Abstract
Loss-of-function mutations in angiogenin (ANG) gene were discovered in amyotrophic lateral sclerosis (ALS) patients and ANG has been shown to prevent neuronal death both in vitro and in vivo. The neuro-protective activity of ANG was brought about partially by inhibiting stress-induced apoptosis. ANG attenuates both the extrinsic and the intrinsic apoptotic signals by activating Nf-κb-mediated cell survival pathway and Bcl-2-mediated anti-apoptotic pathway. Here we report that ANG inhibits nuclear translocation of apoptosis inducing factor (AIF), an important cell death-executing molecule known to play a dominant role in neurodegenerative diseases. ANG inhibits serum withdrawal-induced apoptosis by attenuating a series of Bcl-2-dependent events including caspase-3 activation, poly ADP-ribose polymerase-1 (PARP-1) cleavage, and AIF nuclear translocation.
Kew words: Angiogenin, ribonuclease, apoptosis, stress
Introduction
ANG has been detected in virtually all human organs and tissues (Weiner et al., 1987) although it was originally isolated as a tumor angiogenic protein from the conditioned medium of HT-29 colon adenocarcinoma cells (Fett et al., 1985). ANG is a 14.4 kDa small protein belonging to the vertebrate-specific, secreted ribonuclease superfamily. The widespread expression of ANG suggests that it may play a more universal role than merely stimulating angiogenesis. Indeed, ANG has been shown to exhibit both angiogenic and non-angiogenic activities. For examples, it regulates ribosomal RNA (rRNA) transcription in both cancer and nerve cells, and its up-and down-regulation has been linked to the pathogenesis of cancer and neurodegenerative diseases, respectively (Li and Hu, 2010). ANG has also been shown to mediate the production of tRNA-derived, stress-induced small RNA (tiRNA) in response to stresses (Fu et al., 2009; Yamasaki et al., 2009). tiRNA is a novel class of small RNA that is derived from tRNA and is induced by stress (Thompson et al., 2008). It reprograms protein translation and promotes cell survival under adverse conditions (Emara et al., 2010). ANG is the ribonuclease responsible for cleaving tRNA at the anticodon to produce tiRNA thereby playing an important role in stress response of mammalian cells (Emara et al., 2010; Yamasaki et al., 2009).
Missense mutations in ANG gene have recently been found to be associated with ALS, a fatal neurodegenerative disease caused by the degeneration of motor neurons (Millecamps et al., 2010). Accumulating evidence shows that wild type ANG protects motor neuron degeneration induced by various stresses including excitotoxicity, endoplasmic reticulum stress, as well as hypoxia (Kieran et al., 2008). The neuro-protective activity of ANG has been shown to be mediated, at least in part, by its anti-apoptotic activity (Kieran et al., 2008; Li et al., 2010). Apoptosis of motor neurons has been well-documented in ALS (Friedlander, 2003). Crossbreeding of SOD1G93A mice, the ALS model mice that develop motor impairment, with mice expressing a mutant caspase-1 gene slowed down disease progression by 50% and prolonged survival by 9% (Friedlander et al., 1997). Caspase inhibitor (zVAD-fmk) has been shown to increase the survival of SOD1G93A mice by 22% (Li et al., 2000). Prolonged caspase activations were found in ALS model mice (Friedlander, 2003). Caspase-1 and -3 were found to be activated in the spinal cord of ALS patients (Li et al., 2000; Martin, 1999). Moreover, caspase-9 activation and cytochrome c release have also been documented in ALS model mice (Zhu et al., 2002). Caspase activation in ALS seems to be induced by protein aggregates and can be modulated by Bcl-2 family proteins. For example, blocking the mitochondrial apoptotic pathway preserves motor neuron viability and function in ALS model mice (Reyes et al., 2010). Consistently, SOD1G93A mice carrying a transgenic Bcl-2 gene survive longer (Kostic et al., 1997). All these results indicate that motor neuron apoptosis is an underlying mechanism of ALS pathogenesis. However, genetic deletion of caspase-11, a dual regulator of caspase-1 and -3, in ALS model mice did not have any effects in disease outcome, suggesting that caspase activation is not sufficient for neurodegeneration (Kang et al., 2003).
AIF is another death-executing molecule that can induce caspase-independent cell death (Thress et al., 1998). AIF is a mitochondrial flavoprotein that possesses NADH-dependent oxidoreductase activity (Krantic et al., 2007). Upon an apoptotic insult and permeabilization of outer mitochondrial membrane, AIF undergoes proteolysis, is released from the intermembrane space, and translocated to the nucleus where it triggers chromatin condensation and large-scale DNA degradation in a caspase-independent manner (Cande et al., 2002). AIF nuclear translocation has been shown to be a major mediator of neurodegeneration (Galluzzi et al., 2009). Translocation of AIF into the nucleus has been observed in a variety of neurodegenerative disease models such as brain trauma and ischemia (Cao et al., 2003; Zhang et al., 2002), Parkinson’s disease (Perier et al., 2010), and ALS (Oh et al., 2006). In a previously study (Li et al., 2010), we have shown that ANG prevents serum withdrawal-induced apoptosis of P19 cells, a widely used cell mode for neuroscience research (Bain et al., 1994). We have shown that ANG attenuates both the intrinsic and extrinsic apoptosis signals. It upregulates as well as activates Nf-κB thereby promoting cell survival. It also increases the levels of both mRNA and protein of Bcl-2 thereby preventing mitochondria-mediated apoptosis. In the present study, we investigated the involvement of AIF in the anti-apoptotic activity of ANG. Our results show that ANG prevented serum withdrawal-induced nuclear translocation of AIF. It also prevented PARP-1 cleavage, an upstream event of AIF release. Knockdown of Bcl-2 abolished the preventive activity of ANG toward nuclear translocation of AIF and PARP-1 cleavage. Moreover, we found that the preventive activity of ANG toward caspase-3activation is also Bcl-2-dependent. Taken together, we are presenting a series of sequential events in the anti-apoptotic action of ANG that involves the signal cascade from upregulation of Bcl-2, activation of caspase, cleavage of PARP-1, and nuclear translocation of AIF.
Materials and methods
ANG and cell culture
ANG was prepared as a recombinant protein and purified to homogeneity as described (Shapiro et al., 1988). The ribonucleolytic and angiogenic activities of each preparation were examined by tRNA assay and endothelial cell tube formation assay, respectively (Riordan and Shapiro, 2001). P19 mouse embryonal carcinoma cells were maintained in DMEM plus 10% FBS in the presence of penicillin (100 units/ml) and streptomycin (100 μg/ml). Cells were sub-cultured in a 1:10 ratio every 48 h to maintain exponential growth and to avoid aggregation and differentiation. For serum withdrawal-induced apoptosis, cells were seeded and cultured in DMEM + 10% FBS for 24 h, washed with DMEM three times, and cultured in serum-free DMEM in the presence or absence of 1 μg/ml ANG for the time period indicated.
Bcl-2 knockdown
An empty vector control (pSM) and a mouse Bcl-2-specific shRNA clone targeting the sequence of GTGATGAAGTACATACATT were obtained from Open Biosystems (Huntsville, AL, USA). They were transfected into P19 cells in the presence of Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Stable transfectants were selected with 2 μg/ml puromycin. The pooled populations of the transfectants were used. The protein level of Bcl-2 was determined by Western blotting analysis.
Immunofluorescence (IF) of AIF
Cells were cultured on cover slips placed in 48-well plates. Cells were fixed in methanol at −20 ºC for 10 min, blocked with 1 % BSA at RT for 1 h, and incubated with a rabbit anti-AIF polyclonal IgG at 1:1000 dilution (Cell Signaling, Danvers, MA, USA) at 4 ºC overnight. Incubation with the second antibody Alexa 555-labeled goat F(ab’)2 anti-rabbit IgG (Invitrogen) was carried out at 37 ºC for 1 h. The cover slips were mounted on glass slides and fluorescent images were taken on a Leica fluorescent microscope. Confocal images were takenand analyzed on an Olympus Fluoview Confocal microscope.
Western blotting
Nuclear proteins and total cell lysates were prepared using the Nuclear Extract Kit from Active Motif (Carlsbad, CA, USA). Protein concentrations were determined by a Reducing Agent-Compatible BCA Assays Kit from Pierce (Rockford, IL, USA). Proteins (20 μg) were separated by SDS-PAGE and electrotransfered to a nitrocellulose membrane. The membrane was blocked by 5 % fat-free milk in TBST and incubated with the primary antibodies at 4 ºC overnight. Incubation with the second antibody was carried out at RT for 1 h. The antibodies against AIF, cleaved-caspase-3 (Asp175, 5A1E, #9664), cleaved-PARP-1 (Asp214, D64E10, #5625), and Bcl-2 were from Cell Signaling and were used at a dilution of 1:1000. The anti-β-actin IgG was from Santa Cruz (Santa Cruz, CA, USA), and was used at a dilution of 1:600. Anti-Histone H3 IgG was from Biolegend (San Diego, CA, USA) and was used at a dilution of 1:500. The band intensities were determined with Image J.
Results
ANG inhibits nuclear translocation of AIF
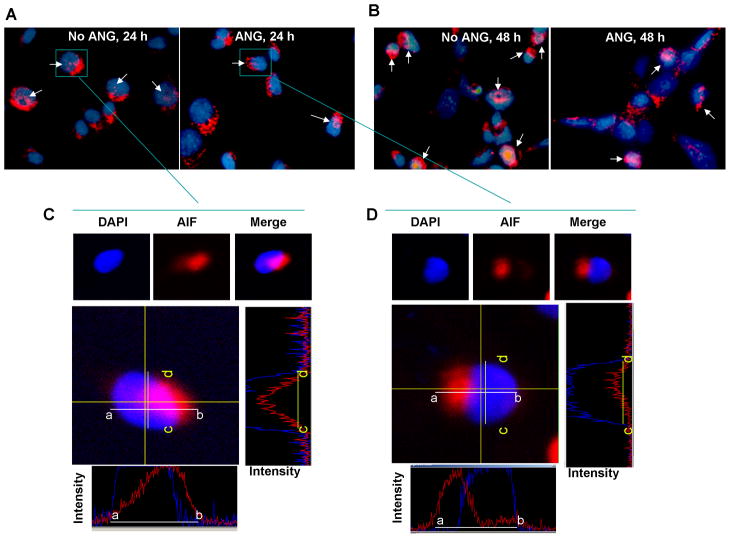
We first examined the effect of ANG on serum withdrawal-induced nuclear translocation of AIF. IF staining with an AIF antibody was used to visualize subcellular localization of AIF. Fig. 1A shows that AIF was detected outside the nuclei in most cells. However, in the absence of exogenous ANG nuclear localization of AIF was detected (indicated by arrows) in 37 ± 7% of the cells after 24 h in serum-free medium (Fig. 1A, left). Nuclear localization of AIF was decreased when the cells were incubated with 1 μg/ml exogenous ANG (Fig. 1A, right). In the presence of ANG, nuclear AIF was found in only 5 ± 1.5% of the cells. A similar effect of ANG on nuclear translocation of AIF was observed in 48 h culture (Fig. 1B). Fig. 1C and 1D show the confocal microscopy images of the selected cells (marked by square). Images shown were from the middle panels of the Z-sections. Fluorescence profiles of the AIF (red) and DNA (blue) in both X (bottom panels, indicated by white bars) and Y (right panels, indicated by yellow bars) axes indicated that AIF staining overlaps with DAPI staining in the absence of ANG (Fig. 1C) but not in the presence of ANG (Fig. 1D). These results indicate that serum withdrawal induced nuclear translocation of AIF and that this event was prevented by ANG.
Fig. 1.
ANG inhibits nuclear translocation of AIF. P19 cells were cultured on cover slips in serum-free medium in the absence or presence of ANG for 24 (A) or 48 h (B). Cells were washed, fixed and proceeded for IF with an anti-AIF IgG. DAPI staining was used to visualize the cell nucleus. The pictures shown are the merged images of AIF and DAPI staining. (C and D), Confocal microscopy images of the selected cells. Top panels show the DAPI and AIF staining of the same cell and the merged images. Middle left panels are the middle panel of the confocal z-sectioning at 0.5 μm interval. Middle right panels are the fluorescent profiles taken at XZ axis indicated as yellow bars (c-d) of the middle panel. Bottom panels show the fluorescent profiles taken at YZ axis indicated as white bars (a-b).
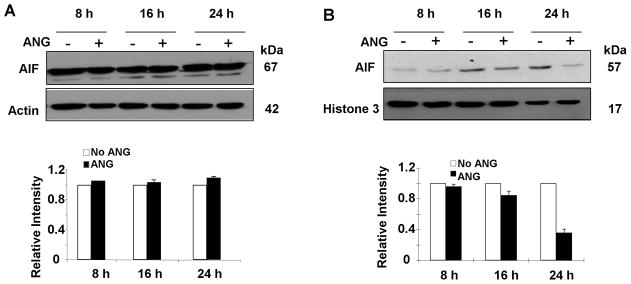
To confirm the IF results, we performed Western blotting analysis of AIF in total cell lysates and in nuclear extracts. The protein level of AIF in total cell lysates did not change after treatment with ANG for 8 h to 24 h (Fig. 2A), indicating that ANG did not alter the expression level of AIF. However, ANG decreased the amount of nuclear AIF in a time-dependent manner (Fig. 2B). The protein levels of nuclear AIF in ANG-treated cells were 96, 85, and 36 % of that in non-treated cells after 8, 16, and 24 h culture, respectively, in serum-free medium (Fig. 2B, bottom panel). These results demonstrated that translocation of AIF from mitochondriato nuclei was inhibited by ANG.
Fig. 2.
ANG blocks nuclear translocation of AIF. Total cell lysates and nuclear fractions were prepared from the cells cultured in serum-free medium in the presence or absence of ANG. (A) Effect of ANG on total AIF protein level in the cell lysate. Top panel are Western blotting results with AIF and β-actin antibodies from a representative experiment. Bottom panel is the relative density of AIF with β-actin as the normalization control. Data shown is the mean ± SD of three independent experiments. (B) Effect of ANG on AIF levels in the nucleus. Top panels are Western blotting film images from a representative experiment. Bottom panel shows the relative density with Histone H3 as the normalization control. Data shown is the mean ± SD of three independent experiments.
ANG Prevents PARP-1 cleavage
PARP-1 catalyzes the elongation and branching of poly (ADP-ribose) polymer and acts as a guard for genomic damage. It participates in many physiological events such as DNA replication and transcription, damage repair and maintenance of genomic integrity (Virag and Szabo, 2002). Proteolytic cleavage (activation) of PARP-1 has been considered as a hallmark biochemical feature of apoptosis. PARP-1activation is required for nuclear translocation of AIF and AIF is an essential downstream effector of PARP-1-mediated cell death (Yu et al., 2003). The effect of ANG on nuclear translocation of AIF prompted us to examine whether PARP-1 cleavage is also altered in the presence of ANG. Fig. 3 shows that PARP-1 cleavage was indeed inhibited by ANG. At 4 and 8 h, the amount of cleaved PARP-1 in the presence of ANG is 57 and 80%, respectively, of that in its absence (Fig. 3, bottom panel). No difference was observed before 4 h and after 16 h, indicating that the effect of ANG on PARP-1 cleavage was transient but occurred earlier than the effect on AIF nuclear translocation. These results are consistent with PARP-1 cleavage being an early event in apoptosis.
Fig. 3.
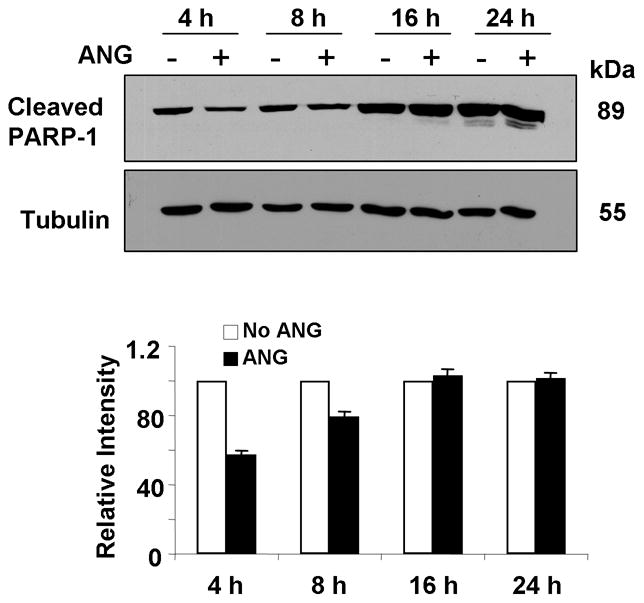
ANG inhibits PARP-1 cleavage. Total cell lysates were prepared from the cells cultured in serum-free medium in the presence or absence of ANG at different time. Top panel are the Western blotting results with cleaved PARP-1 and tubulin antibodies from a representative experiment. Bottom panel is the relative density of cleaved PARP-1 with tubulin as the normalization control. Data shown is the mean ± SD of three independent experiments.
Knockdown of Bcl-2 decreases the protective activity of ANG toward nuclear translocation of AIF
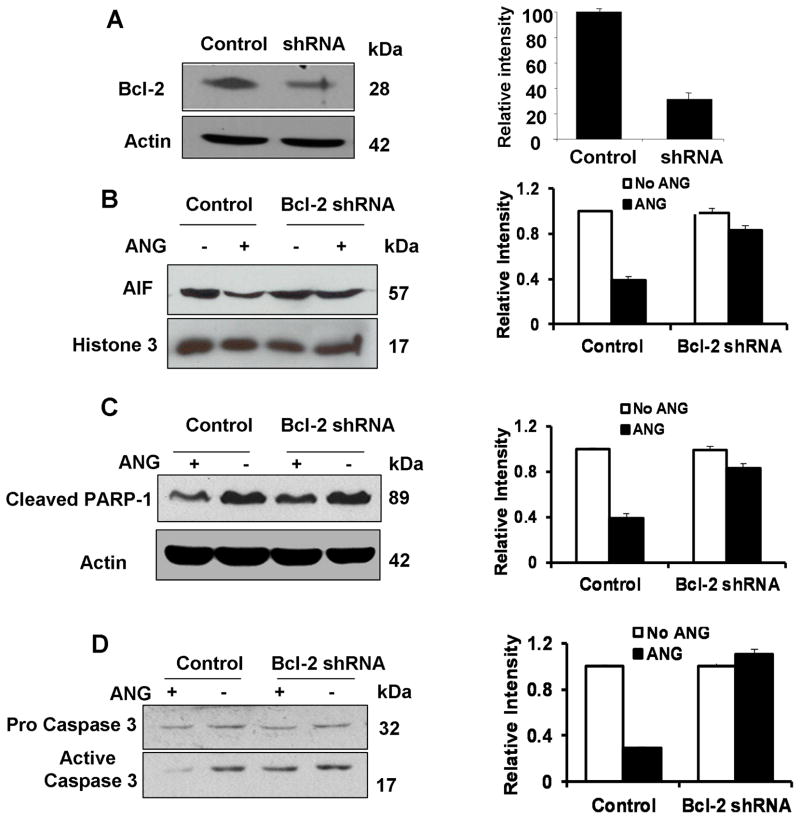
We have previously shown that ANG upregulates Bcl-2 and that Bcl-2 mediates the anti-apoptotic activity of ANG (Li et al., 2010). In order to know whether the preventive effect of ANG on nuclear translocation of AIF is Bcl-2-dependent, we used Bcl-2-specific shRNA to knock-down the expression of Bcl-2 and examined the resultant changes in nuclear translocation of AIF in the presence or absence of ANG. Fig. 4A shows that the Bcl-2 protein level decreased by 69% in the pooled population of Bcl-2-specific shRNA stable transfectants. When the vector control transfectants were cultured in serum-free medium in the presence of ANG, nuclear translocation of AIF was inhibited by 61% (Fig. 4B). However, in Bcl-2 knockdown cells, ANG could only inhibit nuclear translocation of AIF by 17% (Fig. 4B). Thus, knockdown of Bcl-2 inhibited the protective activity of ANG against nuclear translocation of AIF by 72%.
Fig. 4.
Bcl-2 siRNA abolishes the protective activity of ANG. (A) Western blotting analysis of the protein level Bcl-2 in vector control and in Bcl-2 specific shRNA transfectants. The bar graph at right is the relative density of Bcl-2 with β-actin. (B) AIF protein level in the nuclear fractions extracted from vector control and Bcl-2 shRNA transfectants cultured in serum-free medium with or without ANG for 24 h. The left panel is Western blotting film with an anti-AIF IgG. The right panel is relative intensity of AIF with Histone H3 as the normalization control. (C) The protein level of cleaved PARP-1 in the total cell lysates from vector control and Bcl-2 shRNA transfectants incubated with or without ANG for 16 h. The left panel is Western blotting film with anti-cleaved PARP-1 IgG. The right panel is the relative intensity of cleaved PARP-1 with actin as the normalization control. (D) The protein level of pro-caspase-3 and activated caspase-3 in total cell lysate from vector control and Bcl-2 shRNA transfectants incubated with or without ANG for 2 h. The left panel is Western blotting film with anti-caspase-3 IgG. The right panel is the relative density of active caspase-3 vs pro-caspase-3. The Western blotting images were from a representative experiment. The bar graphs shown are mean ± SD of three independent experiments.
Knockdown of BCL-2 attenuates the preventive function of ANG toward PARP-1 cleavage
Similarly, Bcl-2 knockdown also attenuates the protective activity of ANG against PARP-1 cleavage. As shown in Fig. 4C, ANG prevented serum withdrawal-induced PARP-1 cleavage by 46% and 9%, respectively, in vector control and in Bcl-2 shRNA transfectants, indicating that Bcl-2 knockdown inhibited the protective activity of ANG against PARP-1 cleavage by 80%. These results demonstrated that the protective activity of ANG towards PARP-1 cleavage is also dependent on Bcl-2.
Knockdown of Bcl-2 prevents ANG from inhibitingcaspase-3 activation
ANG has been shown to prevent the formation of active caspase-3 from the precursor (Li et al., 2010). In order to know whether this function of ANG is also mediated by Bcl-2, we examined the protein level of active caspase-3 in vector control and in Bcl-2 shRNA transfectants in the presence and absence of ANG. Fig. 4D shows that ANG decreased the level of active caspase-3 by 71% in vector control transfectants but no longer had any effect in Bcl-2 shRNA transfectants, indicating that the protective role of ANG against caspase-3 activation was completely abolished in Bcl-2 knockdown cells. These data demonstrated that ANG prevents caspase-3 activation in a Bcl-2 dependent manner.
Discussion
ANG has been proved to have neuro-protective function against various stressful conditions (Kieran et al., 2008; Sebastia et al., 2009; Subramanian and Feng, 2007). We have reported previously that ANG prevents serum withdrawal-induced apoptosis of P19 embryonal carcinoma cells through inhibition of both mitochondria and death receptor apoptotic pathways (Li et al., 2010). For the role of ANG in mitochondria-mediated apoptosis, we have shown that ANG upregulates both the mRNA and protein of Bcl-2. Knockdown of Bcl-2 with a specific shRNA decreased the anti-apoptotic activity of ANG as shown by flow cytometric analysis. Bcl-2 is located in the ER and nuclear membranes as well as in the outer membranes of the mitochondria. As an anti-apoptosis gene, it is a relative upstream molecule and has been shown to play an important role in ALS pathological process (Pedrini et al., 2010; Reyes et al., 2010). Identification of the downstream targets of Bcl-2 that are involved in the anti-apoptosis function of ANG would help understand the neuro-protective function of ANG. Here, we report that ANG prevents nuclear translocation of AIF in a Bcl-2-dependent manner.
It has been well documented that AIF-induced cell death is associated with the production of large-scale DNA fragments (Krantic et al., 2007). Nuclear translocation of AIF has been considered as a major mechanism in neurodegenerative diseases. AIF cleavage and nuclear translocation can be either caspase-dependent or -independent (Oh et al., 2006). Caspase-independent AIF nuclear translocation appears to be mediated by cleavage by cysteine proteases such as cathepsins and calpains that are different from caspases (Krantic et al., 2007). It is usually a very early event of the cell death cascade (Yu et al., 2003). For example, N-methyl-D-aspartate receptor-induced AIF nuclear translocation in neurons occurs with 2 h (Vosler et al., 2009). However, the preventive activity of ANG toward nuclear translocation of AIF was significant only after 16 h incubation (Fig. 2). We have previously shown that the most significant effect of ANG on caspase-3 activation occurs at 2 h, a relatively early event. Thus, nuclear translocation of AIF tails caspase-3 activation, suggesting that AIF is an effector of caspase-3 in this experimental system. More importantly, we found that the inhibitory activity of ANG toward caspase-3 activation is also Bcl-2 dependent.
Proteolytic cleavage of PARP-1 by caspase into 89- and 24-kDa fragments is an important biochemical feature of apoptosis (Kaufmann et al., 1993). PARP-1 cleavage has been shown to signal nuclear translocation of AIF resulting in nuclear apoptosis (Yu et al., 2003). Thus, AIF could act as a mediator of PARP-1-induced cell death. In other words, PARP-1 activation is required for AIF translocation and AIF is necessary for PARP-1-dependent cell death. Our results are consistent with this notion. First, the peak time of the preventive function of ANG toward PARP-1 cleavage and AIF translocation occurs at 4h and 24h, respectively. Blockage of AIF nuclear translocation is a much later event than prevention of PARP-1 cleavage in responding to ANG treatment. However, both events are dependent to Bcl-2. Thus, our results show that the anti-apoptotic role of ANG is executed by upregulation of Bcl-2 that occurs in less than 1 hour which then leads to caspase-3 activation that occurs within 2 hours. Activated caspase-3 results in PARP-1 cleavage and AIF nuclear translocation that occurs at 8 and 24 h, respectively. Taken together, our data suggest that the anti-apoptosis activity of ANG is executed by a series of sequential events that involve Bcl-2 upregulation, caspase-3 activation, PARP-1 cleavage, and AIF nuclear translocation.
Acknowledgments
Contract grant sponsor: National Institutes of Health
Contract grant numbers: R01NS065237, R01CA105241
Literature Cited
- Bain G, Ray WJ, Yao M, Gottlieb DI. From embryonal carcinoma cells to neurons: the P19 pathway. Bioessays. 1994;16(5):343–348. doi: 10.1002/bies.950160509. [DOI] [PubMed] [Google Scholar]
- Cande C, Cecconi F, Dessen P, Kroemer G. Apoptosis-inducing factor (AIF): key to the conserved caspase-independent pathways of cell death? J Cell Sci. 2002;115(Pt 24):4727–4734. doi: 10.1242/jcs.00210. [DOI] [PubMed] [Google Scholar]
- Cao G, Clark RS, Pei W, Yin W, Zhang F, Sun FY, Graham SH, Chen J. Translocation of apoptosis-inducing factor in vulnerable neurons after transient cerebral ischemia and in neuronal cultures after oxygen-glucose deprivation. J Cereb Blood Flow Metab. 2003;23(10):1137–1150. doi: 10.1097/01.WCB.0000087090.01171.E7. [DOI] [PubMed] [Google Scholar]
- Emara MM, Ivanov P, Hickman T, Dawra N, Tisdale S, Kedersha N, Hu GF, Anderson P. Angiogenin-induced tRNA-derived stress-induced RNAs promote stress-induced stress granule assembly. J Biol Chem. 2010;285(14):10959–10968. doi: 10.1074/jbc.M109.077560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett JW, Strydom DJ, Lobb RR, Alderman EM, Bethune JL, Riordan JF, Vallee BL. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry. 1985;24(20):5480–5486. doi: 10.1021/bi00341a030. [DOI] [PubMed] [Google Scholar]
- Friedlander RM. Apoptosis and caspases in neurodegenerative diseases. N Engl J Med. 2003;348(14):1365–1375. doi: 10.1056/NEJMra022366. [DOI] [PubMed] [Google Scholar]
- Friedlander RM, Brown RH, Gagliardini V, Wang J, Yuan J. Inhibition of ICE slows ALS in mice. Nature. 1997;388(6637):31. doi: 10.1038/40299. [DOI] [PubMed] [Google Scholar]
- Fu H, Feng J, Liu Q, Sun F, Tie Y, Zhu J, Xing R, Sun Z, Zheng X. Stress induces tRNA cleavage by angiogenin in mammalian cells. FEBS Lett. 2009;583(2):437–442. doi: 10.1016/j.febslet.2008.12.043. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Morselli E, Kepp O, Kroemer G. Targeting post-mitochondrial effectors of apoptosis for neuroprotection. Biochim Biophys Acta. 2009;1787(5):402–413. doi: 10.1016/j.bbabio.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Kang SJ, Sanchez I, Jing N, Yuan J. Dissociation between neurodegeneration and caspase-11-mediated activation of caspase-1 and caspase-3 in a mouse model of amyotrophic lateral sclerosis. J Neurosci. 2003;23(13):5455–5460. doi: 10.1523/JNEUROSCI.23-13-05455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann SH, Desnoyers S, Ottaviano Y, Davidson NE, Poirier GG. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993;53(17):3976–3985. [PubMed] [Google Scholar]
- Kieran D, Sebastia J, Greenway MJ, King MA, Connaughton D, Concannon CG, Fenner B, Hardiman O, Prehn JH. Control of motoneuron survival by angiogenin. J Neurosci. 2008;28(52):14056–14061. doi: 10.1523/JNEUROSCI.3399-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic V, Jackson-Lewis V, de Bilbao F, Dubois-Dauphin M, Przedborski S. Bcl-2: prolonging life in a transgenic mouse model of familial amyotrophic lateral sclerosis. Science. 1997;277(5325):559–562. doi: 10.1126/science.277.5325.559. [DOI] [PubMed] [Google Scholar]
- Krantic S, Mechawar N, Reix S, Quirion R. Apoptosis-inducing factor: a matter of neuron life and death. Prog Neurobiol. 2007;81(3):179–196. doi: 10.1016/j.pneurobio.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Li M, Ona VO, Guegan C, Chen M, Jackson-Lewis V, Andrews LJ, Olszewski AJ, Stieg PE, Lee JP, Przedborski S, Friedlander RM. Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science. 2000;288(5464):335–339. doi: 10.1126/science.288.5464.335. [DOI] [PubMed] [Google Scholar]
- Li S, Hu GF. Angiogenin-mediated rRNA transcription in cancer and neurodegeneration. Int J Biochem Mol Biol. 2010;1(1):26–35. [PMC free article] [PubMed] [Google Scholar]
- Li S, Yu W, Kishikawa H, Hu GF. Angiogenin prevents serum withdrawal-induced apoptosis of P19 embryonal carcinoma cells. FEBS J. 2010;277(17):3575–3587. doi: 10.1111/j.1742-4658.2010.07766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ. Neuronal death in amyotrophic lateral sclerosis is apoptosis: possible contribution of a programmed cell death mechanism. J Neuropathol Exp Neurol. 1999;58(5):459–471. doi: 10.1097/00005072-199905000-00005. [DOI] [PubMed] [Google Scholar]
- Millecamps S, Salachas F, Cazeneuve C, Gordon P, Bricka B, Camuzat A, Guillot-Noel L, Russaouen O, Bruneteau G, Pradat PF, Le Forestier N, Vandenberghe N, Danel-Brunaud V, Guy N, Thauvin-Robinet C, Lacomblez L, Couratier P, Hannequin D, Seilhean D, Le Ber I, Corcia P, Camu W, Brice A, Rouleau G, LeGuern E, Meininger V. SOD1, ANG, VAPB, TARDBP, and FUS mutations in familial amyotrophic lateral sclerosis: genotype-phenotype correlations. J Med Genet. 2010;47(8):554–560. doi: 10.1136/jmg.2010.077180. [DOI] [PubMed] [Google Scholar]
- Oh YK, Shin KS, Kang SJ. AIF translocates to the nucleus in the spinal motor neurons in a mouse model of ALS. Neurosci Lett. 2006;406(3):205–210. doi: 10.1016/j.neulet.2006.07.044. [DOI] [PubMed] [Google Scholar]
- Pedrini S, Sau D, Guareschi S, Bogush M, Brown RH, Jr, Naniche N, Kia A, Trotti D, Pasinelli P. ALS-linked mutant SOD1 damages mitochondria by promoting conformational changes in Bcl-2. Hum Mol Genet. 2010;19(15):2974–2986. doi: 10.1093/hmg/ddq202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perier C, Bove J, Dehay B, Jackson-Lewis V, Rabinovitch PS, Przedborski S, Vila M. Apoptosis-inducing factor deficiency sensitizes dopaminergic neurons to parkinsonian neurotoxins. Ann Neurol. 2010;68(2):184–192. doi: 10.1002/ana.22034. [DOI] [PubMed] [Google Scholar]
- Reyes NA, Fisher JK, Austgen K, VandenBerg S, Huang EJ, Oakes SA. Blocking the mitochondrial apoptotic pathway preserves motor neuron viability and function in a mouse model of amyotrophic lateral sclerosis. J Clin Invest. 2010;120(10):3673–3679. doi: 10.1172/JCI42986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan JF, Shapiro R. Isolation and enzymatic activity of angiogenin. Methods Mol Biol. 2001;160:375–385. doi: 10.1385/1-59259-233-3:375. [DOI] [PubMed] [Google Scholar]
- Sebastia J, Kieran D, Breen B, King MA, Netteland DF, Joyce D, Fitzpatrick SF, Taylor CT, Prehn JH. Angiogenin protects motoneurons against hypoxic injury. Cell Death Differ. 2009;16(9):1238–1247. doi: 10.1038/cdd.2009.52. [DOI] [PubMed] [Google Scholar]
- Shapiro R, Harper JW, Fox EA, Jansen HW, Hein F, Uhlmann E. Expression of Met-(−1) angiogenin in Escherichia coli: conversion to the authentic less than Glu-1 protein. Anal Biochem. 1988;175(2):450–461. doi: 10.1016/0003-2697(88)90569-6. [DOI] [PubMed] [Google Scholar]
- Subramanian V, Feng Y. A new role for angiogenin in neurite growth and pathfinding: implications for amyotrophic lateral sclerosis. Hum Mol Genet. 2007;16(12):1445–1453. doi: 10.1093/hmg/ddm095. [DOI] [PubMed] [Google Scholar]
- Thompson DM, Lu C, Green PJ, Parker R. tRNA cleavage is a conserved response to oxidative stress in eukaryotes. RNA. 2008;14(10):2095–2103. doi: 10.1261/rna.1232808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thress K, Henzel W, Shillinglaw W, Kornbluth S. Scythe: a novel reaper-binding apoptotic regulator. Embo J. 1998;17(21):6135–6143. doi: 10.1093/emboj/17.21.6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virag L, Szabo C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54(3):375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- Vosler PS, Sun D, Wang S, Gao Y, Kintner DB, Signore AP, Cao G, Chen J. Calcium dysregulation induces apoptosis-inducing factor release: cross-talk between PARP-1- and calpain-signaling pathways. Exp Neurol. 2009;218(2):213–220. doi: 10.1016/j.expneurol.2009.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner HL, Weiner LH, Swain JL. Tissue distribution and developmental expression of the messenger RNA encoding angiogenin. Science. 1987;237(4812):280–282. doi: 10.1126/science.2440105. [DOI] [PubMed] [Google Scholar]
- Yamasaki S, Ivanov P, Hu GF, Anderson P. Angiogenin cleaves tRNA and promotes stress-induced translational repression. J Cell Biol. 2009;185(1):35–42. doi: 10.1083/jcb.200811106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SW, Wang H, Dawson TM, Dawson VL. Poly(ADP-ribose) polymerase-1 and apoptosis inducing factor in neurotoxicity. Neurobiol Dis. 2003;14(3):303–317. doi: 10.1016/j.nbd.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Zhang X, Chen J, Graham SH, Du L, Kochanek PM, Draviam R, Guo F, Nathaniel PD, Szabo C, Watkins SC, Clark RS. Intranuclear localization of apoptosis-inducing factor (AIF) and large scale DNA fragmentation after traumatic brain injury in rats and in neuronal cultures exposed to peroxynitrite. J Neurochem. 2002;82(1):181–191. doi: 10.1046/j.1471-4159.2002.00975.x. [DOI] [PubMed] [Google Scholar]
- Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, Sarang S, Liu AS, Hartley DM, Wu DC, Gullans S, Ferrante RJ, Przedborski S, Kristal BS, Friedlander RM. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral clerosis in mice. Nature. 2002;417(6884):74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]