Abstract
Several nanoparticle platforms are currently being developed for applications in medicine, including both synthetic materials and naturally-occurring bionanomaterials such as viral nanoparticles (VNPs) and their genome-free counterparts, virus-like particles (VLPs). A broad range of genetic and chemical engineering methods have been established that allow VNP/VLP formulations to carry large payloads of imaging reagents or drugs. Furthermore, targeted VNPs and VLPs can be generated by including peptide ligands on the particle surface. In this article, we highlight state-of-the-art virus engineering principles and discuss recent advances that bring potential biomedical applications a step closer. Viral nanotechnology has now come of age and it will not be long before these formulations assume a prominent role in the clinic.
Keywords: viral nanoparticles, virus-like particles, bioconjugation, genetic engineering, vaccines, phage display, drug delivery, imaging, tissue targeting
1. Classes of nanomaterials for applications in medicine
Nanomedicine refers to the medical application of nanotechnology, and particularly to the development of novel nanomaterials that can be used for disease diagnosis and therapy. The unique properties of nanoparticles promise to deliver a new generation of diagnostic reagents with higher signal-to-noise ratios than current imaging modalities, as well as targeted therapies that are more efficacious than today’s medicines and that have fewer adverse effects. Nanomaterials have a large surface-to-volume ratio compared to traditional delivery vehicles which offers a greater capacity for drugs and/or imaging reagents, and the ability to decorate nanoparticles with specific ligands means these diagnostic and therapeutic payloads can be delivered to particular cells. Several classes of nanomaterials are currently being developed, including synthetic materials and naturally occurring bionanomaterials such as viral nanoparticles (VNPs). Each of these systems has benefits and limitations with regard to pharmacokinetics, toxicity, immunogenicity and specificity for the target tissue [1–7].
Thus far, more than 25 platforms have been approved for clinical use, including PEGylated liposomal doxorubicin (Doxil®/Caelyx®), albumin-bound paclitaxel (Abraxane®), nanoparticle-based contrast agents for use in magnetic resonance imaging (MRI), poly(lactic-co-glycolic acid) (PLGA) formulations for drug delivery, and virus-based vaccines [8–10]. Additional formulations are currently undergoing clinical testing [11]. However, there is a significant lag between the development of novel nanomedical platforms and their translation into the clinic, often because encouraging in vitro results cannot be replicated in vivo, or because the materials are not biodegradable and therefore persist in the body. Research has therefore focused on biocompatible nanoparticle platforms, i.e. bionanomaterials, which are naturally biocompatible and thus promising candidates for in vivo use.
2. Viral nanoparticles (VNPs)
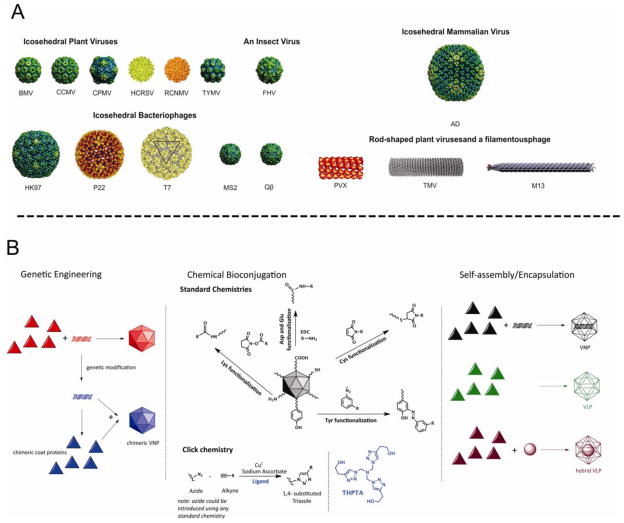
Viruses have evolved naturally to infect specific host cells with great efficiency, and deliver their cargo of genetic material. Viruses therefore provide an ideal basis for the development of targeted drug delivery vehicles or tissue-specific imaging reagents. Interest in the exploitation of viral nanoparticles (VNPs) and virus-like particles (VLPs) has united biologists, chemists, engineers and medical researchers. VLPs are the genome-free counterparts of VNPs, and can be regarded as a subclass of VNPs. VNPs derived from plants and bacteria are particularly valuable because they are not only biocompatible and biodegradable, but they are also considered non-infectious and non-hazardous in humans and other mammals [12,13*]. VNPs are well-characterized, monodisperse structures (many solved at atomic resolution) that can be produced in large quantities. Based on their highly symmetrical structures (Figure 1A) [14–18], VNPs can be regarded as one of the most advanced and versatile nanomaterials produced by nature. The basic VNP structure can be ‘programmed’ in a number of ways so that the internal cavity can be filled with drug molecules, imaging reagents, quantum dots and other nanoparticles, whereas the external surface can be decorated with targeting ligands to allow cell-specific delivery (Figure 1B) [reviewed in 19*].
Figure 1.
A. Viral nanoparticles (VNPs) used in materials science and medicine. Icosahedral plant viruses: Brome mosaic virus (BMV), Cowpea cholorotic mottle virus (CCMV), Cowpea mosaic virus (CPMV), Hibiscus cholorotic ringspot virus (HCRSV), Red clover necrotic mottle virus (RCNMV), Turnip yellow mosaic virus (TYMV). Icosahdral insect virus: Flock House virus (FHV). Icosahedral bacteriophages: HK97, P22, T7, MS2 and Qβ. Note that P22 and T7 are head-tail phages, with the tails not shown. Icosahedral mammalian virus: Adenovirus (Ad). Rod-shaped and filamentous viruses: Potato virus X (PVX), Tobacco mosaic virus (TMV), bacteriophage M13. Images of the following VNPs were reproduced from the VIPER Database; URL: http://www.viperdb.scripps.edu/: BMV, CCMV, CPMV, P22, TYMV, FHV, HK97, MS2, Ad, and Qβ. The structures of HCRSV, RCNMV, T7, PVX and TMV were reproduced from refs [14–18], respectively. B. Genetic, chemical, and self-assembly/encapsulation manipulations of VNPs in biomedical research.
3. Production and engineering of VNPs
3.1 Production of VNPs and VLPs
VNPs can be produced in high titers in their natural hosts whereas VLPs are more suitable for production in heterologous expression systems. VNPs based on plant viruses such as Brome mosaic virus (BMV), Cowpea chlorotic mottle virus (CCMV), Cowpea mosaic virus (CPMV), Potato virus X (PVX) and Tobacco mosaic virus (TMV) can be produced in gram quantities in plants, and similar yields can be achieved with VNPs based on bacteriophages such as Qβ, MS2, HK97 and M13 using cultures of the bacterium Escherichia coli. VLPs and chimeric (mutant) VNPs are often produced in heterologous expression systems such as E. coli and yeast. VLPs based on eukaryotic viruses tend to be produced using baculovirus vectors in insect cells (e.g. for Flock House virus VLPs) [20*] or adenovirus vectors in mammalian cells [21]. VLPs can also be produced directly from VNPs by pH-induced swelling followed by alkaline hydrolysis of the released nucleic acids. Alternatively, intact VNPs can be disassembled into their individual coat protein subunits and reassembled into VLPs once the nucleic acids have been removed (Figure 1B) [22].
3.2 Genetic engineering
VNPs and VLPs are assembled from protein subunits whose structure and physicochemical properties can be modified by genetic engineering. This offers a clear advantage over any synthetic material, because chemical modifications are not 100% efficient. Genetic engineering can be used to insert amino acids that serve as ligation handles for bioconjugation, introduce peptide-based affinity tags and to insert peptides as targeting ligands or as epitopes to stimulate the immune response (if the VNP/VLP is conceived as a vaccine candidate). For example, mutant VNPs containing additional cysteine residues have been generated to allow covalent modification using thiol-selective chemistry [23,24], and both labeling and purification have been simplified by the incorporation of His6 tags, biotin-binding peptides and the B domain of Staphylococcal protein A (SPAB) [25,26*].
3.3 Chemical engineering and cargo encapsulation
VNP/VLP coat proteins can also be chemically modified using bioconjugation protocols (Figure 1B). Amino acids with reactive side chains such as lysine, cysteine, aspartate and glutamate can be functionalized with antibodies, oligonucleotides, peptides, proteins, carbohydrates, fluorescent reagents and drugs using N-hydroxysuccinimidyl ester (NHS), maleimide, isothiocyanate and carbodiimide chemistries. In addition, these methods can be used to incorporate synthetic functional groups to allow the use of click chemistry, tyrosine-diazonium coupling and oxime-hydrozone ligation [19].
Loading the interior cavity of a VLP can be accomplished by diffusion and entrapment making use of swelling mechanisms [27**], or by disassembly and reassembly [28,29]. A wide variety of cargos including enzymes, polymers, drugs and synthetic nanoparticles have been successfully incorporated into VLPs using these approaches (Figure 1B) [30–32].
4. From engineering to potential applications in medicine
Several vaccines for infectious diseases are based on mammalian VLPs and are already used in the clinic [10,33–35], while similar concepts for the prevention of cancer are also in development [36]. State-of-the art viral gene delivery to human patients is achieved using mammalian virus vectors, several formulations of which are currently undergoing clinical trials [37]. Many novel VNP/VLP platforms are filling the development pipeline and the focus has shifted toward the use of bacteriophages and plant viruses because they are considered safer in humans. This is supported by recent studies evaluating the biodistribution, pharmacokinetics and potential toxicity of CPMV, CCMV, Qβ and M13 nanoparticles in mice. In all cases, broad biodistribution was reported with no apparent toxicity [12,38,39].
4.1 Vaccines based on VLPs and chimeric VNPs
Viruses provide a useful development platform for novel vaccines because their highly repetitive proteinaceous structure, particulate nature and pathogen associated molecular patterns (PAMPs) increase their potential immunogenicity. Three basic strategies have been employed: i) VNPs are rendered non-infectious by chemical treatment; ii) genome-free, non-infectious VLPs are produced in heterologous expression systems; and iii) chimeric VNPs are created by genetic engineering, a strategy often used with viruses that do not naturally infect humans [reviewed in 40*]. Although immunogenicity is desirable for the development of vaccines, it reduces the efficacy of nanomaterials used as imaging reagents or for drug delivery. Therefore, efforts have been made to shield VNPs from the immune system, and the most popular approach is the attachment of polyethylene glycol (PEG) chains to the external surface. PEGylation has been shown to reduce the biospecific interactions and immunogenicity of VNPs while increasing plasma circulation time and stability [41–43**].
4.2 Targeted delivery
Targeted nanoparticles are particularly valuable as diagnostic and therapeutic modalities because they increase the signal-to-noise ratio of imaging reagents by avoiding background staining, and they improve the efficacy of drugs and reduce adverse effects by concentrating the therapeutic molecule where it is required. The development of targeted nanoparticles has been facilitated by the use of combinatorial phage-display libraries to discover cell-specific molecular targets and their ligands [44–47**]. In the past two years, several targeted VNP formulations have been engineered to target cancer cells, including CPMV, HCSRV (Hibiscus chlorotic ringspot virus), RCNMV (Red clover necrotic mosaic virus), HK97, and M13 VNPs decorated with folic acid or transferrin [27,48–51**]. Most recently, in vivo tumor targeting has been demonstrated with CPMV particles decorated with peptide ligands. Proof of concept was established by engineering CPMV particles to display peptide F56, which binds specifically to vascular endothelial growth factor receptor 1 (VEGFR-1). These particles were found to localize specifically in tumors overexpressing VEGFR-1 in a preclinical tumor mouse model with human colon carcinoma xenografts derived from HT-29 cells. The CPMV formulations were able to pass through the endothelial layer and localize within the tumor [52**]. These design principles have also been used for in vivo imaging of prostate tumors derived from PC-3 cells using the chick embryo chorioallantoic (CAM) tumor membrane model. CPMV formulations were covalently modified with infrared imaging dyes and bombesin peptides to target gastrin-releasing peptide receptors overexpressed on prostate carcinoma cells (Figure 2) [53**]. These studies demonstrated that VNPs can be targeted efficiently to disease sites and pave the way for the development of further tissue-specific imaging reagents and drugs.
Figure 2.

Intravital imaging of viral nanoparticle uptake in prostate tumors in vivo. A. Intravital fluorescence confocal imaging of PC-3 prostate tumor (green channel) showing uptake of AF647-labeled CPMV-PEG-bombesin (heat map) over time. Images are representative of n=10 experiments. Colors correspond to tumor/stroma ratio (see key). Scale bar = 3 mm. B. Intravital imaging of PC-3 prostate tumor (green channel) showing uptake of AF647-labeled CPMV-PEG (heat map) over time. Images are representative of n=10 experiments. Colors correspond to tumor/stroma ratio (see key). Scale bar = 3 mm. C. Quantitation of tumor uptake of CPMV conjugates over time, n=10 experiments per group. Values expressed as mean tumor/stroma ratio, using GFP channel to delineate tumor. Uptake of CPMV-PEG-bombesin is significantly higher than CPMV-PEG at 2 h and beyond (P<0.0001). D. Accumulation of CPMV conjugates in tumor tissue, measured by fluorescence confocal microscopy of tissue sections. Grayscale and color merged images are provided with PC-3 GFP cells (green) and CPMV-AF647 conjugates (red). Scale bar = 75 μm. Reproduced from Ref 53.
4.3 Therapeutic VNPs and gene-delivery vectors
Drugs such as doxorubicin, paclitaxel, hygromycin and other cytotoxins have been loaded onto targeted RCNMV, HCSRV, MS2 and M13 formulations, and in each case specific cancer cell killing was achieved in cultured cells [27,48–50]. Similarly, targeted chloramphenicol-loaded M13 particles have been used as antibiotic delivery vehicles [54]. Alternative approaches focus on the delivery of photosensitizers for photodynamic therapy (PDT), e.g. through the use of C60 fullerene VNP conjugates using CPMV and Qβ [55**]. Staphylococcus-targeted ruthenium-CCMV conjugates have been developed to treat bacterial infections [56**], and MS2 particles loaded with porphyrins have been targeted to T-cells using receptor- specific aptamers as candidates for PDT in the treatment of leukemia [57**].
The natural role of viruses delivering nucleic acids into cells makes them eminently suitable as vectors for gene therapy, and several mammalian viruses have been studied in clinical trials [37]. However, safety concerns reflecting the tropism of mammalian viruses and the potential toxicity, immunogenicity and reversion to replication competence in vectors derived from adenovirus, adeno-associated virus (AAV), herpesvirus and lentivirus has renewed interest in non-mammalian platforms such as bacteriophages [58–60]. MS2 particles modified with HIV-1 TAT cell penetrating peptides have recently been shown to act as effective gene transfer agents [61]. M13 particles displaying RGD peptides and carrying a chimeric M13-AAV cassette (RGD-4C AAVP) has also shown promising results, specifically targeting solid tumors after systemic administration in mice [101]. The transduction of mammalian cells can be achieved without a helper virus or any trans-acting factors, but allows tumor imaging and the delivery of drugs (Figure 4) [62,63**]. Other developments include combinations of viral and non-viral platforms, such as adenoviruses covered with a lipid bilayer (virosomes) to overcome tissue tropism and hepatoxicitiy [64], and nanovaults containing adenovirus membrane lysis proteins [65].
4.4 Imaging
VNPs can be covalently conjugated to many different imaging reagents, such as fluorescent dyes which can be used for intravital vascular imaging. For example, fluorescent CPMV sensors allow the vasculature and blood flow to be visualized in living mouse and chick embryos to a depth of up to 500 μm and for up to 72 h [43]. These CPMV sensors specifically label endothelial cells by interacting with vimentin displayed on the cell surface, and uptake is enhanced in the tumor endothelium [66]. This CPMV-specific feature has been exploited to image tumor angiogenesis [67**]. The potential to visualize tumors and metastatic cells has been emphasized in a recent study showing that CPMV interacts with a variety of human cancer cell lines and can home in on tumors in vivo [68].
Encapsulation techniques can also be used to design hybrid VNPs with fluorescent cores for use in cellular imaging applications. For example, this has been demonstrated using Simian virus 40 (SV40) VLPs encapsulating quantum dots and tracking them by confocal microscopy [69], and using BMV VLPs encapsulating the cyanine dye indocyanine green and tracking them by infrared optical imaging [70**]. Encapsulation of the imaging agent inside the VLP has the advantage of leaving the external surface available for chemical modification with targeting ligands.
VNPs have also been developed as reagents for non-invasive imaging techniques such as MRI and PET (positron emission tomography). MRI contrast reagents have been developed by decorating CPMV, CCMV and MS2 particles with gadolinium [71–74*]. This approach is useful because the large surface area of VNPs allows decoration with hundreds of Gd ions, and their rigid molecular structures and large rotational correlation offers high relaxivity values. MS2 capsids have also been covalently modified with a xenon host molecule, cryptophane-A, on the capsid interior. The resulting system shows significantly improved 29Xe solubility and very high CEST (chemical exchange saturation transfer) sensitivity in comparison to the free cryptophane-A without the VNP carrier [75].
Most recently, VNPs have been explored as tools for PET imaging applications. MS2 particles have been labeled with [18F]-fluorobenzaldehyde and evaluated in rats. These experiments showed that although [18F]fluorobenzaldehyde is rapidly cleared from the circulation, [18F]-MS2 particles can persist for at least 3 hours (Figure 3) [76**]. In a different study, iron oxide nanoparticles and 18F-fluoride were encapsulated into envelopes derived from Hemagglutinating virus of Japan (HVJ), and achieved a similarly high signal-to-noise imaging resolution in PET. The combined magnetic iron oxide core and 18F imaging reagent will be useful for region-specific delivery by magnetic guidance [77].
Figure 3.

Volume-rendered PET images of male Sprague–Dawley rats injected with [18F]-MS2. Sprague–Dawley rats were anesthetized and placed side-by-side in a PET scanner. The animals were injected at the same time through the tail vein with same amount of either of [18F]-fluorobenzaldehyde or [18F]-MS2. [18F]-fluorobenzaldehyde was rapidly cleared from circulation, with essentially no signal in the heart blood pool after only 15 seconds. In contrast, [18F]-MS2 remained in circulation for the duration of the experiment (3 hours) as seen in the figure. Reproduced with permission from reference [76].
5. Outlook
VNPs and VLPs provide the basis for a diverse range of biomedical applications including disease prevention, diagnosis, monitoring and therapy. Chemical and genetic modification can be used to generate numerous functionalities, e.g. adding drugs, toxins, imaging reagents, epitopes and specific targeting peptides to the internal and external surfaces of the particle. Encapsulation and self-assembly can be used to fill the cavities of such particles with diverse payloads. This unique flexibility provides many advantages over synthetic nanoparticles.
Viral nanotechnology is an emerging field at a relatively early stage of development. However, considering that some recently-approved nanoparticle-based technologies were introduced almost three decades ago, VNPs have taken giant strides towards the clinic and their development is accelerating. One reason for this is that VNP technology offers a highly versatile platform, both in chemical engineering terms and in the context of biomedical development and applications. Additional research is required to develop a comprehensive understanding of the behavioral properties of VNP/VLP platforms in vivo to facilitate the translation of virus-based nanomedicines from the research laboratory to the clinic.
Highlights.
Viral nanoparticles serve as versatile tools for applications in medicine.
Genetically engineered VNPs are used as vaccines.
Chemically engineered VNPs are used in targeted drug-delivery and biomedical imaging.
Acknowledgments
This work was funded by NIH grants R00 EB009105 (PI Nicole F. Steinmetz) and P30 EB011317 (PI Jeffrey Duerk).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Resch-Genger U, Grabolle M, Cavaliere-Jaricot S, Nitschke R, Nann T. Quantum dots versus organic dyes as fluorescent labels. Nat Methods. 2008;5(9):763–775. doi: 10.1038/nmeth.1248. [DOI] [PubMed] [Google Scholar]
- 2.McNerny DQ, Leroueil PR, Baker JR. Understanding specific and nonspecific toxicities. a requirement for the development of dendrimer-based pharmaceuticals. Wires Nanomed Nanobi. 2010;2(3):249–259. doi: 10.1002/wnan.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jesorka A, Orwar O, Liposomes Technologies and Analytical Applications. Annu Rev Anal Chem. 2008;1:801–832. doi: 10.1146/annurev.anchem.1.031207.112747. [DOI] [PubMed] [Google Scholar]
- 4.Giljohann DA, Seferos DS, Daniel WL, Massich MD, Patel PC, Mirkin CA. Gold Nanoparticles for Biology and Medicine. Angew Chem Int Edit. 2010;49 (19):3280–3294. doi: 10.1002/anie.200904359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fortin JP, Wilhelm C, Servais J, Menager C, Bacri JC, Gazeau F. Size-sorted anionic iron oxide nanomagnets as colloidal mediators for magnetic hyperthermia. J Am Chem Soc. 2007;129(9):2628–2635. doi: 10.1021/ja067457e. [DOI] [PubMed] [Google Scholar]
- 6.Qiao RR, Yang CH, Gao MY. Superparamagnetic iron oxide nanoparticles. from preparations to in vivo MRI applications. J Mater Chem. 2009;19(35):6274–6293. [Google Scholar]
- 7.Khemtong C, Kessinger CW, Gao JM. Polymeric nanomedicine for cancer MR imaging and drug delivery. Chem Commun. 2009;24:3497–3510. doi: 10.1039/b821865j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner V, Dullaart A, Bock AK, Zweck A. The emerging nanomedicine landscape. Nat Biotechnol. 2006;24(10):1211–1217. doi: 10.1038/nbt1006-1211. [DOI] [PubMed] [Google Scholar]
- 9.Lu JM, Wang XW, Marin-Muller C, Wang H, Lin PH, Yao QZ, Chen CY. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev Mol Diagn. 2009;9(4):325–341. doi: 10.1586/erm.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peek LJ, Middaugh CR, Berkland C. Nanotechnology in vaccine delivery. Adv Drug Deliver Rev. 2008;60(8):915–928. doi: 10.1016/j.addr.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farrell D, Alper J, Ptak K, Panaro NJ, Grodzinski P, Barker AD. Recent Advances from the National Cancer Institute Alliance for Nanotechnology in Cancer. Acs Nano. 2010;4(2):589–594. doi: 10.1021/nn100073g. [DOI] [PubMed] [Google Scholar]
- 12*.Singh P, Prasuhn D, Yeh RM, Destito G, Rae CS, Osborn K, Finn MG, Manchester M. Bio-distribution, toxicity and pathology of cowpea mosaic virus nanoparticles in vivo. J Control Release. 2007;120(1–2):41–50. doi: 10.1016/j.jconrel.2007.04.003. Describes the in vivo properties of CPMV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13*.Kaiser CR, Flenniken ML, Gillitzer E, Harmsen AL, Harmsen AG, Jutila MA, Douglas T, Young MJ. Biodistribution studies of protein cage nanoparticles demonstrate broad tissue distribution and rapid clearance in vivo. Int J Nanomed. 2007;2(4):715–733. Describes the in vivo properties of CCMV. [PMC free article] [PubMed] [Google Scholar]
- 14.Doan DN, Lee KC, Laurinmaki P, Butcher S, Wong SM, Dokland T. Three-dimensional reconstruction of hibiscus chlorotic ringspot virus. J Struct Biol. 2003;144(3):253–261. doi: 10.1016/j.jsb.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Sherman MB, Guenther RH, Tama F, Sit TL, Brooks CL, Mikhailov AM, Orlova EV, Baker TS, Lommel SA. Removal of divalent cations induces structural transitions in red clover necrotic mosaic virus, revealing a potential mechanism for RNA release. J Virol. 2006;80(21):10395–10406. doi: 10.1128/JVI.01137-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agirrezabala X, Velázquez-Muriel JA, Gómez-Puertas P, Scheres SHW, Carazo JM, Carrascosa JL. Quasi-Atomic Model of Bacteriophage T7 Procapsid Shell. Insights into the Structure and Evolution of a Basic Fold. Structure. 2007;15 (4):461–472. doi: 10.1016/j.str.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Kendall A, McDonald M, Bian W, Bowles T, Baumgarten SC, Shi J, Stewart PL, Bullitt E, Gore D, Irving TC, Havens WM, et al. Structure of flexible filamentous plant viruses. J Virol. 2008;82(19):9546–9554. doi: 10.1128/JVI.00895-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sachse C, Chen JZ, Coureux P-D, Stroupe ME, Fändrich M, Grigorieff N. High-resolution Electron Microscopy of Helical Specimens. A Fresh Look at Tobacco Mosaic Virus. Journal of Molecular Biology. 2007;371(3):812–835. doi: 10.1016/j.jmb.2007.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19*.Pokorski JK, Steinmetz NF. The Art of Engineering Viral Nanoparticles. Mol Pharm. 2010 doi: 10.1021/mp100225y. Review article on the chemical engineering of VNPs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Schneemann A, Young MJ. Viral assembly using heterologous expression systems and cell extracts. Adv Protein Chem. 2003;64:1–36. doi: 10.1016/s0065-3233(03)01001-5. Review article on production of VNPs. [DOI] [PubMed] [Google Scholar]
- 21.Garnier A, Cote J, Nadeau I, Kamen A, Massie B. Scale-up of the adenovirus expression system for the production of recombinant protein in human 293S cells. Cytotechnology. 1994;15(1–3):145–155. doi: 10.1007/BF00762389. [DOI] [PubMed] [Google Scholar]
- 22.Mueller A, Kadri A, Jeske H, Wege C. In vitro assembly of Tobacco mosaic virus coat protein variants derived from fission yeast expression clones or plants. Journal of Virological Methods. 2010;166(1–2):77–85. doi: 10.1016/j.jviromet.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 23.Miller RA, Presley AD, Francis MB. Self-assembling light-harvesting systems from synthetically modified tobacco mosaic virus coat proteins. J Am Chem Soc. 2007;129(11):3104–3109. doi: 10.1021/ja063887t. [DOI] [PubMed] [Google Scholar]
- 24.Wang Q, Lin T, Johnson JE, Finn MG. Natural supramolecular building blocks. Cysteine-added mutants of cowpea mosaic virus. Chemistry & Biology. 2002;9 (7):813–819. doi: 10.1016/s1074-5521(02)00166-7. [DOI] [PubMed] [Google Scholar]
- 25*.Park JS, Cho MK, Lee EJ, Ahn KY, Lee KE, Jung JH, Cho Y, Han SS, Kim YK, Lee J. A highly sensitive and selective diagnostic assay based on virus nanoparticles. Nat Nanotechnol. 2009;4(4):259–264. doi: 10.1038/nnano.2009.38. Describes the use of VNPs in diagnostic assays. [DOI] [PubMed] [Google Scholar]
- 26*.Lee J-H, Kim JS, Park J-S, Lee W, Lee KE, Han S-S, Lee KB, Lee J. A Three-Dimensional and Sensitive Bioassay Based on Nanostructured Quartz Combined with Viral Nanoparticles. Advanced Functional Materials. 2010;20(12):2004–2009. Describes the use of VNPs in diagnostic assays. [Google Scholar]
- 27**.Ren Y, Wong SM, Lim LY. Folic acid-conjugated protein cages of a plant virus. A novel delivery platform for doxorubicin. Bioconjugate Chem. 2007;18(3):836–843. doi: 10.1021/bc060361p. Targeted drug-delivery using VNPs. [DOI] [PubMed] [Google Scholar]
- 28.Sikkema FD, Comellas-Aragones M, Fokkink RG, Verduin BJM, Cornelissen JJLM, Nolte RJM. Monodisperse polymer-virus hybrid nanoparticles. Org Biomol Chem. 2007;5(1):54–57. doi: 10.1039/b613890j. [DOI] [PubMed] [Google Scholar]
- 29.Daniel MC, Tsvetkova IB, Quinkert ZT, Murali A, De M, Rotello VM, Kao CC, Dragnea B. Role of Surface Charge Density in Nanoparticle-Templated Assembly of Bromovirus Protein Cages. Acs Nano. 2010;4(7):3853–3860. doi: 10.1021/nn1005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren YP, Wong SM, Lim LY. In vitro-reassembled plant virus-like particles for loading of polyacids. J Gen Virol. 2006;87:2749–2754. doi: 10.1099/vir.0.81944-0. [DOI] [PubMed] [Google Scholar]
- 31.Hu YF, Zandi R, Anavitarte A, Knobler CM, Gelbart WM. Packaging of a polymer by a viral capsid. The interplay between polymer length and capsid size. Biophys J. 2008;94(4):1428–1436. doi: 10.1529/biophysj.107.117473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dixit SK, Goicochea NL, Daniel MC, Murali A, Bronstein L, De M, Stein B, Rotello VM, Kao CC, Dragnea B. Quantum dot encapsulation in viral capsids. Nano Lett. 2006;6(9):1993–1999. doi: 10.1021/nl061165u. [DOI] [PubMed] [Google Scholar]
- 33.Mcaleer WJ, Buynak EB, Maigetter RZ, Wampler DE, Miller WJ, Hilleman MR. Human Hepatitis-B Vaccine from Recombinant Yeast. Nature. 1984;307(5947):178–180. doi: 10.1038/307178a0. [DOI] [PubMed] [Google Scholar]
- 34.Villa LL, Costa RLR, Petta CA, Andrade RP, Ault KA, Giuliano AR, Wheeler CM, Koutsky LA, Malm C, Lehtinen M, Skjeldestad FE, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6(5):271–278. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 35.Martin SJ, Vyakarnam A, Cheingsongpopov R, Callow D, Jones KL, Senior JM, Adams SE, Kingsman AJ, Matear P, Gotch FM, Mcmichael AJ, et al. Immunization of Human Hiv-Seronegative Volunteers with Recombinant P17/P24-Ty Virus-Like Particles Elicits Hiv-1 P24-Specific Cellular and Humoral Immune-Responses. Aids. 1993;7(10):1315–1323. [PubMed] [Google Scholar]
- 36.Pejawar-Gaddy S, Rajawat Y, Hilioti Z, Xue J, Gaddy DF, Finn OJ, Viscidi RP, Bossis I. Generation of a tumor vaccine candidate based on conjugation of a MUC1 peptide to polyionic papillomavirus virus-like particles. Cancer Immunol Immun. 2010;59(11):1685–1696. doi: 10.1007/s00262-010-0895-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edelstein ML, Abedi MR, Wixon J, Edelstein RM. Gene therapy clinical trials worldwide 1989–2004 - an overview. J Gene Med. 2004;6(6):597–602. doi: 10.1002/jgm.619. [DOI] [PubMed] [Google Scholar]
- 38.Srivastava AS, Kaido T, Carrier E. Immunological factors that affect the in vivo fate of T7 phage in the mouse. Journal of Virological Methods. 2004;115(1):99–104. doi: 10.1016/j.jviromet.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 39.Gatto D, Ruedl C, Odermatt B, Bachmann MF. Rapid response of marginal zone B cells to viral particles. J Immunol. 2004;173(7):4308–4316. doi: 10.4049/jimmunol.173.7.4308. [DOI] [PubMed] [Google Scholar]
- 40*.Plummer EM, Manchester M. Viral nanoparticles and virus-like particles: platforms for contemporary vaccine design. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010 doi: 10.1002/wnan.119. Review describing the applications of VNPs as platforms for vaccine development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raja KS, Wang Q, Gonzalez MJ, Manchester M, Johnson JE, Finn MG. Hybrid virus-polymer materials. 1. Synthesis and properties of PEG-decorated cowpea mosaic virus. Biomacromolecules. 2003;4(3):472–476. doi: 10.1021/bm025740+. [DOI] [PubMed] [Google Scholar]
- 42.Steinmetz NF, Manchester M. PEGylated viral nanoparticles for biomedicine. the impact of PEG chain length on VNP cell interactions in vitro and ex vivo. Biomacromolecules. 2009;10(4):784–792. doi: 10.1021/bm8012742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43**.Lewis JD, Destito G, Zijlstra A, Gonzalez MJ, Quigley JP, Manchester M, Stuhlmann H. Viral nanoparticles as tools for intravital vascular imaging. Nat Med. 2006;12(3):354–360. doi: 10.1038/nm1368. Use of fluorescent VNPs for intravital imaging applications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruoslahti E, Bhatia SN, Sailor MJ. Targeting of drugs and nanoparticles to tumors. J Cell Biol. 2010;188(6):759–768. doi: 10.1083/jcb.200910104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45**.Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Girard OM, Hanahan D, Mattrey RF, Ruoslahti E. Tissue-Penetrating Delivery of Compounds and Nanoparticles into Tumors. Cancer Cell. 2009;16(6):510–520. doi: 10.1016/j.ccr.2009.10.013. Tumor targeting using peptide-engineered VNPs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruoslahti E. Vascular zip codes in angiogenesis and metastasis. Biochem Soc T. 2004;32:397–402. doi: 10.1042/BST0320397. [DOI] [PubMed] [Google Scholar]
- 47.Arap W, Haedicke W, Bernasconi M, Kain R, Rajotte D, Krajewski S, Ellerby HM, Bredesen DE, Pasqualini R, Ruoslahti E. Targeting the prostate for destruction through a vascular address. P Natl Acad Sci USA. 2002;99(3):1527–1531. doi: 10.1073/pnas.241655998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loo L, Guenther RH, Lommel SA, Franzen S. Infusion of dye molecules into Red clover necrotic mosaic virus. Chem Commun. 2008;(1):88–90. doi: 10.1039/b714748a. [DOI] [PubMed] [Google Scholar]
- 49.Acosta-Ramirez E, Perez-Flores R, Majeau N, Pastelin-Palacios R, Gil-Cruz C, Ramirez-Saldana M, Manjarrez-Orduno N, Cervantes-Barragan L, Santos-Argumedo L, Flores-Romo L, Becker I, et al. Translating innate response into long-lasting antibody response by the intrinsic antigen-adjuvant properties of papaya mosaic virus. Immunology. 2008;124(2):186–197. doi: 10.1111/j.1365-2567.2007.02753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50**.Brown WL, Mastico RA, Wu M, Heal KG, Adams CJ, Murray JB, Simpson JC, Lord JM, Taylor-Robinson AW, Stockley PG. RNA bacteriophage capsid-mediated drug delivery and epitope presentation. Intervirology. 2002;45(4–6):371–380. doi: 10.1159/000067930. Targeted drug-delivery using VNPs. [DOI] [PubMed] [Google Scholar]
- 51**.Huang RK, Steinmetz NF, Fu CY, Manchester M, Johnson JE. Transferrin-mediated targeting of bacteriophage HK97 nanoparticles into tumor cells. Nanomedicine (Lond) 2011;6(1):55–68. doi: 10.2217/nnm.10.99. Tumor cell targeting using protein-engineered VNPs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52**.Brunel FM, Lewis JD, Destito G, Steinmetz NF, Manchester M, Stuhlmann H, Dawson PE. Hydrazone Ligation Strategy to Assemble Multifunctional Viral Nanoparticles for Cell Imaging and Tumor Targeting. Nano Lett. 2010;10(3):1093–1097. doi: 10.1021/nl1002526. Tumor targeting using peptide-engineered VNPs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steinmetz NF, Ablack A, Hickey JL, Ablack J, Manocha B, Mymryk JSLGL, Lewis JD. Intravital Imaging of Human Prostate Cancer using Viral Nanoparticles Targeted to Gastrin-Releasing Peptide Receptors. Small. doi: 10.1002/smll.201000435. in press. Tumor targeting using peptide-engineered VNPs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yacoby I, Shamis M, Bar H, Shabat D, Benhar I. Targeting antibacterial agents by using drug-carrying filamentous bacteriophages. Antimicrob Agents Ch. 2006;50 (6):2087–2097. doi: 10.1128/AAC.00169-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55**.Steinmetz NF, Hong V, Spoerke ED, Lu P, Breitenkamp K, Finn MG, Manchester M. Buckyballs Meet Viral Nanoparticles. Candidates for Biomedicine. J Am Chem Soc. 2009;131(47):17093–17095. doi: 10.1021/ja902293w. VNPs as candidates for PDT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56**.Suci PA, Varpness Z, Gillitzer E, Douglas T, Young M. Targeting and photodynamic killing of a microbial pathogen using protein cage architectures functionalized with a photosensitizer. Langmuir. 2007;23(24):12280–12286. doi: 10.1021/la7021424. VNPs as candidates for PDT. [DOI] [PubMed] [Google Scholar]
- 57**.Stephanopoulos N, Tong GJ, Hsiao SC, Francis MB. Dual-Surface Modified Virus Capsids for Targeted Delivery of Photodynamic Agents to Cancer Cells. Acs Nano. 2010;4(10):6014–6020. doi: 10.1021/nn1014769. VNPs as candidates for PDT. [DOI] [PubMed] [Google Scholar]
- 58.Waehler R, Russell SJ, Curiel DT. Engineering targeted viral vectors for gene therapy. Nat Rev Genet. 2007;8(8):573–587. doi: 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tenenbaum L, Lehtonen E, Monahan PE. Evaluation of risks related to the use of adeno-associated virus-based vectors. Current Gene Therapy. 2003;3(6):545–565. doi: 10.2174/1566523034578131. [DOI] [PubMed] [Google Scholar]
- 60.Wu TL, Ertl HCJ. Immune barriers to successful gene therapy. Trends Mol Med. 2009;15(1):32–39. doi: 10.1016/j.molmed.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 61.Wei B, Wei YX, Zhang K, Wang J, Xu RH, Zhan S, Lin GG, Wang W, Liu M, Wang JN, Zhang R, et al. Development of an antisense RNA delivery system using conjugates of the MS2 bacteriophage capsids and HIV-1 TAT cell penetrating peptide. Biomed Pharmacother. 2009;63(4):313–318. doi: 10.1016/j.biopha.2008.07.086. [DOI] [PubMed] [Google Scholar]
- 62**.Hajitou A, Trepel M, Lilley CE, Soghomonyan S, Alauddin MM, Marini FC, Restel BH, Ozawa MG, Moya CA, Rangel R, Sun Y, et al. A hybrid vector for ligand-directed tumor targeting and molecular imaging. Cell. 2006;125(2):385–398. doi: 10.1016/j.cell.2006.02.042. Gene-delivery using engineered VNPs. [DOI] [PubMed] [Google Scholar]
- 63.Trepel M, Stoneham CA, Eleftherohorinou H, Mazarakis ND, Pasqualini R, Arap W, Hajitou A. A heterotypic bystander effect for tumor cell killing after adeno-associated virus/phage-mediated, vascular-targeted suicide gene transfer. Mol Cancer Ther. 2009;8(8):2383–2391. doi: 10.1158/1535-7163.MCT-09-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Singh R, Al-Jamal KT, Lacerda L, Kostarelos K. Nanoengineering artificial lipid envelopes around adenovirus by self-assembly. Acs Nano. 2008;2(5):1040–1050. doi: 10.1021/nn8000565. [DOI] [PubMed] [Google Scholar]
- 65.Lai CY, Wiethoff CM, Kickhoefer VA, Rome LH, Nemerow GR. Vault Nanoparticles Containing an Adenovirus-Derived Membrane Lytic Protein Facilitate Toxin and Gene Transfer. Acs Nano. 2009;3(3):691–699. doi: 10.1021/nn8008504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koudelka KJ, Destito G, Plummer EM, Trauger SA, Siuzdak G, Manchester M. Endothelial Targeting of Cowpea Mosaic Virus (CPMV) via Surface Vimentin. Plos Pathog. 2009;5(5) doi: 10.1371/journal.ppat.1000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67**.Leong HS, Steinmetz NF, Ablack A, Destito G, Zijlstra A, Stuhlmann H, Manchester M, Lewis JD. Intravital imaging of embryonic and tumor neovasculature using viral nanoparticles. Nat Protoc. 2010;5(8):1406–1417. doi: 10.1038/nprot.2010.103. Use of fluorescent VNPs for intravital imaging applications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Steinmetz NF, Cho C-F, Ablack A, Lewis JD, Manchester M. CPMV nanoparticles target surface vimentin on cancer cells. Nanomedicine. 2011 doi: 10.2217/nnm.10.136. in press. [DOI] [PMC free article] [PubMed]
- 69.Li F, Zhang ZP, Peng J, Cui ZQ, Pang DW, Li K, Wei HP, Zhou YF, Wen JK, Zhang XE. Imaging viral behavior in Mammalian cells with self-assembled capsid-quantum-dot hybrid particles. Small. 2009;5(6):718–726. doi: 10.1002/smll.200801303. [DOI] [PubMed] [Google Scholar]
- 70**.Jung B, Rao AL, Anvari B. Optical Nano-Constructs Composed of Genome-Depleted Brome Mosaic Virus Doped with a Near Infrared Chromophore for Potential Biomedical Applications. Acs Nano. 2011 doi: 10.1021/nn1028696. Use of hybrid VNPs for imaging applications. [DOI] [PubMed] [Google Scholar]
- 71*.Datta A, Hooker JM, Botta M, Francis MB, Aime S, Raymond KN. High relaxivity gadolinium hydroxypyridonate-viral capsid conjugates: nanosized MRI contrast agents. J Am Chem Soc. 2008;130(8):2546–2552. doi: 10.1021/ja0765363. VNP-Gd complexes as candidates for MRI. [DOI] [PubMed] [Google Scholar]
- 72*.Allen M, Bulte JWM, Liepold L, Basu G, Zywicke HA, Frank JA, Young M, Douglas T. Paramagnetic viral nanoparticles as potential high-relaxivity magnetic resonance contrast agents. Magnet Reson Med. 2005;54(4):807–812. doi: 10.1002/mrm.20614. VNP-Gd complexes as candidates for MRI. [DOI] [PubMed] [Google Scholar]
- 73*.Anderson EA, Isaacman S, Peabody DS, Wang EY, Canary JW, Kirshenbaum K. Viral nanoparticles donning a paramagnetic coat: Conjugation of MRI contrast agents to the MS2 capsid. Nano Lett. 2006;6(6):1160–1164. doi: 10.1021/nl060378g. VNP-Gd complexes as candidates for MRI. [DOI] [PubMed] [Google Scholar]
- 74*.Prasuhn DE, Yeh RM, Obenaus A, Manchester M, Finn MG. Viral MRI contrast agents: coordination of Gd by native virions and attachment of Gd complexes by azide-alkyne cycloaddition. Chem Commun. 2007;(12):1269–1271. doi: 10.1039/b615084e. VNP-Gd complexes as candidates for MRI. [DOI] [PubMed] [Google Scholar]
- 75.Meldrum T, Seim KL, Bajaj VS, Palaniappan KK, Wu W, Francis MB, Wemmer DE, Pines A. A Xenon-Based Molecular Sensor Assembled on an MS2 Viral Capsid Scaffold. J Am Chem Soc. 2010;132(17):5936–5937. doi: 10.1021/ja100319f. [DOI] [PubMed] [Google Scholar]
- 76**.Hooker JM, O’Neil JP, Romanini DW, Taylor SE, Francis MB. Genome-free viral capsids as carriers for positron emission tomography radiolabels. Mol Imaging Biol. 2008;10(4):182–191. doi: 10.1007/s11307-008-0136-5. VNPs for PET imaging. [DOI] [PubMed] [Google Scholar]
- 77.Flexman JA, Cross DJ, Lewellen BL, Miyoshi S, Kim Y, Minoshima S. Magnetically targeted viral envelopes. A PET investigation of initial biodistribution. Ieee T Nanobiosci. 2008;7(3):223–232. doi: 10.1109/TNB.2008.2002288. [DOI] [PMC free article] [PubMed] [Google Scholar]