Abstract
Oxidative stress and mitochondrial dysfunction occur before apoptosis in many retinal diseases. Under these conditions, a larger fraction of flavoproteins become oxidized and, when excited by blue-light, emit green flavoprotein fluorescence (FPF). In this study, we evaluated the utility of FPF as an early indicator of mitochondrial stress, pre-apoptotic cellular instability, and apoptosis of human retinal pigment epithelial (HRPE) cells subjected to hydrogen peroxide (H2O2) or monocytes (unstimulated or interferon-γ-stimulated) in vitro and of freshly isolated pieces of human and rat neural retina subjected to H2O2 ex vivo. Increased FPF of HRPE cells exposed to H2O2 correlated with reduced mitochondrial membrane potential (ΔΨm) and increased apoptosis in a time- and dose-dependent manner. HRPE cells co-cultured with monocytes had increased FPF that correlated in a time-dependent manner with reduced ΔΨm, increased apoptosis, and early expression of pro-inflammatory chemokines, interleukin-8 (IL8) and monocyte chemotactic factor-1 (MCP1), which are known to be induced by oxidative stress. Increased FPF, reduced ΔΨm, and upregulation of IL8 and MCP1 occurred as early as 1–2 hours after exposure to stressors, while apoptosis did not occur in HRPE cells until later time points. The antioxidant, N-aceytl-cysteine (NAC), inhibited increased FPF and apoptosis of HRPE cells subjected to H2O2. Increased FPF of human and rat neural retina also correlated with increased apoptosis. This study suggests that FPF is a useful measure of mitochondrial function in retinal cells and tissues and can detect early mitochondrial dysfunction that may precede apoptosis.
Keywords: apoptosis, chemokines, flavoproteins, mitochondrial dysfunction, monocytes, oxidative stress, neural retina, retinal pigment epithelium
1. Introduction
Oxidant damage is believed to be a major contributor to the pathogenesis of numerous retinal diseases by participating in mitochondrial stress and apoptotic cell death of neuronal and supportive cells (Lieven et al., 2003; Sano et al., 2006; Armstrong et al., 2004; Jiang et al., 2005). Retinal cell apoptosis involves alterations in mitochondrial membrane potential (ΔΨm) leading to the release of mediators that activate caspases, which initiate downstream damage to cellular components, including nucleic acids and proteins (Lieven et al., 2003; Sano et al., 2006; Armstrong et al., 2004; Jiang et al., 2005). However, reactive oxygen metabolites (ROM), including hydrogen peroxide (H2O2), impair enzymatic complexes of the electron transport chain and reduce ΔΨm before apoptosis occurs (Long et al., 2004). Under these conditions, flavoproteins linked to these enzymes become oxidized, and when excited by blue-light, emit green flavoprotein fluorescence (FPF) (Benson et al., 1979; Chance et al., 1979). Thus, measurement of mitochondrial metabolic activity by FPF may serve as an early indicator of disease (Elner et al., 2008a; Elner et al., 2008b; Field et al., 2008; Field et al., 2009a; Field et al., 2009b).
Exogenous or endogenous ROM, including H2O2, induced by light, toxins, or other stimulants of apoptosis, may cause death of cells within the neurosensory retina or retinal pigment epithelium (RPE) (Jin et al., 2001; Barak et al., 2001; Kannan et al., 2004). In cultured human RPE (HRPE) cells, exogenous H2O2 has also been shown to induce mitochondrial oxidative dysfunction and DNA damage (Ballinger et al., 1999), and elevate retinal FPF (Elner et al., 2008a). Sub-lethal or lethal concentrations of H2O2 reduce the ΔΨm of retinal cells early in the apoptotic process (Cao et al., 1999). In addition to H2O2, unstimulated and interferon (IFN)-γ-stimulated monocytes have been shown to induce generation of retinal ROM when co-cultured with mouse or human RPE cells, which subsequently may undergo apoptosis (Yoshida et al., 2003; Yang et al., 2009). ROM-induced impairment of mitochondrial electron transport results in oxidation of flavoproteins before apoptosis occurs (Long et al, 2004). Thus, FPF may serve as an early signal of cells prone to apoptosis.
ROM have also been shown to mediate production of pro-inflammatory chemokines, interleukin-8 (IL8) and monocyte chemotactic factor-1 (MCP1), also known as CC motif ligand 2 (CCL2) (Zeng et al., 2003; Kina et al., 2009), which modulate ocular inflammatory responses, including leukocyte chemotaxis and activation within the eye (Yoshida et al., 2001a). IL8 and MCP1 are the principal chemokines secreted by HRPE cells and have been implicated in a number of retinal diseases (Yoshida et al., 2001a; Bian et al., 2004). Direct contact between monocytes and HRPE cells upregulates the production of IL8 and MCP1 (Yoshida et al., 2001a; Bian et al., 2004. Thus, it is important to understand the temporal expression of monocyte adhesion-induced HRPE chemokine gene and protein expression with respect to FPF, ΔΨm, and apoptosis.
In this study, we examined the relationship of FPF, ΔΨm, and apoptosis in HRPE cells and fresh rat and human neural retina subjected to 1) lethal or sub-lethal concentrations of H2O2, and 2) stimulated or unstimulated monocytes to demonstrate whether FPF may serve as an early indicator of apoptosis, pre-apoptotic cellular instability, and the induction of pro-inflammatory chemokines.
2. Materials and Methods
2.1. Materials
N-acetyl-cysteine (NAC) and H2O2 were purchased from Sigma-Aldrich (St. Louis, MO). Recombinant IFN-γ was from R&D Systems (Minneapolis, MN). Costar tissue culture 96-well assay plates with black walls and clear bottoms were obtained from Fisher Scientific (Pittsburgh, PA).
2.2. HRPE cell culture
HRPE cells were isolated from donor eyes by enzymatic digestion and cultured as previously described (Elner et al., 1990; Bian et al., 2009; Yang et al., 2011a; 2011b). The protocol adhered to the provisions of the Declaration of Helsinki for the use of human tissue in research. Briefly, the sensory retina was separated gently from the HRPE monolayer, and the HRPE cells were removed from Bruch’s membrane using one hour incubation with papain. Isolated HRPE cells were grown into Falcon Primaria flasks in DMEM/F12 containing 10% fetal bovine serum (FBS), penicillin G (100 U ml−1), streptomycin sulfate (100 μg ml−1), and amphotericin B (0.25 μg ml−1) at 37 °C in a humidified incubator under 5% CO2. In all experiments, parallel assays were performed on the second to fourth passaged HRPE cells. HRPE cells were seeded into tissue culture 96-well assay plates with black walls and clear bottoms (Costar) at the same time and density from the same parent cultures at a density of 5 × 103 cells/well, and grown in phenol red-free complete DMEM/F12 for 7–14 days to 90%–100% confluence. RPE cells were placed in serum-free media for 24 hr before treatments. All experiments were repeated at least three times on three different cell lines.
2.3. Isolation of normal human and rat neural retina
Human neural retina was obtained from orbital exenteration specimens containing lesions not-involving the globe at the University of Michigan. The human retina was isolated from the specimens, cut into pieces and placed in Hanks’ balanced salt solution (HBSS), containing Ca2+ and Mg2+, without phenol red. Specimens were examined for FPF within 30 minutes of exenteration. Human research was approved by the institutional review board (IRB) at the University of Michigan. For isolation of rat neural retina, normal, healthy Sprague-Dawley (SD) rats, less than 6 months old, were obtained from the Unit for Laboratory Animal Medicine at the University of Michigan through the Rodent Recycling Program. Animals were killed by carbon dioxide, and the eyes were removed. The rat neural retina was isolated from each eye and cut into three pieces. The fresh neural retinal pieces were washed with HBSS. Specimens were examined for FPF within 30 minutes of exenteration. Animal research was approved by the University Committee on Use and Care of Animals (UCUCA) at the University of Michigan.
2.4. Monocyte isolation
Human monocytes were freshly isolated from the peripheral blood of healthy volunteers, as described previously (Yoshida et al., 2001b). In brief, peripheral blood was drawn into a heparinized syringe and 1:1 diluted in 0.9% saline. Mononuclear cells were separated by density gradient centrifugation. The cells were washed and then layered onto density gradient (Fico-Lite monocytes, 1.068 g/mL) for the enrichment of monocytes. Freshly-isolated human peripheral monocytes were preincubated with RPMI culture medium or the culture medium containing IFN-γ (500 units/ml) for 12 hr prior to co-culturing with HRPE monolayers.
2.5. HRPE cell and human and rat neural retina incubations
HRPE cells in 96-well plates were washed with HBSS, containing Ca2+ and Mg2+, without phenol red. HRPE cells were stimulated with hydrogen peroxide (H2O2; 0.01, 0.05, 0.1, and 0.2 mM) for 1, 3, 6, and 24 hr. For HRPE cells in monocyte co-culture, enriched unstimulated or IFN-γ-stimulated monocyte populations were overlaid onto near-confluent HRPE cultures for 1, 2, 4, and 24 hours. After co-culture, the monocytes were removed as previously described (Yoshida et al., 2001b), and HRPE cells were subjected to further analyses. To evaluate neural retinal FPF, pieces of human and rat neural retina were incubated for 20 min with H2O2 (0.2 mM) in the presence and absence of N-acetyl-cysteine (NAC; 1 mM). To assess apoptosis, aliquots of human and rat neural retina were exposed to 0.2mM H2O2 with or without NAC for 6 hours.
2.6. Flavoprotein fluorescence measurement
FPF of HRPE cells was measured with a FlexStation Scanning Fluorometer (Molecular Devices, Sunnyvale, CA). Excitation and emission wavelengths were set to 436 nm and 520 nm, respectively (Shiino et al., 1998), with a cutoff of 495 nm. For human and rat neural retina, FPF acquisitions were captured from each neural retinal piece, mounted under a coverslip, using a stereomicroscope (Stereo Lumar.V12, Carl Zeiss, Oberkochen, Germany) equipped with narrow bandwidth excitation and emission filters of 465±3nm and 535±3nm, respectively (Omega Optical, Brattleboro, Vermont), and an electronmultiplying charge-coupled device camera (Photometrics, Tucson, Arizona). The images were stored as 512 × 512 pixel 16-bit grayscale TIFF files. The integrated intensity of FPF was calculated for each acquisition.
2.7. Mitochondrial membrane potential measurement
JC-1 dye was used to monitor ΔΨm, as previously described (Yang et al., 2009). In the undamaged mitochondria, the aggregated dye appears as red fluorescence, whereas in the apoptotic cell with altered ΔΨm, the dye remains as monomers in the cytoplasm with diffuse green fluorescence. The red/green fluorescence ratio is dependent on ΔΨm. HRPE cells were loaded with JC-1 for 1 hour, washed, and exposed to different treatments. Fluorescence intensity was measured with a FlexStation Scanning Fluorometer. For green fluorescence measurements, excitation and emission wavelengths were set to 485 nm and 530 nm, respectively; for red fluorescence measurements, excitation and emission wavelengths were set to 520 nm and 610 nm, respectively.
2.8. Apoptotic cell death detection
Neural retinal and HRPE apoptosis was evaluated by using a Cell Death Detection ELISAPLUS kit (Roche Applied Science, Indianapolis, IN). This photometric enzyme immunoassay provides the quantitative in vitro determination of cytoplasmic histone-associated DNA fragments (mono- and oligonucleosomes) after induced cell death. After various treatments, HRPE cells and human and rat neural retinas were washed, harvested, lysed, centrifuged to remove nuclei, and supernatants were collected. An aliquot of the supernatant from each sample was incubated with immunoreagents (anti-histone-biotin plus anti-DNA-peroxidase-conjugated antibody) in 96-well streptavidin-coated plates on a shaker. After three washes with incubation buffer, ABTS substrate solution was added to each well, and absorbance was read at 405 nm and 495 nm, respectively, in a microplate reader. The absorbance difference between A405nm and A490nm was standardized to wet weight of the neural retina and results normalized to control.
2.9. IL8 and MCP1 ELISA
The levels of antigenic IL8 and MCP1 in the serial dilutions of HRPE supernatants were quantitated by modification of a double-ligand ELISA method as previously described (Bian et al., 2001). Standards included 0.5 log dilutions of recombinant IL8 and MCP1 (R&D Systems) from 5pg to 100ng/well.
2.10. Semiquantitative reverse transcription-polymerase chain reaction (RT-PCR)
Total cellular RNA was isolated from nearly confluent cultures of HRPE cells (TRIzol extraction reagent; Invitrogen) according to the manufacturer’s procedure. Synthethic oligonucleotide primers based on the cDNA sequences of human IL8, MCP1 and β-actin were prepared: IL8, 5′-AAGCTGGCCGTGGCTCTCTTG-3′ and 5′-AGCCCTCTTCAAAAACTTCTC-3′; MCP1, 5′-GCTCATAGCAGCCACCTTCATTC-3′ and 5′-GTCTTCGGAGTTTGGGTTTGC-3′; and β-actin, 5′-GTGGGGCGCCCCAGGCACCA-3′ and 5′-CTCCTTAATGTCACGCACGATTTC-3′. RT-PCR was carried out in a semiquantitative manner, essentially as previously described (Yoshida et al., 1998). Linearity range of the reaction was determined running 15 to 35 cycles. DNA was denatured for 5 minutes at 94°C, followed by 28, 26, and 20 PCR cycles for IL8, MCP1, and β-actin, respectively. Each cycle included a 1-minute denaturation at 94°C, a 1-minute primer annealing at 65°C, and a 2-minute polymerization at 72°C. Each RT-PCR reaction mixture was analyzed by electrophoresis on a 2% agarose gel and stained with ethidium bromide. The intensity of the ethidium bromide luminescence was measured by an image sensor with a computer-controlled display.
2.11. Northern blot analysis
Total cellular RNA was isolated from nearly confluent cultures of HRPE cells as described earlier in this manuscript. The RNA was separated by electrophoresis using 1% formaldehyde-agarose gels and transferred overnight to the positively charged nylon membranes by capillary blotting. The blots were hybridized overnight at 50°C with DIG-labeled oligonucleotide probes (25 ng/mL). Bound probes were detected with an anti-DIG Fab fragment conjugated to alkaline phosphatase with the chemiluminescent substrate CSPD (disodium 3-(4-methoxyspiro {1,2-dioxetane-3, 2′-(5′chloro) tricycle [3.3.1.1.] decan}-4-yl)phenyl phosphate). Specific chemokine mRNA was quantified by laser densitometry. Equivalent amounts of total RNA load per gel lane were assessed by monitoring 18s and 28s ribosomal RNA.
2.12. Statistical analysis
Data in the text and figure legends are expressed as the mean ± SEM. Differences were detected using ANOVA and t-tests and P < 0.05 was considered statistically significant.
3. Results
3.1. In vitro assessment of FPF, ΔΨm, and apoptosis in HRPE cells subjected to oxidative stress
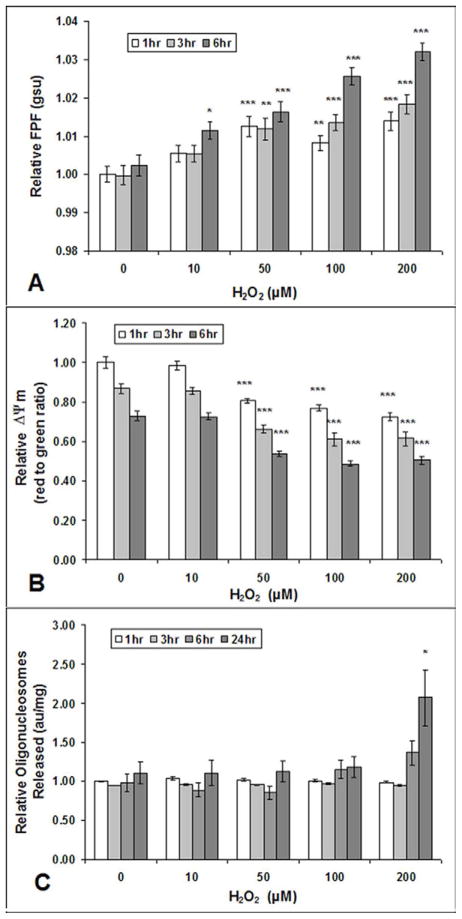
HRPE cells incubated with varying concentrations of sub-lethal and lethal levels of H2O2 showed significantly increased FPF levels when compared to those of control HRPE cells after 6 hours of H2O2 incubation, even with H2O2 concentrations as low as 10 μM (Fig. 1A). HRPE cells incubated with H2O2 concentrations of 50 μM or greater showed significantly increased FPF levels when compared to those of control HRPE cells after only 1 hour of H2O2 incubation. Significant reductions in ΔΨm of HRPE cells were evident after 1, 3, and 6 hours of treatment with H2O2 concentrations of 50, 100, and 200 μM (Fig. 1B). No significant reduction in ΔΨm was detected by the JC-1 assay in HRPE cells treated with 10 μM of H2O2. Apoptosis, measured by the number of oligonucleosomes released, was only significantly increased in HRPE cells incubated with 200 μM of H2O2 for 24 hours when compared to oligonucleosome levels of HRPE control cells (Fig. 1C). Thus, increases in FPF levels and reductions in ΔΨm occur at sub-lethal and lethal H2O2 concentrations. In the latter, the increases in FPF levels and reductions in ΔΨm occur prior to apoptosis.
Fig. 1. FPF, Δψm, and apoptosis in cultured human RPE cells after exposure to H2O2.
Increased FPF (A) and reduced ΔΨm (B) at 1, 3, and 6 hours after cultured human RPE cell exposure to various H2O2 concentrations. Human RPE cells did not show increased levels of apoptosis (released mono- and oligonucleosomes) measured by Cell Detection ELISA, until 24 hours after exposure to 200 μM H2O2 (C). Values are mean (standard error). *P<.05, ***P<.001, compared with corresponding control (0 μM H2O2). Each experiment was conducted on 3 different cell lines, n=9.
3.2. Ex vivo assessment of FPF and ΔΨm in human and rat neural retina subjected to oxidative stress
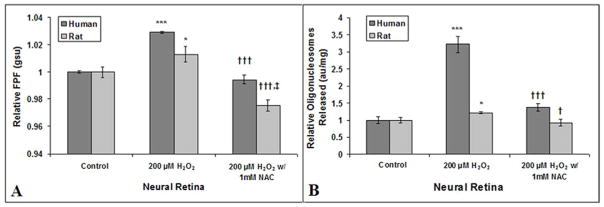
Pieces of fresh human and rat neural retina incubated with 200 μM of H2O2 had significantly increased FPF levels (p-value: <0.001 and <0.05) when compared to FPF levels of control human and rat neural retina (Fig. 2A). This increase in FPF was completely blocked by co-treatment with 1mM of NAC. For rat neural retina incubated with H2O2 + NAC, FPF levels were significantly decreased from that of the control rat neural retina. FPF of the human neural retinal pieces incubated with H2O2 + NAC was 3.5% less than that of control pieces, but did not reach statistical significance (p > 0.05). Apoptosis, measured by the number of oligonucleosomes released, was significantly increased in pieces of fresh human and rat neural retina incubated with 200 μM of H2O2 for 6 hours (p < 0.001 and < 0.05) when compared to FPF of control human and rat neural retina (Fig. 2B). This increase in apoptosis was significantly inhibited in human neural retina and completely inhibited in rat neural retina by co-treatment with 1 mM of NAC.
Fig. 2. Ex vivo FPF assessment of human and rat neural retina mitochondrial stress and correlation with apoptosis.
FPF (A) and apoptotic cell death (B) of human (n=4) and rat neural retina (n=12) incubated with hydrogen peroxide (H2O2), with or without N-acetylcysteine (NAC). Values are mean (standard error). *P<.05, ***P<.001, compared with control; †<.05, †††<.001, compared with H2O2-treated cells; ‡‡<.01, compared with control; gsu indicates grayscale units; au, arbitrary units.
3.3. In vitro assessment of FPF, ΔΨm, and apoptosis in HRPE cells subjected to unstimulated or stimulated monocytes
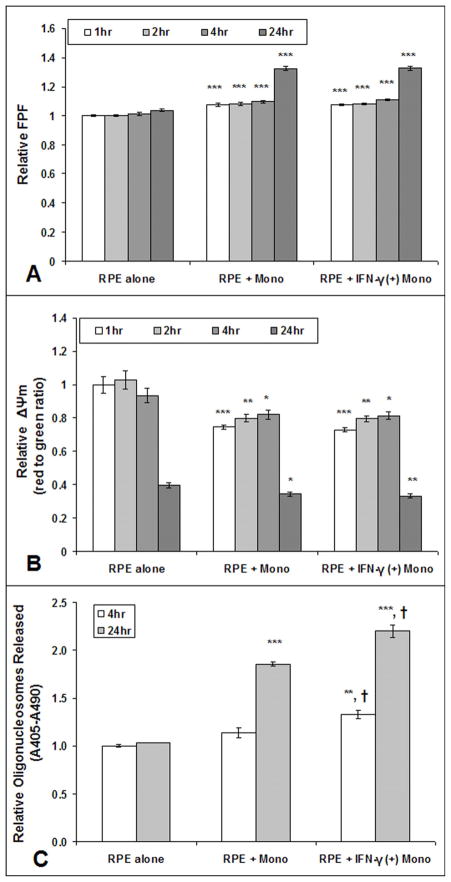
HRPE cells incubated with unstimulated or IFN-γ-stimulated monocytes for 1, 2, 4, or 24 hours showed increased FPF levels and reduced ΔΨm when compared to that of control HRPE cells (Fig. 3A–B), but there was no significant differences in FPF or ΔΨm of HRPE cells co-cultured with unstimulated as compared to stimulated monocytes. Apoptosis was not present in HRPE cells co-cultured with unstimulated monocytes for 4 hours, but apoptosis did occur after co-culture for 24 hours (Fig. 3C). In HRPE cells cocultured with IFN-γ-stimulated monocytes, apoptosis occurred at 4 and 24 hours. There was no apoptosis after 1 or 2 hours in HRPE cells co-cultured with unstimulated or IFN-γ-stimulated monocytes (data not shown). Additionally, HRPE cells co-cultured with IFN-γ-stimulated monocytes had significantly increased apoptosis from that of HRPE cells co-cultured with unstimulated monocytes for 4 and 24 hours.
Fig. 3. FPF, Δψm, and apoptosis in cultured human RPE cells after exposure to unstimulated or IFN-γ-stimulated (+) monocytes.
Increased FPF (A) and reduced ΔΨm (B) at 1, 2, 4 and 24 hours after cultured human RPE cell exposure to unstimulated or IFN-γ-stimulated (+) monocytes. Human RPE cells did not show increased levels of apoptosis (released oligonucleosomes) measured by Cell Detection ELISA, until 4 hours after exposure to IFN-γ-stimulated (+) monocytes or until 24 hours after exposure to unstimulated monocytes(C). Values are mean (standard error). *P<.05, **P<.01, ***P<.001, compared with control RPE alone. †<.05, compared with RPE + unstimulated monocytes. Each experiment was conducted on 3 different cell lines, n=9.
3.4. Gene and protein expression of HRPE cells subjected to unstimulated or stimulated monocytes
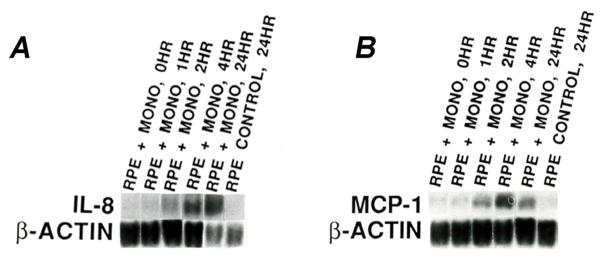
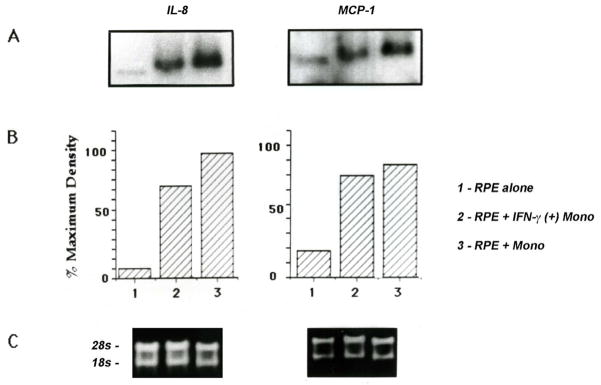
Gene expression of IL8 and MCP1, measured by northern blot analysis, was significantly upregulated after only 2 hours of co-culture with unstimulated monocytes (Fig. 4A–B). RT-PCR (Table 1) and microarray analysis (data not shown) also showed increased IL8 and MCP1 gene expression in HRPE cells co-cultured with unstimulated monocytes. Protein expression of IL8, measured by ELISA, was 1.7x, 8.8x, and 140x greater than basal levels in HRPE cells exposed to unstimulated monocytes for 1, 4, and 24 hours, respectively (Table 1). The same trend was true for MCP1, with increases in protein expression of 4.2x, 8.3x, and 88x over basal levels after exposure to unstimulated monocytes for 1, 4, and 24 hours, respectively. IFN-γ-stimulated monocytes induced less HRPE IL8 and MCP1 gene and protein expression than unstimulated monocytes (Fig. 5, Table 1).
Fig. 4. IL8 and MCP1 gene expression in HRPE cells exposed to unstimulated monocytes.
Northern blot analysis of HRPE IL8 (A) and MCP1 (B) mRNA expression in HRPE cells exposed to unstimulated monocytes for 0, 1, 2, 4, and 24 hours. Unstimulated HRPE cells were used as control. The data are representative of experiments performed 3 times on each of 3 different cell lines, n=9.
Table 1.
Gene (RT-PCR) and protein (ELISA) expression in HRPE cells exposed to monocytes.
| Cell Culture | IL-8 | MCP-1 | |
|---|---|---|---|
| RT-PCRa | RPE + MONO, 4HR | 15.3 | 6.1 |
| RPE + MONO, 24HR | 86.2 | 9.5 | |
|
| |||
| ELISAb | RPE + MONO, 0HR | 0.73 ± 0.13 | 0.3 ± 0.02 |
| RPE + MONO, 1HRc | 1.23 ± 0.02 (p=0.019) | 1.26 ± 0.14 (p=0.0022) | |
| RPE + MONO, 4HRc | 6.45 ± 0.62 (p=0.00082) | 2.49 ± 0.06 (p=0.0000047) | |
| RPE + MONO, 24HRc | 99.80 ± 8.54 (p=0.00021) | 26.29 ± 0.69 (p=0.00040) | |
| RPE + IFN-γ (+) MONO, 24 HRc | 48.61 ± 3.32 (p=0.00032) | 15.80 ± 4.04 (p=0.0000029) | |
Fold increase is compared to RPE + MONO, 0HR
Values are mean concentrations (ng/mL) ± standard error
p-value is compared with control RPE + MONO, 0HR
The data are representative of experiments performed 3 times on each of 3 different cell lines, n=9.
Fig. 5. IL8 and MCP1 gene expression in HRPE cells exposed to unstimulated or IFN-γ-stimulated monocytes.
Northern blot analysis of HRPE IL8 and MCP1 mRNA expression in HRPE cells exposed to unstimulated or IFN-γ-stimulated monocytes for 24 hours (A, B). Unstimulated HRPE cells were used as control. Equivalent total cellular RNA loading per lane is shown by electrophoretic profile of 18s and 28s RNA (C). The data are representative of experiments performed 3 times on each of 3 different cell lines, n=9.
4. Discussion
Our current study substantiates FPF to be an indicator of mitochondrial dysfunction in retinal cells because increases in FPF levels in HRPE cells correlated with reductions in ΔΨm, as measured by the JC-1 assay (Figs. 1–3). With the excitation and emission wavelengths used in this study, increases or decreases in the emitted signal from baseline control should only be due to dynamic changes in the oxidation state of flavoproteins and not from other sources of inherent green emission, including advanced glycation end product (AGE) or lipofuscin autofluorescence, which do not change with acute exposure to various apoptotic stressors or treatments. Moreover, the cultured HRPE cells and rat neural retina were too young to accumulate any significant concentrations of AGEs or lipofuscin. Ex vivo samples of human RPE were not analyzed in this study because of shear stress-induced damage to fresh RPE sheets. It is important to note that there are several potential sources of oxidized flavoproteins in the respiratory chain that are involved in fatty acid and carbohydrate pathways, including lipoamide dehydrogenase (LAD), NADH hydrogenase (electron complex I), succinate dehydrogenase (electron complex II), electron transfer protein, and acyl dehydrogenase. Thus, the FPF signal is not specific to one flavoprotein source but is an amalgam of all of these sources.
In this study, apoptosis did not occur in HRPE cells until incubation with 200 μM of H2O2 for 24 hours, whereas increased FPF and reduced ΔΨm occurred in HRPE cells after only 1 hour of incubation with 50, 100, and 200 μM of H2O2 (Fig. 1). The same trend was seen in HRPE cells incubated with unstimulated or IFN-γ-stimulated monocytes, as increased FPF and reduced ΔΨm was present as early as 1 hour after treatment, but there was no apoptosis at that time (Fig. 3, data not shown). Apoptosis did not occur until HRPE cells were exposed to IFN-γ-stimulated monocytes for 4 hours or unstimulated monocytes for 24 hours, with significantly greater apoptosis occurring in response to stimulated monocytes, corroborating previous findings (Yoshida et al., 2003; Yang et al., 2009). Additionally, increased levels of apoptosis correlated with the more substantial increases in FPF levels and reductions in ΔΨm seen at higher concentrations or longer incubations of H2O2.
In other cell types, impairment of enzymatic complexes II and IV of the electron transport chain and reductions in ΔΨm occur within one hour of H2O2 exposure, before apoptosis commences (Long et al., 2004). Interestingly, electron transport complex II is involved in the redox status of flavin adenine dinucleotide (FAD) that, when oxidized, contributes to the FPF signal. Thus, changes in the mitochondrial electrochemical gradient caused by impaired electron transport and reduced ΔΨm likely result in oxidation of FAD and other flavoproteins. Therefore, FPF and ΔΨm are early indicators of instability, even in cells exposed to low levels of oxidants that are insufficient to cause apoptosis, while more substantial alterations of these indicators are predictive of evolving apoptosis that appears at later time points.
Increases in FPF levels and apoptosis in HRPE cells (Elner et al., 2008a; Field et al., 2009b) and human and rat neural retina (Fig. 2A–B) subjected to lethal levels of H2O2 were significantly inhibited by NAC-antioxidant treatment. In fact, NAC-antioxidant treatment resulted in FPF levels that were significantly below those of control rat neural retina not exposed to H2O2 (Fig. 2A). Although not statistically significant, this trend was also seen in HRPE cell (Elner et al., 2008a; Field et al., 2009b) and human neural retinal (Fig. 2A) FPF levels following NAC-antioxidant treatment. However, NAC did not reduce apoptosis levels back to those of HRPE cells (Field et al., 2009b) and human neural retina (Fig 2B) not exposed to H2O2. One explanation may be that H2O2 was not completely neutralized by NAC and caused substantial cell death before the 24 hour measurements, resulting in fewer live cells to emit the FPF signal. This would imply that clinical quantitative measurement of FPF would be a more accurate indicator of retinal health in early, but not advanced disease. We also tested, but saw no significant decrease in FPF or apoptotic levels of control HRPE cells following treatment with NAC (data not shown).
In this study, we verified that HRPE cells co-cultured with monocytes induce gene and protein expression of pro-inflammatory chemokines, IL8 and MCP1 (Figs. 4 and 5, Table 1) (Yoshida et al., 2001a; Bian et al., 2004). Additionally, we showed that IL8 and MCP1 gene and protein expression increased well before apoptosis occurred, as early as 1–2 hours after HRPE cell co-culture with unstimulated monocytes, and correlated with increases in FPF levels and reductions in ΔΨm (Figs 3–5). One possible explanation for prompt HRPE chemokine induction is the high levels of ROM induced by monocyte-binding to mouse or human RPE cells (Yoshida et al., 2003; Yang et al., 2009), because oxidative stressors have been shown to induce IL8 and MCP1 production in other cell types (Zeng et al., 2003; Kina et al., 2009). Interestingly, production of HRPE MCP1 was significantly inhibited by NAC while that of IL8 was not (data not shown).
This study demonstrates that increased FPF levels in HRPE cells exposed to various stressors correlates with reductions in ΔΨm and upregulation of proinflammatory cytokines, which can all occur well before apoptosis. Current methods of clinical retinal disease assessment are only effective after a large number of cells have been lost to apoptotic cell death. However, cells exhibit disease-related mitochondrial dysfunction well before they are destined to die. Therefore, the ability to non-invasively monitor the relative health of mitochondria rapidly with FPF represents a significant opportunity to diagnose, monitor, and treat the underlying disease.
FPF has previously been used in combination with nicotinamide nucleotide (NAD(P)H) fluorescence to create a ratio that is useful for real-time in vitro quantification of the mitochondrial redox status of many different cell types, including corneal, pancreatic islet, and mesenchymal stem cells (Masters, 1984; Masters et al., 1989; Rocheleau et al., 2004; Tsubota et al., 1998; Reyes et al., 2006; Park et al., 2006). This ratio serves as an indicator of the mitochondrial redox status because NAP(P)H oxidation correlates with FP oxidation but the fluorescence of the two molecules is inversely related. This method may also be used for in vivo experimental measurements and has been used on rabbit hearts and rodent brains during surgery to assess mitochondrial functional changes due to electrical stimulation and ischemia/reperfusion injury (Reinert et al., 2004; Shibuki et al., 2003; Shino et al., 1998; Ranji et al., 2006; Rajpurohit et al., 1999). Recently, Cano et al., 2008, used this technique to show that cultured HRPE cells incubated with increasing concentrations of H2O2 or tert-butyl hydroperoxide had declining NAD(P)H fluorescence and increased FPF, indicative of impaired oxidative phosphorylation, oxidant-induced injury, and cell death.
While measuring NAD(P)H fluorescence, in addition to FPF, is useful to assess changes in mitochondrial redox status and function, NAD(P)H fluorescence cannot be used on human eyes because the excitation ultraviolet wavelengths for NAD(P)H have phototoxic effects on ocular cells, including those of the retina. As a result, we have developed a clinical instrument that non-invasively assesses human retinal FPF. Excitation wavelengths for FPF can be phototoxic to the retina with prolonged exposures, but clinical FPF imaging uses exposures to excitatory blue light that are at least 100 times less than the International Electrotechnical Commission (IEC) maximum permissible energy safety threshold for light damage (International Electrotechnical Commission, 2007). Using this method, we have shown that FPF increases due to increased FP oxidation in eyes of humans with pseudotumor cerebri, diabetes, central serous retinopathy, age-related macular degeneration and retinitis pigmentosa (Elner et al., 2008a; Elner et al., 2008b; Field et al., 2008; Field et al., 2009a). As indicated by our previous work and our data in this study, FPF may prove to be a translational tool to safely, rapidly and non-invasively assess retinal mitochondrial function in vitro, ex vivo, and in vivo.
Highlights.
Flavoprotein fluorescence is an indicator of mitochondrial dysfunction
Oxidative stress and monocytes increase retinal flavoprotein fluorescence
Increased flavoprotein fluorescence correlates with increased chemokine expression
Flavoprotein fluorescence is an early indicator of cells prone to undergo apoptosis
Acknowledgments
Supported by NIH grants EY09441, EY007003, EY019986, and Research to Prevent Blindness Senior Scientific Investigator Award (Victor M. Elner).
Footnotes
Declaration of Interest: Drs. Elner and Petty have a financial interest in the presented material by having founded OcuSciences, Inc., to commercialize the clinical retinal FPF instrument.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armstrong JS, Whiteman M, Yang H, Jones DP, Sternberg P., Jr Cysteine starvation activates the redox-dependent mitochondrial permeability transition in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:4183–89. doi: 10.1167/iovs.04-0570. [DOI] [PubMed] [Google Scholar]
- Ballinger SW, Van Houten B, Jin GF, Conklin CA, Godley BF. Hydrogen peroxide causes significant mitochondrial DNA damage in human RPE cells. Exp Eye Res. 1999;68:765–772. doi: 10.1006/exer.1998.0661. [DOI] [PubMed] [Google Scholar]
- Barak A, Morse LS, Goldkorn T. Ceramide: A potential mediator of apoptosis in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2001;42:247–254. [PubMed] [Google Scholar]
- Benson RC, Meyer RA, Zaruba ME, McKhann GM. Cellular autofluorescence—is it due to flavins? J Histochem Cytochem. 1979;27:44–48. doi: 10.1177/27.1.438504. [DOI] [PubMed] [Google Scholar]
- Bian ZM, Elner SG, Yoshida A, Elner VM. Differential involvement of phosphoinositide 3-kinase/Akt in human RPE MCP-1 and IL-8 expression. Invest Ophthalmol Vis Sci. 2004;45:1887–1896. doi: 10.1167/iovs.03-0608. [DOI] [PubMed] [Google Scholar]
- Bian ZM, Elner SG, Yoshida A, Elner VM. Human RPE-monocyte co-culture induces chemokine gene expression through activation of MAPK and NIK cascade. Exp Eye Res. 2003;76:573–583. doi: 10.1016/s0014-4835(03)00029-0. [DOI] [PubMed] [Google Scholar]
- Bian ZM, Elner VM, Yoshida A, Kunkel SL, Elner SG. Signaling pathways for glycated human serum albumin-induced IL-8 and MCP-1 secretion in human RPE cells. Invest Ophthalmol Vis Sci. 2001;42:1660–1668. [PubMed] [Google Scholar]
- Bian ZM, Elner SG, Elner VM. Dual involvement of caspase-4 in inflammatory and ER stress-induced apoptotic responses in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2009;50:6006–6014. doi: 10.1167/iovs.09-3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Tombran-Tink J, Chen W, Mrazek D, Elias R, McGinnis JF. Pigment epithelium-derived factor protects cultured retinal neurons against hydrogen peroxide-induced cell death. J Neurosci Res. 1999;57:789–800. [PubMed] [Google Scholar]
- Cano MV, Reyes JM, Park CY, Gao X, Mori K, Chuck RS, Gehlbach PL. Demonstration by redox fluorometry that sulforaphane protects retinal pigment epithelial cells against oxidative stress. Invest Ophthalmol Vis Sci. 2008;49:2606–2612. doi: 10.1167/iovs.07-0960. [DOI] [PubMed] [Google Scholar]
- Chance B, Schoener B, Oshino R, Itshak F, Nakase Y. Oxidation-reduction ratio studies of mitochondria in freeze-trapped samples. J Biol Chem. 1979;254:4764–4771. [PubMed] [Google Scholar]
- Elner SG, Elner VM, Field MG, Park S, Heckenlively JR, Petty HR. Retinal flavoprotein autofluorescence as a measure of retinal health. Trans Am Ophthalmol Soc. 2008;106:215–224. [PMC free article] [PubMed] [Google Scholar]
- Elner VM, Park S, Cornblath W, Hackel R, Petty HR. Flavoprotein autofluorescence detection of early ocular dysfunction. Arch Ophthalmol. 2008;126:259–260. doi: 10.1001/archophthalmol.2007.44. [DOI] [PubMed] [Google Scholar]
- Elner VM, Strieter RM, Elner SG, et al. Neutrophil chemotactic factor (IL-8) gene expression by cytokine-treated retinal pigment epithelial cells. Am J Pathol. 1990;136:745–750. [PMC free article] [PubMed] [Google Scholar]
- Field MG, Elner VM, Puro DG, Feuerman JM, Musch DC, Pop-Busui R, Hackel R, Heckenlively JR, Petty HR. Rapid, non-invasive detection of diabetes-induced retinal metabolic stress. Arch Ophthalmol. 2008;126:934–938. doi: 10.1001/archopht.126.7.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field MG, Elner VM, Park S, Hackel R, Heckenlively JR, Elner SG, Petty HR. Detection of retinal metabolic stress due to central serous retinopathy. Retina. 2009;29:1162–1166. doi: 10.1097/IAE.0b013e3181a3b923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field MG, Elner VM, Feuerman JM, Heckenlively JR, Petty HR. Potential Causes of Altered Autofluorescence in Diabetic Persons-Reply. Arch Ophthalmol. 2009;127:943–945. doi: 10.1001/archophthalmol.2009.156. [DOI] [PubMed] [Google Scholar]
- International Electrotechnical Commission. IEC 60825-1. 2.0 2007. Safety of laser products – Part 1: Equipment classification and requirements. [Google Scholar]
- Jiang S, Moriarty-Craige SE, Orr M, Cai J, Sternberg P, Jr, Jones DP. Oxidant-induced apoptosis in human retinal pigment epithelial cells: dependence on extracellular redox state. Invest Ophthalmol Vis Sci. 2005;46:1054–1061. doi: 10.1167/iovs.04-0949. [DOI] [PubMed] [Google Scholar]
- Jin GF, Hurst JS, Godley BF. Hydrogen peroxide stimulates apoptosis in cultured human retinal pigment epithelial cells. Curr Eye Res. 2001;22:165–173. doi: 10.1076/ceyr.22.3.165.5517. [DOI] [PubMed] [Google Scholar]
- Kannan R, Jin M, Gamulescu MA, Hinton DR. Ceramide-induced apoptosis: role of catalase and hepatocyte growth factor. Free Rad Biol Med. 2004;37:166–175. doi: 10.1016/j.freeradbiomed.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Kina S, Nakasone T, Takemoto H, Matayoshi A, Makishi S, Sunagawa N, Liang F, Phonaphonh T, Sunakawa H. Regulation of chemokine production via oxidative pathway in HeLa cells. Mediators Inflamm. 2009:183760. doi: 10.1155/2009/183760. Epub 2010 Jan 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieven CJ, Vrabec JP, Levin LA. The effects of oxidative stress on mitochondrial transmembrane potential in retinal ganglion cells. Antioxid Redox Sign. 2003;5:641–646. doi: 10.1089/152308603770310310. [DOI] [PubMed] [Google Scholar]
- Long X, Goldenthal MJ, Wu GM, Marin-Garcia J. Mitochondrial Ca2+ flux and respiratory enzyme activity decline are early events in cardiomyocyte response to H2O2. J Mol Cell Cardiol. 2004;37:63–70. doi: 10.1016/j.yjmcc.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Masters BR. Noninvasive corneal redux fluorometry. Curr Top Eye Res. 1984;4:139–200. [PubMed] [Google Scholar]
- Masters BR, Ghosh AK, Wilson J, Matschinsky FM. Pyridine nucleotides and phosphorylation potential of rabbit corneal epithelium and endothelium. Invest Ophthal Vis Sci. 1989;30:861–868. [PubMed] [Google Scholar]
- Park CY, Zhu Z, Zhang C, Moon CS, Chuck RS. Cellular redox state predicts in vitro corneal endothelial cell proliferation capacity. Exp Eye Res. 2006;83:903–910. doi: 10.1016/j.exer.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Rajpurohit R, Mansfield K, Ohyama K, Ewert D, Shapiro IM. Chondrocyte death is linked to development of a mitochondrial membrane permeability transition in the growth plate. J Cell Physiol. 1999;179:287–296. doi: 10.1002/(SICI)1097-4652(199906)179:3<287::AID-JCP6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Ranji M, Kanemoto S, Matsubara M, et al. Fluorescence spectroscopy and imaging of myocardial apoptosis. J Biomed Opt. 2006;11:064036. doi: 10.1117/1.2400701. [DOI] [PubMed] [Google Scholar]
- Reinert KC, Dunbar RL, Gao W, Chen G, Ebner TJ. Flavoprotein autofluorescence imaging of neuronal activation in the cerebellar cortex in vivo. J Neurophysiol. 2004;92:199–211. doi: 10.1152/jn.01275.2003. [DOI] [PubMed] [Google Scholar]
- Reyes JM, Fermanian S, Yang F, et al. Metabolic changes in mesenchymal stem cells in osteogenic medium measured by autofluorescence spectroscopy. Stem Cells. 2006;24:1213–1317. doi: 10.1634/stemcells.2004-0324. [DOI] [PubMed] [Google Scholar]
- Rocheleau JV, Head WS, Piston DW. Quantitative NAD(P)H/flavoprotein autofluorescence imaging reveals metabolic mechanisms of pancreatic islet pyruvate response. J Biol Chem. 2004;279:31780–1787. doi: 10.1074/jbc.M314005200. [DOI] [PubMed] [Google Scholar]
- Ryu SY, Peixoto PM, Teijido O, Dejean LM, Kinnally KW. Role of mitochondrial ion channels in cell death. BioFactors. 2010;36:255–263. doi: 10.1002/biof.101. [DOI] [PubMed] [Google Scholar]
- Sano Y, Furuta A, Setsuie R, Kikuchi H, Wang YL, Sakurai M, Kwon J, Noda M, Wada K. Photoreceptor cell apoptosis in the retinal degeneration of Uchl3-deficient mice. Am J Pathol. 2006;169:132–141. doi: 10.2353/ajpath.2006.060085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuki K, Hishida R, Murakami H, et al. Dynamic imaging of somatosensory cortical activity in the rat visualized by flavoprotein autofluorescence. J Physiol. 2003;549:919–927. doi: 10.1113/jphysiol.2003.040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiino A, Matsuda M, Handa J, Chance B. Poor recovery of mitochondrial redox state in CA1 after transient forebrain ischemia in gerbils. Stroke. 1998;29:2421–2425. doi: 10.1161/01.str.29.11.2421. [DOI] [PubMed] [Google Scholar]
- Tsubota K, Laing RA, Chiba K, Hanninen LA, Kenyon KR. Non-invasive metabolic analyses of preserved rabbit cornea. Arch Ophthalmol. 1998;106:1713–1717. doi: 10.1001/archopht.1988.01060140885034. [DOI] [PubMed] [Google Scholar]
- Yang D, Elner SG, Lin LR, Reddy VN, Petty HR, Elner VM. Association of superoxide anions with retinal pigment epithelial cell apoptosis induced by mononuclear phagocytes. Invest Ophthalmol Vis Sci. 2009;50:4998–5005. doi: 10.1167/iovs.09-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Elner SG, Clark AJ, Hughes BA, Petty HR, Elner VM. Activation of P2X Receptors Induces Apoptosis in Human Retinal Pigment Epithelium. Invest Ophthalmol Vis Sci. 2011a;52:1522–1530. doi: 10.1167/iovs.10-6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Elner SG, Chen X, Field MG, Petty HR, Elner VM. MCP-1-activated monocytes induce apoptosis in human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2011b doi: 10.1167/iovs.10-7023. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Elner SG, Bian ZM, Kindezelskii AL, Petty HR, Elner VM. Activated monocytes induce human retinal pigment epithelial cell apoptosis through caspase-3 activation. Lab Invest. 2003;83:1117–1129. doi: 10.1097/01.lab.0000082393.02727.b5. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Elner SG, Bian ZM, Kunkel SL, Lukacs NW, Elner VM. Differential chemokine regulation by TH2 cytokines during human RPEmonocyte coculture. Invest Ophthalmol Vis Sci. 2001;42:1631–1638. [PubMed] [Google Scholar]
- Yoshida A, Elner SG, Bian ZM, Kunkel SL, Lukacs NW, Elner VM. Thrombin regulates chemokine induction during human retinal pigment epithelial cell/monocyte interaction. Am J Pathol. 2001;159:1171–1180. doi: 10.1016/S0002-9440(10)61793-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Yoshida S, Khalil AK, Ishibashi T, Inomata H. Role of NF-kappaB- mediated interleukin-8 expression in intraocular neovascularization. Invest Ophthalmol Vis Sci. 1998;39:1097–1106. [PubMed] [Google Scholar]
- Zeng X, Dai J, Remick DG, Wang X. Homocysteine mediated expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human monocytes. Cir Res. 2003;93:311–320. doi: 10.1161/01.RES.0000087642.01082.E4. [DOI] [PubMed] [Google Scholar]