Pro software. A minimum of 1×104 gated events were acquired per trial.
Oxidative stress assay
Intracellular reactive oxygen species (ROS) level was assessed via flow cytometry using the CM-H2DCFDA (5-[and-6]-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester) assay (Molecular Probes, USA). Cells were seeded onto sterile flat-bottom six-well plates (Corning) and grown overnight to achieve the desired confluence. Following treatment, cells were washed with PBS and stained for 30 min with CM-H2DCFDA under standard incubation conditions (including hypoxia samples). Stained cells were washed with PBS, detached from the plate surface using trypsin and suspended in PBS for analysis using the FL1-H channel of a BD FACSCalibur Flow Cytometer (BD Biosciences) supported by BD CellQuest Pro software. A minimum of 1×104 gated events were acquired per trial.
Antioxidant assay
Superoxide dismutase (SOD) activity was assessed via spectrophotometry using the Superoxide Dismutase Assay (Trevigen, USA). Cells were seeded into sterile 150 cm2 culture flasks (Corning) and grown overnight to achieve the desired confluence. Immediately following treatment, pelleted cell samples were lysed and their total protein quantitated via DC Protein Assay (Bio-Rad, USA). Samples were assayed as per the manufacturer’s instructions and the absorbance was read at 550 nm using a PharmaSpec UV-1700 Visible Spectrophotometer (Shimadzu, USA). Briefly, SOD activity was calculated by measuring absorbance at 330 s subtracted by absorbance at 30 s; these values were converted to units of SOD per volume by reference to an SOD inhibition curve that was generated in parallel.
Mitochondrial activity assay
Mitochondrial membrane potential was assessed via flow cytometry using the MitoProbe JC-1 Assay Kit for Flow Cytometry (Molecular Probes). Cells were seeded onto sterile flat-bottom six-well plates (Corning) and grown overnight to achieve the desired confluence. Following hypoxia, cells were washed with PBS and stained for 30 min with JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzimidazolylcarbocyanineiodide). Stained cells were then detached from the plate surface via trypsinization, then were suspended in PBS for analysis via flow cytometry using the FL1-H (monomer) and FL2-H (aggregate) channels of a BD FACSCalibur Flow Cytometer (BD Biosciences) supported by BD CellQuest Pro software. A minimum of 1×104 gated events were acquired per trial. The ratio of red fluorescence (potential-dependent generation of J-aggregates) to green fluorescence (nonconverted, monomeric form) was used as per the manufacturer’s instructions to describe the relative status of mitochondrial electric potential.
Statistical analysis
Data were presented as mean ± SEM, and all data presented represent n≥3 independent experiments. Statistical analyses were performed using GraphPad Prism software (GraphPad Software Inc, USA). Student’s t test was used throughout, with P<0.05 considered to be significant.
RESULTS
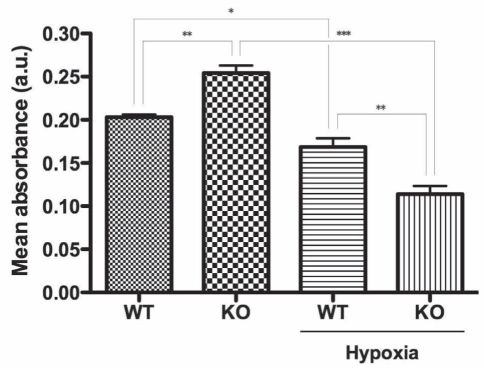
The present study evaluated the responses of SMCs from CSE-WT and CSE-KO mice to hypoxic stress. Cell proliferation/viability was assessed via the MTT assay (Figure 1) – a quantitative index of the activity of dehydrogenases, which in living cells, reduce the yellow tetrazolium salt to produce a purple formazan dye that can be measured spectrophotometrically. CSE-KO SMCs exhibited significantly greater proliferation/viability versus their CSE-WT counterparts as evidenced by increased MTT reduction (125.4±5.9%, P<0.01) (Figure 1). By contrast, hypoxia (12 h, 1% O2) caused significantly decreased MTT reduction in both cell lines versus their respective controls, but a much greater decrease was observed in the hypoxic CSE-KO (45.2±4.8%, P<0.001) than in the hypoxic CSE-WT cells (83.2±5.6%, P<0.05) (Figure 1).
Figure 1).
Cellular proliferation/viability assay (MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]). Colorimetric MTT assay indicating cystathionine γ-lyase (CSE)-wild-type (WT) and CSE-knockout (KO) smooth muscle cell proliferation at 12 h hypoxia. *P<0.05; **P<0.01; ***P<0.001. a.u. Arbitrary units
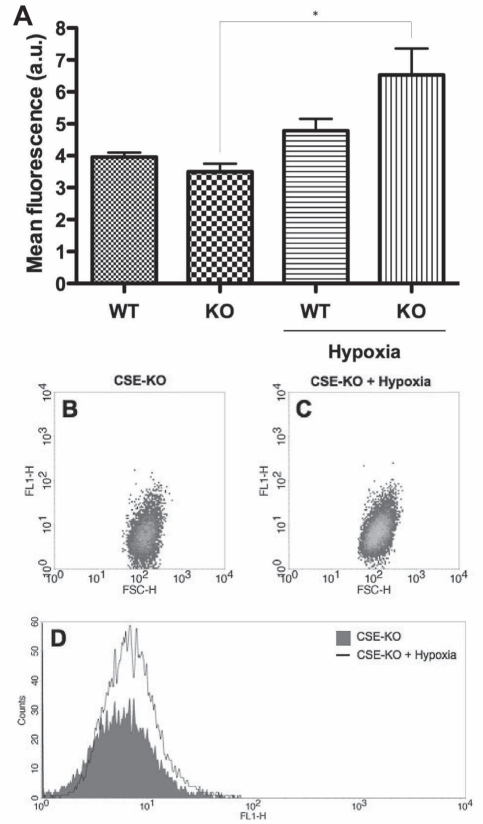
Apoptosis was measured via fluorescence flow cytometry using the CaspaTag Caspase-3/7 assay (Figure 2); this employs a cell-permeable, carboxyfluorescein-labelled fluoromethyl ketone peptide inhibitor that covalently binds to a reactive cysteine residue that resides on the large subunit of the active caspase heterodimer, thereby inhibiting further enzymatic activity and serving as a direct measure of the amount of active caspase-3/7 present in the cell. Consistent with the marked decrease in CSE-KO proliferation/viability on hypoxic insult, a significant increase in positive staining for active caspases-3/7 was evident in hypoxic CSE-KO cells versus control, but not in the CSE-WT cell line (Figure 2).
Figure 2).
Apoptosis assay (CaspaTag 3/7, Chemicon International, USA). A Fluorescence flow cytometry CaspaTag 3/7 assay indicating an abundance of active caspase-3 and caspase-7 in cystathionine γ-lyase (CSE)-wild-type (WT) and CSE-knockout (KO) smooth muscle cells at 12 h hypoxia. *P<0.05. a.u. Arbitrary units. B and C Representative dot plots indicating FL1 fluorescence versus forward scatter (FSC) in the cell populations of control and hypoxic KO samples. D Representative histogram indicating FL1 fluorescence versus cell counts of control and hypoxic KO samples. H Height
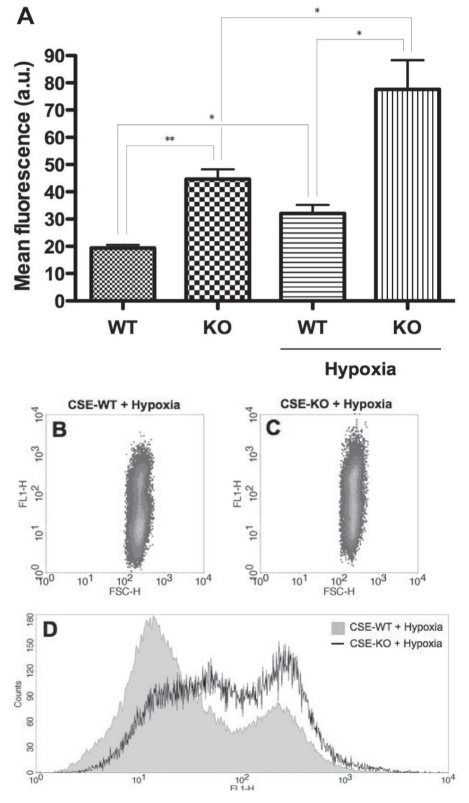
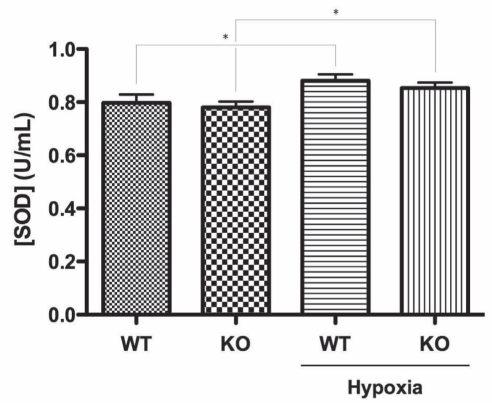
To explore whether oxidative stress was a mechanism underlying the observed susceptibility of CSE-WT SMCs to hypoxia, intracellular ROS level was assessed via fluorescence flow cytometry using the CM-H2DCFDA assay (Figure 3), wherein the namesake dye molecule remains nonfluorescent until the acetate groups are removed by intracellular esterases as oxidation occurs within the cell. It was found that even under basal conditions, CSE-KO SMCs featured greater ROS levels than CSE-WT cells (231.2±12.8%, P<0.01) (Figure 3). Furthermore, while hypoxia induced similar ROS increases in both the CSE-WT (165.4±8.5%, P<0.05) and CSE-KO cell lines (172.3±9.6%, P<0.05) versus their respective controls, hypoxic CSE-KO cells exhibited significantly greater absolute ROS levels versus hypoxic CSE-WT SMCs (243.6±28.1%, P<0.05) (Figure 3). In light of these findings, potential differences in antioxidant capacity were examined via spectrophotometric measurement of SOD activity (Figure 4). While hypoxia induced similar, significant increases in SOD activity in both CSE-WT (110.6±2.9%, P<0.05) and CSE-KO (109.4±2.9%, P<0.05) versus their respective controls, no inherent differences between the two cell lines were observed under basal or hypoxic conditions (Figure 4).
Figure 3).
Intracellular reactive oxygen species (ROS) assay (CM-H2DCFDA). A Fluorescence flow cytometry CM-H2DCFDA assay indicating intracellular ROS levels in cystathionine γ-lyase (CSE)-wild-type (WT) and CSE-knockout (KO) smooth muscle cells at 12 h hypoxia. *P<0.05; **P<0.01. a.u. Arbitrary units. B and C Representative dot plots indicating FL1 fluorescence versus forward scatter (FSC) in the cell populations of hypoxic WT and KO samples. D Representative histogram indicating FL1 fluorescence versus cell counts of hypoxic WT and KO samples. H Height
Figure 4).
Colorimetric superoxide dismutase (SOD) activity assay indicating SOD activity in cystathionine γ-lyase (CSE)-wild-type (WT) and CSE-knockout (KO) smooth muscle cells at 12 h hypoxia. *P<0.05
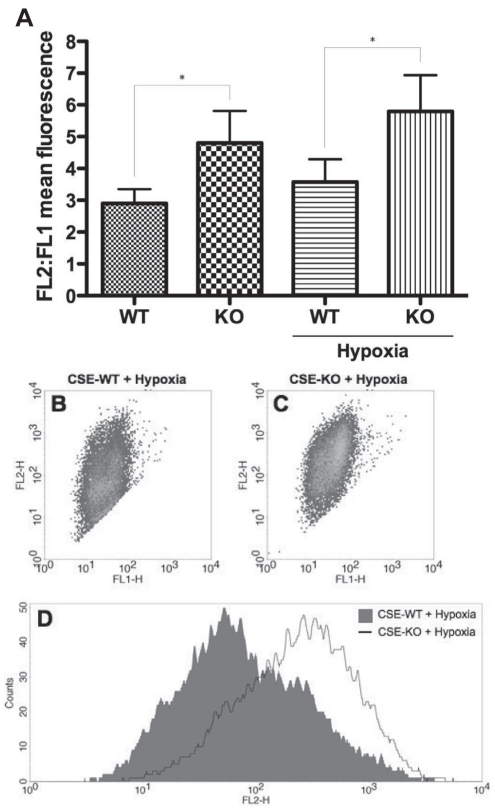
To investigate whether differences in mitochondrial activity contributed to the inherent redox imbalance and increased susceptibility of CSE-KO SMCs to hypoxia versus their CSE-WT counterparts, mitochondrial membrane potential was assessed via fluorescence flow cytometry using the JC-1 assay (Figure 5). The cationic dye JC-1 exhibits potential-dependent accumulation in mitochondria. CSE-KO SMCs featured a significantly increased FL2:FL1 mean fluorescence ratio versus CSE-WT cells under both basal (169.6±11.7%, P<0.05) and hypoxic (164.2±19.9%, P<0.05) conditions (Figure 5). However, no significant hypoxia-induced increases in mitochondrial activity were observed in either cell line (Figure 5).
Figure 5).
Mitochondrial membrane potential assay (JC-1, Molecular Probes, USA). A Fluorescence flow cytometry JC-1 assay indicating mitochondrial membrane potential in cystathionine γ-lyase (CSE)-wild-type (WT) and CSE-knockout (KO) smooth muscle cells at 12 h hypoxia. *P<0.05. B and C Representative dot plots indicating FL2 versus FL1 fluorescence in the cell populations of hypoxic WT and KO samples. D Representative histogram indicating FL2 fluorescence versus cell counts of hypoxic WT and KO samples. H Height
DISCUSSION
The arrival of gasotransmitter biology has greatly influenced our understanding of vascular physiology and pathophysiology, from the Nobel prize-winning discovery that nitric oxide is the endothelium-derived relaxing factor to the recent discovery that the newest gasotransmitter, H2S, may be an equally significant physiological vasorelaxant. Indeed, Yang et al (2) demonstrated that mice that were genetically deficient in CSE displayed marked hypertension comparable with that of endothelial nitric oxide synthase-deficient mice, and established CSE as the physiological source of H2S in multiple tissues including smooth muscle.
Using SMCs derived from the mesenteric artery of these CSE-WT and CSE-KO mice, we recently demonstrated that CSE-deficient SMCs lacked CSE messenger RNA and protein expression, and featured severely decreased H2S production (15). Given that abnormal CSE/H2S pathway had been demonstrated in vascular diseases including hypertension and atherosclerosis (2–8), we elected to compare the cellular responses of these CSE-WT and CSE-KO SMCs to hypoxia – a ubiquitous feature of cardiovascular disease and an important contributor to vascular remodelling in hypertension and atherosclerosis. Consistent with our previous finding of increased proliferation of CSE-deficient versus wild-type SMCs (15), CSE-KO cells exhibited significantly greater proliferation/viability under basal conditions versus their CSE-WT counterparts. Hypoxic insult caused significantly decreased proliferation/viability in both CSE-WT and CSE-KO cell lines, but a substantially larger decrease in the CSE-deficient SMCs. This hypoxia-induced change was also reflected as a significant increase in apoptosis of CSE-KO but not CSE-WT cells versus control. Taken together, these data indicate that the endogenous CSE/H2S pathway is essential for regulation of cell growth, and it plays a cytoprotective role against hypoxia-induced depression of metabolic activity and apoptosis.
Both exogenously applied H2S and inhibition of endogenous H2S production have been shown to regulate cell growth and death in various systems including the cardiovascular (12,13,31), immune (32,33), pancreatic (34,35) and respiratory systems (36). Yang et al (13) demonstrated that H2S induced apoptosis of human aorta SMCs through phosphorylation of ERK1/2 and its subsequent activation of caspase-3 – an effect that was greatly enhanced on inhibition of the endogenous CSE/H2S pathway. Similarly, overexpression of CSE resulted in increased endogenous H2S that stimulated apoptosis of SMCs with inhibited basal H2S levels (12). Moreover, we recently showed that CSE-deficient SMCs featured reduced phosphorylation of ERK1/2, were more susceptible to H2S-induced apoptosis, and exhibited greater proliferation than their CSE-WT counterparts (15). These CSE-deficient SMCs also showed decreased p21Cip/WAF-1 level and cyclin D1 expression (15), indicating H2S-mediated regulation of cell cycle progression. Recent findings point to an essential role for the endogenous CSE/H2S pathway in maintaining SMC phenotype (16). Thus, a phenomenon has been demonstrated whereby the endogenous H2S level is critical for the balance between apoptosis and cell proliferation in SMCs (26). Our present findings are consistent with this concept in that the lack of an endogenous CSE/H2S pathway rendered SMCs more susceptible to hypoxia-induced cell death. This susceptibility of CSE-deficient SMCs to hypoxia may be due to impairment of H2S-mediated stimulation of stress-responsive, mitogen-activated protein kinases and phosphorylation of specific transcriptional factors including hypoxia-inducible factor (HIF)-1α and specificity protein-1 that regulate cellular processes of proliferation, differentiation and apoptosis. Indeed, given the established cytoprotective role for H2S in models of ischemic pre- and postconditioning (29), and the recent finding that H2S induces HIF-1 nuclear localization and transcription of HIF-1 targets (37), it is plausible that our findings indicate a key role for the endogenous CSE/H2S pathway in the protective hypoxic stress response in SMCs.
Oxidative stress is an important contributing factor in a variety of pathophysiological conditions including hypertension, atherosclerosis, ischemia-reperfusion injury, hypertrophy and heart failure (17,18). Considering the known antioxidant effects of H2S in the vasculature (23–25), and given the ROS-inducing nature of hypoxic stress (38), we investigated whether differences in redox status underlie the observed susceptibility of CSE-deficient SMCs to hypoxia. Even under basal conditions, CSE-KO cells featured much higher intracellular ROS levels versus CSE-WT cells. Hypoxia caused significantly increased ROS elaboration in both cell lines, but the stark difference between CSE-WT and CSE-KO cells persisted, with hypoxic CSE-KO SMCs exhibiting much higher intracellular ROS levels than hypoxic CSE-WT cells. To explore whether concomitant changes in anti-oxidant capacity were also present, the activity of SOD was also examined. We found that hypoxia induced similar increases in SOD activity in both CSE-WT and CSE-KO cells versus their respective controls, but no differences between the cell lines were evident under basal or hypoxic conditions. Taken together, these data suggest that the endogenous CSE/H2S pathway plays a role in regulating redox status, and that an inherent redox imbalance in the CSE-deficient SMCs may have contributed to the hypoxia-induced death of CSE-KO cells. Indeed, given the large body of evidence of ROS-induced apoptosis in cardiovascular disease (17,39), our observation of an inherent redox imbalance in the CSE-deficient SMCs could plausibly underlie the hypoxia-induced depression of metabolic activity and increased apoptosis therein. Importantly, this is consistent with our recent demonstration that hydrogen peroxide decreased cell proliferation in both the CSE-WT and CSE-KO cell lines, but significantly more so in the CSE-deficient SMCs, suggesting that these cells were more sensitive to oxidative stress (15).
At the cellular level, hypoxia limits O2 availability for the electron transport chain, enhancing electron leak and causing mitochondria to accumulate oxidative damage, leading to dysfunction and disease (28,38). Increasing evidence points to an important regulatory role for H2S on mitochondrial function (19,21,40–42), impairment of which is associated with ROS-induced cell damage and death (21,38,40,41). To ascertain whether our findings could be traced to differences in mitochondrial activity, we measured mitochondrial membrane potential in the CSE-WT and CSE-KO SMCs. Remarkably, the CSE-deficient cells showed significantly greater mitochondrial activity than their CSE-WT counterparts under both basal and hypoxic conditions, but no significant hypoxia-induced changes were observed in either cell line. These data indicate aberrant mitochondrial activity in the CSE-deficient SMCs that paralleled the observed redox imbalance in those cells, suggesting a possible mitochondria-driven, ROS-enhancing mechanism at play. Commensurate with just such a scenario of increased cellular respiration and/or mitochondrial activity – and, thus, increased byproduction of ROS – both intracellular ROS levels and mitochondrial membrane potential were significantly higher in CSE-KO versus CSE-WT SMCs. Importantly, these data are consistent with the previously described protective roles for the CSE/H2S pathway in regulating redox balance (7,19,21,22,25) and mitochondrial function (19,21,40–42). Indeed, Elrod et al (41) demonstrated that H2S was cardioprotective against myocardial ischemia-reperfusion injury via stabilization of mitochondrial structure and function – a finding recently supported in a rat model of heart failure (40) and in an in vitro model of hypoxic rat cardiomyocytes (21). H2S was also shown to induce a suspended animation-like state in mice through its action on cytochrome c oxidase (43) that was protective against hypoxic damage (42). Moreover, Kimura et al (44) demonstrated that H2S protects neurons from oxidative stress by increasing both the production and redistribution of glutathione to mitochondria (19).
In summary, the present findings suggest a compromised ability of CSE-deficient vascular SMCs to regulate proliferation, redox levels and/or the byproduction of ROS that may result from deficient H2S-mediated mitochondrial regulation versus their CSE-WT counterparts. CSE-KO cells were more susceptible to hypoxia-induced death than CSE-WT cells, indicating an essential contribution of the endogenous CSE/H2S pathway to the protective hypoxia stress response. These findings support the concept that H2S is a critical regulator of vascular homeostasis, a deficiency of which is associated with pathologies including atherosclerosis and hypertension.
CONCLUSION
The results of our study indicate that vascular SMCs deficient in the H2S-generating enzyme, CSE, exhibit redox imbalance and aberrant mitochondrial activity versus their CSE-WT counterparts. CSE-deficient SMCs were also more susceptible to hypoxia-induced cell death, indicating an essential contribution of the endogenous CSE/H2S pathway to the protective hypoxia stress response. These findings support the concept that H2S is a crucial regulator of vascular homeostasis, a deficiency of which is associated with various pathologies.
Acknowledgments
This study was supported by the Northern Ontario School of Medicine (NK) and the Canadian Institutes of Health Research (RW). SB was supported by a studentship award from the Heart and Stroke Foundation of Ontario. GY was supported by a New Investigator Award from the Heart and Stroke Foundation of Canada.
REFERENCES
- 1.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20:6008–16. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang G, Wu L, Jiang B, et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–90. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang R. Two’s company, three’s a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–8. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 4.Wang R. The gasotransmitter role of hydrogen sulfide. Antioxid Redox Signal. 2003;5:493–501. doi: 10.1089/152308603768295249. [DOI] [PubMed] [Google Scholar]
- 5.Wang R. Is H2S a stinky remedy for atherosclerosis? Arterioscler Thromb Vasc Biol. 2009;29:156–7. doi: 10.1161/ATVBAHA.108.180190. [DOI] [PubMed] [Google Scholar]
- 6.Meng QH, Yang G, Yang W, et al. Protective effect of hydrogen sulfide on balloon injury-induced neointima hyperplasia in rat carotid arteries. Am J Pathol. 2007;170:1406–14. doi: 10.2353/ajpath.2007.060939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi YX, Chen Y, Zhu YZ, et al. Chronic sodium hydrosulfide treatment decreases medial thickening of intramyocardial coronary arterioles, interstitial fibrosis, and ROS production in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;293:H2093–100. doi: 10.1152/ajpheart.00088.2007. [DOI] [PubMed] [Google Scholar]
- 8.Lu M, Liu YH, Goh HS, et al. Hydrogen sulfide inhibits plasma renin activity. J Am Soc Nephrol. 2010;21:993–1002. doi: 10.1681/ASN.2009090949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feihl F, Liaudet L, Levy B, Waeber B. Hypertension and microvascular remodelling. Cardiovasc Res. 2008;78:274–85. doi: 10.1093/cvr/cvn022. [DOI] [PubMed] [Google Scholar]
- 10.Lee R, Owens G, Scott-Burden T. Pathophysiology of smooth muscle in hypertension. Can J Physiol Pharmacol. 1995;73:574–84. doi: 10.1139/y95-073. [DOI] [PubMed] [Google Scholar]
- 11.Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: Roles of apoptosis, inflammation, and fibrosis. Hypertens. 2001;38:581–7. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- 12.Yang G, Wu L, Wang R. Pro-apoptotic effect of endogenous H2S on human aorta smooth muscle cells. FASEB J. 2006;20:553–5. doi: 10.1096/fj.05-4712fje. [DOI] [PubMed] [Google Scholar]
- 13.Yang G, Sun X, Wang R. Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of mitogen-activated protein kinases and caspase-3. FASEB J. 2004;18:1782–4. doi: 10.1096/fj.04-2279fje. [DOI] [PubMed] [Google Scholar]
- 14.Li W, Jin HF, Liu D, et al. Hydrogen sulfide induces apoptosis of pulmonary artery smooth muscle cell in rats with pulmonary hypertension induced by high pulmonary blood flow. Chin Med J. 2009;122:3032–8. [PubMed] [Google Scholar]
- 15.Yang G, Wu L, Bryan S, Khaper N, Mani S, Wang R. Cystathionine gamma-lyase deficiency and overproliferation of smooth muscle cells. Cardiovasc Res. 2010;86:487–95. doi: 10.1093/cvr/cvp420. [DOI] [PubMed] [Google Scholar]
- 16.Yang G, Pei Y, Teng H, Cao Q, Wang R. Specificity protein-1 as a critical regulator of human cystathionine gamma-lyase in smooth muscle cells. J Biol Chem. 2011;286:26450–60. doi: 10.1074/jbc.M111.266643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khaper N, Bryan S, Dhingra S, et al. Targeting the vicious inflammation-oxidative stress cycle for the management of heart failure. Antioxid Redox Signal. 2010;13:1033–49. doi: 10.1089/ars.2009.2930. [DOI] [PubMed] [Google Scholar]
- 18.Fukai T, Ushio-Fukai M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15:1583–606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura Y, Goto YI, Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal. 2010;12:1–13. doi: 10.1089/ars.2008.2282. [DOI] [PubMed] [Google Scholar]
- 20.Whiteman M, Armstrong JS, Chu SH, et al. The novel neuromodulator hydrogen sulfide: An endogenous peroxynitrite ‘scavenger’? J Neurochem. 2004;90:765–8. doi: 10.1111/j.1471-4159.2004.02617.x. [DOI] [PubMed] [Google Scholar]
- 21.Yang Z, Yang C, Xiao L, et al. Novel insights into the role of HSP90 in cytoprotection of H2S against chemical hypoxia-induced injury in H9c2 cardiac myocytes. Int J Mol Med. 2011;28:397–403. doi: 10.3892/ijmm.2011.682. [DOI] [PubMed] [Google Scholar]
- 22.Mishra PK, Tyagi N, Sen U, Givvimani S, Tyagi S. H2S ameliorates oxidative and proteolytic stresses and protects the heart against adverse remodeling in chronic heart failure. Am J Physiol Heart Circ Physiol. 2010;298:H451–6. doi: 10.1152/ajpheart.00682.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan SK, Chang T, Wang H, Wu L, Wang R, Meng QH. Effects of hydrogen sulfide on homocysteine-induced oxidative stress in vascular smooth muscle cells. Biochem Biophys Res Commun. 2006;351:485–91. doi: 10.1016/j.bbrc.2006.10.058. [DOI] [PubMed] [Google Scholar]
- 24.Jeney V, Komódi E, Nagy E, et al. Supression of hemin-mediated oxidation of low-density lipoprotein and subsequent endothelial reactions by hydrogen sulfide (H(2)S) Free Radic Biol Med. 2009;46:616–23. doi: 10.1016/j.freeradbiomed.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muzaffar S, Shukla N, Bond M, et al. Exogenous hydrogen sulfide inhibits superoxide formation, NOX-1 expression and Rac1 activity in human vascular smooth muscle cells. J Vasc Res. 2008;45:521–8. doi: 10.1159/000129686. [DOI] [PubMed] [Google Scholar]
- 26.Wang R. Signaling pathways for the vascular effects of hydrogen sulfide. Curr Opin Nephrol Hypertens. 2011;20:107–12. doi: 10.1097/MNH.0b013e3283430651. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Zhao X, Jin H, et al. Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2009;29:173–9. doi: 10.1161/ATVBAHA.108.179333. [DOI] [PubMed] [Google Scholar]
- 28.Simon MC, Liu L, Barnhart BC, et al. Hypoxia-induced signaling in the cardiovascular system. Annu Rev Physiol. 2008;70:51–71. doi: 10.1146/annurev.physiol.70.113006.100526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lavu M, Bhushan S, Lefer DJ. Hydrogen sulfide-mediated cardioprotection: Mechanisms and therapeutic potential. Clin Sci. 2011;120:219–29. doi: 10.1042/CS20100462. [DOI] [PubMed] [Google Scholar]
- 30.Olson KR, Whitfield NL, Bearden SE, et al. Hypoxic pulmonary vasodilation: A paradigm shift with a hydrogen sulfide mechanism. Am J Physiol Regul Integr Comp Physiol. 2010;298:R51–60. doi: 10.1152/ajpregu.00576.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baskar R, Sparatore A, Del Soldato P, et al. Effect of S-diclofenac, a novel hydrogen sulfide releasing derivative inhibit rat vascular smooth muscle cell proliferation. Eur J Pharmacol. 2008;594:1–8. doi: 10.1016/j.ejphar.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 32.Mariggiò MA, Minunno V, Riccardi S, et al. Sulfide enhancement of PMN apoptosis. Immunopharmacol Immunotoxicol. 1998;20:399–408. doi: 10.3109/08923979809034822. [DOI] [PubMed] [Google Scholar]
- 33.Rinaldi L, Gobbi G, Pambianco M, Micheloni C, Mirandola P, Vitale M. Hydrogen sulfide prevents apoptosis of human PMN via inhibition of p38 and caspase 3. Lab Invest. 2006;86:391–7. doi: 10.1038/labinvest.3700391. [DOI] [PubMed] [Google Scholar]
- 34.Cao Y, Adhikari S, Ang AD, et al. Mechanism of induction of pancreatic acinar cell apoptosis by hydrogen sulfide. Am J Physiol Cell Physiol. 2006;291:C503–10. doi: 10.1152/ajpcell.00547.2005. [DOI] [PubMed] [Google Scholar]
- 35.Yang G, Yang W, Wu L, et al. H2S, endoplasmic reticulum stress, and apoptosis of insulin-secreting beta cells. J Biol Chem. 2007;282:16567–76. doi: 10.1074/jbc.M700605200. [DOI] [PubMed] [Google Scholar]
- 36.Baskar R, Li L, Moore PK. Hydrogen sulfide-induces DNA damage and changes in apoptotic gene expression in human lung fibroblast cells. FASEB J. 2007;21:247–55. doi: 10.1096/fj.06-6255com. [DOI] [PubMed] [Google Scholar]
- 37.Budde MW, Roth MB. Hydrogen sulfide increases hypoxia-inducible factor-1 activity independently of von Hippel-Lindau tumor suppressor-1 in C. elegans. Mol Biol Cell. 2010;21:212–7. doi: 10.1091/mbc.E09-03-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy E, Steenbergen C. Preconditioning: The mitochondrial connection. Annu Rev Physiol. 2007;69:51–67. doi: 10.1146/annurev.physiol.69.031905.163645. [DOI] [PubMed] [Google Scholar]
- 39.Di Lisa F, Kaludercic N, Carpi A, Menabo R, Giorgio M. Mitochondria and vascular pathology. Pharmacol Rep. 2009;61:123–30. doi: 10.1016/s1734-1140(09)70014-3. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Wang Q, Guo W, et al. Hydrogen sulfide attenuates cardiac dysfunction in a rat model of heart failure: A mechanism through cardiac mitochondrial protection. Biosci Rep. 2011;31:87–98. doi: 10.1042/BSR20100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elrod JW, Calvert JW, Morrison J, et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci. 2007;104:15560–5. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blackstone E, Roth MB. Suspended animation-like state protects mice from lethal hypoxia. Shock. 2007;27:370–2. doi: 10.1097/SHK.0b013e31802e27a0. [DOI] [PubMed] [Google Scholar]
- 43.Blackstone E, Morrison M, Roth MB. H2S induces a suspended animation-like state in mice. Science. 2005;308:518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- 44.Kimura Y, Dargusch R, Schubert D, et al. Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid Redox Signal. 2006;8:661–70. doi: 10.1089/ars.2006.8.661. [DOI] [PubMed] [Google Scholar]