Abstract
WLBU2 is a peptide antibiotic designed for broad antimicrobial activity, including bacteria associated with periodontal disease. Although periodontitis is associated with various systemic conditions, ranging from cardiovascular disease to preterm birth, local therapy is needed to treat the source of infection. Biodegradable polymers are often used to control locally the amount and rate of delivery of drugs. In the present study, a bioerodible association polymer comprising cellulose acetate phthalate (CAP) and Pluronic® F-127 (PF-127) was explored for its interaction with WLBU2. The intrinsic antimicrobial activity of CAP/PF-127 and the combined effects of the polymer and WLBU2 were examined using Streptococcus gordonii, a species involved in early colonisation of tooth surfaces. The polymer blend alone had dose-dependent bacteriostatic properties, resulting in a ≥2 log decrease in colonies at the highest concentrations tested, possibly due to the hydrophobicity of CAP disrupting the surface of bacteria. When WLBU2 was combined with CAP/PF-127, an apparent binding of peptide to polymer significantly decreased the activity compared with free WLBU2, which functions like other cationic peptides by destabilising the bacterial membrane. Formulation with sucrose as an excipient, which reduced the interaction between WLBU2 and polymer, restored the bactericidal activity of the peptide antibiotic as reflected by a >3 log decrease in S. gordonii. WLBU2 can be locally delivered using CAP/PF-127 as a release vehicle, with the peptide’s bactericidal activity dominating the polymer’s bacteriostatic effect.
Keywords: Anti-infectives, Antimicrobial peptide, Periodontitis, Polymeric drug carrier, Streptococcus gordonii, WLBU2
1. Introduction
Bacteria and bacterially-derived products stimulate inflammation and lead to the destruction of structural tissues supporting teeth and implants in conditions such as periodontitis and peri-implantitis. Although other bacterial types will also be present, streptococci dominate as early colonisers of tooth surfaces, constituting up to 90% of the bacteria that quickly attach following professional cleaning [1].
Systemic delivery of antibiotics to treat oral infections can cause a number of adverse effects, including fetal toxicity, teratogenesis, discoloration and hyperplasia of teeth, and depressed skeletal growth [2]. Localised delivery, however, can not only reduce side effects but will also reduce the amount of drug used. An obstacle to effectively implementing therapeutic use of many biomolecules is development of an appropriate delivery system.
Concerns about the emergence of antibiotic-resistant bacteria necessitate the consideration of alternative treatments. Cationic antimicrobial peptides (AMPs) often have broad antimicrobial activity and can kill or neutralise Gram-negative and Gram-positive bacteria, fungi and parasites [3]. AMPs are natural products in sites likely to encounter pathogens, such as the skin, ears, eyes and various epithelial surfaces. WLBU2 is a 24-amino acid peptide derived from lentivirus lytic peptide 1 that was designed for broad activity both against Gram-positive and Gram-negative bacteria [4]. More recently, WLBU2 has been shown to be effective against oral bacteria [5]. Cationic amphiphilic peptides function by destabilising the bacterial membrane following electrostatic binding to lipopolysaccharide, after which the peptides can interact with cytoplasmic components [3,4].
In this project, the bioactivity of WLBU2 peptide antibiotic was studied in conjunction with a bioerodible polymer capable of localised drug delivery. In particular, the association polymer blend system of cellulose acetate phthalate (CAP) and Pluronic® F-127 (PF-127) was used because it provides zero-order kinetics and has been shown to be useful for releasing different biomolecules, including small-molecule drugs, peptides and proteins [6,7]. In contrast to most biodegradable materials used for drug delivery, CAP/PF-127 physically erodes following deprotonation of the carboxyl groups of CAP, which disrupts hydrogen bonding with the ether oxygens of PF-127.
2. Materials and methods
2.1. Microsphere fabrication and degradation
CAP/PF-127 microspheres were made by a water–acetone–oil–water triple emulsion process as reported elsewhere [6,7]. A 70:30 (w/w) CAP:PF-127 blend ratio was used because it degrades by surface erosion under a variety of conditions. Microspheres were sterilised with ultraviolet radiation in a laminar flow hood for 30 min. CAP/PF-127 degradation products (1.5 mg/mL) were obtained by eroding polymer in 150 mM phosphate-buffered saline (pH 7.4) during incubation at 37 °C.
2.2. Antimicrobial activity
The antibacterial effects of CAP/PF-127 and WLBU2 on Streptococcus gordonii (as an important Gram-positive, commensal microorganism responsible for early colonisation of teeth) and Escherichia coli (as a positive control) were determined using a standard broth dilution assay as described previously [5]. In brief, bacteria were propagated to mid-log phase, washed with 10 mM phosphate buffer (PB) and re-suspended in PB to achieve an initial density of 106–107 colony-forming units/mL in the killing assay. Bacterial cultures were incubated at 37 °C with two-fold dilutions of CAP/PF-127 degradation products or WLBU2 peptide under appropriate growth conditions for 30 min. This time was selected because it exceeds the minimum time needed for WLBU2 to kill in serum, which is analogous to the subgingival transudate. Survival of bacteria was measured by plating 10-fold serial dilutions of the treated suspensions on blood agar and counting colonies after incubating for 24 h.
2.3. Statistical analysis
Results (mean ± standard deviation) were calculated from triplicate samples. Following one-way analysis of variance (ANOVA), post-hoc comparisons were made using the Tukey–Kramer test when the P-value was significant (P < 0.05).
3. Results and discussion
3.1. Effects of CAP/PF-127 polymer on Escherichia coli and Streptococcus gordonii
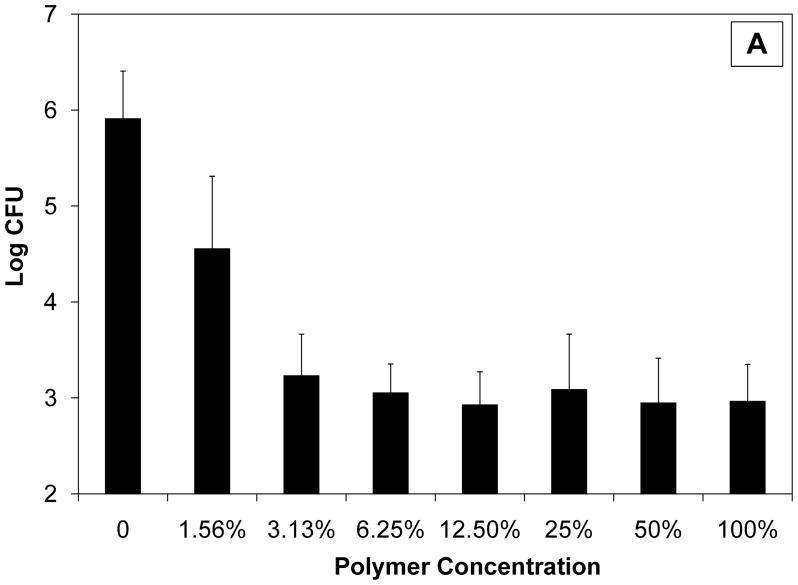
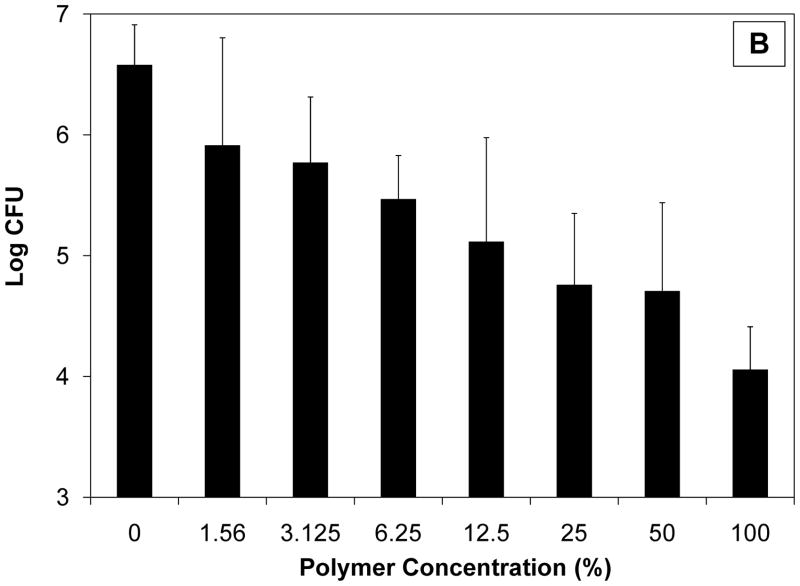
As shown in Fig. 1, even in the absence of an antimicrobial agent, CAP/PF-127 resulted in a statistically significant, concentration-dependent inhibition both of E. coli (P < 0.001) and S. gordonii (P < 0.01). The overall magnitude of the effect as well as the sensitivity differed for the two types of bacteria. For E. coli, the number of colonies was nearly 1000 times lower than in control cultures at 3.125% (47 μg/mL) polymer degradation products or higher. Even at the lowest concentration tested (1.56%), growth was still more than 10 times less than in controls. Streptococcus gordonii was less sensitive to the polymer, with a 2.5 log reduction observed at only the highest (100%; 1.5 mg/mL) concentration; a reduction of less than one order of magnitude was observed at the lowest concentration of polymer tested. Micronised CAP formulated into a cream, however, has been reported to have microbicidal properties, including blocking human immunodeficiency virus type 1 (HIV-1) and herpes virus infectivity as well as activity against bacteria associated with vaginosis [8,9]. Although the mechanism of bacteriostatic activity of CAP and/or PF-127 in these studies is unknown, two possibilities are described in the literature [8,9]. First, micronised CAP has been associated with reduced environmental pH, but the buffered system used for the present experiments probably prevented acidification. Second, and more likely, the hydrophobicity of CAP may have disrupted the surface of bacteria similar to what would occur with a detergent.
Fig. 1.
Bacteriostatic effect of cellulose acetate phthalate/Pluronic® F-127 (CAP/PF-127) polymer erosion byproducts on (a) Escherichia coli and (b) Streptococcus gordonii. Statistically significant concentration-dependent effects were seen both for E. coli (P < 0.001) and S. gordonii (P < 0.01). Data are the mean ± standard deviation (n = 3). CFU, colony-forming units.
3.2. Effects of WLBU2 without and with polymer on Escherichia coli and Streptococcus gordonii
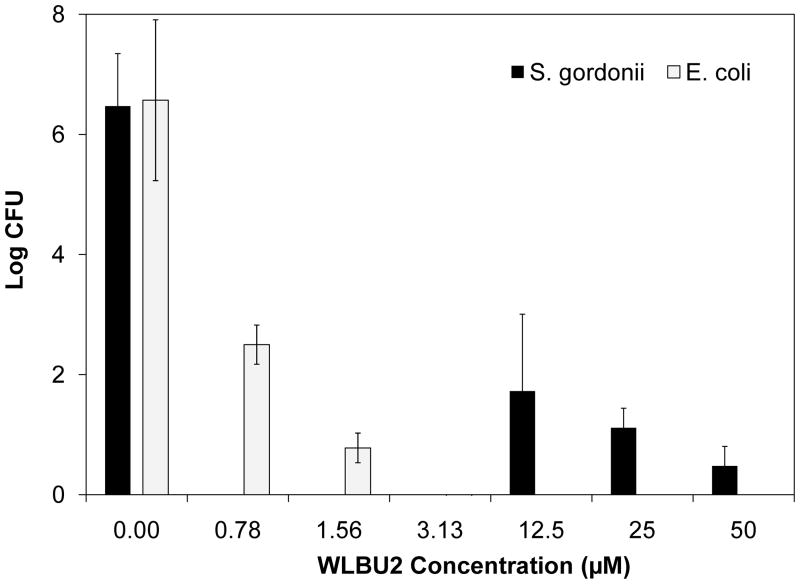
In the absence of CAP/PF-127 polymer, the WLBU2 AMP was more effective against E. coli at significantly lower concentrations (P < 0.001) than it was against S. gordonii (Fig. 2). Whereas nearly 6 log reduction of E. coli occurred at 1.56 μM WLBU2, this was not seen for S. gordonii until 50 μM. The differential sensitivity is likely related to the different cell wall structures of the Gram-positive and Gram-negative bacteria. Streptococcus gordonii was previously shown to be less susceptible to WLBU2 than was Gram-negative Fusobacterium nucleatum [5].
Fig. 2.
Bactericidal effect of WLBU2 peptide on Escherichia coli and Streptococcus gordonii. Results are shown only for concentrations that caused a ≥3 log reduction. The peptide was effective against E. coli at significantly lower concentrations (P < 0.001) than it was against S. gordonii. Data are the mean ± standard deviation (n = 3). CFU, colony-forming units.
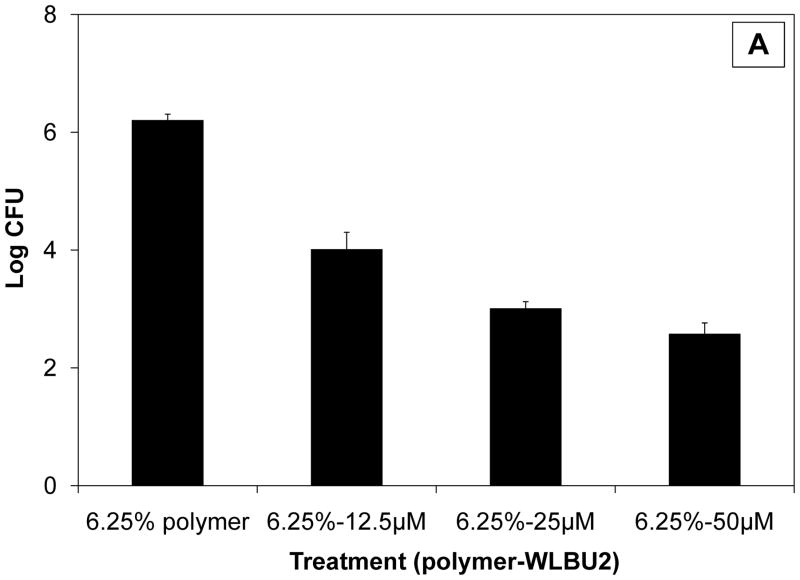
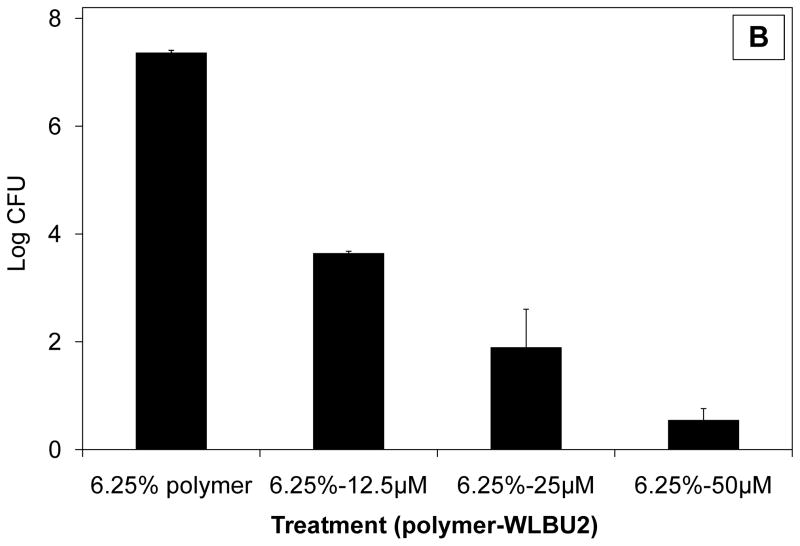
Based on their individual effects, combinations of (bacteriostatic) polymer and (bactericidal) peptide were next investigated for their activity against S. gordonii. At least an additive, if not synergistic, effect was hypothesised, as was recently reported for exogenous antibiotics plus a cathelicidin AMP [10]. For experiments combining CAP/PF-127 and WLBU2, a polymer concentration of 6.25% was selected because its modest effect (ca. 1 log reduction) permitted a large margin for further bactericidal activity. As shown in Fig. 3a, 6.25% polymer plus 12–50 μM WLBU2 dose-dependently reduced the number of bacteria. At the highest peptide concentration, >3 log killing was obtained, but the overall effect was reduced ca 100-fold compared with peptide alone (P < 0.01). Interestingly, the interaction between polymer and the AMP appeared to be reducing the activity of WLBU2. This finding is consistent with those for polymers and surfaces inhibiting the activity of peptides and enzymes. For example, the proteolytic activity of trypsin was inhibited by binding to carboxylic polymers [11], and the ability of cecropin P1, another AMP, to kill E. coli was adversely affected depending on how it was attached to a surface [12]. In addition, immobilisation decreased the activity of a model cationic (KLAL) and a magainin-derived (MK5E) peptide without adversely affecting their spectrum of activity [13].
Fig. 3.
Antibacterial effect of WLBU2 peptide on Streptococcus gordonii when combined with cellulose acetate phthalate/Pluronic® F-127 (CAP/PF-127) polymer. (A) Reduced bioactivity of peptide in the absence of sucrose. The overall effect was reduced ca. 100-fold compared with peptide alone (P < 0.01). (B) Restoration of the bactericidal effect in the presence of an equimolar amount of sucrose. Data are the mean ± standard deviation (n = 3). CFU, colony-forming units.
Use of excipients is widespread in pharmaceutical formulations for protecting biomolecules [14,15]. Common additives include carbohydrates, such as sucrose, trehalose and dextrans. Therefore, to protect WLBU2, the peptide was mixed with an equimolar amount of sucrose before addition to the polymer solution. Fig. 3b shows that addition of sucrose largely restored the activity of the AMP against S. gordonii to levels without polymer, with the exception of 12.5 μM WLBU2 (P < 0.05). At the lower peptide concentration, the amount of polymer may have been high enough to overwhelm the protective effect of sucrose and prevent WLBU2 from exerting its bactericidal activity.
4. Conclusion
The present study showed that interaction between the AMP WLBU2 and the CAP/PF-127 polymer blend reduced the bactericidal effect of the peptide on the oral bacterium S. gordonii, even though the polymer itself is bacteriostatic. Formulation with sucrose, however, reduces peptide–polymer binding and restored the bactericidal activity of WLBU2. Overall, WLBU2 can be locally delivered using CAP/PF-127 as a release vehicle, with the peptide’s bactericidal activity dominating the polymer’s bacteriostatic effect.
Acknowledgments
The authors thank Tim Mietzner and Kazi Islam (University of Pittsburgh, Pittsburgh, PA) for synthesis and characterisation of the WLBU2 peptide.
Funding
This work was supported by the National Institutes of Health (DE018177) and the US Army Medical Research Acquisition Activity (W81XWH-09-1-0461). The contents herein do not necessarily represent the position or policy of the US Government and no official endorsement should be inferred. JRM was supported by a fellowship from the University of Kentucky’s AMSTEMM Program.
Footnotes
Competing interests
None declared.
Ethical approval
Not required.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levy RM, Giannobile WV, Feres M, Haffajee AD, Smith C, Socransky SS. The short-term effect of apically repositioned flap surgery on the composition of the subgingival microbiota. Int J Periodontics Restorative Dent. 1999;19:555–67. [PubMed] [Google Scholar]
- 2.Slots J, Ting M. Systemic antibiotics in the treatment of periodontal disease. Periodontol 2000. 2002;28:106–76. doi: 10.1034/j.1600-0757.2002.280106.x. [DOI] [PubMed] [Google Scholar]
- 3.Hancock RE, Scott MG. The role of antimicrobial peptides in animal defenses. Proc Natl Acad Sci U S A. 2000;97:8856–61. doi: 10.1073/pnas.97.16.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deslouches B, Phadke SM, Lazarevic V, Cascio M, Islam K, Montelaro RC, et al. De novo generation of cationic antimicrobial peptides: influence of length and tryptophan substitution on antimicrobial activity. Antimicrob Agents Chemother. 2005;49:316–22. doi: 10.1128/AAC.49.1.316-322.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Novak KF, Diamond WJ, Kirakodu S, Peyyala R, Anderson KW, Montelaro RC, et al. Efficacy of the de novo-derived antimicrobial peptide WLBU2 against oral bacteria. Antimicrob Agents Chemother. 2007;51:1837–9. doi: 10.1128/AAC.00924-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raiche AT, Puleo DA. Association polymers for modulated release of bioactive proteins. IEEE Eng Med Biol Mag. 2003;22:37–41. doi: 10.1109/memb.2003.1256270. [DOI] [PubMed] [Google Scholar]
- 7.Jeon JH, Puleo DA. Alternating release of different bioactive molecules from a complexation polymer system. Biomaterials. 2008;29:3591–8. doi: 10.1016/j.biomaterials.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neurath AR, Li YY, Mandeville R, Richard L. In vitro activity of a cellulose acetate phthalate topical cream against organisms associated with bacterial vaginosis. J Antimicrob Chemother. 2000;45:713–4. doi: 10.1093/jac/45.5.713. [DOI] [PubMed] [Google Scholar]
- 9.Neurath AR, Strick N, Li YY, Debnath AK. Cellulose acetate phthalate, a common pharmaceutical excipient, inactivates HIV-1 and blocks the coreceptor binding site on the virus envelope glycoprotein gp120. BMC Infect Dis. 2001;1:17. doi: 10.1186/1471-2334-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leszczynska K, Namiot A, Janmey PA, Bucki R. Modulation of exogenous antibiotic activity by host cathelicidin LL-37. APMIS. 2010;118:830–6. doi: 10.1111/j.1600-0463.2010.02667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luessen HL, Verhoef JC, Borchard G, Lehr CM, de Boer AG, Junginger HE. Mucoadhesive polymers in peroral peptide drug delivery. II. Carbomer and polycarbophil are potent inhibitors of the intestinal proteolytic enzyme trypsin. Pharm Res. 1995;12:1293–8. doi: 10.1023/a:1016213405081. [DOI] [PubMed] [Google Scholar]
- 12.Strauss J, Kadilak A, Cronin C, Mello CM, Camesano TA. Binding, inactivation, and adhesion forces between antimicrobial peptide cecropin P1 and pathogenic E. coli. Colloids Surf B Biointerfaces. 2010;75:156–64. doi: 10.1016/j.colsurfb.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 13.Bagheri M, Beyermann M, Dathe M. Immobilization reduces the activity of surface-bound cationic antimicrobial peptides with no influence upon the activity spectrum. Antimicrob Agents Chemother. 2009;53:1132–41. doi: 10.1128/AAC.01254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cleland JL, Powell MF, Shire SJ. The development of stable protein formulations: a close look at protein aggregation, deamidation, and oxidation. Crit Rev Ther Drug Carrier Syst. 1993;10:307–77. Erratum in: Crit Rev Ther Drug Carrier Syst 1994, 11, 60. [PubMed] [Google Scholar]
- 15.Krishnamurthy R, Manning MC. The stability factor: importance in formulation development. Curr Pharm Biotechnol. 2002;3:361–71. doi: 10.2174/1389201023378229. [DOI] [PubMed] [Google Scholar]