Abstract
Autonomic reactivity was studied in individuals with fragile X syndrome (FXS), a genetic disorder partially characterized by abnormal social behavior. Relative to age-matched controls, the FXS group had faster baseline heart rate and lower amplitude respiratory sinus arrhythmia (RSA). In contrast to the typically developing controls, there was a decrease in RSA with age within the FXS group. Moreover, within the FXS group heart rate did not slow with age. The FXS group also responded with an atypical increase in RSA to the social challenge, while the control group reduced RSA. In a subset of the FXS group, the autonomic profile did not change following 2 months and 1 year of lithium treatment. The observed indices of atypical autonomic regulation, consistent with the Polyvagal Theory, may contribute to the deficits in social behavior and social communication observed in FXS.
Keywords: fragile X syndrome, respiratory sinus arrhythmia, heart rate, heart rate variability, social challenge, Polyvagal Theory
Fragile X syndrome (FXS) is the most common inherited form of intellectual disability. The syndrome is caused by an unstable trinucleotide repeat expansion mutation of >200 CGG repeats in the promoter of the FMR1 (Fragile X Mental Retardation-1) gene (Verkerk, Pieretti, Sutcliffe, Fu, Kuhl, Pizzuti, Reiner, Richards, Victoria, Zhang, Eussen, van Ommen, Blonden, Riggins, Chastain, Kunst, Galjaard, Caskey, Nelson, Oostra, & Warren, 1991) on the long arm of the X chromosome. The expansion results in hypermethylation of the FMR1 promoter, transcriptional inactivation of the gene and failure to produce the gene product, fragile X mental retardation protein (FMRP). The prevalence rate is approximately 1/4000 males and females (Turner, Webb, Wake, & Robinson, 1996).
While the cognitive and social deficits of individuals with FXS are well documented (Lesniak-Karpiak, Mazzocco, & Ross, 2003; Cohen, Fisch, Sudhalter, Wolf-Schein, Hanson, Hagerman, Jenkins, & Brown, 1988; Simon & Finucane, 1996; Turk & Cornish, 1998; Wishart, Cebula, Willis, & Pitcairn, 2007), less is known about physiological state regulation and reactivity. Since physiological state regulation is a major modulator of emergent social, emotional, cognitive, and motor behaviors, an understanding of autonomic state regulation may provide useful insights into the behavioral repertoire of the individual with FXS and provide a justification and/or index of specific interventions including pharmacological treatments that may impact on physiological regulation.
Relationship between physiological state and emergent behavior
The Polyvagal Theory (Porges, 1995, 2001, 2003, 2007) provides a biobehavioral framework to link the atypical social behavior observed in FXS with the neural regulation of the autonomic nervous system. The theory proposes that specific mechanisms in neural regulation of the autonomic nervous system are neuroanatomically and neurophysiologically linked to the regulation of the striated muscles of the face and head involved in social engagement behaviors. Consistent with the theory, the atypical behavioral state regulation and social behavior frequently observed in individuals with FXS may be paralleled by an atypical neural regulation of the autonomic nervous system.
The Polyvagal Theory describes an integrated Social Engagement System (SES) regulating both the striated muscles of the face and head (i.e., via the special visceral efferent pathways of CN V, VII, VIII, X, XI) and the heart and bronchi (i.e., via the myelinated vagal efferent pathways originating in the nucleus ambiguus). Thus, the neural mechanisms regulating behavioral state and fostering social engagement behaviors (i.e., the calm physiological states mediated by the vagus) are neuroanatomically and neurophysiologically linked to the nerves regulating the muscles necessary for generating facial expressions (i.e., facial muscles), listening to human voice (i.e., middle ear muscles), gesturing (i.e., muscles of the neck and head for nodding), ingestion, and vocalization (i.e., laryngeal and pharyngeal muscles). When both the visceral (i.e., vagal) and somatomotor (muscles of face and head) components of the SES are functioning properly, the person is able to regulate behavioral state and to engage in appropriate spontaneous social behavior. Since the functioning of the SES is dependent on underlying neurophysiological systems and neuroanatomical structures, individuals with FXS, who typically present with state regulation difficulties and limited social engagement behaviors, may have deficiencies in the neural regulation of the Social Engagement System.
Measurement of respiratory sinus arrhythmia (RSA), a periodic heart rate pattern at the frequencies of spontaneous breathing, provides an opportunity to monitor the dynamic impact of myelinated vagal pathways to the heart (e.g. the visceromotor component of the SES). In general, independent of age, higher amplitude RSA is typically associated with a slower heart rate, since myelinated vagal efferent pathways are inhibitory on the sino-atrial node.
Low amplitude RSA has been associated with several psychiatric and medical disorders (e.g., Rottenberg, Clift, Bolden, & Salomon, 2007; Brosschot, Van Dijk, & Thayer, 2007; Masi, Hawkley, Rickett, & Cacioppo, 2007). Studies have reported that individuals with difficulty in social behavior and/or behavioral state regulation have lower RSA and difficulties in regulating RSA during social and cognitive challenges (Dale, O’Hara, Keen, & Porges, 2010; Calkins, 1997; Porges, Doussard-Roosevelt, Portales, & Greenspan, 1996; Blair & Peters, 2003). Thus, a depressed influence of myelinated vagal efferent pathways on the heart provides a hypothetical mechanism to explain the high heart rates and lower thresholds of autonomic and behavioral reactivity observed in these clinical populations. Based on these findings with other clinical populations, we hypothesize that individuals with FXS, who also express difficulty with social behavior, might also have atypical regulation of the underlying biobehavioral social engagement system.
Although the literature during early development identifies an increase in RSA with age (Porges et al., 1994; Stifter & Jain, 1996; Suess et al., 1994; Alkon et al., 2003; Marshall & Stevenson-Hinde, 1998), maturational influences on the autonomic nervous system appear to be an under explored area in studies of FXS. Unlike studies of development in a typically developing population, due to difficulties in recruiting large samples within a specified age range, studies of FXS often recruit participants across a broad age range. Although control groups in FXS studies frequently consist of typically developing children that have been carefully age matched, the reported diagnostic group differences might be confounded by age differences.
Physiological functioning in individuals with FXS
Although the literature evaluating physiological reactivity in individuals with FXS is sparse, the available literature suggests atypical physiological regulation as indexed by cardiac activity, skin conductance and/or cortisol levels.
Cardiac activity
The few studies examining baseline cardiac activity in FXS provide inconclusive findings. However, a closer investigation of these studies identifies that the studies differ along a variety of dimensions including age range, sex, and testing context. Keysor, Mazzocco, McLeod and Hoehn-Saric (2002) reported no differences between FXS and control females (13–22 years of age) on baseline heart rate or RSA. However, Roberts, Boccia, Bailey, Hatton, and Skinner (2001) reported significantly faster heart rate and lower amplitude RSA in FXS males (1–11 years of age) during a baseline task while watching a video. Moreover, in a recent study, Hall, Lightbody, Huffman, Lazzeroni and Reiss (2009) reported that males with FXS (8–20 years of age) had higher heart rate, lower amplitude RSA and lower heart rate variability during both a baseline and social interaction, relative to their typically developing siblings. In the Hall et al. study, females with FXS (5–19 years of age) had lower amplitude vagal tone and lower heart rate variability, but comparable heart rate to typically developing siblings. Roberts et al. (2001) reported that heart rate and RSA were not responsive in the FXS group to the experimental protocol (i.e., alternating 5-minute segments of videos and IQ testing). In contrast, the age-matched control group responded to the protocol with increases in heart rate and decreases in RSA amplitude during cognitive tasks. Roberts, Mazzocco, Murphy and Haehn-Saric (2008) reported that heart rate and RSA reactivity during a cognitive task did not differ between age-matched females with and without FXS. However, only within the FXS group (i.e., females between the ages of 13 and 22 years) was there greater heart rate reactivity with better performance on a cognitive task (i.e., mental arithmetic).
Skin conductance
Keysor and colleagues (2002) reported that females with FXS have a higher mean skin conductance range than control subjects. Using the same database, Roberts and others reported that the FXS group had smaller skin conductance reactions during a mental arithmetic task (Roberts et al., 2008). Miller, McIntosh, McGrath, Shyu, Lampe, Taylor, Tassone, Neitzel, Stackhouse, & Hagerman (1999) reported that FXS males, relative to typically developing males, reacted with larger electrodermal responses during a sensory stimulation paradigm when contrasted with a typically developing control group. However, Hagerman, Miller, McGrath-Clarke, Riley, Goldson, Harris, Simon, Church, Bonnell, Ognibene, and McIntosh (2002) reported that a developmental delay group (including both males and females) responded similarly to the FXS group (including both males and females). When the FXS group was given stimulant medication, excess electrodermal reactivity decreased.
Salivary cortisol
Wisbeck, Huffman, Freund, Gunnar, Davis, and Reiss (2000) reported that male and female children with FXS, when contrasted to typically developing children, had higher cortisol levels at lunchtime and bedtime. FXS males, in particular, had higher cortisol levels 30 minutes post social stressor (Wisbeck et al., 2000). Hessl, Glaser, Dyer-Friedman, Blasey, Hastie, Gunnar, and Reiss (2002) reported that children with FXS, especially males, had higher levels of salivary cortisol. Within the FXS group there was a relationship between higher cortisol levels and more withdrawn behavior. Although Hessl, Glaser, Dyer-Friedman, and Reiss (2006) reported no group differences in cortisol reactivity, their data demonstrated that for the FXS group, increases in gaze aversion were associated with decreases in cortisol and increases in eye contact were associated with increases in cortisol. In contrast, for the control group, increases in gaze aversion were associated with increases in cortisol. Inconsistent with the above, Hall, DeBernardis, and Reiss (2006) reported that for males with FXS, higher levels of cortisol were related to decreased levels of eye contact.
Summary
The findings of studies investigating autonomic state regulation in FXS are inconsistent, although there appears to be a tendency for vagal regulation of the heart to be reduced (i.e., increased heart rate and reduced RSA and HRV) and a complimentary increase in sympathetic-adrenal reactivity (e.g., increases in electrodermal activity and cortisol level). Inspection of these studies identifies several factors including age range, sex, and context that preclude a definitive interpretation.
The Polyvagal Theory would lead to specific predictions regarding FXS and social behavior. The theory predicts that individuals who have difficulties in social behavior would have decreased vagal regulation of the heart (i.e., lower RSA) and increased sympathetic-adrenal activity (i.e., increased skin conductance and increased cortisol). The inconsistency of findings regarding autonomic regulation in the FXS literature may be, in part, due the broad age range and sex of the participants. For example, the physiological measures, especially measures of vagal regulation manifested in beat-to-beat heart rate, are age-dependent. Thus, it is possible that inconsistency across studies might be due to either the differences in age range between the studies or the use of broad age ranges within the studies. In addition, since the functional effects of FXS have a greater detrimental effect on males, males may express greater atypical autonomic regulation.
Lithium as treatment
Lithium has been proposed as a treatment to improve the physiological, cognitive and behavioral deficits demonstrated by individuals with FXS (Berry-Kravis, Sumis, Hervey, Nelson, Porges, Weng, Weiler, & Greenough, 2008). Lithium acts as a negative modulator of metabotropic glutamate receptor 5 (mGluR5)-mediated protein synthesis, which has been shown to be enhanced in the FXS knockout (KO) mouse (Huber, Gallagher, Warren, & Bear, 2002; Bear, Huber, Warren, 2004). A recent paper, reporting on the cohort of FXS participants studied in the current paper, suggested that lithium may provide behavioral and cognitive benefits in individuals with FXS (Berry-Kravis et al., 2008. However, it is not known whether these observed improvements are paralleled by changes in physiological state.
Hypotheses
The study evaluated whether male participants with FXS have an atypical autonomic profile that may interfere with spontaneous social engagement and fosters mobilization and defensive behaviors (e.g., faster heart rate, lower RSA, faster breathing). Follow-up sessions (conducted 2 months and 1 year following beginning of lithium treatment) evaluated whether lithium influences the autonomic profiles of Fragile X individuals and whether lithium influences the autonomic response profile of Fragile X individuals to a social stressor. It was hypothesized that the FXS group would have lower amplitude RSA, faster heart rate during baselines and a dampened physiological response during the social stressor. In addition, it was hypothesized that the behavioral improvements observed with lithium would be paralleled by increases in RSA and decreases in heart rate.
Methods
Participants
Fragile X
Eighteen male 6–23 year olds were recruited from the Fragile X Clinic at Rush University Medical Center. All participants were diagnosed with fragile X syndrome and had a fragile X full mutation (CGG expansion >200 repeats) with at least partial methylation on FMR1-DNA analysis1. Scores ranged from 47–61 on the Stanford Binet ABIQ [Mean(SD) = 51.40 (5.38)] and from 48–54 on the Stanford Binet working memory [Mean(SD) = 48.60 (1.90)]. All participants had a Clinical Global Impression-Severity (CGI-S) score of 4 or greater (indicating moderate or greater severity of behavioral problems) and an Aberrant-Behavior Checklist-Irritability Scale score of 12 or greater (top 50th percentile). Individuals who displayed persistent psychotic symptoms or with symptom severity likely judged to endanger personal safety or safety of others were excluded. At least one parent or guardian was present during the research session and spoke fluent English with a reading level of 6th grade or greater. Analyses were conducted on participants who had complete data throughout the protocol (N=12, mean (SD) age=12.00 (4.84) years). Autonomic data from six participants were not collected or analyzed due to either equipment failure or non-compliance. Several of the FXS participants were receiving medication at the time of their participation in the experiment. The dose and effects of these drugs on the autonomic nervous system were monitored during routine clinic visits and consistent with the literature (Hall et. al., 2009), did not have noticeable effects on heart rate.
Comparison group
Data from twenty-one2 typically developing male participants were collected using identical procedures as those for the Fragile X participants. The mean age of the comparison group was not statistically different from the mean age of the FXS group [FXS group: mean (SD) age = 12.00 (4.84) years; Comparison group: mean (SD) age = 11.48 (3.28) years]. The comparison participants were recruited from the Chicago area via public solicitation (e.g., newspaper, magazine, internet advertisements). Individuals in the comparison group had no current diagnosis of a mental disorder, were not taking medications, and did not have a medical condition that could interfere with their participation in the experimental protocol. The protocols for both the Fragile X and the Comparison Group were approved by the Institutional Review Board at the University of Illinois at Chicago and Rush University Medical Center.
Physiological measures
Cardiac data
Physiological data were recorded using the LifeShirt® (Vivometrics). The LifeShirt® is a non-invasive, continuous ambulatory monitoring system that collects cardiac, ventilatory and activity data. Heart rate data were continuously recorded using three self-adhering electrodes (Meditrace) placed directly onto the upper chest and on the lateral surface of the abdomen. Heart rate data from the LifeShirt® has been benchmarked against laboratory equipment and provides measures with sufficient accuracy to generate sensitive indices of autonomic regulation (see Heilman & Porges, 2007).
Heart rate data were visually inspected and edited off-line with CardioEdit software (Brain-Body Center, University of Illinois at Chicago). Editing consisted of integer arithmetic (i.e., dividing intervals between heart beats when detections of R-wave from the ECG were missed or adding intervals when spuriously invalid detections occurred). RSA was calculated with CardioBatch software (Brain-Body Center, University of Illinois at Chicago) consistent with the procedures developed by Porges (1985). The Porges method quantifies the amplitude of RSA with age-specific parameters that are sensitive to the maturational shifts in the frequency of spontaneous breathing. The method includes the following steps: (1) R-R intervals are timed to the nearest msec to produce a time series of sequential heart periods; (2) sequential heart periods are resampled into 250 msec intervals to produce time-based data; (3) the time-based series is detrended by a 51-point cubic moving polynomial (Porges & Bohrer, 1990) that is stepped through the data to create a smoothed template and the template is subtracted from the original time-based series to generate a detrended residual series; (4) the detrended time series is bandpassed to extract the variance in the heart period pattern associated with spontaneous breathing3; and (f) the natural logarithm of the variance of the bandpassed time series is calculated as the measure of the amplitude of RSA (Riniolo & Porges, 1997). These procedures are statistically equivalent to frequency domain methods (i.e., spectral analysis) for the calculation of the amplitude of RSA when heart period data are stationary (Porges & Byrne, 1992). Two minutes of heart rate data during each baseline condition, the first five minutes of heart rate data collected during the social challenge (SC1) and the second five minutes of heart rate data collected during the social challenge (SC2) were edited. RSA and heart rate were quantified during each sequential 30-sec epoch and the averages within each condition were used in the data analyses.
Respiratory data
Respiratory rate was continuously recorded using the LifeShirt®. Embedded in the LifeShirt® are Respibands, consisting of a sinusoidal arrangement of electrical wires. One Respiband is sewn into the shirt at the level of the ribcage and another at the level of the abdomen. The respiratory parameters are monitored when chest wall movements stretch the Respibands, which are activated by an extremely low current, electrical oscillator circuit.
Activity data
Activity data were collected continuously, via an accelerometer embedded in the LifeShirt® on the anterior surface. The dual axis accelerometer acquires motion and posture information along the x, y and z axes. Posture and motion information are derived by decomposing the 3 raw signals into their respective motion and posture components. The posture signal (the DC component) is obtained by low-pass filtering to remove the motion signal (AC component). The motion signal is obtained by subtracting the posture component from the raw signal. The activity parameter used in this study was the absolute value of motion defined as the summation of x- and y-motion axes.
Procedures
Parental consent was obtained prior to the start of the study. After obtaining parental consent and participant assent/consent (when applicable), the physiological monitoring equipment (LifeShirt®) was demonstrated to the parents and participants, and the equipment was attached to the participant. After ensuring that the parent/guardian and participant were comfortable with the equipment, the study proceeded.
An initial baseline measure of heart rate (2 minutes) was collected while the participant sat in a chair at a table. During this and subsequent baselines, the participant was encouraged to sit as quietly as possible, and to minimize motor activity.
At the beginning of the social challenge, the parent/guardian was asked to leave the research room while an unfamiliar researcher entered the room. Thus, the social challenge involved both absence of parent, and introduction of an unfamiliar person to the participant. The parent/guardian was able to watch the social challenge through video monitoring equipment in an adjacent room. During the social challenge, the researcher administered two auditory tests (data not reported here). While participants were asked to repeat words that were presented via headphones during the first auditory test, the participants were not required to face the researcher once the researcher confirmed the participant understood, and was comfortable, with the auditory testing. The social challenge ended when the parent returned to the room with the original researcher, and the unfamiliar researcher exited. The post-social challenge baseline was then recorded as the final task.
After completion of the research, the physiological sensors were removed from the participant and the parent/guardian and participant were thanked and dismissed.
Procedures: Follow-up sessions
For the follow-up sessions, participants in the Fragile X group were reassessed 2 months and 1 year following the initial research session to evaluate the effect of lithium treatment on autonomic measures. Participants began lithium treatment4 on the day of the initial research session. The protocol for the 2-month and 1 year assessments was identical to the protocol for the initial assessment.
Results
Baseline physiological measures
Descriptive statistics (mean, standard deviation) for each physiological variable are presented in Table 1. Univariate analyses of variance (ANOVA) demonstrated significant group differences in baseline RSA, heart rate, and respiration rate. The FXS group had lower amplitude RSA, faster heart rate, and faster respiration rate than the comparison group. Baseline heart rate and RSA were correlated in both the FXS (r = −.68, p < .02) and the comparison (r = −.46, p < .03) groups.
Table 1.
Descriptive statistics [mean (SD)] of RSA, heart rate (HR), respiration rate and motor activity during baseline
| FXS (n=12) | Comparison (n=21) | Significance | |
|---|---|---|---|
| RSA [ln(msec)2] | 6.27 (.76) | 7.56 (.87) | F(1, 32) = 18.41, p<.000 |
| HR (bpm) | 96.44 (13.90) | 77.19 (12.91) | F(1, 32) = 16.07, p<.000 |
| Respiration rate (breaths/min)1 | 23.30 (6.18) | 19.08 (2.68) | F(1, 31) = 7.34, p<.01 |
| Motor activity | .91 (.86) | .72 (.42) | F(1, 32) = .75, p<.39 |
Due to equipment error, one FXS participant did not have respiration rate for baseline.
Effect of age on physiological measures
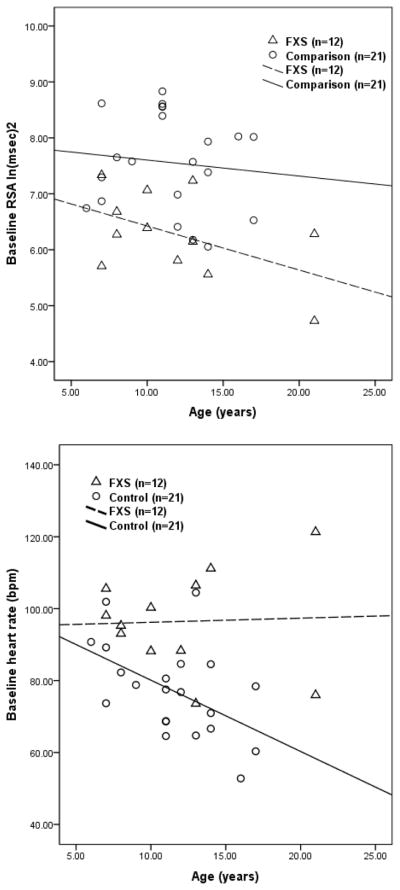
Given the low frequency of FXS in the population, participants were recruited across a broad range of ages. Since it is well known that autonomic parameters (e.g., Gunther & Morgado, 2005) change with maturation (e.g., heart rate and breathing rate slow), analyses were conducted to evaluate the maturational trends in the FXS and comparison groups. Correlations between the baseline physiological measures and age are reported in Table 2. Tests of parallelism were calculated to determine group differences in the regression slopes. Significant group differences in the slopes were observed for RSA, heart rate, and respiratory rate (see Table 2). As illustrated in Figure 1, the control group exhibited the well-documented maturational trend of heart rate slowing across the age range of the participants tested in this study. However, the FXS group did not follow this predicted trend. The atypical age-related decrease in RSA observed in the FXS group (see Figure 1) may provide a mechanism contributing to this apparent violation of an allometric law relating body size, which increases with age during childhood, to heart rate. Thus, the progressive age-related decrease in neural regulation of the heart via the vagus manifested in RSA may offset the allometric relation between increases in body size and the slowing of the heart.
Table 2.
Correlations between age and baseline physiological measures and results of tests of parallelism
| FXS (n=12) | Comparison (n=21) | Significance | |
|---|---|---|---|
| RSA [ln(msec)2] | r = −.50, p<.10 | r = −.11, p<.64 | |
| Regression equation | ŷ = 7.21 − .08x | ŷ = 7.89 − .03x | F(1, 30) = 18.06, p<.000 |
| HR (bpm) | r = .04, p<.90 | r = −.50, p<.02 | |
| Regression equation | ŷ = 95.06 + .12x | ŷ = 99.93 − 1.98x | F(1, 30) = 22.33, p<.000 |
| Respiration rate (breaths/min)1 | r = −.39, p<.23 | r = −.29, p<.21 | |
| Regression equation | ŷ = 29.01 − .48x | ŷ = 21.77 − .24x | F(1, 29) = 6.04, p<.02 |
| Motor activity | r = −.19, p<.55 | r = −.18, p<.44 | |
| Regression equation | ŷ = 1.34 − .04x | ŷ = .98 − .02x | F(1, 30) = .66, p<.42 |
Due to equipment error, one FXS participant did not have respiration rate for baseline.
Figure 1.
Age-related changes in physiological measures
Change in physiological reactivity across the protocol
RSA
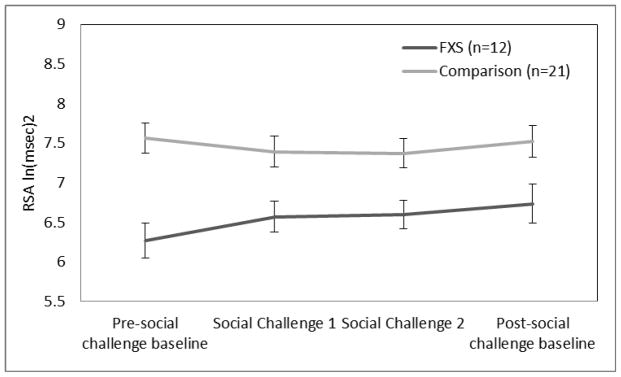
Repeated measures ANOVA [Condition (pre-social challenge baseline, SC1, SC2, post-social challenge baseline) X Group (FXS, comparison) demonstrated a group effect, F(1, 31) = 11.59, p<.002. The FXS group had lower RSA than the comparison group. As illustrated in Figure 2, there was a significant interaction, F(3, 93) = 2.69, p<.05, characterized by group differences in the direction of RSA change to SC1.
Figure 2.
RSA reactivity during social challenge.
Heart rate
Repeated measures ANOVA [Condition (pre-social challenge baseline, SC1, SC2, post-social challenge baseline) X Group (FXS, comparison) demonstrated a group effect, F(1, 31) = 18.10, p<.000. The FXS group had faster heart rate than the comparison group.
Respiration rate
Repeated measures ANOVA [Condition (pre-social challenge baseline, SC1, SC2, post-social challenge baseline) X Group (FXS, comparison) demonstrated a group effect, F(1, 30) = 10.59, p<.003. The FXS group had faster respiration rate than the comparison group. There was also a significant condition effect, F(2.15, 64.62) = 4.89, p<.009, characterized by an increase in respiration rate from pre-social challenge baseline to SC1.
Physiological reactivity to and recovery from the social challenge
Reactivity values were computed for each participant by differencing the average physiological value during the baseline immediately preceding the social challenge from the average physiological value during the first 5 minutes of the social challenge. Recovery values were computed by differencing the average physiological value during the first 5 minutes of the social challenge from the average physiological value during the baseline immediately following the social challenge. There were significant group differences in only RSA reactivity, F (1,32) = 6.27, p <.02. In response to the social challenge, RSA increased in the FXS group and decreased in the comparison group (see Figure 2).
Effect of age on physiological reactivity to/recovery from the social challenge between groups
Analyses were conducted to determine whether the maturational trends in autonomic reactivity and recovery to the social challenge were similar in the FXS and comparison groups. Only in the comparison group did the older children respond with larger heart rate responses to the social challenge, r(21) = .51, p<.02.
2-Month Lithium Follow-up
Autonomic variables and activity were evaluated during baseline, reactivity, and recovery in a subset of the Fragile X participants following 2 months of lithium treatment. The protocol, described above, was re-administered to 8 subjects. The age range of participants in the 2 month follow-up (at the time of the initial session) was 7–21 years (M = 11.63, SD = 4.72).
Baseline, reactivity, and recovery measures
Procedures similar to those applied to the initial test session (see above) were used to quantify the dependent variables. There was no significant session effect (initial versus 2-month follow-up) in any of the physiological baseline, reactivity, or recovery measure, thus, indicating that 2 months of lithium treatment did not result in a reliable effect on the physiological measures.
1-Year Lithium Follow-up
Autonomic and activity were evaluated during baseline, reactivity, and recovery in a subset of the Fragile X participants following 1 year of lithium treatment. The same protocol, as described above, was re-administered to 6 subjects who also participated in the initial session. The age range of participants in the 1-year follow-up (at the time of the initial session) was 7–21 years (M = 12.5, SD = 5.21).
Baseline, reactivity, and recovery measures
Procedures similar to those applied to the initial test session (see above) were used to quantify the dependent variables. There was no significant session effect (initial versus 1-year follow-up) in any of the physiological baseline, reactivity, or recovery measure, indicating that 1 year of lithium treatment did not result in a reliable effect on the physiological measures.
Discussion
The results confirm that FXS males have atypical autonomic activity and reactivity. During baseline, the FXS group might be characterized as being in a physiological state that would support and prepare the child to mobilize (e.g., fight and flight behaviors) and is not in a physiological state that would support calm social interactions. Specifically, the FXS group, relative to the comparison group, had less vagal regulation of the heart (i.e., faster heart rate and lower amplitude RSA) and faster breathing rates. These results are consistent with findings reported by Roberts and colleagues (2001) and Hall and colleagues (2009). Thus, consistent with predictions from the Polyvagal Theory, a physiological state characterized by low vagal regulation of the heart would support states of high activity levels and greater reactivity and defensive behaviors. This behavioral profile would be consistent with the observed difficulties in social behavior and behavioral state regulation observed in FXS.
The FXS group did not exhibit a RSA decrease during social challenge. This transitory withdrawal of the vagal brake, observed in the comparison group and lacking in the FXS group, may represent an adaptive precautionary vigilance response preparing the individual to mobilize if the novel person would become threatening (Porges et al., 1996). A decrease in RSA in response to a social stimulus has been proposed as a positive indicator of social behavior and may reflect the sequential and contingent withdrawal and reinstatement of the vagal brake during social engagement behaviors. A similar atypical RSA response to a social challenge was reported by Dale and colleagues (2010), who reported an increase in RSA in response to a cognitive task uniquely in infants with regulatory disorders.
Prior to the Polyvagal Theory (Porges, 1995), suppression of RSA and other components of heart rate variability were interpreted as indices of mental effort with little interest directed at understanding the neural mechanisms mediating these autonomic responses. The Polyvagal Theory placed an emphasis on neural mechanisms and on the adaptive function of these physiological shifts (see Porges, 2007). From the polyvagal perspective, the suppression of RSA represents an adaptive shift in visceral state characterized by a withdrawal of cardiac vagal tone. Functionally, the transitory removal of the vagal brake provides a physiological state to facilitate efficient mobilization. Thus, the withdrawal of vagal tone associated with sustained attention may characterize a physiological state that promotes vigilance as an intermediary precautionary psychological process to monitor the risk in the environment. If the cues in the environment necessitate defensive behaviors, then being in a mobilized physiological state (i.e., vagal withdrawal) will reduce the latency to express defensive behaviors (i.e., fight and flight). If defensive behaviors are not necessary to maintain or to negotiate safety, then the rapid vagal regulatory mechanisms are reinstated to immediately calm and self-soothe. Support for this interpretation can be seen in the infant data in which suppression of RSA is correlated with maternal reports of longer attention spans and being more easily soothed (see Huffman, Bryan, del Carmen, Pedersen, Doussard-Roosevelt, & Porges, 1998) and documentation that suppression of RSA during social/attentive tasks in infants is a predictor of positive social behavior and state regulation in young children (see Porges et al., 1996). Thus, a lack of this adaptive response to evaluate the risk in the environment through a well-modulated neural system (i.e., transitory withdrawal of the vagal brake) places the FXS individuals at a disadvantage in evaluating environmental risk and identifying the appropriate social cues in safe social contexts.
The research uncovered a unique and potentially important finding relating autonomic function to maturation in FXS. Due to the low frequency of FXS in the population, most studies recruit participants across a broad age range. This occurred in several of the cited studies that evaluated autonomic function. However, when a broad age range is used with children, it provides an opportunity to evaluate the developmental trend in autonomic function. In typically developing children, paralleling increasing age are decreases in heart rate. The slowing of heart rate typically observed follows the well-known allometric law relating heart rate to body mass. Although the comparison group exhibited these predicted age-related responses, the FXS did not. This difference in baseline activity could not be attributed to group differences in motor activity.
Moreover, the FXS group did not demonstrate the age-related profiles of heart rate regulation that were exhibited by the comparison group. In the typically developing comparison group, there were significantly larger increases in heart rate with age during the social challenge that were not present in the FXS group. As described above, these results indicate that the FXS group, without having age-appropriate tonic levels of vagal regulation of the heart (i.e., baseline RSA) and age-appropriate regulation of the vagal brake, may have a maladaptive response to evaluate risk in the environment and to facilitate the detection of social cues.
The atypical relationship between autonomic activity and age in the FXS group could be the result of several factors. First, individuals with FXS may experience a plateau in the development of vagal regulation that, due to an inability to regulate behavioral state, may contribute to the frequently reported plateau or decline in cognitive ability and adaptive functioning (e.g., Fisch, Carpenter, Howard-Peebles, Holden, Tarleton, Simensen, & Nance, 2007; Wright-Talamante, Cheema, Riddle, Luckey, Taylor, & Hagerman, 1996; Fisch, Carpenter, Howard-Peebles, Maddalena, Simensen, Tarleton, Julien-Inalsingh, Chalifoux, & Holden, 1996; Bailey, Mesibov, Hatton, Clark, Roberts, & Mayhew, 1998; Glaser, Hessl, Dyer-Friedman, Johnston, Wisbeck, Taylor, & Reiss, 2003; Dykens, Ort, Cohen, Finucane, Spiridigliozzi, Lachiewicz, Reiss, Freund, Hagerman, & O’Connor, 1996). Second, the reports of deficits in the white matter tracts in FXS (Barnea-Goraly, Eliez, Hedeus, Menon, White, Moseley, & Reiss, 2003) might reflect a developmental progression of white matter defects in the specific tracts connecting higher brain structures to the source nuclei in the brainstem that control autonomic function. Third, there may be moderating or mediating variables that are interfering with state regulation, such as context, medication treatment, intervention strategies and/or anxiety. For example, individuals with FXS may show an increase in anxiety with age that would interfere with state regulation. However, a deficit in either the central regulation of autonomic state (e.g., via white matter tract alterations) or in the function of the myelinated vagal efferent pathways would both be manifested in RSA and may provide a more parsimonious explanation of the reports of progressive anxiety with age. Within the FXS sample, the individual drugs and doses suggest, consistent with the literature (Hall et al., 2009), that these treatments would not have major effects on the autonomic nervous system.
Lithium has been reported to improve behaviors in FXS, as evidenced by significant improvement on the Aberrant Behavior Checklist-Community Edition (ABC-C), clinical global improvement scale (CGI), visual analog scale for behavior (VAS), Vineland Adaptive Behavior Scale (VABS), The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) List Learning and enhanced ERK activation rate (Berry-Kravis et al., 2008). Since lithium had little effect on the domains of autonomic activity monitored in this study, lithium may be working through another pathway. Alternatively, if lithium influences vagal function indirectly, the statistical power of our design may have been insufficient to detect an effect with only 8 participants being evaluated after 2 months and only 6 subjects after 1 year of lithium treatment.
The study design included additional tasks to assess the functioning of the Social Engagement System including tympanometry to assess the functioning of the middle ear muscles and tests of auditory processing, eye gaze, and emotion recognition tests. Unfortunately, these tasks proved too difficult for most individuals in the FXS sample to complete. Since difficulties in social engagement behavior are a common feature of FXS, alternative techniques need to be developed that are appropriate for the ability level and attentional limitations of this population. By assessing multiple indices, it will be possible to get a more complete profile of both the autonomic and the behavioral components of the social engagement system in individuals with FXS.
The study is limited due to the small number of FXS participants. Testing a larger sample across a broad age range will be critical in replicating and further examining the atypical developmental features of the autonomic nervous system that have been observed in this study. Unfortunately, due to the difficulties in recruiting samples of FXS children, the limited literature describing autonomic responses in FXS children is represented by several studies with samples sizes similar to this study. Even when the samples are small, the participants usually represent a broad age range.
In summary, the data indicate that individuals with FXS have difficulty with state regulation and autonomic reactivity, which, according to the Polyvagal Theory, may underlie deficits in social behavior and the expression of behavioral symptoms of anxiety. The observed autonomic response profiles (i.e., low RSA and difficulties in regulating the vagal brake) in the FXS group is consistent with studies evaluating autonomic indicators of individuals with social behavior problems across other diagnostic categories and independent of known genetic mechanisms. These findings inform the Polyvagal Theory and provide additional support linking atypical autonomic regulation with difficulties in expressing effective and spontaneous social engagement behaviors. The findings suggest that monitoring autonomic activity during a social challenge, such as the one used in this study, may provide an objective laboratory protocol to monitor individual differences and potentially assess features of treatment in individuals with FXS. Moreover, the study identifies for the first time an atypical developmental trend in autonomic regulation in FXS children including age-related decreases in vagal regulation of the heart and a lack of heart rate slowing with development.
Acknowledgments
The project described was supported, in part, by a grant from the FRAXA Research Foundation (EBK/SWP), and by grant HD 53570 from the National Institute of Child Health and Human Development (SWP). We would like to thank the parents and children who participated in the study, the researchers at the Rush Fragile X clinic for coordinating appointments, and the researchers at the Brain-Body Center who assisted with data collection.
Footnotes
Of the 18 participants who were recruited, 2 had a mosaic pattern and 16 had a fully methylated full mutation. Of the 12 participants whose data were included in the initial session, 2 had a mosaic pattern and 10 had a fully methylated full mutation. Of the 8 participants included in the 2 month follow-up session, 1 had a mosaic pattern and 7 had a fully methylated full mutation. All of the participants in the 1 year follow-up had a fully methylated full mutation.
Data from two participants in the comparison group were not included in the analyses because of scores in the clinical range on the ASEBA. Two additional participants in the comparison group were not included in the analyses due to equipment problems
In the current study, the frequency band of spontaneous breathing was expanded to include age-appropriate breathing frequencies for children and adults, 0.12–1.00 Hz.
Participants weighing more than 50 kg were started on lithium carbonate 300 mg TID (orally). Participants less than 50 kg were started on lithium carbonate 20 mg/kg/day rounded to the nearest 150 mg increment and divided in a TID dosing schedule to a maximum of 300 mg TID. After the lithium level was obtained from a 2 week follow-up visit (clinic only), the dose was titrated up to achieve a predicted level of 0.8–1.2, or was titrated down if there were side effects or if the level was in the upper end of the therapeutic range.
Contributor Information
Keri J. Heilman, Email: kheilman@psych.uic.edu.
Emily R. Harden, Email: eharden@psych.uic.edu.
Danielle M. Zageris, Email: dzageris@psych.uic.edu.
Elizabeth Berry-Kravis, Email: Elizabeth_M_berry-kravis@rush.edu.
Stephen W. Porges, Email: sporges@psych.uic.edu.
References
- Bailey DB, Jr, Mesibov GB, Hatton DD, Clark RD, Roberts JE, Mayhew L. Autistic behavior in young boys with fragile X syndrome. Journal of Autism and Developmental Disorders. 1998;28:499–508. doi: 10.1023/a:1026048027397. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Eliez S, Hedeus M, Menon V, White CD, Moseley M, Reiss AL. White matter tract alterations in fragile X syndrome: preliminary evidence from diffusion tensor imaging. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2003;118:81–8. doi: 10.1002/ajmg.b.10035. [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends in Neurosciences. 2004;27:370–7. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Sumis A, Hervey C, Nelson M, Porges SW, Weng N, Weiler IJ, Greenough WT. Open-label treatment trial of lithium to target the underlying defect in fragile X syndrome. Journal of Developmental &Behavioral Pediatrics. 2008;29(4):293–302. doi: 10.1097/DBP.0b013e31817dc447. [DOI] [PubMed] [Google Scholar]
- Blair C, Peters R. Physiological and neurocognitive correlates of adaptive behavior in preschool among children in Head Start. Developmental Neuropsychology. 2003;24:479–97. doi: 10.1207/S15326942DN2401_04. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Van Dijk E, Thayer JF. Daily worry is related to low heart rate variability during waking and the subsequent nocturnal sleep period. International Journal of Psychophysiology. 2007;63:39–47. doi: 10.1016/j.ijpsycho.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Calkins SD. Cardiac vagal tone indices of temperamental reactivity and behavioral regulation in young children. Developmental Psychobiology. 1997;31:125–35. doi: 10.1002/(sici)1098-2302(199709)31:2<125::aid-dev5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Cohen IL, Fisch GS, Sudhalter V, Wolf-Schein EG, Hanson D, Hagerman R, Jenkins EC, Brown WT. Social gaze, social avoidance, and repetitive behavior in fragile X males: a controlled study. American Journal of Mental Retardation. 1988;92:436–46. [PubMed] [Google Scholar]
- Dale LP, O’Hara EA, Keen J, Porges SW. Infant regulatory disorders: Temperamental, physiological and behavioral features. J Dev Behav Pediatr. 2010 doi: 10.1097/DBP.0b013e3181e32c4f. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykens E, Ort S, Cohen I, Finucane B, Spiridigliozzi G, Lachiewicz A, Reiss A, Freund L, Hagerman R, O’Connor R. Trajectories and profiles of adaptive behavior in males with fragile X syndrome: multicenter studies. Journal of Autism and Developmental Disorders. 1996;26:287–301. doi: 10.1007/BF02172475. [DOI] [PubMed] [Google Scholar]
- Fisch GS, Carpenter N, Howard-Peebles PN, Holden JJ, Tarleton J, Simensen R, Nance W. Studies of age-correlated features of cognitive-behavioral development in children and adolescents with genetic disorders. American Journal of Medical Genetics: Part A. 2007;143:2478–89. doi: 10.1002/ajmg.a.31915. [DOI] [PubMed] [Google Scholar]
- Fisch GS, Carpenter N, Howard-Peebles PN, Maddalena A, Simensen R, Tarleton J, Julien-Inalsingh C, Chalifoux M, Holden JJ. Lack of association between mutation size and cognitive/behavior deficits in fragile X males: a brief report. American Journal of Medical Genetics. 1996;64:362–4. doi: 10.1002/(SICI)1096-8628(19960809)64:2<362::AID-AJMG25>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Glaser B, Hessl D, Dyer-Friedman J, Johnston C, Wisbeck J, Taylor A, Reiss A. Biological and environmental contributions to adaptive behavior in fragile X syndrome. American Journal of Medical Genetics: Part A. 2003;117:21–9. doi: 10.1002/ajmg.a.10549. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Miller LJ, McGrath-Clarke J, Riley K, Goldson E, Harris SW, Simon J, Church K, Bonnell J, Ognibene TC, McIntosh DN. Influence of stimulants on electrodermal studies in Fragile X syndrome. Microscopy Research and Technology. 2002;57:168–173. doi: 10.1002/jemt.10067. [DOI] [PubMed] [Google Scholar]
- Hall S, DeBernardis M, Reiss A. Social escape behaviors in children with fragile X syndrome. Journal of Autism and Developmental Disorders. 2006;36:935–47. doi: 10.1007/s10803-006-0132-z. [DOI] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, Huffman LC, Lazzeroni LC, Reiss AL. Physiological correlates of social avoidance behavior in chidren and adolescents with Fragile X Syndrome. Journal of American Academy of Child and Adolescent Psychiatry. 2009;48:320–329. doi: 10.1097/CHI.0b013e318195bd15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman KJ, Porges SW. Accuracy of the LifeShirt (Vivometrics) in the detection of cardiac rhythms. Biological Psychology. 2007;75:300–5. doi: 10.1016/j.biopsycho.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessl D, Glaser B, Dyer-Friedman J, Blasey C, Hastie T, Gunnar M, Reiss AL. Cortisol and behavior in fragile X syndrome. Psychoneuroendocrinology. 2002;27:855–72. doi: 10.1016/s0306-4530(01)00087-7. [DOI] [PubMed] [Google Scholar]
- Hessl D, Glaser B, Dyer-Friedman J, Reiss AL. Social behavior and cortisol reactivity in children with fragile X syndrome. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2006;47:602–10. doi: 10.1111/j.1469-7610.2005.01556.x. [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:7746–50. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman LC, Bryan YE, del Carmen R, Pedersen FA, Doussard-Roosevelt JA, Porges SW. Infant temperament and cardiac vagal tone: assessments at twelve weeks of age. Child Development. 1998;69:624–35. [PubMed] [Google Scholar]
- Keysor CS, Mazzocco MM, McLeod DR, Hoehn-Saric R. Physiological arousal in females with fragile X or Turner syndrome. Developmental Psychobiology. 2002;41:133–46. doi: 10.1002/dev.10060. [DOI] [PubMed] [Google Scholar]
- Lesniak-Karpiak K, Mazzocco MM, Ross JL. Behavioral assessment of social anxiety in females with Turner or fragile X syndrome. Journal of Autism and Developmental Disorders. 2003;33:55–67. doi: 10.1023/a:1022230504787. [DOI] [PubMed] [Google Scholar]
- Masi CM, Hawkley LC, Rickett EM, Cacioppo JT. Respiratory sinus arrhythmia and diseases of aging: Obesity, diabetes mellitus, and hypertension. Biological Psychology. 2007;74:212–223. doi: 10.1016/j.biopsycho.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LJ, McIntosh DN, McGrath J, Shyu V, Lampe M, Taylor AK, Tassone F, Neitzel K, Stackhouse T, Hagerman RJ. Electrodermal responses to sensory stimuli in individuals with Fragile X syndrome. American Journal of Medical Genetics. 1999;83:268–279. [PubMed] [Google Scholar]
- Porges SW. Method and apparatus for evaluating rhythmic oscillations in aperiodic physiological response systems. 4520944 United States Patent no. 1985
- Porges SW. Orienting in a defensive world: mammalian modifications of our evolutionary heritage. A Polyvagal Theory. Psychophysiology. 1995;32:301–18. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. The Polyvagal Theory: phylogenetic substrates of a social nervous system. International Journal of Psychophysiology. 2001;42:123–46. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Porges SW. The Polyvagal Theory: phylogenetic contributions to social behavior. Physiology & Behavior. 2003;79:503–13. doi: 10.1016/s0031-9384(03)00156-2. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–43. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Bohrer Robert E. The analysis of periodic processes in psychophysiological research. In: Cacioppo JT, Tassinary LG, editors. Principles of Psychophysiology: Physical, Social, and Inferential Elements. New York: Cambridge University Press; 1990. pp. 708–753. [Google Scholar]
- Porges SW, Byrne EA. Research methods for measurement of heart rate and respiration. Biological Psychology. 1992;34:93–130. doi: 10.1016/0301-0511(92)90012-j. [DOI] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales AL, Greenspan SI. Infant regulation of the vagal “brake” predicts child behavior problems: a psychobiological model of social behavior. Developmental Psychobiology. 1996;29:697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Riniolo T, Porges SW. Inferential and descriptive influences on measures of respiratory sinus arrhythmia: sampling rate, R-wave trigger accuracy, and variance estimates. Psychophysiology. 1997;34:613–21. doi: 10.1111/j.1469-8986.1997.tb01748.x. [DOI] [PubMed] [Google Scholar]
- Roberts J, Mazzocco MM, Murphy MM, Hoehn-Saric R. Arousal modulation in females with fragile X or Turner Syndrome. Journal of Autism and Developmental Disorders. 2008;38:20–7. doi: 10.1007/s10803-007-0356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Boccia ML, Bailey DB, Jr, Hatton DD, Skinner M. Cardiovascular indices of physiological arousal in boys with fragile X syndrome. Developmental Psychobiology. 2001;39:107–23. doi: 10.1002/dev.1035. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Symons FJ, Johnson AM, Hatton DD, Boccia ML. Blink rate in boys with fragile X syndrome: preliminary evidence for altered dopamine function. Journal of Intellectual Disability Research. 2005;49:647–56. doi: 10.1111/j.1365-2788.2005.00713.x. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Clift A, Bolden S, Salomon K. RSA fluctuation in major depressive disorder. Psychophysiology. 2007;44:450–8. doi: 10.1111/j.1469-8986.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- Simon EW, Finucane BM. Facial emotion identification in males with fragile X syndrome. American Journal of Medical Genetics. 1996;67:77–80. doi: 10.1002/(SICI)1096-8628(19960216)67:1<77::AID-AJMG13>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Turk J, Cornish K. Face recognition and emotion perception in boys with fragile-X syndrome. Journal of Intellectual Disability Research. 1998;42:490–9. doi: 10.1046/j.1365-2788.1998.4260490.x. [DOI] [PubMed] [Google Scholar]
- Turner G, Webb T, Wake S, Robinson H. Prevalence of fragile X syndrome. American Journal of Medical Genetics. 1996;64:196–7. doi: 10.1002/(SICI)1096-8628(19960712)64:1<196::AID-AJMG35>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Verkerk AJMH, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DPA, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang F, Eussen BE, van Ommen GJB, Blonden LAJ, Riggins GJ, Chastain JL, Kunst CB, Galjaard H, Caskey CT, Nelson DL, Oostra BA, Warren ST. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in Fragile X Syndrome. Cell. 1991;65:905–14. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Wisbeck JM, Huffman LC, Freund L, Gunnar MR, Davis EP, Reiss AL. Cortisol and social stressors in children with fragile X: a pilot study. Journal of Developmental and Behavioral Pediatrics. 2000;21:278–82. doi: 10.1097/00004703-200008000-00004. [DOI] [PubMed] [Google Scholar]
- Wishart JG, Cebula KR, Willis DS, Pitcairn TK. Understanding of facial expressions of emotion by children with intellectual disabilities of differing aetiology. Journal of Intellectual Disability Research. 2007;51:551–63. doi: 10.1111/j.1365-2788.2006.00947.x. [DOI] [PubMed] [Google Scholar]
- Wright-Talamante C, Cheema A, Riddle JE, Luckey DW, Taylor AK, Hagerman RJ. A controlled study of longitudinal IQ changes in females and males with fragile X syndrome. American Journal of Medical Genetics. 1996;64:350–5. doi: 10.1002/(SICI)1096-8628(19960809)64:2<350::AID-AJMG23>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]