Abstract
Sepsisis a clinical syndrome characterized by a multi-system response to a microbial pathogenic insult consisting of a mosaic of interconnected biochemical, cellular, and organ-organ interaction networks. A central thread that connects these responses is inflammation, which, while attempting to defend the body and prevent further harm, causes further damage through the feed-forward, pro-inflammatory effects of damage-associated molecular pattern molecules. In this review, we address the epidemiology and current definitions of sepsis, and focus specifically on the biological cascades that comprise the inflammatory response to sepsis. We suggest that attempts to improve clinical outcomes by targeting specific components of this network have been unsuccessful due to the lack of an integrative, predictive, and individualized systems-based approach to define the time-varying, multi-dimensional state of the patient. We highlight the translational impact of computational modeling and other complex systems approaches as applied to sepsis, including in silico clinical trials, patient-specific models, and complexity-based assessments of physiology.
Keywords: Sepsis, Inflammatory Response, Physiologic Variability, Mathematical Model
INTRODUCTION
Sepsis is a significant public health concern1–3. Though “septicemia” accounts for approximately 1% of overall deaths in the U.S.4, the number is much larger (nearly 10%) when factoring in deaths from pneumonia and other causes of severe sepsis. Sepsis affects persons of all ages,5 is the leading cause of morbidity and mortality for patients admitted to an intensive care unit (ICU), and may be considered the 10th leading cause of death overall in the United States. The incidence of sepsis is projected to increase by 1.5% per year, rising to more than 1,110,000 cases or more annually by 20202. Sepsis also reduces the quality of life of many of those who survive.
Despite a large body of scientific literature concerning individual mechanisms of disease in sepsis – implicating organ dysfunction caused by failure of key processes in epithelial cells and involving various biological mechanisms from endothelial defects to dysregulated inflammation and the associated complement and coagulation networks - there are few therapies and relatively imprecise diagnostics for sepsis. In the present review, we suggest that our view of sepsis has evolved from a general concept, to that of rigidly defined (but seldom absolute) diagnoses, to a more fluid perception of sepsis as a dynamic progression of host-pathogen interactions that can be assessed by examining the dynamic, multi-dimensional state of the patient (Fig. 1). By “dynamic” we mean time-varying, and by “state” we mean literally the compendium of all relevant variables that can inform a clinician about the pathophysiology and biological state of a patient at a given instant. Importantly, a characterization of this “dynamic state” could be considered sufficiently complete when the data contained therein are sufficient for prediction of the future course of the patient. Thus, the concept of “dynamic state” encompasses the idea that the data that make up this state are sufficient to predict the progression of a patient for a clinically appropriate future duration, and that these data are of sufficient granularity to reflect all relevant biological and physiological processes and therefore amenable for analysis using predictive computational models and algorithms. Though potentially daunting in scope, we suggest that the concept of the dynamic state underlies a modern, mechanistic view of a very old disease, one that better encompasses the progression of this disease in individual patients rather than focusing on rigid, pre-defined diagnoses.
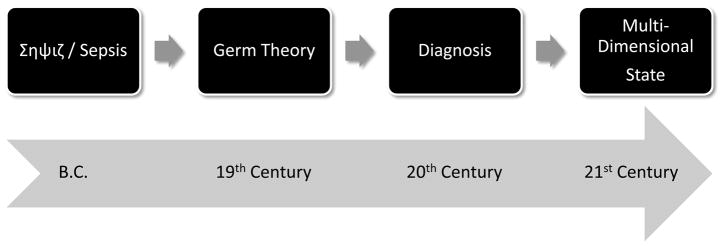
Figure 1. Sepsis: A brief history.
Our concept of sepsis has progressed from phenomenological description (antiquity) to rigid diagnostic criteria (20th century). The future holds the potential for individualized, predictive, and multi-dimensional description of the patient’s state.
SEPSIS: A BRIEF HISTORY
The difficulty in developing mechanistically-based diagnoses has hampered our understanding of the pathophysiology of sepsis, and to a certain degree has impaired the development of successful therapies. In beginning to unravel the mechanistic underpinnings of sepsis with the goals of improved diagnosis and therapy, it is useful to trace the dynamic evolution of our concepts concerning this disease. Starting from the origins of the word “sepsis” approximately 2700 years ago in the Greek word “σηψιs” (the “decomposition of animal or vegetable organic matter in the presence of bacteria”19), our view of sepsis has progressed through various stages. The initial view of the process was encapsulated in the Germ Theory, in which pathogens were the sole causes of sepsis20–22. Subsequent advances led to the development of fairly rigid diagnostic guidelines based on the host’s response to infection, guidelines developed in part in response to the inability to curb sepsis solely through therapy aimed at the pathogen (see below). The most recent development has been the emergence of a multi-scale, systems perspective of host-pathogen interactions at the organ, tissue, cellular, and molecular levels (Fig. 1)23–31. Below, we focus on this most recent development.
By 1990, after approximately 20 years of intensive care and 40 years of anti-sepsis therapies based on the Germ Theory suggested that many patients could die despite antibiotics and life support, the notion emerged that the host’s intertwined inflammatory and physiological responses to the pathogen were at least as much to blame as the pathogen itself32. In 1991, the American College of Chest Physicians (ACCP) and the Society of Critical Care Medicine (SCCM) agreed on a new definition of sepsis as the development of a systemic inflammatory response syndrome (SIRS) due to infection. The severity of sepsis was graded based on the development of hemodynamic compromise and associated organ dysfunction as follows: severe sepsis, septic shock, and multiple organ dysfunction syndrome (MODS)33. These definitions served as a more uniform reference point for performing clinical trials, facilitating hypothesis generation, and establishing guidelines for the care of the septic patient (Fig. 1). The 1991 North American Consensus Conference concept of SIRS is now considered outdated, however, and the four SIRS criteria have been expanded to a longer list of possible signs of sepsis in the latest definitions34. These were developed in 2001, under the auspices of the SCCM, the European Society of Intensive Care Medicine, the ACCP, and the Surgical Infection Societies. The conferees concluded that the diagnostic criteria for SIRS were overly sensitive and nonspecific, and that a more comprehensive list of signs and symptoms that may accompany sepsis would better reflect the clinical response to infection34. In addition, a staging system was proposed for the purpose of incorporating both host factors and response to a particular infectious insult. This concept, termed PIRO (predisposition, infection, response, organdysfunction )34 represents an attempt to include the patient’s response to treatment in the diagnosis.
As important as these advances have been, we suggest that these metrics and criteria remain too imprecise to move beyond identifying population tendencies, and are removed from the increasing mechanistic knowledge being generated. We have progressed in our understanding of sepsis to include high-dimensional genomic and proteomic datasets, signal processing techniques that assist in creating diagnostic sense from chaotic physiological data, and mechanistic mathematical modeling based on pre-clinical and clinical data. This increased resolution of knowledge regarding the pathophysiology of sepsis has offered the promise of more precise characterization of the disease. These advances have also raised the possibility of defining the multi-dimensional “state” of an individual sepsis patient, based on direct measurements of the molecules that orchestrate the interplay among infection, inflammation, and organ dysfunction (Fig. 1)46. As we discuss below, these emerging approaches may help define sepsis in a more precise fashion (Fig. 2) that includes detailed, dynamic physiologic and molecular characteristics of patient sub-groups, and, eventually, of individuals.
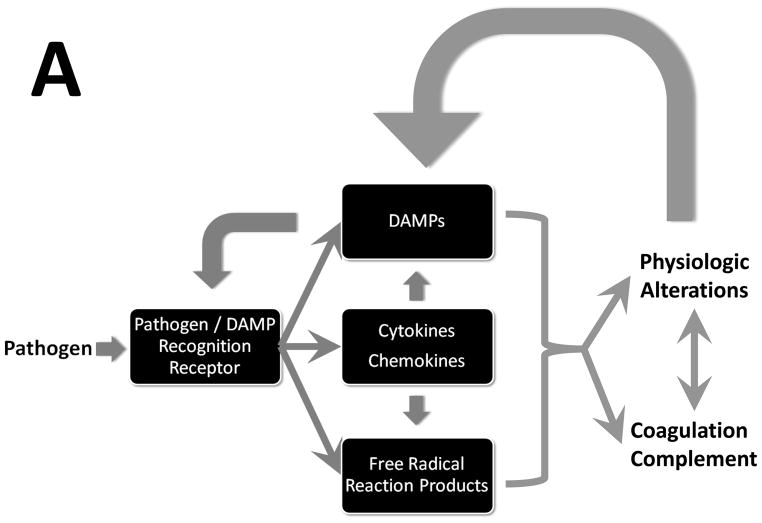
Figure 2. Sepsis: A process flow.
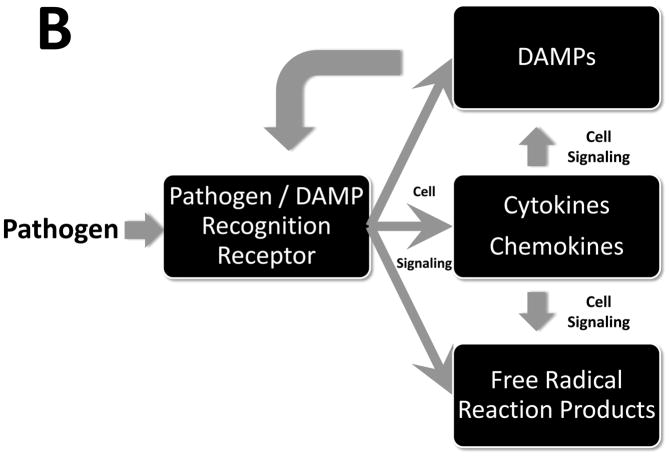
Upon stimulation by pathogens, a multifaceted inflammatory response ensues, driven by cytokines, free radical reaction products, and damage-associated molecular patterns (DAMPs). Panel A: The inflammatory response affects, and is affected by, interactions with physiological systems (manifest as reduced physiological variability) and the coagulation and complement cascades. Panel B: The acute inflammatory response is sensed via defined receptors for both pathogen-derived products and DAMPs, and modulated via intracellular signaling pathways.
SEPSIS: A PROCESS FLOW
Just as the conceptual evolution of sepsis has been dynamic, we now appreciate that the pathogenesis of sepsis involves a dynamic, complex process of cellular activation resulting in the the activation of neutrophils, monocytes and microvascular endothelial cells; the triggering of neuroendocrine mechanisms; and activation of the complement, coagulation, and fibrinolytic systems. Acute inflammation is a central mechanism that helps connect these processes across time and space (Fig. 2A). The innate immune response recognizes the presence of invading pathogens, acts towards initial containment, recruits additional cells to eliminate the pathogens and, concurrently, involves feedback mechanisms that serve to limit and restrict the pro-inflammatory component such that homeostatic dynamic equilibrium can be re-established49. These factors function in a series of interlinked and overlapping networks, suggesting that “inflammation is communication”50. Like any situation that involves communication, the content, tone, and context matters a great deal. On the one hand, an appropriately robust inflammatory response is necessary to survive diverse insults both in the very short and long term51. It is important to note that though organs obtained from sepsis patients post mortem may not exhibit histological damage52, these organs are nonetheless dysfunctional as a result of various defects that manifest, at the cellular level, in both epithelial53 and endothelial cells. We suggest that this dysfunction occurs due to a positive feedback loop in which inflammation induced by pathogen-derived signals leads to the release from epithelial and endothelial cells of Damage-Associated Molecular Pattern (DAMP) molecules, the molecular messengers of tissue damage. In turn, these “danger signals” stimulate nearby inflammatory cells to produce more of the classical inflammatory mediators, leading to further release of DAMP’s and ultimately to self-maintaining inflammation even after the pathogen has been cleared (Fig. 2B). The body is equipped to suppress inflammation and drive cell/tissue/organ healing both through the production of anti-inflammatory mediators as well as through an inherent suppression of pro-inflammatory signaling (referred to as tolerance or desensitization). However, in progressive sepsis, these anti-inflammatory influences are either insufficient to suppress self-maintaining inflammation, or are over-produced and lead to an immunosuppressed state.
In the following sections, we will describe some of these components and place them into an appropriate context. It should be noted that presenting the information requires a linear structure; this should in no way obscure the complex dynamic actuality of the system in reality (Fig. 2). We suggest that the key to developing effective diagnostics and treatments for sepsis requires effective characterization of the architecture and dynamics of the inflammatory system from a mechanistic standpoint.
Pathogen Recognition
The innate immune system is a highly evolutionarily conserved host defense mechanism against pathogens57, though an alternative viewpoint suggests that this system evolved in order to respond to trauma and injury (see below)58. Innate immune responses to pathogens are initiated by pattern recognition receptors (PRRs), which recognize specific structures of microorganisms (Fig. 2). At least four families of PRRs are recognized: Toll-like receptors (TLRs); nucleotide oligomerization domain leucine-rich repeat (NOD-LRR) proteins; cytoplasmic caspase activation and recruiting domain helicases such as retinoic-acid-inducible gene I (RIG-I)-like helicases (RLHs); and C-type lectin receptors expressed on dendritic and myeloid cells. Bacteria and viruses have molecular structures that are: generally not shared with their host, common among related pathogens, and invariant. These molecular signatures are also expressed by nonpathogenic and commensal bacteria59 and are now referred to as pathogen-associated molecular pattern (PAMP) molecules or microbial-associated molecular pattern (MAMP) molecules. Though further studies are necessary to fully elucidate this phenomenon, the degree to which a given sepsis patient responds to a given PAMP may be in part controlled by single nucleotide polymorphisms (SNPs) in PRR’s such as TLR4 and related molecules such as CD14. In general, all sepsis patients manifest robust inflammatory responses to PAMPs, unless they have specific genetic deficiencies in relevant intracellular signaling molecules66.
Inflammatory Signal Transduction
Inflammatory signals are transduced by a series of adaptor molecules that bind to the PRRs and protein kinases and phosphatases that control signal propagation in the cytoplasm, culminating either in the rapid, post-transcriptional or post-translational modulation of a variety of inflammatory mediators, or in the activation of various transcription factors (Fig. 2B). These factors include nuclear factor-κB (NF-κB), activator protein-1 (AP-1), members of the CCAAT-enhancer-binding protein (C/EBP) family, Early Growth Response Protein 1 (EGR-1), p53 and Signal Transducer and Activator of Transcription 1 (STAT1). These mechanisms have been the subject of considerable study and have been reviewed extensively elsewhere.
Production of “Classical” Inflammatory Mediators
A wide variety of cytokines and effector molecules such as reactive oxygen and nitrogen species are produced as a consequence of the receptor binding and signaling events described above (Fig. 2B). Many of these mediators and their actions in sepsis have been studied for two decades and have been discussed extensively elsewhere. It is important to note that among the earliest mediators to be tested both experimentally and clinically in sepsis, tumor necrosis factor alpha (TNF-α) continues to be of interest from a systems perspective, since it is central to a well-studied positive feedback loop that augments both further production of TNF-α as well as numerous other inflammatory mediators. TNF-α also helps drive the production of anti-inflammatory cytokines. In patients with established SIRS, both pro-inflammatory cytokines and anti-inflammatory species co-exist in the circulation in markedly increased amounts, the simultaneous presence of both pro-inflammatory cytokines and their counter-regulators has been associated with adverse outcomes. Though the phenotype of systemic inflammation can be recapitulated by exogenous administration of TNF-α, a series of failed anti-cytokine clinical trials - as well as subsequent studies demonstrating the beneficial and necessary roles of TNF-α in a well-balanced inflammatory response – has led to a retrospective recognition of the individual- and context-specific interplay of pro- and anti-inflammatory mediators.
DAMP’s and “late mediators” of sepsis
As has been recently appreciated, intracellular molecules (e.g. intracellular proteins or fragments thereof, DNA, and even inorganic crystals) that are expressed or released following host tissue injury are endogenous equivalents of PAMPs. These molecules are known as alarmins or Damage-associated Molecular Patterns (DAMPs). DAMPs, like PAMPs, also bind to PRRs either expressed on the surface of immune cells or present intracellularly (Fig. 2). In the setting of sepsis, high mobility group protein B1 (HMGB1), a nuclear protein that stabilize nucleosome formation in almost all eukaryotic cells, is a key DAMP85–87 and a therapeutic target. HMGB1 is but one member of a growing list of DAMP’s89. For example, mitochondrial DNA and other products were recently reported to be DAMPs in the setting of trauma.90
While extensive, reductionist studies have yielded a tremendous amount of data and the potential for sepsis therapies directed against inflammatory cytokines74, TLR’s91–93 DAMP’s such as HMGB194–96, and mediators of inflammatory signal transduction97–99, we suggest that the development and implementation of such therapies will require an understanding of the complexity of the myriad actions and interactions of these ligands and receptors (Fig. 3)100–102.
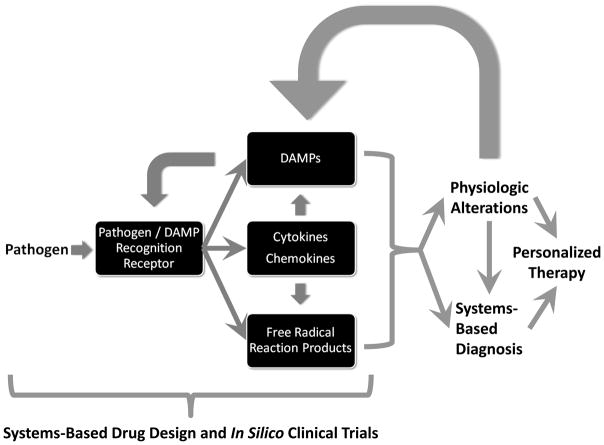
Figure 3. Towards multi-dimensional, individualized description of patient state.
The future of sepsis diagnosis and therapy will depend on a growing understanding of the cellular and molecular mechanisms of inflammation by which pathogens are sensed and eliminated, along with the effects of inflammation on physiology and vice versa. These interactions will form the basis of computational models used for rational design of drugs and the clinical trials by which those drugs are tested. Multi-dimensional analysis of inflammation biomarkers and physiologic waveforms, along with mechanistic mathematical modeling, may aid in discerning individual patient states for the purposes of diagnosis and therapy.
CHANGES IN PHYSIOLOGY DURING SEPSIS: INSIGHTS FROM COMPLEX SYSTEMS
Inflammation-induced organ dysfunction is a hallmark of sepsis. From a systems perspective, it has been hypothesized for over 15 years that these oscillatory systems are coupled, and that the disruption of this coupling is a hallmark of sepsis38. Both experimental and clinical studies have suggested that one measure of this disrupted oscillatory coupling is reduced variability (or increased regularity) in various physiologic signals, chief among them being heart rate (Fig. 3)103–105. Time-domain analysis of heart rate variability (HRV) has subsequently evolved as a potential non-invasive diagnostic modality for sepsis106. These data can also be used indirectly to detect variability attributed to sympathetic and parasympathetic branches of the autonomic nervous system as well as other physiological processes that affect heart rate, including respiration, blood pressure, and temperature106. Using these sophisticated signal processing techniques, various studies have reported that a decrease in HRV indices may be potentially diagnostic of higher morbidity and mortality in critically ill patients. In addition to HRV, examination of other physiologic parameters from a complex systems approach has also yielded valuable insights into the physiology of sepsis. For example, changes in ventilation and breath-to-breath variability occur with sepsis, particularly in the setting of respiratory failure. Multiple mechanisms have been implicated including increased central drive and increased metabolic requirements115, as well the cyclooxygenase pathway116. Furthermore, these changes in breathing and heart rate variability have implications for heart-lung interactions in sepsis. However, we are still far from a complete understanding of the iterative, recursive interactions between inflammation and HRV. Based on prior work in animal models of sepsis (F.J. Jacono and T.E. Dick, unpublished observations), we hypothesize that pro-inflammatory cytokines such as IL-1β and TNF-α act to decrease HRV and breathing pattern variability by affecting the central nervous system in septic patients, and that in turn reduced physiological variability further stimulates inflammation. If this hypothesis is correct, a systems understanding may allow us to unify the pattern-based, diagnostically relevant use of physiological waveforms with the increasingly detailed, mechanistic understanding of acute inflammation in order to improve therapy for sepsis (Fig. 3).
Renewed interest in the diagnostic utility of metrics such as HRV in the setting of trauma and sepsis suggests that these methods may reach clinical utility in the near future (see the recent 9th International Conference on Complexity in Acute Illness; http://www.iccai.org/sci_info_2010.php). We suggest that in order for the analyses of physiologic variability to progress beyond pattern analysis, a mechanistic understanding of how sepsis results in reduced physiologic variability is required. We further suggest that the inflammatory mechanisms described above will affect physiologic function (and will therefore manifest as changes in indices of physiologic variability); in turn, these changes in physiology will impact the inflammatory response (Fig. 3).
DECIPHERING THE NONLINEAR PROCESS FLOW OF SEPSIS VIA COMPUTATIONAL SIMULATIONS
Despite promising results at the basic science and pre-clinical level, large-scale trials of therapies targeted at inhibiting specific inflammatory mediators have generally failed to improve survival118. Above, we have discussed the growing recognition that inflammation and the physiology to which it is coupled demonstrates complex, nonlinear behavior. This property significantly limits the intuitive extrapolation to system/patient level effects of mechanistic knowledge derived from basic science. Reductionism has been successful when applied to systems whose behavior can be reduced to a “linear” (i.e. single direct relationship) representation such that the results of various independent experiments can be aggregated additively to obtain and predict the behavior of the system as a whole28. However, systems such as the acute inflammatory response, that have multiple feedback loops and saturating dose response kinetics, are inherently nonlinear. The nonlinear interactions among pathogen recognition elements, signal transduction pathways, inflammatory mediators, DAMP’s, and the physiologic processes that they collectively impact (Fig. 2A) require more sophisticated mathematical representation for their characterization23–31.
Systems biology approaches may offer a solution to understanding these interactions. For addressing complex biological processes such as the acute inflammatory response in sepsis28, both the NIH in its Roadmap Initiative (http://nihroadmap.nih.gov/) and the FDA in its “Critical Path” document have called for the use of in silico (computer) models to augment preclinical animal studies in order to develop novel therapies120. In silico studies use the growing power of digital computers to mine large databases in search of patterns that either elucidate mechanisms or that are diagnostically useful121. Mathematical models of physiology characterize the evolution of observables over time, and are therefore dynamic. Their purpose is predictive description—to provide entailment and insight into what the future state of the system might be, given knowledge of the current state of the system. This dynamic property suggests that mathematical models can be considered as testable hypotheses. When a mathematical model predicts measurable behavior that emulates the physiology under study, one can reasonably infer that the mathematical model has, in fact, captured potentially useful interrelations28. Conversely, when model and experiment disagree, the assumptions encapsulated in the model must be reassessed (it should be noted that this process is not limited to mathematical models). Therefore, transparency in model construction is critical, such that the assumptions underlying the model can be examined in detail. Since behavioral nonlinearities and high-dimensional parameter spaces represent challenges in the calibration of such models to experimental data, and since such data fitting is further impaired by uncertainty and variability of sparse observations (especially in settings of preclinical and clinical studies)122, the formalisms associated with mathematical models may provide a framework in which underlying hypotheses can be more effectively examined and modified.
There have been notable successes in the translational applications of mechanistic mathematical models of acute inflammation as applied to sepsis, trauma, and wound healing. On a purely theoretical level, simple models of acute inflammation have suggested that morbidity and mortality in sepsis may arise from diverse insult- and patient-specific circumstances such as pathogen number and virulence (i.e. degree to which pathogens stimulate a pro-inflammatory response), as well as the degree to which DAMPs are produced in response to both the pathogen and pro-inflammatory mediators123. Mathematical models of some of the inflammatory signal-transduction cascades described above may help drive mechanism-based drug discovery and device development, namely for the demonstration of likely efficacy throughout the development process; augmentation of and integration with existing experimental data sets directed towards drug/device development; and the execution of simulated clinical trials, both to facilitate the planning of future clinical trials.124–126 (Fig. 3). Other mathematical models were used to yield insights into the acute inflammatory response in diverse shock states (most importantly the suggestion that a common “wiring framework” but different initial conditions could account for diverse manifestations of endotoxemic vs. hemorrhagic shock)127–132, as well as the responses to anthrax133 and necrotizing enterocolitis134. At the pre-clinical level, mathematical modeling has helped define and predict the acute inflammatory responses of experimental animals and humans138, all crucial advances if we are to bridge the gap from imperfect pre-clinical animal models to the setting of human sepsis.
Initial translational successes of mathematical models involved the ability to reproduce (and suggest improvements to) clinical trials in sepsis (Fig. 3); these successes have been extended to the design of prospective clinical trials. One in silico clinical trial platform (Immunetrics, Inc.) was recently augmented to include a multi-scale, equation-based mechanistic simulation that encompasses dynamic interactions among multiple tissues, immune cells, and inflammatory mediators, along with a “virtual clinician” (an automated system to examine simulated patients’ status at clinically relevant intervals and administer standard of care interventions as necessary). This mathematical model was fit to time course data consisting of various biomarkers and clinical markers, both inflammatory and physiologic variables from published human endotoxemia studies141 as well and community-acquired severe sepsis patients in the Genetic and Inflammatory Markers of Sepsis (GenIMS)77 study. This model was capable of reproducing the entire spectrum of patients in the GenIMS study by altering only a handful of parameters related to pathogen, antibiotic efficacy and baseline patient status. The model simulations provided evidence of changes in disease progression and likelihood of survival as a function of various treatments, patient stratification on the basis of outcome and time of death, and predictive ability for patient outcome beyond hospital discharge. Model training and optimization led to the identification of a minimum set of temporal analyte data required to predict future trajectories and outcomes of patients (S. Chang, Y. Vodovotz, J.A. Kellum, and D.C. Angus, unpublished observations). We suggest that computational platforms such as this one could usher in a new era of rationally-designed drugs, as well as informing the design of future clinical trials (Fig. 3).
From a potentially diagnostic standpoint (and in accord with clinical findings described above), studies involving mechanistic mathematical models of sepsis suggest that detrimental outcomes are accompanied by the simultaneous elevation of both pro- and anti-inflammatory mediators. Moreover, recent mathematical modeling studies have begun to lay the foundation for a similar mechanistic underpinning for the interactions between inflammation and HRV. Furthermore, insights provided by data-driven modeling approaches such as Principal Component Analysis (PCA) in individual trauma/sepsis patients suggest that characteristic profiles of pro- and anti-inflammatory cytokines are both central drivers of the pathology of severely ill patients, and that Principal Cytokine “barcodes” may be of diagnostic potential even though raw cytokine data may not. These advances in the use of mathematical modeling and related systems approaches hold the potential to change the way drugs and diagnostics are developed and clinical trials are designed and carried out, all based on rational, mechanistic, and, in a sense “predictable” underpinnings.
CONCLUSIONS AND FUTURE PROSPECTS
We have come quite a long way since the early attempts at mathematical modeling in sepsis were met with both hope145 and skepticism121. The septic response, a complex chain of events involving pro- and anti-inflammatory processes, humoral and cellular reactions, and microcirculatory alterations, requires that we move away from a biomarker search (whether biological or physiological158–161). Although many of these individual markers have shown merit in defined cohorts, we suggest that merely sorting through an ever-growing array of biomarkers and metrics of physiological signals will not solve the problem of accurate diagnosis or prediction of treatment efficacy. Rather, mechanistically-oriented computational simulation and modeling may be a means for reconciling the diverse attempts at diagnosis of sepsis, as well as providing a rational framework for the design of new, personalized therapies (Fig. 3). Though substantial additional work is needed, we suggest that computational modeling can facilitate the transition from static diagnosis towards a dynamic definition of the state of the individual septic patient.
Acknowledgments
This work was supported in part by the National Institutes of Health grants R01GM67240, P50GM53789, R33HL089082, R01HL080926, R01AI080799, R01HL76157, 3R01GM034695-25S1, and R01GM082974; National Institute on Disability and Rehabilitation Research grant H133E070024; the VA Research Service; as well as grants from the Commonwealth of Pennsylvania, the Pittsburgh Lifesciences Greenhouse, and the Pittsburgh Tissue Engineering Initiative.
ABBEREVIATIONS
- ICU
Intensive care unit
- HIV
Human immunodeficiency virus
- ACCP
American College of Chest Physicians
- SCCM
Society of Critical Care Medicine
- MODS
Multiple Organ Dysfunction Syndrome
- SIRS
Systemic Inflammatory Response Syndrome
- PIRO
predisposition; infection; response; organ dysfunction
- PRRs
Pattern recognition receptors
- PAMPs
Pathogen-associated molecular patterns
- MAMPs
Microbial-associated molecular patterns
- DAMPs
Damage-associated molecular patterns
- AIR
Acute inflammatory response
- HRV
Heart rate variability
Footnotes
DISCLOSURES
Drs. Vodovotz and Billiar are co-founders of and stakeholders in Immunetrics, Inc., which has licensed from the University of Pittsburgh the rights to commercialize aspects of the mathematical modeling of inflammation. Dr. An is a consultant to Immunetrics, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 4.Heron M, Hoyert DL, Murphy SL, et al. Deaths: final data for 2006. NatlVital StatRep. 2009;57:1–134. [PubMed] [Google Scholar]
- 5.Weycker D, Akhras KS, Edelsberg J, et al. Long-term mortality and medical care charges in patients with severe sepsis. Crit Care Med. 2003;31:2316–2323. doi: 10.1097/01.CCM.0000085178.80226.0B. [DOI] [PubMed] [Google Scholar]
- 6.Wheeler AP, Bernard GR. Treating patients with severe sepsis. N Engl J Med. 1999;340:207–214. doi: 10.1056/NEJM199901213400307. [DOI] [PubMed] [Google Scholar]
- 7.Angus DC, Wax RS. Epidemiology of sepsis: an update. Crit Care Med. 2001;29:S109–S116. doi: 10.1097/00003246-200107001-00035. [DOI] [PubMed] [Google Scholar]
- 8.Sands KE, Bates DW, Lanken PN, et al. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA. 1997;278:234–240. [PubMed] [Google Scholar]
- 9.Hoyert DL, Arias E, Smith BL, et al. Deaths: final data for 1999. NatlVital StatRep. 2001;49:1–113. [PubMed] [Google Scholar]
- 10.Increase in National Hospital Discharge Survey rates for septicemia--United States, 1979–1987. MMWR Morb Mortal Wkly Rep %19. 1990;39:31–34. [PubMed] [Google Scholar]
- 11.Heyland DK, Hopman W, Coo H, et al. Long-term health-related quality of life in survivors of sepsis. Short Form 36: a valid and reliable measure of health-related quality of life. Crit Care Med. 2000;28:3599–3605. doi: 10.1097/00003246-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Perl TM, Dvorak L, Hwang T, et al. Long-term survival and function after suspected gram-negative sepsis. JAMA. 1995;274:338–345. [PubMed] [Google Scholar]
- 13.Protti A, Singer M. Bench-to-bedside review: potential strategies to protect or reverse mitochondrial dysfunction in sepsis-induced organ failure. Crit Care. 2006;10:228. doi: 10.1186/cc5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balestra GM, Legrand M, Ince C. Microcirculation and mitochondria in sepsis: getting out of breath. CurrOpinAnaesthesiol. 2009;22:184–190. doi: 10.1097/ACO.0b013e328328d31a. [DOI] [PubMed] [Google Scholar]
- 15.Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101:3765–3777. doi: 10.1182/blood-2002-06-1887. [DOI] [PubMed] [Google Scholar]
- 16.Ait-Oufella H, Maury E, Lehoux S, et al. The endothelium: physiological functions and role in microcirculatory failure during severe sepsis. Intensive Care Med. 2010;36:1286–1298. doi: 10.1007/s00134-010-1893-6. [DOI] [PubMed] [Google Scholar]
- 17.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. NatRevImmunol. 2008;8:776–787. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levi M, van der Poll T. Inflammation and coagulation. Crit Care Med. 2010;38:S26–S34. doi: 10.1097/CCM.0b013e3181c98d21. [DOI] [PubMed] [Google Scholar]
- 19.Geroulanos S, Douka ET. Historical perspective of the word "sepsis". Intensive Care Med. 2006;32:2077. doi: 10.1007/s00134-006-0392-2. [DOI] [PubMed] [Google Scholar]
- 20.Ewald PW. Evolution of virulence. InfectDisClinNorth Am. 2004;18:1–15. doi: 10.1016/S0891-5520(03)00099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vincent JL. Clinical sepsis and septic shock--definition, diagnosis and management principles. Langenbecks ArchSurg. 2008;393:817–824. doi: 10.1007/s00423-008-0343-1. [DOI] [PubMed] [Google Scholar]
- 22.Schottmueller H. Wesen und Behandlung der Sepsis. Inn Med. 2009;31:257–280. [Google Scholar]
- 23.An G. Agent-based computer simulation and sirs: building a bridge between basic science and clinical trials. Shock. 2001;16:266–273. doi: 10.1097/00024382-200116040-00006. [DOI] [PubMed] [Google Scholar]
- 24.Buchman TG, Cobb JP, Lapedes AS, et al. Complex systems analysis: a tool for shock research. Shock. 2001;16:248–251. doi: 10.1097/00024382-200116040-00002. [DOI] [PubMed] [Google Scholar]
- 25.Kitano H. Systems biology: a brief overview. Science. 2002;295:1662–1664. doi: 10.1126/science.1069492. [DOI] [PubMed] [Google Scholar]
- 26.Neugebauer EA, Willy C, Sauerland S. Complexity and non-linearity in shock research: reductionism or synthesis? Shock. 2001;16:252–258. doi: 10.1097/00024382-200116040-00003. [DOI] [PubMed] [Google Scholar]
- 27.Tjardes T, Neugebauer E. Sepsis research in the next millennium: concentrate on the software rather than the hardware. Shock. 2002;17:1–8. doi: 10.1097/00024382-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Vodovotz Y, Clermont G, Chow C, et al. Mathematical models of the acute inflammatory response. CurrOpinCrit Care. 2004;10:383–390. doi: 10.1097/01.ccx.0000139360.30327.69. [DOI] [PubMed] [Google Scholar]
- 29.Vodovotz Y, Csete M, Bartels J, et al. Translational systems biology of inflammation. PLoSComputBiol. 2008;4:e1000014. doi: 10.1371/journal.pcbi.1000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen MJ, Grossman AD, Morabito D, et al. Identification of complex metabolic states in critically injured patients using bioinformatic cluster analysis. Crit Care. 2010;14:R10. doi: 10.1186/cc8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.An G. In-silico experiments of existing and hypothetical cytokine-directed clinical trials using agent based modeling. Crit Care Med. 2004;32:2050–2060. doi: 10.1097/01.ccm.0000139707.13729.7d. [DOI] [PubMed] [Google Scholar]
- 32.Strieter RM, Lynch JP, III, Basha MA, et al. Host responses in mediating sepsis and adult respiratory distress syndrome. SeminRespirInfect. 1990;5:233–247. [PubMed] [Google Scholar]
- 33.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 34.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 35.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 36.Chung TP, Laramie JM, Province M, et al. Functional genomics of critical illness and injury. Crit Care Med. 2002;30:S51–S57. [PubMed] [Google Scholar]
- 37.Cobb JP, O’Keefe GE. Injury research in the genomic era. Lancet. 2004;363:2076–2083. doi: 10.1016/S0140-6736(04)16460-X. [DOI] [PubMed] [Google Scholar]
- 38.Godin PJ, Buchman TG. Uncoupling of biological oscillators: a complementary hypothesis concerning the pathogenesis of multiple organ dysfunction syndrome. Crit Care Med. 1996;24:1107–1116. doi: 10.1097/00003246-199607000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Buchman TG, Cobb JP, Lapedes AS, et al. Complex systems analysis: a tool for shock research. Shock. 2001;16:248–251. doi: 10.1097/00024382-200116040-00002. [DOI] [PubMed] [Google Scholar]
- 40.Buchman TG. Nonlinear dynamics, complex systems, and the pathobiology of critical illness. CurrOpinCrit Care. 2004;10:378–382. doi: 10.1097/01.ccx.0000139369.65817.b6. [DOI] [PubMed] [Google Scholar]
- 41.Seely AJ, Macklem PT. Complex systems and the technology of variability analysis. Crit Care. 2004;8:R367–R384. doi: 10.1186/cc2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vodovotz Y, Csete M, Bartels J, et al. Translational systems biology of inflammation. PLoSComputBiol. 2008;4:1–6. doi: 10.1371/journal.pcbi.1000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vodovotz Y, Constantine G, Rubin J, et al. Mechanistic simulations of inflammation: Current state and future prospects. MathBiosci. 2009;217:1–10. doi: 10.1016/j.mbs.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ventetuolo CE, Levy MM. Biomarkers: diagnosis and risk assessment in sepsis. ClinChest Med. 2008;29:591–603. vii. doi: 10.1016/j.ccm.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Claus RA, Otto GP, Deigner HP, et al. Approaching clinical reality: markers for monitoring systemic inflammation and sepsis. CurrMolMed. 2010;10:227–235. doi: 10.2174/156652410790963358. [DOI] [PubMed] [Google Scholar]
- 46.Bone RC, Grodzin CJ, Balk RA. Sepsis: a new hypothesis for pathogenesis of the disease process. Chest. 1997;112:235–243. doi: 10.1378/chest.112.1.235. [DOI] [PubMed] [Google Scholar]
- 47.Vincent JL, Abraham E. The last 100 years of sepsis. AmJ RespirCrit Care Med. 2006;173:256–263. doi: 10.1164/rccm.200510-1604OE. [DOI] [PubMed] [Google Scholar]
- 48.Cinel I, Opal SM. Molecular biology of inflammation and sepsis: a primer. Crit Care Med. 2009;37:291–304. doi: 10.1097/CCM.0b013e31819267fb. [DOI] [PubMed] [Google Scholar]
- 49.Vodovotz Y, Chow CC, Bartels J, et al. In silico models of acute inflammation in animals. Shock. 2006;26:235–244. doi: 10.1097/01.shk.0000225413.13866.fo. [DOI] [PubMed] [Google Scholar]
- 50.Mi Q, Li NYK, Ziraldo C, et al. Translational systems biology of inflammation: Potential applications to personalized medicine. Personalized Medicine. 2010;7:549–559. doi: 10.2217/pme.10.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Namas R, Ghuma A, Torres A, et al. An adequately robust early TNF-a response is a hallmark of survival following trauma/hemorrhage. PLoS ONE. 2009;4:e8406. doi: 10.1371/journal.pone.0008406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. NEnglJ Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 53.Fink MP, Delude RL. Epithelial barrier dysfunction: a unifying theme to explain the pathogenesis of multiple organ dysfunction at the cellular level. Crit Care Clin. 2005;21:177–196. doi: 10.1016/j.ccc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 54.Marshall JC. Inflammation, coagulopathy, and the pathogenesis of multiple organ dysfunction syndrome. Crit Care Med. 2001;29:S99–106. doi: 10.1097/00003246-200107001-00032. [DOI] [PubMed] [Google Scholar]
- 55.Jarrar D, Chaudry IH, Wang P. Organ dysfunction following hemorrhage and sepsis: mechanisms and therapeutic approaches (Review) IntJ MolMed. 1999;4:575–583. doi: 10.3892/ijmm.4.6.575. [DOI] [PubMed] [Google Scholar]
- 56.Crouser E, Exline M, Knoell D, et al. Sepsis: links between pathogen sensing and organ damage. CurrPharmDes. 2008;14:1840–1852. doi: 10.2174/138161208784980572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Creagh EM, O’Neill LA. TLRs, NLRs and RLRs: a trinity of pathogen sensors that cooperate in innate immunity. Trends Immunol. 2006;27:352–357. doi: 10.1016/j.it.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 58.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 59.Granucci F, Foti M, Ricciardi-Castagnoli P. Dendritic cell biology. AdvImmunol. 2005;88:193–233. 193–233. doi: 10.1016/S0065-2776(05)88006-X. [DOI] [PubMed] [Google Scholar]
- 60.Nduka OO, Parrillo JE. The pathophysiology of septic shock. Crit Care Clin. 2009;25:677–702. vii. doi: 10.1016/j.ccc.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 61.Cinel I, Dellinger RP. Advances in pathogenesis and management of sepsis. CurrOpinInfectDis. 2007;20:345–352. doi: 10.1097/QCO.0b013e32818be70a. [DOI] [PubMed] [Google Scholar]
- 62.Opitz B, Eitel J, Meixenberger K, et al. Role of Toll-like receptors, NOD-like receptors and RIG-I-like receptors in endothelial cells and systemic infections. ThrombHaemost. 2009;102:1103–1109. doi: 10.1160/TH09-05-0323. [DOI] [PubMed] [Google Scholar]
- 63.Arcaroli J, Fessler MB, Abraham E. Genetic polymorphisms and sepsis. Shock. 2005;24:300–312. doi: 10.1097/01.shk.0000180621.52058.e1. [DOI] [PubMed] [Google Scholar]
- 64.Gibot S, Cariou A, Drouet L, et al. Association between a genomic polymorphism within the CD14 locus and septic shock susceptibility and mortality rate. Crit Care Med. 2002;30:969–973. doi: 10.1097/00003246-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 65.Paterson HM, Murphy TJ, Purcell EJ, et al. Injury primes the innate immune system for enhanced Toll-like receptor reactivity. J Immunol. 2003;171:1473–1483. doi: 10.4049/jimmunol.171.3.1473. [DOI] [PubMed] [Google Scholar]
- 66.Vogel SN, Awomoyi AA, Rallabhandi P, et al. Mutations in TLR4 signaling that lead to increased susceptibility to infection in humans: an overview. JEndotoxinRes. 2005;11:333–339. doi: 10.1179/096805105X58724. [DOI] [PubMed] [Google Scholar]
- 67.Pritts T, Hungness E, Wang Q, et al. Mucosal and enterocyte IL-6 production during sepsis and endotoxemia--role of transcription factors and regulation by the stress response. AmJ Surg. 2002;183:372–383. doi: 10.1016/s0002-9610(02)00812-7. [DOI] [PubMed] [Google Scholar]
- 68.van der Poll T, van Deventer SJ. Cytokines and anticytokines in the pathogenesis of sepsis. InfectDisClinNorth Am. 1999;13:413–426. ix. doi: 10.1016/s0891-5520(05)70083-0. [DOI] [PubMed] [Google Scholar]
- 69.Adrie C, Pinsky MR. The inflammatory balance in human sepsis. Intensive Care Med. 2000;26:364–375. doi: 10.1007/s001340051169. [DOI] [PubMed] [Google Scholar]
- 70.Maier RV. Pathogenesis of multiple organ dysfunction syndrome - endotoxin, inflammatory cells, and their mediators: cytokines and reactive oxygen species. SurgInfect(Larchmt) 2000;1:197–205. doi: 10.1089/109629600750018123. [DOI] [PubMed] [Google Scholar]
- 71.Ulloa L, Tracey KJ. The "cytokine profile": a code for sepsis. Trends MolMed. 2005;11:56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 72.Jawa RS, Kulaylat MN, Baumann H, et al. What is new in cytokine research related to trauma/critical care. J Intensive Care Med. 2006;21:63–85. doi: 10.1177/0885066605284325. [DOI] [PubMed] [Google Scholar]
- 73.Pinsky MR. Sepsis and multiple organ failure. ContribNephrol. 2007;156:47–63. doi: 10.1159/000102070. [DOI] [PubMed] [Google Scholar]
- 74.Cai B, Deitch EA, Ulloa L. Novel insights for systemic inflammation in sepsis and hemorrhage. MediatorsInflamm. 2010;2010:642462. doi: 10.1155/2010/642462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–1635. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- 76.Hoffmann A, Levchenko A, Scott ML, et al. The IkB-NFkB signaling module: Temporal control and selective gene activation. Science. 2002;298:1241–1245. doi: 10.1126/science.1071914. [DOI] [PubMed] [Google Scholar]
- 77.Kellum JA, Kong L, Fink MP, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. ArchInternMed. 2007;167:1655–1663. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goldie AS, Fearon KC, Ross JA, et al. Natural cytokine antagonists and endogenous antiendotoxin core antibodies in sepsis syndrome. The Sepsis Intervention Group. JAMA. 1995;274:172–177. [PubMed] [Google Scholar]
- 79.van der Poll T, de Waal MR, Coyle SM, et al. Antiinflammatory cytokine responses during clinical sepsis and experimental endotoxemia: sequential measurements of plasma soluble interleukin (IL)-1 receptor type II, IL-10, and IL-13. J InfectDis. 1997;175:118–122. doi: 10.1093/infdis/175.1.118. [DOI] [PubMed] [Google Scholar]
- 80.Abraham E, Anzueto A, Gutierrez G, et al. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. NORASEPT II Study Group. Lancet. 1998;351:929–933. [PubMed] [Google Scholar]
- 81.Reinhart K, Menges T, Gardlund B, et al. Randomized, placebo-controlled trial of the anti-tumor necrosis factor antibody fragment afelimomab in hyperinflammatory response during severe sepsis: The RAMSES Study. Crit Care Med. 2001;29:765–769. doi: 10.1097/00003246-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 82.Pinsky MR. Dysregulation of the immune response in severe sepsis. AmJMedSci. 2004;328:220–229. doi: 10.1097/00000441-200410000-00005. [DOI] [PubMed] [Google Scholar]
- 83.van der Poll T, Opal SM. Host-pathogen interactions in sepsis. Lancet InfectDis. 2008;8:32–43. doi: 10.1016/S1473-3099(07)70265-7. [DOI] [PubMed] [Google Scholar]
- 84.dib-Conquy M, Cavaillon JM. Stress molecules in sepsis and systemic inflammatory response syndrome. FEBS Lett. 2007;581:3723–3733. doi: 10.1016/j.febslet.2007.03.074. [DOI] [PubMed] [Google Scholar]
- 85.Wang H, Bloom O, Zhang M, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 86.Sunden-Cullberg J, Norrby-Teglund A, Rouhiainen A, et al. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit Care Med. 2005;33:564–573. doi: 10.1097/01.ccm.0000155991.88802.4d. [DOI] [PubMed] [Google Scholar]
- 87.Angus DC, Yang L, Kong L, et al. Circulating high-mobility group box 1 (HMGB1) concentrations are elevated in both uncomplicated pneumonia and pneumonia with severe sepsis. Crit Care Med. 2007;35:1061–1067. doi: 10.1097/01.CCM.0000259534.68873.2A. [DOI] [PubMed] [Google Scholar]
- 88.Yang H, Ochani M, Li J, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. ProcNatlAcadSciUSA. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Skoberne M, Beignon AS, Bhardwaj N. Danger signals: a time and space continuum. Trends MolMed. 2004;10:251–257. doi: 10.1016/j.molmed.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 90.Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leon CG, Tory R, Jia J, et al. Discovery and development of toll-like receptor 4 (TLR4) antagonists: a new paradigm for treating sepsis and other diseases. PharmRes. 2008;25:1751–1761. doi: 10.1007/s11095-008-9571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hennessy EJ, Parker AE, O’Neill LA. Targeting Toll-like receptors: emerging therapeutics? NatRevDrug Discov. 2010;9:293–307. doi: 10.1038/nrd3203. [DOI] [PubMed] [Google Scholar]
- 93.Wittebole X, Castanares-Zapatero D, Laterre PF. Toll-like receptor 4 modulation as a strategy to treat sepsis. MediatorsInflamm. 2010;2010:568396. doi: 10.1155/2010/568396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Klune JR, Dhupar R, Cardinal J, et al. HMGB1: endogenous danger signaling. MolMed. 2008;14:476–484. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang H, Ward MF, Sama AE. Novel HMGB1-inhibiting therapeutic agents for experimental sepsis. Shock. 2009;32:348–357. doi: 10.1097/SHK.0b013e3181a551bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang H, Tracey KJ. Targeting HMGB1 in inflammation. BiochimBiophysActa. 2010;1799:149–156. doi: 10.1016/j.bbagrm.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Adams JL, Badger AM, Kumar S, et al. p38 MAP kinase: molecular target for the inhibition of pro-inflammatory cytokines. ProgMedChem. 2001;38:1–60. doi: 10.1016/s0079-6468(08)70091-2. [DOI] [PubMed] [Google Scholar]
- 98.Calzado MA, Bacher S, Schmitz ML. NF-kappaB inhibitors for the treatment of inflammatory diseases and cancer. CurrMedChem. 2007;14:367–376. doi: 10.2174/092986707779941113. [DOI] [PubMed] [Google Scholar]
- 99.Uwe S. Anti-inflammatory interventions of NF-kappaB signaling: potential applications and risks. BiochemPharmacol. 2008;75:1567–1579. doi: 10.1016/j.bcp.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 100.Namas R, Ghuma A, Hermus L, et al. The acute inflammatory response in trauma/hemorrhage and traumatic brain injury: Current state and emerging prospects. Libyan JMed. 2009;4:136–148. doi: 10.4176/090325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vodovotz Y, An G. Systems Biology and Inflammation. In: Yan Q, editor. Systems Biology in Drug Discovery and Development: Methods and Protocols. Totowa, NJ: Springer Science & Business Media; 2009. pp. 181–201. [Google Scholar]
- 102.Vodovotz Y, Constantine G, Faeder J, et al. Translational systems approaches to the biology of inflammation and healing. ImmunopharmacolImmunotoxicol. 2010;32:181–195. doi: 10.3109/08923970903369867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Annane D, Trabold F, Sharshar T, et al. Inappropriate sympathetic activation at onset of septic shock: a spectral analysis approach. AmJ RespirCrit Care Med. 1999;160:458–465. doi: 10.1164/ajrccm.160.2.9810073. [DOI] [PubMed] [Google Scholar]
- 104.Korach M, Sharshar T, Jarrin I, et al. Cardiac variability in critically ill adults: influence of sepsis. Crit Care Med. 2001;29:1380–1385. doi: 10.1097/00003246-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 105.Piepoli M, Garrard CS, Kontoyannis DA, et al. Autonomic control of the heart and peripheral vessels in human septic shock. Intensive Care Med. 1995;21:112–119. doi: 10.1007/BF01726532. [DOI] [PubMed] [Google Scholar]
- 106.Fairchild KD, Saucerman JJ, Raynor LL, et al. Endotoxin depresses heart rate variability in mice: cytokine and steroid effects. AmJ Physiol RegulIntegrComp Physiol. 2009;297:R1019–R1027. doi: 10.1152/ajpregu.00132.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. EurHeart J. 1996;17:354–381. [PubMed] [Google Scholar]
- 108.Kleiger RE, Stein PK, Bigger JT., Jr Heart rate variability: measurement and clinical utility. Ann NoninvasiveElectrocardiol. 2005;10:88–101. doi: 10.1111/j.1542-474X.2005.10101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pomeranz B, Macaulay RJ, Caudill MA, et al. Assessment of autonomic function in humans by heart rate spectral analysis. AmJ Physiol. 1985;248:H151–H153. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- 110.Godin PJ, Fleisher LA, Eidsath A, et al. Experimental human endotoxemia increases cardiac regularity: results from a prospective, randomized, crossover trial. Crit Care Med. 1996;24:1117–1124. doi: 10.1097/00003246-199607000-00009. [DOI] [PubMed] [Google Scholar]
- 111.Barnaby D, Ferrick K, Kaplan DT, et al. Heart rate variability in emergency department patients with sepsis. AcadEmergMed. 2002;9:661–670. doi: 10.1111/j.1553-2712.2002.tb02143.x. [DOI] [PubMed] [Google Scholar]
- 112.Chen WL, Kuo CD. Characteristics of heart rate variability can predict impending septic shock in emergency department patients with sepsis. AcadEmergMed. 2007;14:392–397. doi: 10.1197/j.aem.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 113.Pontet J, Contreras P, Curbelo A, et al. Heart rate variability as early marker of multiple organ dysfunction syndrome in septic patients. J Crit Care. 2003;18:156–163. doi: 10.1016/j.jcrc.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 114.Ahmad S, Ramsay T, Huebsch L, et al. Continuous multi-parameter heart rate variability analysis heralds onset of sepsis in adults. PLoS ONE. 2009;4:e6642. doi: 10.1371/journal.pone.0006642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Magder S. Bench-to-bedside review: ventilatory abnormalities in sepsis. Crit Care. 2009;13:202. doi: 10.1186/cc7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Preas HL, Jubran A, Vandivier RW, et al. Effect of endotoxin on ventilation and breath variability: role of cyclooxygenase pathway. AmJ RespirCrit Care Med. 2001;164:620–626. doi: 10.1164/ajrccm.164.4.2003031. [DOI] [PubMed] [Google Scholar]
- 117.Cancio LC, Batchinsky AI, Salinas J, et al. Heart-rate complexity for prediction of prehospital lifesaving interventions in trauma patients. JTrauma. 2008;65:813–819. doi: 10.1097/TA.0b013e3181848241. [DOI] [PubMed] [Google Scholar]
- 118.Bone RC. Why sepsis trials fail. JAMA. 1996;276:565–566. [PubMed] [Google Scholar]
- 119.Seely AJ, Christou NV. Multiple organ dysfunction syndrome: exploring the paradigm of complex nonlinear systems. Crit Care Med. 2000;28:2193–2200. doi: 10.1097/00003246-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 120.Vodovotz Y. Deciphering the complexity of acute inflammation using mathematical models. ImmunolRes. 2006;36:237–245. doi: 10.1385/IR:36:1:237. [DOI] [PubMed] [Google Scholar]
- 121.Marshall JC. Through a glass darkly: the brave new world of in silico modeling. Crit Care Med. 2004;32:2157–2159. doi: 10.1097/01.ccm.0000142935.34916.b5. [DOI] [PubMed] [Google Scholar]
- 122.Vodovotz Y, Clermont G, Hunt CA, et al. Evidence-based modeling of critical illness: an initial consensus from the Society for Complexity in Acute Illness. J Crit Care. 2007;22:77–84. doi: 10.1016/j.jcrc.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kumar R, Clermont G, Vodovotz Y, et al. The dynamics of acute inflammation. JTheoretical Biol. 2004;230:145–155. doi: 10.1016/j.jtbi.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 124.Gibbs JB. Mechanism-based target identification and drug discovery in cancer research. Science. 2000;287:1969–1973. doi: 10.1126/science.287.5460.1969. [DOI] [PubMed] [Google Scholar]
- 125.Faeder JR, Hlavacek WS, Reischl I, et al. Investigation of early events in Fc epsilon RI-mediated signaling using a detailed mathematical model. JImmunol. 2003;170:3769–3781. doi: 10.4049/jimmunol.170.7.3769. [DOI] [PubMed] [Google Scholar]
- 126.RiviŠre B, Epshteyn Y, Swigon D, et al. A simple mathematical model of signaling resulting from the binding of lipopolysaccharide with Toll-like receptor 4 demonstrates inherent preconditioning behavior. MathBiosci. 2009;217:19–26. doi: 10.1016/j.mbs.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chow CC, Clermont G, Kumar R, et al. The acute inflammatory response in diverse shock states. Shock. 2005;24:74–84. doi: 10.1097/01.shk.0000168526.97716.f3. [DOI] [PubMed] [Google Scholar]
- 128.Reynolds A, Rubin J, Clermont G, et al. A reduced mathematical model of the acute inflammatory response: I. Derivation of model and analysis of anti-inflammation. JTheorBiol. 2006;242:220–236. doi: 10.1016/j.jtbi.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 129.Day J, Rubin J, Vodovotz Y, et al. A reduced mathematical model of the acute inflammatory response: II. Capturing scenarios of repeated endotoxin administration. JTheorBiol. 2006;242:237–256. doi: 10.1016/j.jtbi.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 130.Torres A, Bentley T, Bartels J, et al. Mathematical modeling of post-hemorrhage inflammation in mice: Studies using a novel, computer-controlled, closed-loop hemorrhage apparatus. Shock. 2009;32:172–178. doi: 10.1097/SHK.0b013e318193cc2b. [DOI] [PubMed] [Google Scholar]
- 131.Foteinou PT, Calvano SE, Lowry SF, et al. Modeling endotoxin-induced systemic inflammation using an indirect response approach. MathBiosci. 2009;217:27–42. doi: 10.1016/j.mbs.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Foteinou PT, Calvano SE, Lowry SF, et al. In silico simulation of corticosteroids effect on an NFkB- dependent physicochemical model of systemic inflammation. PLoS ONE. 2009;4:e4706. doi: 10.1371/journal.pone.0004706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kumar R, Chow CC, Bartels J, et al. A mathematical simulation of the inflammatory response to anthrax infection. Shock. 2008;29:104–111. doi: 10.1097/SHK.0b013e318067da56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Arciero J, Rubin J, Upperman J, et al. Using a mathematical model to analyze the role of probiotics and inflammation in necrotizing enterocolitis. PLoS ONE. 2010;5:e10066. doi: 10.1371/journal.pone.0010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Prince JM, Levy RM, Bartels J, et al. In silico and in vivo approach to elucidate the inflammatory complexity of CD14-deficient mice. MolMed. 2006;12:88–96. doi: 10.2119/2006-00012.Prince. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lagoa CE, Bartels J, Baratt A, et al. The role of initial trauma in the host’s response to injury and hemorrhage: Insights from a comparison of mathematical simulations and hepatic transcriptomic analysis. Shock. 2006;26:592–600. doi: 10.1097/01.shk.0000232272.03602.0a. [DOI] [PubMed] [Google Scholar]
- 137.Daun S, Rubin J, Vodovotz Y, et al. An ensemble of models of the acute inflammatory response to bacterial lipopolysaccharide in rats: Results from parameter space reduction. JTheorBiol. 2008;253:843–853. doi: 10.1016/j.jtbi.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 138.Foteinou PT, Calvano SE, Lowry SF, et al. Multiscale model for the assessment of autonomic dysfunction in human endotoxemia. Physiol Genomics. 2010;42:5–19. doi: 10.1152/physiolgenomics.00184.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Clermont G, Bartels J, Kumar R, et al. In silico design of clinical trials: a method coming of age. Crit Care Med. 2004;32:2061–2070. doi: 10.1097/01.ccm.0000142394.28791.c3. [DOI] [PubMed] [Google Scholar]
- 140.An G, Bartels J, Vodovotz Y. In silico augmentation of the drug development pipeline: Examples from the study of acute inflammation. Drug DevRes. 2010;72:1–14. doi: 10.1002/ddr.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Andreasen AS, Krabbe KS, Krogh-Madsen R, et al. Human endotoxemia as a model of systemic inflammation. CurrMedChem. 2008;15:1697–1705. doi: 10.2174/092986708784872393. [DOI] [PubMed] [Google Scholar]
- 142.Clermont G, Bartels J, Kumar R, et al. In silico design of clinical trials: a method coming of age. Crit Care Med. 2004;32:2061–2070. doi: 10.1097/01.ccm.0000142394.28791.c3. [DOI] [PubMed] [Google Scholar]
- 143.Foteinou PT, Calvano SE, Lowry SF, et al. Shock. 2010. A physiological model for autonomic heart rate regulation in human endotoxemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vodovotz Y. Translational systems biology of inflammation and healing. WoundRepair Regen. 2010;18:3–7. doi: 10.1111/j.1524-475X.2009.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Buchman TG. In vivo, in vitro, in silico. Crit Care Med. 2004;32:2159–2160. doi: 10.1097/01.ccm.0000142900.95726.7f. [DOI] [PubMed] [Google Scholar]
- 146.Gullo A, Bianco N, Berlot G. Management of severe sepsis and septic shock: challenges and recommendations. Crit Care Clin. 2006;22:489–501. ix. doi: 10.1016/j.ccc.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 147.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 148.Lobo SM, Lobo FR, Bota DP, et al. C-reactive protein levels correlate with mortality and organ failure in critically ill patients. Chest. 2003;123:2043–2049. doi: 10.1378/chest.123.6.2043. [DOI] [PubMed] [Google Scholar]
- 149.Ugarte H, Silva E, Mercan D, et al. Procalcitonin used as a marker of infection in the intensive care unit. Crit Care Med. 1999;27:498–504. doi: 10.1097/00003246-199903000-00024. [DOI] [PubMed] [Google Scholar]
- 150.Casey LC, Balk RA, Bone RC. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med. 1993;119:771–778. doi: 10.7326/0003-4819-119-8-199310150-00001. [DOI] [PubMed] [Google Scholar]
- 151.Damas P, Ledoux D, Nys M, et al. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg. 1992;215:356–362. doi: 10.1097/00000658-199204000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Friedman G, Jankowski S, Marchant A, et al. Blood interleukin 10 levels parallel the severity of septic shock. J Crit Care. 1997;12:183–187. doi: 10.1016/s0883-9441(97)90030-7. [DOI] [PubMed] [Google Scholar]
- 153.Pinsky MR, Vincent JL, Deviere J, et al. Serum cytokine levels in human septic shock. Relation to multiple-system organ failure and mortality. Chest. 1993;103:565–575. doi: 10.1378/chest.103.2.565. [DOI] [PubMed] [Google Scholar]
- 154.Marshall JC, Foster D, Vincent JL, et al. Diagnostic and prognostic implications of endotoxemia in critical illness: results of the MEDIC study. J InfectDis. 2004;190:527–534. doi: 10.1086/422254. [DOI] [PubMed] [Google Scholar]
- 155.Gibot S, Kolopp-Sarda MN, Bene MC, et al. Plasma level of a triggering receptor expressed on myeloid cells-1: its diagnostic accuracy in patients with suspected sepsis. Ann Intern Med. 2004;141:9–15. doi: 10.7326/0003-4819-141-1-200407060-00009. [DOI] [PubMed] [Google Scholar]
- 156.Dempfle CE, Lorenz S, Smolinski M, et al. Utility of activated partial thromboplastin time waveform analysis for identification of sepsis and overt disseminated intravascular coagulation in patients admitted to a surgical intensive care unit. Crit Care Med. 2004;32:520–524. doi: 10.1097/01.CCM.0000110678.52863.F3. [DOI] [PubMed] [Google Scholar]
- 157.Toh CH, Ticknor LO, Downey C, et al. Early identification of sepsis and mortality risks through simple, rapid clot-waveform analysis. Implications of lipoprotein-complexed C reactive protein formation. Intensive Care Med. 2003;29:55–61. doi: 10.1007/s00134-002-1557-2. [DOI] [PubMed] [Google Scholar]
- 158.Seely AJE, Christou NV. Multiple organ dysfunction syndrome: exploring the paradigm of complex nonlinear systems [Review] Crit Care Med. 2000;28:2193–2200. doi: 10.1097/00003246-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 159.Schmidt HB, Werdan K, Muller-Werdan U. Autonomic dysfunction in the ICU patient. CurrOpinCrit Care. 2001;7:314–322. doi: 10.1097/00075198-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 160.Moorman JR, Lake DE, Griffin MP. Heart rate characteristics monitoring for neonatal sepsis. IEEE TransBiomedEng. 2006;53:126–132. doi: 10.1109/TBME.2005.859810. [DOI] [PubMed] [Google Scholar]
- 161.Werdan K, Schmidt H, Ebelt H, et al. Impaired regulation of cardiac function in sepsis, SIRS, and MODS. CanJPhysiol Pharmacol. 2009;87:266–274. doi: 10.1139/Y09-012. [DOI] [PubMed] [Google Scholar]