Abstract
Objectives
The purpose of this study was to investigate which histological changes associated with risk factors could contribute to the progression from the initial atherosclerotic lesions including fatty streaks to the advanced lesions.
Methods
We examined the associations of histomorphometric findings in the determined anatomical sites of mid-thoracic aortas (TAs) and left coronary arteries (LADs) with major risk factors for atherosclerosis, using a young autopsied series from the Pathobiological Determinants of Atherosclerosis in Youth study. The histological classification by the American Heart Association was graded for 1,013 TAs and 1,009 LADs. Histometric study, including immunohistochemistry, was performed in type 2 lesions (fatty streaks) of TAs from 59 subjects and LADs from 45 ones.
Results
For the progression from the initial lesions into the advanced atherosclerotic lesions, the most effective lipid profiles were low plasma HDL-C in TA and elevated serum non-HDL-C in LAD. This lipid profile of each artery correlated with number or density of intimal smooth muscle cell-derived foam cells, respectively. The serum concentration of non-HDL-C correlated with macrophage foam cells in TAs. Hypertension and hyperglycemia were associated with increase of intimal area and/or collagen content in both arteries, but not with either types of foam cell proliferation. Smoking correlated with increased collagen content in TAs.
Conclusion
There were histologically different ways of progressing from fatty streaks to advanced atherosclerotic lesions depending on the risk factors. For the atherosclerosis progression from type 2 lesions to advanced lesions, increase in number of smooth muscle cell-derived foam cells could be an important indicator.
Keywords: Coronary heart disease, aorta, fatty streaks, histomorphometry, immunohistochemistry, risk factors, smooth muscle foam cells
Introduction
Atherosclerosis begins in childhood or adolescence and progresses during the young adult years to cause clinical coronary heart disease (CHD) in older individuals. The progression from AHA type 2 lesions (fatty streaks) with numerous macrophage foam cells in the intima into AHA type 3 lesions (preatheromas) containing one or more pools of extracellular lipid in intima is the process that transforms reversible, initial lesions into lesions which progress to irreversible, clinically significant lesions. This progression is accompanied by the chemical conversion of intimal lipid [1,2] and by changes in the effects of risk factors demonstrated by macroscopic and microscopic autopsy studies [3–5].
The Pathobiological Determinants of Atherosclerosis in Youth (PDAY) study was a multi-institutional project conducted to investigate atherosclerosis in juvenile and young adult autopsied subjects [6]. Our previous study using PDAY materials revealed the diversity of susceptibility to the progression of fatty streaks into preatheromas according to microscopic topographical differences in the thoracic aorta (TA) and the left coronary artery (LAD). In summary, based on differences in the histological features, the LAD was more vulnerable to lesion progression than the TA [7].
Associations between traditional risk factors and atherosclerotic lesions in the PDAY study have been previously published [3,5,8–10]. However, to date, the association among histomorphometric findings (intimal area and specific tissue components such as foam cells, macrophage, smooth muscle cells, lymphocytes and collagen) and risk factors associated with the progression of atherosclerotic lesions has not been systematically explored. In the present study, we focused on the correlation of histological features to the risk factors which we have shown to be associated with type 2 lesions because the progression of type 2 lesions to the more advanced type lesions leads to clinically significant atherosclerosis,.
Methods
Study subjects
This study was conducted using material derived from the multi-institutional PDAY project [6]. PDAY subjects consist of individuals 15–34 years of age who died of external causes (accidents, homicides, suicides). In the present study, 1,013 TAs and 1,009 left anterior descending (LAD) coronary arteries were available for analysis. Among these subjects, 59 sections of TA from male subjects and 45 ones of LAD from both sexes subjects, demonstrating all type 2 lesions on ORO stained slides were randomly selected for the histological analyses.
Measurement of risk factors
Risk factors were determined and have been previously described in detail [8–10]. Briefly, regarding blood pressure, normotensives and hypertensives were differentiated by an algorithm that predicts mean blood pressure from histological measurements in sections of kidney examined using a method developed by Tracy et a1 [11]. Determination of total serum cholesterol and high density lipoprotein cholesterol (HDL-C) was accomplished using blood collected at autopsy from the vena cava, heart, or aorta. Non-HDL-C was calculated by subtracting HDL-C from total cholesterol. Smoking status was determined based on the levels of post-mortem serum thiocyanate [10]. Hyperglycemia was defined as elevated glycohemoglobin values of ≥8% [9].
Preparation of arteries for microscopic analyses
The methods for dissection and preservation of arteries have previously been detailed [3,5–6]. In summary, a segment of the right half of the thoracic aorta between the 8th and 9th intercostal arteries was evaluated microscopically. The standardized LAD regions included in the present study were located immediately distal to the bifurcation of the circumflex artery. The proximal half of this standardized arterial segment was sectioned on a freezing microtome and stained with Oil Red O (ORO). The distal half was embedded in paraffin in preparation for special stains (Gomori-Trichrome Aldehyde Fuschin (GTAF)) and immunohistochemical analysis [6].
Classification of arterial lesions
For microscopic lesion classification, the ORO and GTAF stained sections were used. Each case was classified using a system developed by the Committee on Vascular Lesions of the Council of the American Heart Association [12–13] according to the consensus of the three pathologist.
The AHA classification of lesions is defined as follows: Type 0 lesions consist of normal intima with no intimal lipid, with or without adaptive intimal thickening; type 1 lesions consist of isolated lipid-laden macrophages; type 2 lesions consist of numerous macrophage foam cells and fine particles of extracellular lipid, but no pools of extracellular lipid; type 3 lesions consist of one or more pools of extracellular lipid, but no well-defined core of lipid, and represent the intermediate or transitional lesion; type 4 lesions consist of a well-defined core of extracellular lipid covered by normal intima; type 5 lesions consist of one or more cores of extracellular lipid plus a reactive fibrous cap, vascularization, or calcification; type 6 lesions consist of an intimal surface defect. Type 6 lesions were not encountered in the PDAY samples.
Case distributions by 10 year age groups, ethnic groups, gender, risk factor profile and the American Heart Association (AHA) classification are shown in Table 1.
Table 1.
Distribution by age group, risk factors and microscopic AHA lesion type in the thoracic aorta and left anterior descending coronary artery (LAD).
| Risk Factors & AHA Lesion Type | Group | Thoracic Aorta (N=1013) | LAD (N=1009) |
|---|---|---|---|
| Age | 15–24 | 521 | 492 |
| 25–34 | 492 | 517 | |
|
| |||
| Ethnicity | Black | 571 | 547 |
| White | 442 | 462 | |
|
| |||
| Gender | Male | 756 | 754 |
| Female | 257 | 255 | |
|
| |||
| Smoker | Non smoker | 592 | 593 |
| Smoker | 421 | 416 | |
|
| |||
| Hypertension | No | 947 | 946 |
| Yes | 66 | 63 | |
|
| |||
| HDL-C ≤ 35 mg/dL | No | 826 | 822 |
| Yes | 187 | 187 | |
|
| |||
| Non-HDL-C ≥ 130 mg/dL | No | 516 | 510 |
| Yes | 497 | 499 | |
|
| |||
| Hyperglycemia | No | 971 | 969 |
| Yes | 42 | 40 | |
|
| |||
| AHA Lesion Type | 0 | 261 | 511 |
| 1 | 82 | 177 | |
| 2 | 659 | 190 | |
| 3 | 10 | 70 | |
| 4 | 0 | 34 | |
| 5 | 1 | 27 | |
Immunohistochemical staining
The procedures for immunohistochemical staining used in the present study were previously shown [7]. Two dual immunohistochemical stains were conducted for each sample: (1) macrophages (HAM-56) and alpha smooth muscle actin (1A4, Carpinteria); and (2) T-lymphocytes (anti-CD3, PS1) and B-lymphocytes (anti-CD20, L26) (Supplementary Table 1).
Image Analysis
A 150μm-length area of the TA and a 50μm-length area of the LAD with the greatest number of total foam cells along the intimal surface was selected for analysis. Details of the methods used for image analysis were previously described [7,14]. Briefly, JPEG image were captured using a light microscope at 200 × magnification with a digital camera. The images were imported and merged as 24-BMP mode into Adobe Photoshop (Version 8.0; Adobe Systems; San Jose, AC). The area of intimal surface of each slide was isolated. The cell counts and intimal area were determined. The total count and density of each cell including macrophages, smooth muscle cells (SMCs), T-and B-lymphocytes were calculated and expressed as a percent of the total area. To calculate the percent of collagen, the number of pixels corresponding to the colored area of interest in the GTAF staining was expressed as a ratio of the total number of pixels within the observed area.
Statistical Analyses
Tests for associations among AHA lesion progression in the TA samples and LAD samples and age and risk factor groups were performed using logistic regression analyses. Correlation of each histological component in the TA and LAD with age and risk factor groups was determined using stepwise multiple regression analyses. The comparisons of histological elements between each risk factor group and quartile groups were examined by Student’s t-test.
Results
Progression on AHA classification of lesions by age and risk factor groups
Table 1 shows that type 2 lesions were more prevalent in the TA than in the LAD, although the LAD had a more distributed range of lesions from type 0 to type 5. Table 2 demonstrates the association of risk factors with lesion progression from initial atherosclerotic lesions (≤ AHA type 2) to advanced lesions (≥ AHA type 3) in each artery by stepwise multiple logistic analyses. In the TA, low serum levels of HDL-C indicated the strongest correlation with the lesion progression from initial to advanced ones. High level of non-HDL-C was also effective for the lesion progression. In the LAD, age and male gender showed the strongest association with the lesion progression. Among the risk factors other than age and male sex, elevation of serum non-HDL-C affected the lesion progression the most, and low serum HDL-C was also a significant factor in the LAD.
Table 2.
Stepwise logistic analyses of atherosclerotic risk factors with lesion progression from the initial atherosclerotic lesions (≤ AHA type 2) to the advanced lesions (≥ AHA type 3) in the thoracic aorta and the left anterior descending coronary artery
| Thoracic Aorta | ||||
|---|---|---|---|---|
| Variable | Coefficient | SE | Odd Ratio | P |
| HDL-C ≤ 35 mg/dL | 1.303 | 0.613 | 3.681 | 0.034 |
| Non-HDL-C ≥ 130 mg/dL | 1.540 | 0.785 | 4.662 | 0.049 |
χ2=9.241
Multiple regression analyses of consecutive values for AHA type of lesions on risk factors within the LAD indicated age (p<0.0001), non-HDL-C (p<0.0001), male sex (p=0.0002), low level of HDL-C (p=0.0121) as significant factors. In TA, the same analyses did not show any significant associations between risk factors and the progression of lesions, probably because there was only one subject showing the lesion more severe than type 3.
Comparison of Histological Components by Risk Factor Group in Type 2 lesions
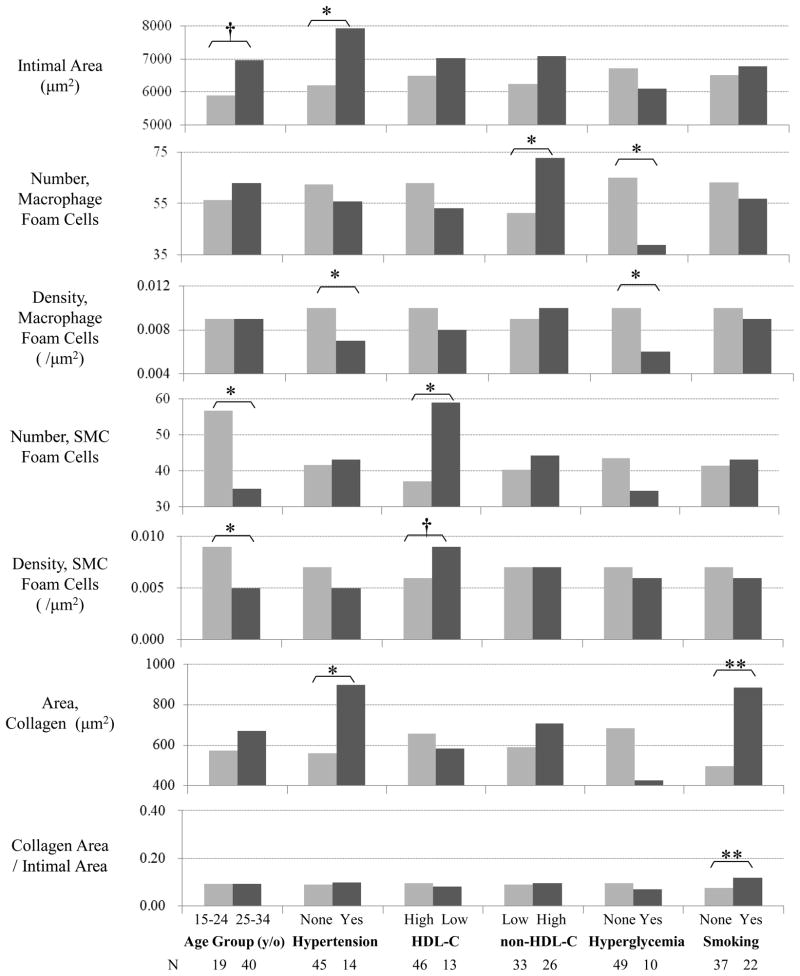
Figure 1 shows the associations among the histological and cellular components in type 2 lesions by age and risk factor groups in the TA. Intimal area tended to be greater in the older group compared to the younger group (P=0.093). The number and density of SMC foam cells, defined as actin-positive and ORO-positive foamy cells, were greater in the younger age group than in the older group (P=0.028 and P=0.010, respectively). Hypertensives exhibited greater areas of intima thickness (P=0.013) and collagen (P=0.034) than non-hypertensives. The density of macrophage foam cells, defined as HAM56-positive and ORO-positive foamy cells was lower in the hypertensives than the non-hypertensives (P=0.022). Both the number and density of SMC foam cells in the subjects with low HDL-C (≤35 mg/dL) were greater compared to those with normal HDL-C (P=0.046 and P=0.061, respectively). These values for SMC foam cells were particularly greater in the subjects with the lowest quartile group of serum HDL-C (≤ 36 mg/dL) than in those with other quartile groups of serum HDL-C (> 36 mg/dL) (data not shown). Subjects with high serum concentration of non-HDL-C showed an increased number of macrophage foam cells (P=0.029). In contrast to the associations of the values for SMC foam cells with serum HDL-C, macrophage foam cells increased consecutively with elevation of non-HDL-C (data not shown). Hyperglycemic subjects had fewer macrophage foam cells (P=0.043). Smokers exhibited a greater area and density of collagen (P=0.005 and P=0.003, respectively). The density of all SMCs containing both foamy and non-foamy SMCs in hyperglycemics (0.033/μm2) tended to be greater than those in normoglycemics (0.026/μm2) (P=0.052), and the density of T-cells was greater in younger age group (0.004/μm2) than in older age group (0.002/μm2) (P=0.045).
Figure 1.
Comparisons of immunohistochemically stained cellular components and GTAF stained histological components in AHA type 2 lesions (fatty streaks) of a 150μm-length area of the thoracic aorta by age and risk factor groups. Collagen Area/Intimal Area indicates the ratio of collagen area to intimal area in the evaluated region. (**, p-value < 0.01; *, p-value < 0.05; †, p-value < 0.1)
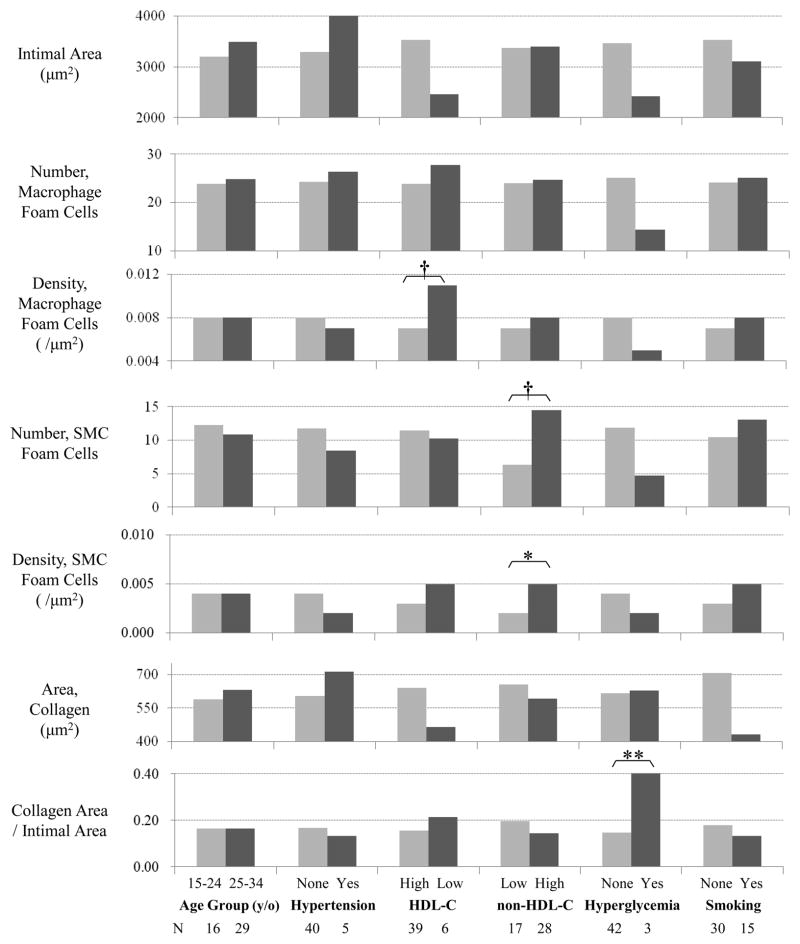
Figure 2 demonstrates the same comparisons but in the LAD. The density of SMC foam cells was greater in the subjects with high serum non-HDL-C than in those with normal level of non-HDL-C (P=0.028). Although the values for SMC foam cells of the first and second quartile groups of non-HDL-C were almost the same, they increased consecutively after the second quartile (non-HDL-C ≥ 158 mg/dL) (data not shown). The density of macrophage tended to be greater in low HDL-C group than in normal HDL-C group (P=0.094). Hyperglycemic subjects exhibited a greater density of collagen (P=0.002). There were no associations among all SMCs, T-lymphoid cells and any risk factors in the LAD.
Figure 2.
Comparisons of immunohistochemically stained cellular components and GTAF stained histological components in AHA type 2 lesions (fatty streaks) of a 50 μm-length area of the left anterior descending coronary artery by age and risk factor groups. Collagen Area/Intimal Area indicates the ratio of collagen area to intimal area in the evaluated region. (**, p-value < 0.01; *, p-value < 0.05; †, p-value < 0.1)
Multiple Regression Analyses of Histological Components with risk factors
Stepwise regression analyses of variable histological parameters in the TA and the LAD with risk factors indicated almost the same correlations as shown in Fig. 1 and Fig. 2 (Supplementary Table 2). In the TA, there was an association between intimal area and hypertension (P=0.013). The number of macrophage foam cells had associations with elevated non-HDL-C (P=0.030) and lower glycohemoglobin (P=0.045). The density of macrophage foam cells correlated negatively with hypertension (P=0.002) and hyperglycemia (P=0.002). The number of SMC foam cells correlated negatively with HDL-C (P=0.019) and age (P=0.011). The density of SMC foam cells also showed negative associations with HDL-C (P=0.023) and age (P=0.004). In addition, smokers demonstrated a greater area and density of intimal collagen (P=0.005 and P=0.003, respectively). Density of T-cell showed a negative correlation with age (P=0.045). In the LAD, non-HDL-C correlated with density of SMC foam cells (P=0.028). The ratio of SMC foam cells to all SMCs was associated with smoking (data not shown). White subjects demonstrated a greater density of all SMCs and collagen (P=0.048 and P=0.049, respectively). Hyperglycemic subjects also indicated a greater density of collagen fibers (P=0.003). B-lymphoid cells were rare and significant correlations with risk factors were not present.
Discussion
In the TA, low HDL-C was determined to be the most significant factor for the lesion progression from initial atherosclerotic lesions (≤AHA type 2) to advanced lesions (≥AHA type 3), however high non-HDL-C was significant as well. Within the LAD, elevated non-HDL-C demonstrated the most significant association, however low HDL-C was also significant. In type 2 lesions, an increase in intimal SMC foam cells was associated with low HDL-C in the TA and with non-HDL-C in the LAD. Macrophage foam cells correlated with non-HDL-C in TA and with low HDL-C in LAD, respectively. Thus, although the most effective lipid profiles for the progression from initial lesions to advanced ones were different in the TA and LAD, they are both highly associated with the proliferation of SMC foam cells in type 2 lesions of each artery. However, the reason why they differ by topographical sites is unclear. Autopsy studies [3,15] have revealed that the extent of gross atherosclerotic lesions vary according to risk factors and topographic arterial segments. One of the reasons for this topographic diversity on the histological effect of risk factors may be due to inherent differences in tissue composition and embryonic properties of SMCs [16].
A previous histological study using PDAY materials revealed that increased collagen content and extracellular lipid deposition occurred simultaneously [17]. Perhaps increase of collagen may promote extracellular lipid deposition through an elevated inflammatory reaction by activation of myeloperoxidase [18]. Furthermore, an increase of proteoglycan, another major component of intimal hyperplasia, which is generated by activated SMCs with oxidized LDL, could result in increased lipid deposition by lipid trapping [19,20]. There is a greater intimal thickness in the coronary artery compared to aorta [7], which may facilitate lipid deposition without accumulation of intracellular lipid.
Many previous studies, including those using PDAY materials, have shown that fatty streaks do not always increase in the subjects with non-lipid risk factors even though advanced atherosclerotic lesions are more extensive in subjects with non-lipid risk factors [3,8–10]. In the present study, neither of the non-lipid risk factors increased either macrophage or SMC foam cells. Instead, one of the non-lipid risk factors, hypertension promotes intimal hyperplasia with an increase in collagen. Smokers demonstrated an increase in collagen content in the aorta. Hyperglycemia encouraged increase of collagen content in the LAD. Both hypertension and hyperglycemia were associated with decreased macrophage foam cells. The growth in atherosclerosis from initial lesion to advanced lesion loaded by non-lipid risk factors does not progress by the way of the classical histological feature of fatty streaks with abundant foam cells.
In aorta, an increase in intimal thickening by age [4,5,21,22] should cause the reduction of both of SMC foam cell count and density, and T-cell density in the older age group. Decreased vascular SMC foam cells may be related to the specific phenotype of SMCs potential to foam cells [23].
Previous studies, including both clinical and autopsied ones, revealed that low HDL-C is a powerful independent risk factor [15,24]. One explanation is that decreased HDL-C results in the reduction of excretion of excessive lipid from the arterial wall to blood stream [24,25].
An anti-inflammatory effect is known as one of the pleiotropic effects of HDL [26]. Elevated inflammation induced by low HDL is related to an increase in SMC foam cells by activating inflammatory cytokines and growth factors [27,28]. Indeed, serum C-reactive protein (CRP) levels are associated with the progression of lesions [29]. Inflammatory cytokines, such as TNF-α and IFN-γ, which regulate production of extracellular matrix by promoting SMC phenotypic change, enhance uptake of modified lipoproteins and formation of SMC foam cells [28]. The mechanism for assembling collagen and fibronectin of SMCs was disrupted by lipid accumulation into SMCs [30]. Therefore, an increase in SMC foam cells with low HDL-C develops particular histological features, namely abundant SMC foam cells with less collagen content and small intimal thickness.
Hyperglycemia showed negative correlation with increase of macrophage foam cells. Although accumulated C-peptide, an inflammatory biomarker, in intima of early atherosclerotic lesions may promote monocyte migration in diabetic subjects [31], no findings indicating elevated inflammatory response such as increased T-cell lymphocytes in the intima of the hyperglycemic subjects were observed in the present study. Hyperglycemia induces increase of transforming growth factor (TGF)-β, a potent regulator of extracellular matrix synthesis including proteoglycan by SMCs [32], which could lead to increased lipid deposition before infiltration and accumulation of macrophages [19]. Thus, hyperglycemia may promote advanced atherosclerotic lesions showing large extracellular deposition without or before definite infiltration of inflammatory cells including monocytes.
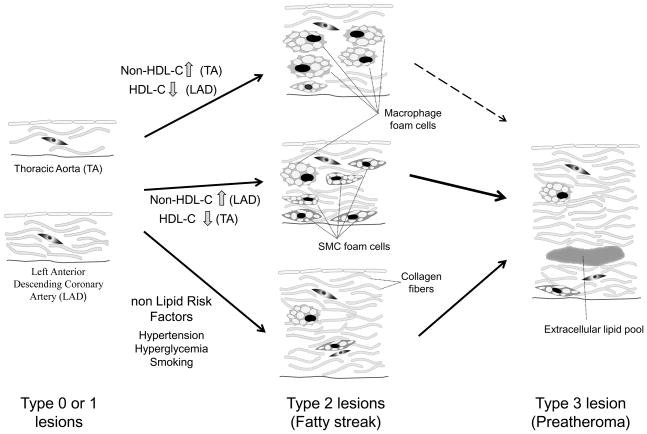
Our present study indicated that there were histologically different ways of progressing from fatty streaks to advanced atherosclerotic lesions depending on the risk factors (Figure 3). Among various histological changes, either an increase in SMC foam cells or intimal hyperplasia, including collagen deposition, is critical for the progression from early atherosclerotic lesions to the more advanced lesions. Low HDL-C is particularly an important risk factor for primary prevention, because it exhibits a powerful effect on the progression of early atherosclerotic lesions, even in the arterial sections such as thoracic aorta, which typically are resistant to atherosclerosis progression.
Figure 3.
Model of histological atherosclerosis progression from AHA type 0 and 1 lesions, initial lesions (left) to AHA type 3 lesions (preatheroma), a first step of advanced lesions (right) through type 2 lesions (fatty streaks, middle) by different risk factors, suggested by the present and other studies. All types of lesions indicate inherently thicker intima in coronary arteries (left, bottom) than in thoracic aorta (left, top). Elevated serum non-HDL-C in thoracic aorta (TA) and low serum HDL-C in left anterior descending coronary artery (LAD) lead to the type 2 lesions with abundant macrophage foam cells (middle, up). These lesions showing typical histological fatty streaks are hard to progress into type 3 lesions especially in TA. Elevated serum non-HDL-C in LAD and low serum HDL-C in TA indicate the type 2 lesions with relatively increased SMC foam cells (middle). These lesions with increased SMC foam cells progress early into type 3 lesions (preatheroma). In contrast to lipid risk factors, non lipid risk factors including hypertension, hyperglycemia and smoking lead to the advanced lesions through type 2 lesions showing a few of both types of foam cells, sometimes accompanying intimal thickening composed of increased collagen fibers and/or proteoglycans (middle, bottom). The figures indicating type 2 lesions with a few foam cells, but with intimal thickness or proliferation of collagen fibers, are supposed to exert easily the progression into type 3 lesions by lipid trapping with collagen fibers and/or proteoglycans synthesized by activated SMCs.
Supplementary Material
Table 3.
| Left Anterior Descending Coronary Artery | ||||
|---|---|---|---|---|
| Variable | Coefficient | SE | Odd Ratio | P |
| Age Group | 1.499 | 0.232 | 4.477 | < 0.001 |
| Male sex | 0.837 | 0.266 | 2.308 | 0.002 |
| Non-HDL-C ≥ 130 mg/dL | 0.474 | 0.200 | 1.607 | 0.018 |
| HDL-C ≤ 35 mg/dL | 0.491 | 0.228 | 1.634 | 0.031 |
χ2=76.350
Acknowledgments
None
Sources of Funding: This study was supported in part by NHLBI Grant # HL60808.
Footnotes
Disclosure: Not applicable
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Katz SS, Shipley GG, Small DM. Physical chemistry of the lipids of human atherosclerotic lesions. Demonstration of a lesion intermediate between fatty streaks and advanced plaques. J Clin Invest. 1976;58:200–1. doi: 10.1172/JCI108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Small DM, Bond MG, Waugh D, Prack M, Sawyer JK. Physicochemical and histological changes in the arterial wall of nonhuman primates during progression and regression of atherosclerosis. J Clin Invest. 1984;73:1590–605. doi: 10.1172/JCI111366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strong JP, Malcom GT, McMahan CA, Tracy RE, Newman WP, Herderick EE, Cornhill JF. Prevalence and extent of atherosclerosis in adolescents and young adults. Implications for prevention from the Pathobiological Determinants of Atherosclerosis in Youth Study. JAMA. 1999;281:727–35. doi: 10.1001/jama.281.8.727. [DOI] [PubMed] [Google Scholar]
- 4.Homma S, Ishii T, Tsugane S, Hirose N. Different effects of hypertension and hypercholesterolemia on the natural history of aortic atherosclerosis by the stage of intimal lesions. Atherosclerosis. 1997;128:85–95. doi: 10.1016/s0021-9150(96)05970-9. [DOI] [PubMed] [Google Scholar]
- 5.Homma S, Ishii T, Malcom GT, Zieske AW, Strong JP, Tsugane S, Hirose N. Histopathological modifications of early atherosclerotic lesions by risk factors -findings in PDAY subjects. Atherosclerosis. 2001;156:389–99. doi: 10.1016/s0021-9150(00)00669-9. [DOI] [PubMed] [Google Scholar]
- 6.Wissler RW. USA multicenter study of the pathobiology of atherosclerosis in youth. Ann NY Acad Sci. 1991;623:26–39. doi: 10.1111/j.1749-6632.1991.tb43716.x. [DOI] [PubMed] [Google Scholar]
- 7.Homma S, Troxclair DA, Zieske AW, Malcom GT, Strong JP for the Pathological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Histological topographical comparisons of atherosclerosis progression in juveniles and young adults. Atherosclerosis. 2008;197:791–8. doi: 10.1016/j.atherosclerosis.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGill HC, Jr, Strong JP, Tracy RE, McMahan CA, Oalmann MC. Relation of a postmortem renal index of hypertension to atherosclerosis in youth. Arterioscler Thromb Vasc Biol. 1995;15:2222–8. doi: 10.1161/01.atv.15.12.2222. [DOI] [PubMed] [Google Scholar]
- 9.McGill HC, Jr, McMahan CA, Malcom GT, Oalmann MC, Strong JP. Relation of glycohemoglobin and adiposity to atherosclerosis in youth. Arterioscler Thromb Vasc Biol. 1995;15:431–40. doi: 10.1161/01.atv.15.4.431. [DOI] [PubMed] [Google Scholar]
- 10.McGill HC, Jr, McMahan CA, Malcom GT, Oalmann MC, Strong JP. Effect of serum lipoprotein and smoking on atherosclerosis in young men and women. The PDAY Research Group. Pathobiological determinants of atherosclerosis in youth. Arterioscler Thromb Vasc Biol. 1997;17:95–106. doi: 10.1161/01.atv.17.1.95. [DOI] [PubMed] [Google Scholar]
- 11.Tracy RE, Durban MV, Heigle T, Oalmann MC. Two variants of nephrosclerosis separately related to age and blood pressure. Am J Pathol. 1988;131:270–82. [PMC free article] [PubMed] [Google Scholar]
- 12.Stary HC, Chandler B, Glagov S, Guyton JR, Insull W, Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner WD, Wissler RW. A definition of the intima of the initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the committee on vascular lesions of the council on atherosclerosis. American Heart Association. Arterioscler Thromb. 1994;14:840–56. doi: 10.1161/01.atv.14.5.840. [DOI] [PubMed] [Google Scholar]
- 13.Stary HC, Chandler B, Dinsmore RE, Fuster V, Glagov S, Insull W, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of the intima of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the committee on vascular lesions of the council on atherosclerosis. American Heart Association. Circulation. 1995;92:1355–74. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 14.Taajes DJ, Wadsworth MP, Schneider DJ, Sobel BE. Improved quantitative characterization of atherosclerotic plaque composition with immunohistochemistry, confocal fluorescence microscopy, and computer-assisted image analysis. Histochem Cell Biol. 2000;113:161–73. doi: 10.1007/s004180050435. [DOI] [PubMed] [Google Scholar]
- 15.Solberg LA, Strong JP. Risk factors and atherosclerotic lesions. A review of autopsy studies. Arteriosclerosis. 1983;3:187–98. doi: 10.1161/01.atv.3.3.187. [DOI] [PubMed] [Google Scholar]
- 16.Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27:1248–58. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- 17.Malcom GT, McMahan CA, McGill HC, Jr, Herderick EE, Tracy RE, Troxclair DA, Strong JP Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Associations of arterial tissue lipids with coronary heart disease risk factors in young people. Atherosclerosis. 2009;203:515–21. doi: 10.1016/j.atherosclerosis.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Shao B, Oda MN, Oram JF, Heinecke JW. Myeloperoxidase: an inflammatory enzyme for generating dysfunctional high density lipoprotein. Curr Opin Cardiol. 2006;21:322–8. doi: 10.1097/01.hco.0000231402.87232.aa. [DOI] [PubMed] [Google Scholar]
- 19.Nakashima Y, Fujii H, Sumiyoshi S, Wight TN, Sueishi K. Early human atherosclerosis: accumulation of lipid and proteoglycans in intimal thickenings followed by macrophage infiltration. Arterioscler Thromb Vasc Biol. 2007;27:1159–65. doi: 10.1161/ATVBAHA.106.134080. [DOI] [PubMed] [Google Scholar]
- 20.Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:812–19. doi: 10.1161/ATVBAHA.107.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stary HC. Evolution and progression of atherosclerotic lesions in coronary arteries of children and young adults. Arteriosclerosis. 1989;9:119–32. [PubMed] [Google Scholar]
- 22.Homma S, Hirose N, Ishida H, Ishii T, Araki G. Carotid plaque and intima-media thickness assessed by B-mode ultrasonography in subjects ranging from young adults to centenarians. Stroke. 2001;32:830–5. doi: 10.1161/01.str.32.4.830. [DOI] [PubMed] [Google Scholar]
- 23.Korshunov VA, Berk BC. Smooth muscle apoptosis and vascular remodeling. Curr Opin Hematol. 2008;15:250–4. doi: 10.1097/MOH.0b013e3282f97d71. [DOI] [PubMed] [Google Scholar]
- 24.Assmann G, Gotto AM., Jr HDL cholesterol and protective factors in atherosclerosis. Circulation. 2004;109:III8–14. doi: 10.1161/01.CIR.0000131512.50667.46. [DOI] [PubMed] [Google Scholar]
- 25.Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab. 2008;7:365–75. doi: 10.1016/j.cmet.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–72. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 27.Luechtenborg B, Hofnagel O, Weissen-Plenz G, Severs NJ, Robenek H. Function of scavenger receptor class A type I/II is not important for smooth muscle foam cell formation. Eur J Cell Biol. 2008;87:91–9. doi: 10.1016/j.ejcb.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Freeman MW, Libby P. Regulation of smooth muscle cell scavenger receptor expression in vivo by atherogenic diets and in vitro by cytokines. J Clin Invest. 1995;95:122–33. doi: 10.1172/JCI117628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zieske AW, Tracy RP, McMahan CA, Herderick EE, Homma S, Malcom GT, McGill HC, Jr, Strong JP for the Pathological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Elevated serum C-reactive protein levels and advanced atherosclerosis in youth. Arterioscler Thromb Vasc Biol. 2005;25:1237–43. doi: 10.1161/01.ATV.0000164625.93129.64. [DOI] [PubMed] [Google Scholar]
- 30.Frontini MJ, O’Neil C, Sawyez C, Chan BM, Huff MW, Pickering JG. Lipid incorporation inhibits Src-dependent assembly of fibronectin and type I collagen by vascular smooth muscle cells. Circ Res. 2009;104:832–41. doi: 10.1161/CIRCRESAHA.108.187302. [DOI] [PubMed] [Google Scholar]
- 31.Marx N, Walcher D, Raichle C, Aleksic M, Bach H, Grüb M, Hombach V, Libby P, Zieske A, Homma S, Strong J. C-peptide colocalizes with macrophages in early arteriosclerotic lesions of diabetic subjects and induces monocyte chemotaxis in vitro. Arterioscler Thromb Vasc Biol. 2004;24:540–5. doi: 10.1161/01.ATV.0000116027.81513.68. [DOI] [PubMed] [Google Scholar]
- 32.Yang SNY, Burch ML, Tannock LR, Evanko S, Osman N, Little PJ. Transforming growth factor-β regulation of proteoglycan synthesis in vascular smooth muscle: Contribution to lipid binding and accelerated atherosclerosis in diabetes. J Diabetes. 2010;2:233–42. doi: 10.1111/j.1753-0407.2010.00089.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.