Abstract
Purpose
To systematically review the current evidence for effects of platelet concentrates on (1) graft maturation and (2) graft-bone interface healing in ACL reconstruction in human, controlled trials, and for ensuing differences in clinical outcomes.
Methods
A systematic search of PubMed, CINAHL, EMBASE, CCTR and CDSR was performed for controlled trials of human ACL reconstruction with and without platelet concentrates. Data validity was assessed and data were collected on graft maturation, graft-bone interface healing and clinical outcome.
Results
Eight studies met the inclusion criteria. Seven studies reported on graft maturation with significantly better outcomes in the platelet groups in four, and large differences in means in two (underpowered) studies. Five studies report on tunnel healing, but four found no difference between groups. Three studies assessed clinical outcome but found no differences, regardless whether they had shown a benefical (1/3) or no effect (2/3) of platelets on graft and tunnel healing.
Conclusion
The current best evidence suggests that the addition of platelet concentrates to ACL reconstruction may have a beneficial effect on graft maturation and could improve it by 20–30% on average, but with substantial variability. The most likely mode of action is that treatment with platelets accelerates graft repopulation and remodeling, and this interpretation is supported by the existing data and biologically plausible. However, the current evidence also shows only a very limited influence of platelet concentrates on graft-bone interface healing and no significant difference in clinical outcomes.
Clinical Relevance
This systematic review collected evidence that the use of platelet concentrates may be a safe and inexpensive way to optimize graft maturation after ACL reconstruction, but there is no evidence for improved graft-bone interface healing or a significant difference in clinical outcomes.
Level of Evidence
Level IV, systematic review of Level II, III, and IV studies.
1. Introduction
1.1. Rationale
Platelet concentrates are popular tool in clinical orthopedics and orthopedic research(1, 2). As a rich source of a plethora of growth factors, platelets are used in fracture healing, spine fusion, as local injections in tendinopathies, and as adjuncts in tendon repair procedures(3–10). One promising fields of platelet concentrate use, however, is sports medicine(1, 2). Platelets, often in combination with scaffolds, are employed in the management of anterior cruciate ligament ruptures and rotator cuff tears(11, 12). A number of new tissue engineering-based regenerative treatments, such as bio-enhanced ACL repair, are being investigated (11, 12), but in this study we want to focus on the effectiveness of clinically available platelet-based treatments for the ACL.
The current gold standard for treating ACL tears is ACL reconstruction, using autogeneic or allogeneic tendon grafts(13, 14). While a number of techniques for tendon preparation and fixation are available, all methods share a common postoperative scenario consisting of two main biologic processes: graft-to-bone healing in the femoral and tibial tunnels(15, 16), and ligamentization of the intra-articular portion of the tendon graft(17, 18). The success of ACL reconstruction depends heavily on these processes, and to improve their outcomes and assure optimal clinical results, platelet concentrates are being used. The rationale behind the use of platelets is the assumption of improved tunnel healing and faster and/or better graft remodeling due to the growth factors released from activated platelets(1, 19, 20). It was the aim of this study to systematically review the current literature for evidence that would substantiate this assumption.
1.2. Objective
The primary objective of this study was to systematically accrue evidence for effects of platelet concentrates on (1) graft maturation and (2) graft-bone interface healing in ACL reconstruction in human, controlled trials. Our primary hypothesis was that the addition of platelet concentrates improves both graft maturation and graft-bone interface healing,
Furthermore, data were collected for clinical outcomes, if provided in the same studies, to assess associations between of differences in clinical outcome with differences in maturation and interface healing based on platelet use. We hypothesized that the improvement in graft maturation and graft-bone interface healing would reflect in improved clinical function in the immediate postoperative phase.
2. Materials and Methods
This systematic review was performed following the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) statement (21). The PRISMA statement (www.prisma-statement.org), put forward by the CONSORT group (www.consort-statement.org) is a evidence-based guideline for conducting and reporting systematic reviews, and was formerly known as the QUOROM (Quality Of Reporting Of Meta-analysis) statement(22).
2.1 Eligibility criteria
Studies were included if they reported on graft maturation and/or graft-bone interface healing in human patients undergoing ACL reconstruction augmented with platelet concentrates. All types of ACL reconstruction were eligible. Platelets could be applied as a liquid or gel, with or without a carrier scaffold to the graft or tunnel(s) or both at the time of surgery. Repeated applications, postoperative injections or combination of platelets with other chemicals or bioactive reagents (growth factors, cytokines, etc) were not eligible. Animal studies were not eligible. No limit was set for minimum follow-up duration since it is sensible to assume the effects of platelet concentrates to be strongest in the short-term and plateau in the long-term. Reporting of clinical data was searched for, but not an inclusion criterion.
2.2 Data sources
The online databases PubMed, CINAHL, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), and Cochrane Database of Systematic Reviews (CDSR) were searched for relevant publications. All dates and languages were included. The last search was performed on May 1st, 2011.
2.3 Search Strategy
The search algorithm was “(((ACL)OR (anterior cruciate ligament))AND (reconstruction))OR (ACL reconstruction)) AND platelets” and was replicated using the keywords as MeSH terms as well. The search algorithm was intentionally kept fairly general to maximize return. All searches were unlimited, i.e., considering publications in all languages and regardless of publication date. In addition to the online searches, the bibliographies of the included studies were reviewed to identify additional publications.
2.4 Study selection
Studies were excluded if title and abstract clearly refuted eligibility. Full texts were obtained for all studies matching the inclusion criteria and all with unclear eligibility. The obtained full texts were reviewed to re-confirm eligibility. All study selections were done independently in duplicate and cross-referenced. Disagreement was resolved by consensus.
2.5 Data items and collection process
Data were extracted for the endpoints maturation and graft-bone interface healing. Eligible methods for assessment of graft maturation were radiological imaging and histology of graft biopsies. Eligible methods for assessment of graft-bone interface healing were histology, MRI, and CT. For the secondary endpoint, clinical outcome, all reported endpoints (clinical scores, AP laxity, subjective patient evaluation, etc) were admitted. All data were extracted independently and in duplicate. Duplicate data extractions were compared for difference and disagreement was resolved by consensus.
2.6 Risk of bias in individual studies
The risk of bias was assessed through categorization by level-of-evidence. Additionally a modified Jadad Score was used(23–25). This score gives one point each for randomization, concealed allocation, and reporting of sample size calculation/attrition. Three points (pts) is the best possible outcome, zero the worst (26).
2.7 Data synthesis
We did not perform a quantitative data analysis because of the clinical heterogeneity, such as different types of platelet concentration systems (with different anticoagulants), different platelet concentrations, different ACL techniques, and different methods of outcome assessment. However, all studies used a platelet concentrate in a human ACL autograft model with imaging (MRI/CT) assessment of graft maturation and/or tunnel healing between 3 and 24 months, which is sufficient similarity to allow direct, descriptive comparison.
3. Results
3.1 Study selection
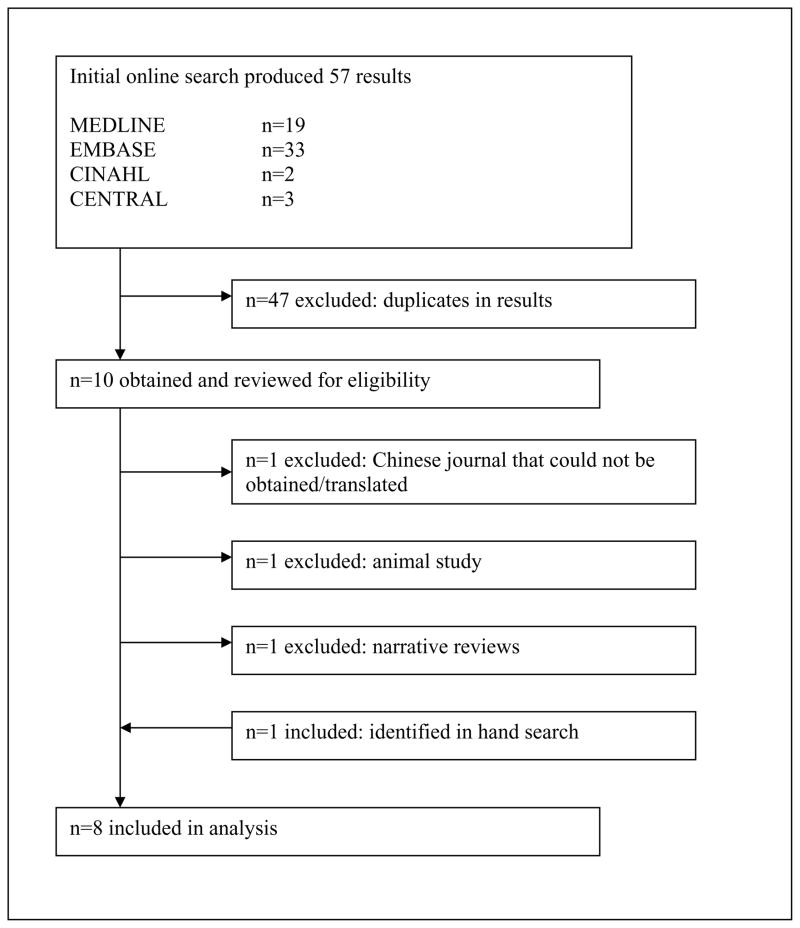
Our online search produced 57 results in total. All 57 publications were obtained and reviewed based on the criteria described above. One paper was published in a Chinese medical journal and could not be obtained for this study(27). One additional paper was identified by cross-referencing the bibliographies of the included papers. Finally, 8 papers reporting on a total of 380 patients (198 ACL reconstructions with platelets, 182 ACL reconstructions without platelets) were included in this analysis(28–35). (Figure 1) These papers were published between 2005 and 2010 in English.
Figure 1.
Trial flow
3.2 Characteristics of the included studies
The average patient age across all studies was 28.9 ± 3.4 years, the patients in the control group were slightly younger by 1.5 ± 2.9 years on average (p=0.451). A average of 26 ± 12 patients were included per study. 23 ± 13% of patients were female. The longest follow-up was at, on average, 10.5±9.5 (range 1.5 to 24) months after ACL reconstruction. Table 1 summarizes the characteristics of these studies. All reports used bone-tendon-bone or hamstring autografts. (Table 2) Four papers report the use of a GPS® system (Gravitational Platelet Separation System, Biomet, Warsaw, IN)(30, 31, 33, 35), two used the Magellan® system (Medtronic, Minneapolis, MN)(28, 34), one used the BTI system II® (BTI Biotechnology Institute, Vitoria, Spain)(32) and one group did not use a commercial system but a Beckman (J-6B, Beckman Coulter Spain, Madrid Spain) centrifuge to create a platelet concentrate of 4.9-fold concentration(29). The concentration of platelets in the concentrates relative to whole blood was approximately 9× for the GPS®, 12× for the Magellan®, and 3× for the BTI® system. Throughout this paper, these platelet concentrations will be referred to as 3×, 5×, 9×, and 12×. (Table 3)
Table 1.
Characteristics of the included studies
| Author | randomized* | blinded assessment* | power calculation* | Jadad Score | LoE | follow- up (mo) | Mean Age Platelet Group | Mean Age Control Group | % female | n Platelet Group | n Contro Group |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ventura et al. 2005 | yes | no | no | 1 | 2 | 6 | 36.6 | 30.2 | 0.1 | 10 | 10 |
| Orrego et al. 2008 | yes | yes | yes | 3 | 2 | 6 | 30 | 30 | 0.15 | 26 | 27 |
| Nin et al. 2009 | yes | yes | no | 2 | 1 | 24 | 26.1 | 26.6 | 0.22 | 50 | 50 |
| Silva et al. 2009 | yes | no | no | 1 | 2 | 3 | 26.8 | 26.8 | 0.05 | 10 | 10 |
| Vogrin et al. 2010 | yes | yes | no | 2 | 1 | 3 | 37.2 | 32.6 | 0.43 | 25 | 25 |
| Figueroa et al. 2010 | yes | yes | yes | 3 | 3 | 6 | 26.8 | 23.6 | 0.34 | 30 | 20 |
| Sanchez et al. 2010 | no | no | no | 0 | 3 | 24 | 28 | 28 | 0.3 | 22 | 15 |
| Radice et al. 2010 | no | yes | no | 1 | 3 | 9 | 30 | 32 | 0.22 | 25 | 25 |
whether reported in paper.
Table 2.
Surgical Parameters
| Author | Graft | Femoral Fixation | Tibial Fixation | Rehabilition Program* |
|---|---|---|---|---|
| Ventura et al. 2005 | Quadrupled Hamstring | Cross Pin | Bioabsorbable Interference Screw | immediate mobilization w/o brace, protected weight bearing for 3 weeks, return to sports @ 6 months |
| Orrego et al. 2008 | Quadrupled Hamstring | Cross Pin | Bioabsorbable Interference Screw | accelerated rehab protocol |
| Nin et al. 2009 | Bone-Patellar Tendon-Bone | Cross Pin | Bioabsorbable Interference Screw | immediate mobilization w/o brace, cycling @ 2–3 months, runnning @ 4 month, return to sports @ 6 months |
| Silva et al. 2009 | Hamstring - Double Bundle | Extracortical Button | Bioabsorbable Interference Screw | Immobilization with brace for 1st week, progressive ROM and protected weight bearing until 5th week |
| Vogrin et al. 2010 | Double-looped Hamstring | Cross Pin | Bioabsorbable Interference Screw | immediate mobilization w/o brace, closed kinetic chain exercises, runnning @ 12 weeks, return to sports @ 6 months |
| Figueroa et al. 2010 | Hamstrings | Cross Pin | Bioabsorbable Interference Screw | accelerated rehab protocol |
| Sanchez et al. 2010 | Hamstrings | Screw | Staples | not reported |
| Radice et al. 2010 | Bone-Patellar Tendon-Bone OR Hamstring | Metallic Interference Screw OR Cross Pin | Metallic Interference Screw ± Staples | not reported |
abbreviated,
Table 3.
Platelet Concentrate Parameters
| Author | PRP Technique | PRP Concentration | PRP location (volume) | Volume Blood Drawn | Anticoagulant | Comments |
|---|---|---|---|---|---|---|
| Vogrin et al. 2010 | Magellan | 12× | graft (4mL) and both tunnels (1mL) | 52 mL | Calcium Citrate (8 mL) | |
| Figueroa et al. 2010 | Magellan | 12× | graft (4 mL) both tunnels(3 mL) | 55 mL | Citrate (5 mL) | |
| Nin et al. 2009 | 5× home made | 5× | graft and tibial tunnel | 40 mL | ? | |
| Silva et al. 2009 | Mini GPS III | 9× | femoral tunnel (1.5m)l | 27 mL | Citirc Acid (3mL) | |
| Orrego et al. 2008 | GPS II | 9× | graft (5 mL) and femoral tunnel (1 mL) | 57 mL | unknown 3 mL | |
| Sanchez et al. 2010 | BTI System II | 3× | graft (6 mL) and both tunnels | 65 mL | Sodium Citrate(3.8%) | |
| Radice et al. 2010 | GPS | 9× | graft (5 mL) | 60 mL | ? | with biomaterial |
| Ventura et al. 2005 | GPS | 9× | both tunnels | 54 mL | Citirc Acid(6 mL) |
The earliest study by far was published by Ventura et al in 2005(35). 20 patients were randomly assigned to ACL reconstruction with (n=10) or without (n=10) a 9× platelet concentrate (level-II evidence). All patients received a quadrupled hamstring autograft that was fixed with BioTransFix (Arhtrex, Naples, FL) proximally and interference screws (BioRCI, Smith and Nephew, Andover, MA) distally. 3mL of platelet concentrate were placed in both tunnels directly with autologous thrombin and KOOS, KT-1000, Tegner and CT were used for outcome assessment at 6 months. A limited number of patients was assessed with MRI additionally.
Orrego et al, in 2008 level-II study, prospectively assessed 108 patients randomly allocated to receive ACL reconstruction alone (n=27), or ACL reconstruction with a platelet concentrate of 9× (n=26) placed on the graft (5mL) and into the femoral tunnel (1mL) after the graft was placed and secured (30). Another 55 patients were treated with a bone-plug and therefore excluded from this study. ACL reconstruction was done by four different surgeons using a quadrupled semitendinosus-gracilis graft fixed with biodegradable pins (Biotransfix, Arthrex, Naples, FL) proximally and biodegradable interference screws (Biodelta, Arthrex, Naples, FL) distally. The concentrate was added between the strands of the hamstring graft and once a firm adhering clot had formed, the graft was pulled into the tunnel. At 6 months MRI imaging was done to blindly assess graft maturation and tunnel healing, as well as clinical assessment using the Lysholm and IKDC scores.
Silva et al published a prospective level-II comparison of ACL reconstruction with and without 9× platelet-rich plasma (PRP) of 10 and 10 patients in 2009.(33) Another 20 patients received PRP three times over 4 weeks or with thrombin and were therefore not eligible for this review. They used a double-bundle technique (semitendinous AM, gracilis PL) with Endobutton (Smith and Nephew, Andover, MA) fixation proximally and bioabsorbable interference screws (Smith and Nephew, Andover, MA) distally. The platelet concentrate was placed between the strands of the implant after it had been placed and secured. Outcome assessment was done at 3 months with MRI to establish the graft signal intensity relative to surrounding tissue as measure of ligamentization.
During that same year, a randomized controlled study of 100 patients undergoing ACL reconstruction (bone-tendon-bone with femoral RigidFix (DePuy Mitek, Raynham, MA) and tibial biodegradable interference screw fixation) with and without a platelet concentrate (n=50 each) was published by Nin et al.(29) The 5× platelet concentrate was allowed to gel and placed on the graft before it was folded and tubularized, so that the gel would remain at the center of the implant during passage. The remaining gel was placed in the tibial tunnel after the graft had been placed. This level-I study presented blinded MRI assessment as well as clinical evaluation at 24 month as endpoints.
In 2010, Vogrin et al compared 20 ACL reconstructions with 21 ACL reconstructions with 4mL of a 12× platelet concentration on the graft and 1 mL in both tunnels in a level-I randomized, controlled, double-blind study(34). The surgical technique used was a double-looped semitendinosus-gracilis graft with crosspins and a tibial bioscrew (both Mitek), all prodcedures were performed by the same surgeon. The graft was covered with platelet gel before being pulled into the tunnels. After graft fixation the remaining gel was placed in the tunnels. Outcome was assessed by MRI 3 months post-operatively.
Three more studies were published in 2010, all of level-III evidence. Radice et al present a prospective, single-blinded study of two groups of 50 athletes each(31). As a multi-center study, different types of ACL reconstruction were used: Bone-tendon-bone (metallic interference screws) for rugby and soccer players and hamstrings (TransFix and tibial screws with metallic staples, both Arthrex, Naples, FL) for skiing, hockey, tae kwan do, and volleyball. For the experimental group, 5mL of platelet concentrate were soaked in a absorbable, porcine, gelatin sponge (Gelfoam, Pfizer, New York, NY) which was either wrapped around the bone-tendon-bone graft or placed in-between strands of hamstrings before implantation. MRI assessment was performed monthly from 3 to 9 month by a blinded radiologist.
Figueroa et al report on a non-randomized comparison of MRI results 14 month after ACL reconstruction in 20 patients with ACL reconstruction with a platelet concentrate in 30 controls(28). Hamstring grafts fixed with TransFix (Arthrex, Naples, FL) and Delta screws (Arthrex, Naples, FL) were used by two different surgeons and, in the experimental group, augmented with 4mL of a 12× concentrate on the graft and 3mL in the femoral and tibial tunnel each using, under arthroscopy, a long needle after the graft was placed and fixed. This is the only study that reports a formal sample size assessment.
The only study without imaging results was published by Sanchez et al, who instead focused on gross morphology and histology of biopsies (3mm × 5–10 mm from proximal end) from second look arthroscopies after hamstring ACL reconstruction with screw fixation proximally and distally(32). 22 patients had received a 12× platelet concentrate by injection of 6 mL into the graft and letting it soak in platelet concentrate before implantation, 15 patients without such treatment served as controls. All second look procedures were done between 6 and 24 months after reconstruction.
3.3 Risk of bias in the included studies
Two studies were blinded, randomized controlled trials with less than 20% attrition and narrow confidence intervals and thus qualify as level-I evidence(29, 34). Three studies were classified as level-II(30, 33, 35), and the remaining three as level-3 comparative studies(28, 31, 32). The average Jadad score was 1.6±1.1 points. No other reasons, such as potential selection bias or attrition bias, for a systematic deviation from the truth were observed. (Table 1)
3.4 Graft Maturation
Six publications provide data on MRI assessment of maturation, assuming that homogenously low signals, similar to native ligaments, are a sign of a maturing graft. Orrego et al classified the graft as high-intensity (similar to synovial fluid) or low-intensity (similar to native posterior cruciate ligament) and found 100% low-intense grafts in the platelet group but only 78% in the control group (p=0.036) at 6 months(30). Figuero et al compared the graft to the semitendinous muscle tissue at 6 months postoperatively and found 63.2% hypointense grafts in the platelet group, but only 42.11% hypointense grafts in the control group without platelets (p=0.316)(28). Nin et al found a 21% lower intensity on PD- and 23% lower intensity on T2-weighted MRI images in the platelet group at 24 months (p=0.454 and 0.100) (29). These papers show a consistent 20–30% difference between ACL reconstruction with and without platelets, even though this difference was not always statistically significant, most likely because of the rather small sample sizes and considerable variability.
Radice et al focused on the time course of graft homogenization and showed that the addition of platelet concentrates reduced this time from 369 days to 177 days on average(31). Vogrin et al, in turn, focused on vascularization in their MRI analysis but found no differences of the intra-articular portion of ACL grafts treated with platelets and without such treatment at 12 weeks(34).
Sanchez et al assessed ACL grafts in gross morphology and histologically. Arthroscopic gross evaluation based on synovium coverage, thickness, and tension revealed excellent ratings for 57.1% of platelet treated ACL grafts, but only 33.3% in the control group (p=0.051)(32). Histological assessment revealed a significantly better maturity index for ACL grafts (12 pts vs 14 pts, p=0.024), and more newly developed synovial tissue enveloping the platelet treated grafts (77% of cases) compared to the control grafts without platelets (40%), which is consistent with a statistically significant difference (p=0.023). Also, in the platelet group, the maturation of this synovial envelope was correlated with healing time (p=0.014 for correlation with follow-up duration), but haphazard in the control group (p=0.674 for correlation with follow-up duration).
Ventura et al presented CT data, which showed a significant difference between groups (p<0.01) with platelet treated ACL graft similar to native PCL tissue, while untreated grafts showed a more heterogeneous nature(35).
3.5 Graft-Bone Interface Healing
Five studies present data on the effect(s) of platelet concentrates on tunnel healing. Silva, in a double-bundle study with 6 months follow-up found no significant difference between PD-weighted and T1-weighted tunnels and showed 31% hypointense interfaces for both groups in the posterolateral tunnels and 28.6%, again for both groups, in the anteromedial tunnels at the earliest follow-up of 3 months(33). The study by Orrego and coworkers showed no osteoligamentous interface in 67% of controls and 88% of platelet treated knees (p=0.107) at 6 months(30). There was no difference in the occurrence of tunnel widening at the same follow-up (p=0.452). Figueroa et al. found no synovial fluid at the tendon-bone interface in 86.8% of patients receiving platelets and 94.7% of the controls (p=0.720) at 6 months of follow-up(28). Vogrin et al assessed vascularization of the interface and found a significantly better score for the group treated with platelets (p<0.001)(34). Ventura et al were not able to assess the interface on CT because of artifacts from bony sclerosis about the tunnels(35).
3.6 Clinical results
Three studies report clinical evaluations in addition to graft and tunnel assessment(29, 30, 35). Ventura et al found no differences in KOOS (83 vs 84 pts), KT-1000 (0.8 mm vs 1.2 mm) or Tegner scores (0.9 pts vs 0.8 pts difference pre- to postoperative) between the platelet treated group and controls at 6 months but had seen a significant difference in graft homogeneity on CT (35). Orrego et al, at 6 months post-operatively, found no difference in Lysholm or IKDC scores either, although they did find a difference in graft maturation(30). Nin et al found no difference in inflammatory markers in peripheral blood, nor differences in range of motion, VAS, KT-1000, or IKDC score, in analogy with no difference seen in radiological outcomes at 24 months of follow-up(29).
4. Discussion
4.1 Summary of evidence
This systematic review assessed the effects of platelet concentrates on graft maturation and graft-bone interface healing in ACL reconstruction in human patients. The, somewhat limited, evidence from the current literature suggests that platelet concentrates may improve the rate at which grafts achieve a low signal on T2 imaging and histologic evidence of graft remodeling but have only little to no effect on tunnel healing. (Table 4)
Table 4.
Conclusion presented in included studies
| Authors | Imaging - Graft | Imaging - Tunnel | Histology - Graft |
|---|---|---|---|
| Vogrin et al. 2010 | …without a statistically signficance between both groups. | …enhances early revascularization in the interface… | |
| Figueroa et al. 2010 | … with MRI at 6 months after reconstruction, we did not find any statistically significant benefit in the APC group in terms of integration assessment and graft maturation | ||
| Nin et al. 2009 | … use of PDGF i[…] has no discernable clinical or biomechanical effect at 2 years’ follow-up. | ||
| Silva et al. 2009 | …use of PRP […] does not seem to accelerate tendon integration. | ||
| Orrego et al. 2008 | …enhancing effect on the graft maturation process… | …without showing a significant effect in the osteoligamentous interface or tunnel widening… | |
| Sanchez et al. 2010 | …resulting in more remodeling compared with untreated grafts… | ||
| Radice et al. 2010 | … a time shortening of 48%… | ||
| Ventura et al. 2005 | …transformation from autologous QHTG to new ACL was faster in the GF-treated group than in controls | ||
Currently, it is estimated that 125,000 ACL reconstructions are performed per year in the US alone(36, 37). The outcome of such a procedure depends on two biological events that occur after the implantation–maturation of the graft and integration and secure fixation of the graft into the osseous tunnel. After implantation, the tendon graft matures by changing into a more ACL-like structure by changes in ultrastructure(38–40), vascularization(41, 42), and innervation(43–47). During the first two weeks after implantation there is histological evidence for central necrosis and subsequent hypocellularity in the graft, followed by a phase of vascularization and repopulation by host cells from week 4 to 10. Finally, the graft becomes more similar to the native ACL over 4 to 6 months. During this time the biomechanical strength of the graft is significantly compromised, but recovers over 6 to 12 months. At the same time, a fibrous interface develops between the implant and the surrounding bone. Weiler et al defined this interface as a disorganized, highly cellular and vascular granulation tissue(15, 16). Over time this tissue matures into a hypocellular and hypovascular dense connective tissue with Sharpey-like collagen fibres. Platelet concentrates are being used to stimulate both processes.
The first outcome for which we collected evidence in this study was graft maturation. Seven studies report on this outcome, but use different methods, different scores, and different follow-up time points. For those that use MRI, it is noteworthy that a healthy ligament shows a homogenous, low intensity signal in T2- and PD-weighted images. Generally, four out of seven studies observed positive effects of platelets on the intra-articular grafts. Such effects included reaching a higher level of tissue homogeneity on MRI or in histology, or reaching such a state earlier. Maybe more interesting is taking a look at three studies that did not find a difference in graft maturation. Nin et al measured graft intensity on proton density-weighted (PD) and T2-weighted images. While they found no significant differences, the mean scores were 230 vs 190 on PD and 75 vs 61 on T2 images for platelet treated grafts and controls, respectively, consistent with a 20–25% improvement based on platelet use. This numbers suggest the possibility of a type-II error, or “not seeing what truly is there”, based on insufficient sample size(48, 49). Figueroa et al found generally low numbers of hypotense grafts on MRI for both groups (63.2% in the platelet group, 42.11% in the control group) despite the relatively long follow-up of 6 months, but again found a 20% difference and the same argument of low power made for Nin et al above can be made here.(28). Vogrin et al, in turn, focused specifically on difference in vascularization, not overall graft maturation, and found no significant difference in this parameter between ACL reconstructions with or without platelets(34). Interestingly, the studies that did not find a difference had various lengths of follow-ups (3, 6, and 24 months) and high concentrations of platelets (12× and 5×), ruling these parameters out as reasons for different outcomes. One explanation that is in agreement with all the available evidence and biologically plausible is that the addition of platelet concentrates, i.e. a source of growth factors, speeds up the maturation process, including cellular repopulation and remodeling, as shown by Orrego, Radice, and Ventura, as well as animal studies(30, 31). However, the beneficial effect of platelets wears off rapidly and no difference can be seen at later time points, as reported by Figueroa and Nin, other than on the microscopic level as demonstrated by Sanchez et al(32). Also, some (late stage) ligamentization processes, such as vascularization, seem not to be affected by platelets, as Vorgin and coworkers observed at 3 and 6 months(34).
The second determinant of success of ACL reconstruction studied herein was graft-bone interface healing. Data are available from 5 studies, but only two found a beneficial effect of platelets. Vogrin et al saw a higher level of interface vascularization, which is essential for bone remodeling, at 3 months, but not 6 months(34). This “early stage” effect is consistent with the mode of action posited above. Orrego et al found a borderline significant positive effect of platelets at six months postoperatively, while all other studies reporting on tunnel healing found no differences (30). All studies but Vogrin et al assess tunnel healing at 6 months of follow-up, which is an interesting fact because earlier studies have suggested that graft integration is largely complete by 12 weeks(50).
Finally, we were interested in assessing whether differences in graft and tunnel healing, or the lack thereof, would translate into differences in clinical outcomes. The three studies that evaluated clinical outcomes used different assessment tools but uniformly reported no differences between groups. Two issues should be considered when interpreting these data. First, the rationale for the use of platelets is to improve (the speed of) graft maturation, which is not necessarily visible in clinical scores focusing on pain, range of motion, and activities-of-daily-living. Second, considering that the most likely “clinical” effect of improved graft and tunnel healing is reduced rates of clinical graft failure, the current studies would be critically underpowered to assess differences in this rare outcome (even if they had included assessment of such outcomes). However, the current best evidence indicates no difference in clinical outcomes between ACL reconstruction with or without platelet concentrates.
After summarizing the findings from the included studies, we also want to focus on the second, no less important task of a good systematic review, identifying blank spots in the literature and describing ways to address them and to improve and expand the available evidence. One of the most obvious differences and the most commonly raised question in the included primary studies was the concentration of the used platelet concentrate. On the one hand, if a beneficial effect is to be assumed, one could also assume a dose-response relationship and opt for higher dosages to maximize the treatment effect. On the other hand, overdosing of platelet-released growth factors can be detrimental for two reasons, first, because of an over stimulation of cells leading to a poorly differentiated, chaotic scar, and second, because some of the released growth factors might precipitate adverse events, such as suppression of osteoclast generation (51). While, to the best of our knowledge, there are no human studies to assess the dose-response relationship of platelet concentrates and ACL healing, data is available from large animal models, suggesting that 3× and 5× concentrates lead to equivalent biomechanical outcomes(52). Thus reducing platelet concentrations would be a safe way to minimize the potential for detrimental effects and the volume of blood needed from the patient without jeopardizing mechanical outcomes.
4.2 Limitations
This study has potential shortcomings. Like any systematic review the quality of this study depends on the quality of the primary data. We assessed the validity of these 8 studies based on levels-of-evidence and the Jadad score. Both methods are composite scores that focus on study design, such as randomization, concealed allocation, and attrition. The included studies scored in the middle to high range, which is representative for orthopedic research studies. However, neither score gives a comprehensive assessment of study validity, as they do not include parameters such as sample size estimation.
Beyond study design, another potential shortcoming is the clinical heterogeneity of the included studies. While age and gender distribution were uniform among all groups, as were postoperative rehabilitation algorithms, there were considerable differences in the used surgical techniques as well as the choice and placement of platelet concentrates. While some of these differences are only little, some might very well have major implications on healing, such as adding the platelet concentrate by injecting it on the graft under arthroscopy versus wrapping the graft in a biomaterial soaked in a platelet gel. As a matter of fact, these differences spurred a number of letters criticizing the lack of clarity when it comes to the description of the platelet concentrates uses (53)–after some authors have used platelet concentrates but referred to them as, for example, PDGF(29, 54). Interestingly, the authors of the included studies wrote letters commenting on the methods used in other studies, also included here, showing the extent of disagreement among investigators as what platelet concentration to use, and how to assess its effects(54). These differences stopped us from conducting a formal quantitative data synthesis, but it is still possible and valid to deduce evidence for a general, overall effect of platelet concentrates.
5. Conclusion
The current best evidence suggests that the addition of platelet concentrates to ACL reconstruction may have a beneficial effect on graft maturation and could improve it by 20–30% on average, but with substantial variability. The most likely mode of action is that treatment with platelets accelerates graft repopulation and remodeling, and this interpretation is supported by the existing data and biologically plausible. However, the current evidence also shows only a very limited influence of platelet concentrates on graft-bone interface healing and no significant difference in clinical outcomes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hall MP, Band PA, Meislin RJ, Jazrawi LM, Cardone DA. Platelet-rich plasma: current concepts and application in sports medicine. J Am Acad Orthop Surg. 2009 Oct;17(10):602–8. doi: 10.5435/00124635-200910000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez M, Anitua E, Orive G, Mujika I, Andia I. Platelet-rich therapies in the treatment of orthopaedic sport injuries. Sports Med. 2009;39(5):345–54. doi: 10.2165/00007256-200939050-00002. [DOI] [PubMed] [Google Scholar]
- 3.El-Sharkawy H, Kantarci A, Deady J, et al. Platelet-rich plasma: growth factors and pro- and anti-inflammatory properties. J Periodontol. 2007 Apr;78(4):661–9. doi: 10.1902/jop.2007.060302. [DOI] [PubMed] [Google Scholar]
- 4.Cenni E, Perut F, Ciapetti G, et al. In vitro evaluation of freeze-dried bone allografts combined with platelet rich plasma and human bone marrow stromal cells for tissue engineering. J Mater Sci Mater Med. 2009 Jan;20(1):45–50. doi: 10.1007/s10856-008-3544-9. [DOI] [PubMed] [Google Scholar]
- 5.Kasten P, Vogel J, Geiger F, Niemeyer P, Luginbuhl R, Szalay K. The effect of platelet-rich plasma on healing in critical-size long-bone defects. Biomaterials. 2008 Oct;29(29):3983–92. doi: 10.1016/j.biomaterials.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Akeda K, An HS, Okuma M, et al. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage. 2006 Dec;14(12):1272–80. doi: 10.1016/j.joca.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Ishida K, Kuroda R, Miwa M, et al. The regenerative effects of platelet-rich plasma on meniscal cells in vitro and its in vivo application with biodegradable gelatin hydrogel. Tissue Eng. 2007 May;13(5):1103–12. doi: 10.1089/ten.2006.0193. [DOI] [PubMed] [Google Scholar]
- 8.Akeda K, An HS, Pichika R, et al. Platelet-rich plasma (PRP) stimulates the extracellular matrix metabolism of porcine nucleus pulposus and anulus fibrosus cells cultured in alginate beads. Spine. 2006 Apr 20;31(9):959–66. doi: 10.1097/01.brs.0000214942.78119.24. [DOI] [PubMed] [Google Scholar]
- 9.Nagae M, Ikeda T, Mikami Y, et al. Intervertebral disc regeneration using platelet-rich plasma and biodegradable gelatin hydrogel microspheres. Tissue Eng. 2007 Jan;13(1):147–58. doi: 10.1089/ten.2006.0042. [DOI] [PubMed] [Google Scholar]
- 10.Everts PA, Knape JT, Weibrich G, et al. Platelet-rich plasma and platelet gel: a review. J Extra Corpor Technol. 2006 Jun;38(2):174–87. [PMC free article] [PubMed] [Google Scholar]
- 11.Vavken P, Murray MM. Translational studies in anterior cruciate ligament repair. Tissue Eng Part B Rev. 2010 Feb;16(1):5–11. doi: 10.1089/ten.teb.2009.0147. [DOI] [PubMed] [Google Scholar]
- 12.Vavken P, Murray MM. The Potential for Primary Repair of the ACL. Sports Med Arthrosc. 2011 Mar;19(1):44–9. doi: 10.1097/JSA.0b013e3182095e5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE., Jr Anterior Cruciate Ligament Reconstruction Autograft Choice: Bone-Tendon-Bone Versus Hamstring: Does It Really Matter? A Systematic Review. Am J Sports Med. 2004 December 1;32(8):1986–95. doi: 10.1177/0363546504271211. [DOI] [PubMed] [Google Scholar]
- 14.Spindler KP, Wright RW. Clinical practice. Anterior cruciate ligament tear. N Engl J Med. 2008 Nov 13;359(20):2135–42. doi: 10.1056/NEJMcp0804745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiler A, Hoffmann RF, Bail HJ, Rehm O, Sudkamp NP. Tendon healing in a bone tunnel. Part II: Histologic analysis after biodegradable interference fit fixation in a model of anterior cruciate ligament reconstruction in sheep. Arthroscopy. 2002 Feb;18(2):124–35. doi: 10.1053/jars.2002.30657. [DOI] [PubMed] [Google Scholar]
- 16.Weiler A, Peine R, Pashmineh-Azar A, Abel C, Sudkamp NP, Hoffmann RF. Tendon healing in a bone tunnel. Part I: Biomechanical results after biodegradable interference fit fixation in a model of anterior cruciate ligament reconstruction in sheep. Arthroscopy. 2002 Feb;18(2):113–23. doi: 10.1053/jars.2002.30656. [DOI] [PubMed] [Google Scholar]
- 17.Amiel D, Kleiner JB, Akeson WH. The natural history of the anterior cruciate ligament autograft of patellar tendon origin. Am J Sports Med. 1986 Nov-Dec;14(6):449–62. doi: 10.1177/036354658601400603. [DOI] [PubMed] [Google Scholar]
- 18.Amiel D, Kleiner JB, Roux RD, Harwood FL, Akeson WH. The phenomenon of “ligamentization”: anterior cruciate ligament reconstruction with autogenous patellar tendon. J Orthop Res. 1986;4(2):162–72. doi: 10.1002/jor.1100040204. [DOI] [PubMed] [Google Scholar]
- 19.Alsousou, Thompson, Hulley, Noble, Willett The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: a review of the literature. The Journal of bone and joint surgery British volume. 2009 Aug 1;91(8):987–96. doi: 10.1302/0301-620X.91B8.22546. [DOI] [PubMed] [Google Scholar]
- 20.Taylor DW, Petrera M, Hendry M, Theodoropoulos JS. A Systematic Review of the Use of Platelet-Rich Plasma in Sports Medicine as a New Treatment for Tendon and Ligament Injuries. Clinical journal of sport medicine: official journal of the Canadian Academy of Sport Medicine. 2011 May 10; doi: 10.1097/JSM.0b013e31821d0f65. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999 Nov 27;354(9193):1896–900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 23.Jadad A, Moore R, Carrol D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trial. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 24.Jadad AR, Cook DJ, Browman GP. A guide to interpreting discordant systematic reviews. CMAJ. 1997 May 14;156(10):1411–6. [PMC free article] [PubMed] [Google Scholar]
- 25.Jadad AR, Cook DJ, Jones A, et al. Methodology and reports of systematic reviews and meta-analyses: a comparison of Cochrane reviews with articles published in paper-based journals. JAMA. 1998 Jul 15;280(3):278–80. doi: 10.1001/jama.280.3.278. [DOI] [PubMed] [Google Scholar]
- 26.Emerson J, Burdick E, Hoaglin D, Mosteller F, Chalmers T. An empirical study of the possible relation of treatment differences to quality scores in controlled randomized clinical trials. Control Clin Trial. 1990;11(5):339–52. doi: 10.1016/0197-2456(90)90175-2. [DOI] [PubMed] [Google Scholar]
- 27.Zhao Y, Zhai W. [Histological observation of tendon-bone healing after anterior cruciate ligament reconstruction by platelet-rich plasma combined with deproteinized bone of calf] Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011 Nov;24(11):1323–9. [PubMed] [Google Scholar]
- 28.Figueroa D, Melean P, Calvo R, et al. Magnetic resonance imaging evaluation of the integration and maturation of semitendinosus-gracilis graft in anterior cruciate ligament reconstruction using autologous platelet concentrate. Arthroscopy. 2010 Oct;26(10):1318–25. doi: 10.1016/j.arthro.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Nin JR, Gasque GM, Azcarate AV, Beola JD, Gonzalez MH. Has platelet-rich plasma any role in anterior cruciate ligament allograft healing? Arthroscopy. 2009 Nov;25(11):1206–13. doi: 10.1016/j.arthro.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Orrego M, Larrain C, Rosales J, et al. Effects of platelet concentrate and a bone plug on the healing of hamstring tendons in a bone tunnel. Arthroscopy. 2008 Dec;24(12):1373–80. doi: 10.1016/j.arthro.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 31.Radice F, Yanez R, Gutierrez V, Rosales J, Pinedo M, Coda S. Comparison of magnetic resonance imaging findings in anterior cruciate ligament grafts with and without autologous platelet-derived growth factors. Arthroscopy. 2010 Jan;26(1):50–7. doi: 10.1016/j.arthro.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez M, Anitua E, Azofra J, Prado R, Muruzabal F, Andia I. Ligamentization of tendon grafts treated with an endogenous preparation rich in growth factors: gross morphology and histology. Arthroscopy. 2010 Apr;26(4):470–80. doi: 10.1016/j.arthro.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 33.Silva A, Sampaio R, Pinto E. Femoral tunnel enlargement after anatomic ACL reconstruction: a biological problem? Knee Surg Sports Traumatol Arthrosc. 2010 Sep;18(9):1189–94. doi: 10.1007/s00167-010-1046-z. [DOI] [PubMed] [Google Scholar]
- 34.Vogrin M, Rupreht M, Dinevski D, et al. Effects of a platelet gel on early graft revascularization after anterior cruciate ligament reconstruction: a prospective, randomized, double-blind, clinical trial. Eur Surg Res. 2010;45(2):77–85. doi: 10.1159/000318597. [DOI] [PubMed] [Google Scholar]
- 35.Ventura A, Terzaghi C, Bordo E, Verdoia C, Gallazi M, Failoni S. Use of growth factors in ACL surgery: preliminary study. J Orthopaed Traumatol. 2005;6:76–9. [Google Scholar]
- 36.Griffin L, Albohm M, Arendt E. Understanding and preventing noncontact anterior cruciate ligament injuries. Am J Sports Med. 2006;34:1512–32. doi: 10.1177/0363546506286866. [DOI] [PubMed] [Google Scholar]
- 37.Silvers HJ, Mandelbaum BR. Prevention of anterior cruciate ligament injury in the female athlete. Br J Sports Med. 2007 Aug;41(Suppl 1):i52–9. doi: 10.1136/bjsm.2007.037200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abe S, Kurosaka M, Iguchi T. Light and electron microscopic study of remodeling and maturation process in autogenous graft for anterior cruciate ligament reconstruction. Arthroscopy. 1993;9:394–405. doi: 10.1016/s0749-8063(05)80313-5. [DOI] [PubMed] [Google Scholar]
- 39.Johnson L. The outcome of a free autogenous semitendinosus tendon graft in human anterior cruciate reconstructive surgery: a histological study. Arthroscopy. 1993;9:131–42. doi: 10.1016/s0749-8063(05)80362-7. [DOI] [PubMed] [Google Scholar]
- 40.Cho S, Muneta T, Ito S. Electron microscopic evaluation of two-bundle anatomically reconstructed anterior cruciate ligament graft. J Orthop Sci. 2004;9:296–301. doi: 10.1007/s00776-004-0779-2. [DOI] [PubMed] [Google Scholar]
- 41.Falconiero R, DiStefano V, Cook T. Revascularization and ligamentization of autogenous anterior cruciate ligament grafts in humans. Arthroscopy. 1998;14:197–205. doi: 10.1016/s0749-8063(98)70041-6. [DOI] [PubMed] [Google Scholar]
- 42.Howell S, Knox K, Farley T, Aylor TM. Revascularization of a human anterior cruciate ligament graft during the first two years of implantation. Am J Sports Med. 1995;23(42–49) doi: 10.1177/036354659502300107. [DOI] [PubMed] [Google Scholar]
- 43.Gomez-Barrena E, Bonsfills N, Martin JG, Ballesteros-Masso R, Foruria A, Nunez-Molina A. Insufficient recovery of neuromuscular activity around the knee after experimental anterior cruciate ligament reconstruction. Acta Orthop. 2008 Feb;79(1):39–47. doi: 10.1080/17453670710014743. [DOI] [PubMed] [Google Scholar]
- 44.Lanzetta A, Corradini C, Verdoia C, Miani A, Castano S, Castano P. The nervous structures of anterior cruciate ligament of human knee, healthy and lesioned, studied with confocal scanning laser microscopy. Ital J Anat Embryol. 2004 Jul-Sep;109(3):167–76. [PubMed] [Google Scholar]
- 45.Adachi N, Ochi M, Uchio Y, Iwasa J, Ryoke K, Kuriwaka M. Mechanoreceptors in the anterior cruciate ligament contribute to the joint position sense. Acta Orthop Scand. 2002 Jun;73(3):330–4. doi: 10.1080/000164702320155356. [DOI] [PubMed] [Google Scholar]
- 46.Ochi M, Iwasa J, Uchio Y, Adachi N, Sumen Y. The regeneration of sensory neurones in the reconstruction of the anterior cruciate ligament. J Bone Joint Surg Br. 1999 Sep;81(5):902–6. doi: 10.1302/0301-620x.81b5.9202. [DOI] [PubMed] [Google Scholar]
- 47.Valeriani M, Restuccia D, Di Lazzaro V, Franceschi F, Fabbriciani C, Tonali P. Clinical and neurophysiological abnormalities before and after reconstruction of the anterior cruciate ligament of the knee. Acta Neurol Scand. 1999 May;99(5):303–7. doi: 10.1111/j.1600-0404.1999.tb00680.x. [DOI] [PubMed] [Google Scholar]
- 48.Altman DG, Bland JM. Absence of evidence is not evidence of absence. BMJ. 1995 Aug 19;311(7003):485. doi: 10.1136/bmj.311.7003.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lochner HV, Bhandari M, Tornetta P., 3rd Type-II error rates (beta errors) of randomized trials in orthopaedic trauma. J Bone Joint Surg Am. 2001 Nov;83-A(11):1650–5. doi: 10.2106/00004623-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Ekdahl M, Wang JH, Ronga M, Fu FH. Graft healing in anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2008 Oct;16(10):935–47. doi: 10.1007/s00167-008-0584-0. [DOI] [PubMed] [Google Scholar]
- 51.Cenni E, Avnet S, Fotia C, Salerno M, Baldini N. Platelet-rich plasma impairs osteoclast generation from human precursors of peripheral blood. Journal of Orthopaedic Research2010 Jun Res. 2010 Jun;28(6):792–7. doi: 10.1002/jor.21073. [DOI] [PubMed] [Google Scholar]
- 52.Mastrangelo A, Vavken P, Fleming B, Harrison S, Murray M. Reduced platelet concentration does not harm PRP effectiveness for ACL repair in a porcine in vivo model. J Orthop Res. 2011;20(7):1002–7. doi: 10.1002/jor.21375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dines JS. Growth factor confusion. Arthroscopy. Sep;26(9):1144. doi: 10.1016/j.arthro.2010.06.020. author reply 5–6. [DOI] [PubMed] [Google Scholar]
- 54.Sanchez M, Anitua E, Andia I. Poor standardization in platelet-rich therapies hampers advancement. Arthroscopy. Jun;26(6):725–6. doi: 10.1016/j.arthro.2010.03.002. author reply 6. [DOI] [PubMed] [Google Scholar]