Abstract
Background
Cardiac manifestations of neonatal lupus (cardiac-NL) include conduction disease and rarely an isolated cardiomyopathy. This study was initiated to determine the mortality and morbidity of cardiac-NL and associated risk factors in a multi-racial/ethnic US-based registry to provide insights into the pathogenesis of antibody mediated injury and data for counseling.
Methods and Results
Three hundred and twenty-five offspring exposed to maternal anti-SSA/Ro antibodies with cardiac-NL met entry criteria. Maternal, fetal echocardiographic, and neonatal risk factors were assessed for association with mortality. Fifty-seven (17.5%) died; 30% in utero. The probability of in utero death was 6%. The cumulative probability of survival at 10 years for a child born alive was 86%. Fetal echocardiographic risk factors associated with increased mortality in a multivariable analysis of all cases included hydrops and endocardial fibroelastosis (EFE). Significant predictors of in utero death were hydrops and earlier diagnosis, and for postnatal death, hydrops, EFE, and lower ventricular rate. Isolated heart block was associated with a 7.8% case fatality rate whereas the concomitant presence of dilated cardiomyopathy or EFE quadrupled the case fatality rate. There was a significantly higher case fatality rate in minorities compared to Caucasians, who were at a lower risk of hydrops and EFE. Pacing was required in 70% and cardiac transplantation in four children.
Conclusion
Nearly one-fifth of fetuses who develop cardiac-NL die from complications which are predicted by echocardiographic abnormalities consistent with antibody associated disease beyond the AV node. The disparity in outcomes observed between minorities and Caucasians warrants further investigation.
Keywords: heart block, antibodies, cardiomyopathy, morbidity, mortality
Neonatal lupus (NL) was initially described in the late 1970s and represents a pathologic readout of passively acquired autoimmunity [1–4]. Identification of advanced fetal heart block, in the absence of structural abnormalities, predicts the presence of maternal autoantibody responses against the ribonucleoproteins SSA/Ro and SSB/La in greater than 85% of cases [5]. Of the affected offspring, 10–15% will have a life-threatening cardiomyopathy, occasionally without associated conduction disease [6–9]. Prospective studies of pregnancies in women with the candidate antibodies have estimated the risk of cardiac-NL at approximately 2% if the mother has had no previously affected pregnancies [10–13]. Recurrence rates in subsequent pregnancies are approximately eight- to nine-fold this risk [14–19]. In addition, the occurrence rate of cardiac-NL following a child with cutaneous-NL is about 6-fold higher [20]. Maternal health status does not appear to be a contributing factor to the risk of having a child with cardiac-NL but the relationship to severity of disease has not been addressed [14, 21].
Available data on estimates of the morbidity and mortality associated with cardiac-NL have been derived from several groups in different countries spanning two decades [5, 14, 15, 22–26]. These studies differ in cohort size, ranging from 55 [14] to 175 fetuses [26]. The overall case fatality rates range from 10% [25] to 29% [5]. The percentages of children receiving pacemakers vary from 63% [15] to 93% [23]. However, these studies did not uniformly require the presence of maternal anti-SSA/Ro or SSB/La antibodies as an inclusion criterion. For several studies, up to 40% of the cases included were not associated with maternal antibodies [5, 23–25]. Recognizing that heart block may have different etiologies, this latter point is relevant since conclusions may have been drawn on distinct nosologic conditions. Moreover, these studies do not provide maternal racial/ethnic breakdowns which could impact outcomes.
Accordingly, this study was initiated to determine the mortality and morbidity of cardiac-NL in a large US-based cohort inclusive of different racial backgrounds in which cardiac phenotype is well defined and exposure to maternal anti-SSA/Ro and/or anti-SSB/La is universal. It is anticipated that these data and any identified risk factors will have a significant impact on physician counseling and ultimate decision making by parents prospectively facing cardiac-NL or who have an affected offspring.
METHODS
Study population
Cardiac-NL cases were identified from the Research Registry for Neonatal Lupus (RRNL), which was established in 1994. Evaluation of de-identified information has approval from the IRB of the New York University (NYU) School of Medicine. Enrollment of a family in the RRNL requires verification of maternal anti-SSA/Ro or SSB/La antibodies (with the exception of anti-RNP antibodies in mothers of children with cutaneous NL) and documentation that at least one child has NL. The affected children were born between January 1963 and April 2010.
Inclusion/Exclusion Criteria
Three hundred and twenty-five children met the following inclusion criteria: a) enrollment in the RRNL by September 30, 2010; b) documentation of maternal antibodies reactive with SSA/Ro and/or SSB/La (based on results from a commercial or hospital laboratory, or performed in the research laboratory of JPB); c) confirmation of cardiac-NL defined herein as the presence of high grade heart block (2nd or 3rd degree) documented by electrocardiogram or echocardiogram, history of pacemaker, or statement in the medical record; and/or presence of cardiac injury or cardiomyopathy which specifically included evidence of a mononuclear infiltrate in the endocardium, myocardium and pericardium, endocardial fibroelastosis (EFE), and/or dilated cardiac chambers with evidence of decreased cardiac output on echocardiogram. Children born with isolated 1st degree heart block (n=8) or isolated sinus bradycardia (n=2) were excluded from this study given the low likelihood of death or requirement of a pacemaker.
Study Design and Data Collection
This was a retrospective study analysis based on review of medical records in the RRNL. The date of death and etiology of the death were recorded. Maternal risk factors analyzed included: mother’s age at time of birth, mother’s race/ethnicity, and maternal health status as assessed by rheumatologists’ notes in the charts. Maternal use of non-fluorinated steroids, fluorinated steroids, beta-agonists such as terbutaline, and hydroxychloroquine during pregnancy were also noted. Neonatal risk factors analyzed included: presence of associated cutaneous-NL (described as annular or elliptical lesions on the face, scalp, trunk, or extremities and verified by medical records, biopsy results and/or photographs [20]), the presence of hepatic and/or hematological laboratory abnormalities, which may be attributed to anti-SSA/SSB antibodies [27], gender, date of birth, gestational age at diagnosis and delivery, and method of delivery. In addition, the presence of carditis was also noted and defined as follows: presence of mononuclear infiltrate on the histologic preparation of the fetal endocardium, myocardium, or pericardium from an autopsy or cardiac biopsy. Fetal echocardiographic parameters analyzed included: ventricular rate at detection and lowest documented ventricular rate. The presence of EFE was identified when there were abnormal areas of echogenicity on the endocardial surface of the cardiac chambers and/or valve leaflets on echocardiogram, or endocardial fibrosis on biopsy or autopsy. Dilated cardiomyopathy (DCM) was defined as increased size of the left ventricle or multiple chambers in the absence of chamber wall hypertrophy with associated decreased contractility on echocardiogram. Hydrops fetalis was defined as an abnormal accumulation of fluid in at least two fetal compartments, including subcutaneous tissue, pleura, pericardium, or the abdominal cavity. The identification of valvular disease was based on in utero or neonatal echocardiography demonstrating moderate to severe stenosis and/or regurgitation involving the aortic, mitral, or pulmonic valves (tricuspid regurgitation was excluded due to its functional relationship to the underlying cardiac disease). In addition, the presence of an atrial septal defect (ASD), ventricular septal defect (VSD), or patent ductus arteriosus (PDA) were also noted if they were identified on an echocardiogram more than 28 days after birth or required surgical intervention. Morbidity assessments included whether the child received a pacemaker and the timing of initial placement as well as information on cardiac transplantation.
Maternal Antibody Testing
Maternal antibody status was established at either the NYU School of Medicine Immunology Laboratory or another CLIA approved outside laboratory. However, 80% of the antibody testing was done at NYU since the RRNL strongly encourages the collection of blood samples for research purposes. In addition, received samples were tested in the laboratory of JPB, using recombinant proteins (52Ro) in an ELISA as previously described [28].
Statistical Analysis
Survival distributions for mortality were estimated by the Kaplan-Meier method using approximate weeks since conception as the time scale for deaths which occurred in utero and years after a live birth in the live analysis. The distribution of time to pacemaker implantation was estimated taking into account death as a competing risk event [29]. To identify potential risk factors for mortality and estimate corresponding hazard ratios, the method of Lee, Wei, and Amato [30] for clustered survival data was applied to account for the correlation in data among multiple offspring from the same mother. Multivariable models were fit using a backward elimination approach based on an initial model which included all covariates which were significant at the p<0.20 level in bivariate analyses. Separate survival analyses were also performed on deaths which occurred in utero versus after a live birth. For the former, gestational age was used as the time scale and neonates which were born alive were censored at the time of birth. For the analysis of deaths following a live birth, weeks since birth (i.e., age in weeks) was used as the time scale and only live births were included in the analysis. Two-sided p values less than 0.05 were considered statistically significant.
RESULTS
Patient Demographics
A total of 325 children from 297 mothers met the final inclusion criteria. Twenty-one (6.5%) had 2nd degree heart block, 257 (79.1%) had 3rd degree heart block and 34 (10.5%) had periods of both 2nd and 3rd degree heart block. In addition, eight (2.5%) had an isolated cardiomyopathy and five (1.5%) had a cardiomyopathy associated with 1st degree heart block. Of thirteen 2nd degree heart block cases exposed to dexamethasone in utero, four reversed to 1st degree heart block or NSR, and only three required permanent pacemaker placement. Of eight 2nd degree heart block cases not exposed to dexamethasone in utero, one reverted spontaneously, and four required pacemakers.
The maternal demographics, health status and antibody status are listed in Table 1. The majority of mothers were Caucasian (75.1%). Overall, most mothers were either asymptomatic or had insufficient symptoms for a formal diagnosis of either Systemic Lupus Erythematosus (SLE) or Sjögren’s Syndrome (SS) at the time of pregnancy. Thus, for many of these women, anti-SSA/Ro and/or SSB/La antibodies were first identified only after the diagnosis of cardiac-NL in the fetus. Antibodies to SSA/Ro occurred in 100% and SSB/La antibodies accompanied the anti-SSA/Ro response in 64.3%. In addition, of 250 mothers in which the anti-52kD Ro antibody status was tested, 227 (90.8%) were positive.
Table 1.
Maternal Demographics
| Race/Ethnicity (N=297) | N | % |
|---|---|---|
| Caucasian | 223 | 75.1% |
| Black | 27 | 9.1% |
| Hispanic | 26 | 8.8% |
| Asian | 14 | 4.7% |
| Mixed Race | 7 | 2.4% |
|
| ||
| Diagnosis at Time of Pregnancy (N=325) | N | % |
|
| ||
| Asymptomatic/UAS | 176 | 54.2% |
| SLE | 45 | 13.8% |
| SS | 75 | 23.1% |
| SLE/SS | 29 | 8.9% |
|
| ||
| Maternal Antibody Status | N | % |
|
| ||
| Anti-SSA/Ro antibody (N=297) | 297 | 100% |
| Anti-SSB/La antibody (N=294) | 189 | 64.3% |
| Anti-52kD Ro antibody (N=250) | 227 | 90.8% |
SLE=Systemic Lupus Erythematosus, SS=Sjögren’s Syndrome, UAS = Undifferentiated Autoimmune Syndrome. Maternal health status was defined at the time of each pregnancy. Since 325 cases came from 297 mothers, the health status of the mother may have progressed from one pregnancy to a subsequent pregnancy, thus maternal diagnoses at the time of pregnancy for all 325 offspring are included. The race/ethnicity and antibody status were only included once
Case Fatality Rate and Etiology of Death
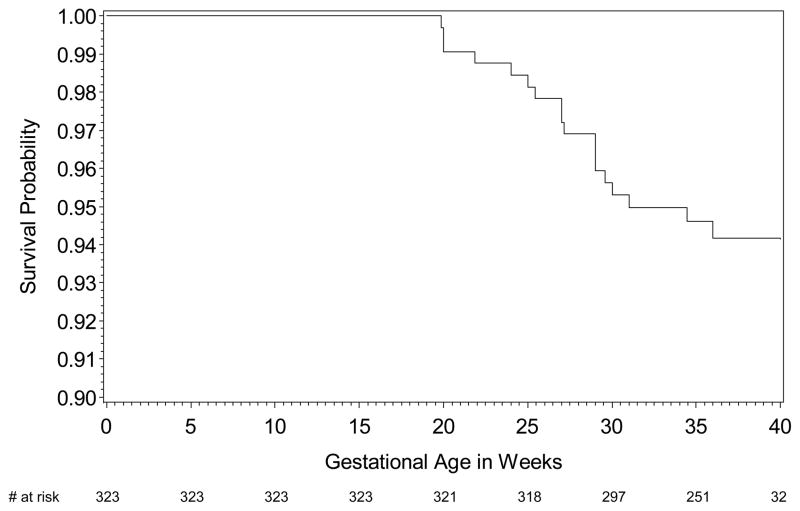
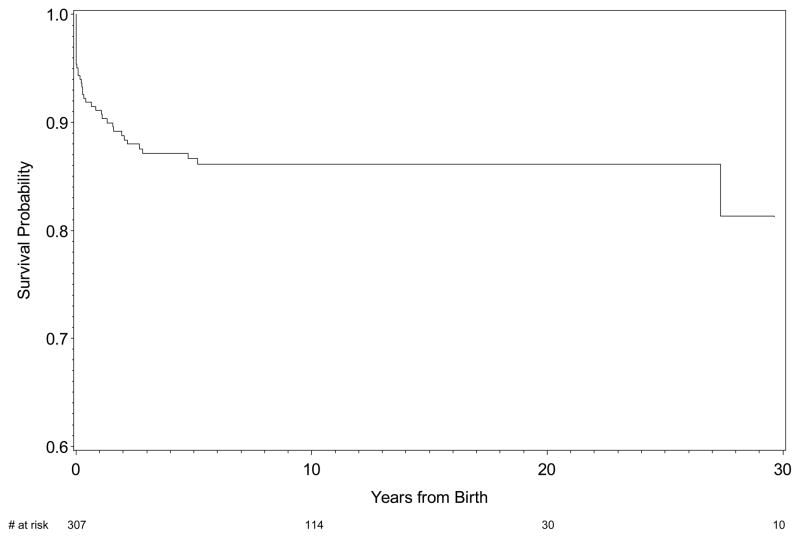
Overall, there were 57 deaths (17.5%) among the 325 children with cardiac-NL. Eighteen fetuses died in utero, the majority prior to thirty weeks of gestation (Figure 1A). At 35 weeks of gestation, 251, (77.7%) cases of cardiac-NL were alive in utero. The probability of dying in utero was 6%. Of 307 children (251 plus 56 delivered before 35 weeks of gestational age) born alive, 39 died after birth with the majority occurring prior to one year postpartum (Figure 1B). The cumulative probability of survival at ten years for a child born alive was 86% (n = 114).
Figure 1.
Figure 1A. Kaplan-Meier survival estimates of fetal cardiac-NL in utero. Of note, the exact gestational age at birth was unknown for two cases which were excluded from the analysis. The X axis represents the number of cases at the corresponding gestational age.
Figure 1B. Kaplan-Meier survival estimates of children with cardiac-NL born alive.
The etiology of the 57 cardiac neonatal deaths was considered primarily cardiac related in 40 (70.2%). Of these 40, 37 were complications of a cardiomyopathy, 1 was a transplant rejection and 2 were secondary to pacemaker complications. In four fetuses (7.0%) the pregnancy was electively terminated after identification of hydrops. Infection was considered the primary cause of death in five (8.8%), with respiratory syncytial virus accounting for three and bacterial pneumonia and sepsis accounting for the remaining two. In eight cases (14.0%), medical records were insufficient to determine the cause of death (Table 2).
Table 2.
Etiology of Cardiac-NL Death
| Outcome (N=57) | N | % |
|---|---|---|
| Cardiac Related | 40 | 70.2% |
| Cardiomyopathy (Hydrops/EFE/DCM) | 37 | |
| Transplant Rejection | 1 | |
| Complications from Pacemaker | 2 | |
| Infectious Complications | 5 | 8.8% |
| RSV | 3 | |
| Pneumonia/Sepsis | 2 | |
| Unknown | 8 | 14.0% |
| Elective Pregnancy Termination with Evidence of Hydrops on Echocardiography | 4 | 7.0% |
EFE=endocardial fibroelastosis, DCM= dilated cardiomyopathy, RSV=respiratory syncytial virus
Maternal, Fetal Echocardiographic and Neonatal Risk Factors Associated with Mortality
Table 3 displays the results of the bivariate analysis of maternal, neonatal and fetal echocardiographic data variables and risk of death occurring both in utero and after birth, as well as the overall risk of death and the associated hazard ratios (HR) for all pregnancies. In the overall analysis, Caucasians were less likely to die (HR=0.47, p=0.005). In addition, there was a trend towards increased mortality in those offspring whose mothers had an established diagnosis of SLE and/or SS at the time of pregnancy (HR=1.57, p=0.095) and the presence of anti-SSB/La antibody (HR=1.68, p=0.093). The presence of carditis was associated with an increase in mortality (HR=8.40, p<.0001). Several fetal echocardiographic risk factors were associated with a statistically significant increase in mortality, including the presence of hydrops (HR=15.37, p=<.0001), DCM (HR=6.65, p<.0001), EFE (HR=6.45, p<.0001) and the presence of valvular diseases (HR=4.50, p=.0001). A higher ventricular nadir rate was protective (HR=0.95, p=0.003). In a multivariable analysis using Cox models, the only significant overall predictors of mortality were hydrops (HR=15.1, p<0.0001), the presence of carditis (HR=4.60, p<0.0002) EFE (HR = 3.69, p<0.0001) and maternal diagnosis of SLE and/or SS (HR=3.44, p=0.0001).
Table 3.
Maternal, Fetal Echocardiographic and Neonatal Mortality Risk Factors
| Mortality and Maternal Risk Factors
| |||||
| Maternal Risk Factors | Cardiac-NL Pregnancies (N=325) | HR | HR | HR | |
| Deceased (N=57) Living (N=268) | Overall | IU | AB | ||
|
| |||||
| Maternal age (mean, yr)x N=325 | 29.5 (57) | 29.8 (268) | 1.00 (.95–1.05) | 1.03 (.95–1.11) | 0.99 (.92–1.06) |
|
| |||||
| Caucasian N=325 | 61.4% (57) | 78.4% (268) | 0.47¥ (.27–.80) | 0.49 (.18–1.29) | 0.45¥ (.24–.85) |
| Maternal anti-La N=322 | 75.4% (57) | 63.8% (265) | 1.68Ω (.92–3.08) | 1.37 (.48–3.90) | 1.85 (.87–3.90) |
| Maternal anti-52kD Ro N=275 | 89.4% (47) | 91.7% (228) | 0.90 (.38–2.14) | N/Dα | 0.59 (.24–1.44) |
| Maternal diagnosis of SLE or SS N=325 | 56.1% (57) | 43.7% (268) | 1.57Ω (.92–2.67) | 1.18 (.45–3.09) | 1.78Ω (.93–3.39) |
| Non-fluorinated steroids N=313 | 23.2% (56) | 14.8% (257) | 1.63 (.88–3.02) | 1.66 (.53–5.21) | 1.58 (.76–3.28) |
| Fluorinated steroids N=318 | 50.9% (57) | 47.1% (261) | 1.27 (.75–2.18) | 3.95¥ (1.26–12.38) | 0.81 (.42–1.56) |
| Terbutaline N=314 | 18.9% (53) | 15.3% (261) | 1.36 (.69–2.67) | 2.48Ω (.93–6.67) | 0.95 (.37–2.41) |
| Hydroxychloroquine† N=318 | 1.8% (57) | 3.4% (261) | N/D | N/D | N/D |
|
| |||||
| Neonatal Risk Factors and Cardiac-NL Mortality
| |||||
| Neonatal Risk Factors | Cardiac-NL Pregnancies (N=325) | HR | HR | HR | |
| Deceased (N=57) Living (N=268) | Overall | IU | AB | ||
|
| |||||
| Female Sex N=323 | 52.7% (55) | 56.0% (268) | 1.04 (.62–1.75) | 0.81 (.33–1.96) | 1.16 (.61–2.20) |
| Associated NL* Rash N=323 | 14.3% (35) | 14.1% (256) | N/A | N/A | 0.96 (.37–2.47) |
| Associated Liver/Heme NL* N=290 | 30.6% (36) | 7.1% (254) | N/A | N/A | 4.47φ (2.15–9.32) |
| Carditis N= 251 | 31.0% (41) | 2.4% (209) | 8.40φ (4.49–15.72) | 6.62φ (2.25–19.48) | 10.38φ (4.73–22.78) |
| Gestational Age at Detection (weeks) N=321 | 24.8 (56) | 26.9 (265) | 0.98 (.95–1.01) | 0.86¥ (.77–.96) | 0.99 (.97–1.01) |
| Delivery by C-section* N=297 | 79.5% (39) | 78.7% (258) | N/A | N/A | 1.31 (.58–2.95) |
| Preterm Delivery (<37 Weeks)* N=307 | 70.0% (40) | 41.2% (267) | N/A | N/A | 3.18φ (1.66–6.10) |
| Gestational Week of Delivery* N=306 | 34.1 (40) | 36.9 (266) | N/A | N/A | 0.78φ (.71–.85) |
| Year of Birthx N=325 | 1996 | 1997 | 1.01 (.98–1.04) | 1.09¥ (1.03–1.16) | .98 (95–1.01) |
|
| |||||
| Fetal Echocardiographic Risk Factors for Cardiac-NL Mortality
| |||||
| Fetal US Risk Factors | Cardiac-NL Pregnancies (N=325) | HR | HR | HR | |
| Deceased (N=57) Living (N=268) | Overall | IU | AB | ||
|
| |||||
| Atrial Rate Nadir N=178 | 128.6 (28) | 125.7 (150) | 1.01 (.98–1.04) | 1.00 (.96–1.04) | 1.02 (.99–1.06) |
| Ventricular Rate at Detection N=196 | 62.5 (36) | 65.0 (160) | 0.99 (.96–1.02) | 0.99 (.96–1.03) | 0.99 (.95–1.03) |
| Ventricular Rate at Nadir N=222 | 46.3 (38) | 53.7 (184) | 0.95¥ (.92–.98) | 1.00 (.93–1.07) | 0.92φ (.89–.94) |
| Endocardial Fibro-elastosis N=254 | 32.6% (43) | 6.2% (211) | 6.45φ (3.3–12.61) | 3.06Ω (.97–9.63) | 9.91φ (4.43–22.17) |
| Dilated Cardiomyopathy N=257 | 53.3% (45) | 9.9% (212) | 6.65φ (3.70–11.95) | 6.49φ (2.33–18.11) | 6.80φ (3.37–13.70) |
| Hydrops N=257 | 57.4% (47) | 4.8% (210) | 15.37φ (8.36–28.24) | 26.60φ (8.04–87.82) | 12.21φ (5.88–25.36) |
| Valvular Disease N=255 | 17.8% (45) | 2.9% (210) | 4.50φ (2.10–9.63) | 2.46 (.55–11.06) | 6.21φ (2.36–16.39) |
| Atrial Septal Defect N=221* | 12.5% (16) | 8.8% (205) | N/A | N/A | 1.40 (.32–6.21) |
| Ventricular Septal Defect N=224† | 0.0% (16) | 3.4% (208) | N/D | N/D | N/D |
| Patent Ductus Arteriosus N=221* | 11.8% (17) | 6.9% (204) | N/A | N/A | 1.72 (.38–7.72) |
Numbers under each variable denote the number of patients for whom the variable was available for analysis. Parentheses adjacent to the percentages denote the number of patients among those alive or dead that had the parameter available for evaluation. The Hazard Ratios (HR) for the overall, in utero evaluation (HR IU), and after birth evaluation (HR AB), are reported with their respective 95% confidence intervals.
N/A=non-applicable.
Variable only evaluated in live births
Hydroxychloroquine and Ventricular Septal Defect were uncommon and therefore statistical analysis for these variables was considered unreliable and not done (N/D)
Analysis was considered unreliable secondary to all the deaths occurring in the anti-52kD Ro positive patients and none in the anti-52kD Ro negative patients
HR calculated per increase in maternal age or year of birth
P<.10
P<.05
P<.001
Separate bivariate analyses were performed and the respective hazard ratios of fetuses dying in utero (HR IU) and those born alive (HR AB) are reported in Table 3. For the in utero deaths, several variables were significantly associated with an increased mortality in bivariate analyses: hydrops (HR=26.60, p=<0.0001), the presence of carditis (HR=6.62, p=0.0006), DCM (HR=6.49, p=0.0004), a more recent year of pregnancy (HR=1.09, p=0.0042), and use of fluorinated steroids (HR=3.95, p=0.02). Trends were also seen for an increased mortality with the presence of EFE (HR=3.06, p=0.06) and the use of terbutaline (HR=2.48, p=0.07). In contrast, a later gestational age at initial detection of cardiac-NL (HR=0.86, p=0.007) was protective. In a multivariable analysis using Cox models, the only significant predictors of mortality for in utero deaths were hydrops (HR=29.67, p<0.0001), the presence of carditis (HR=8.66, p<.0001), a more recent year of pregnancy (HR=1.10, p=0.01) and a later gestational age at diagnosis of cardiac-NL (HR=0.80, p=0.02).
For those children who died after birth the following variables were significantly associated with an increased mortality in bivariate analyses: hydrops (HR=12.21, p<0.0001), the presence of carditis (HR=10.38, p<.0001), EFE (HR=9.91, p<0.0001), dilated cardiomyopathy (HR= 6.80, p<0.0001), presence of associated hepatic/hematologic abnormalities (HR=4.47, p<0.0001), valvular disease (HR=6.21, p=0.0002), and preterm delivery (HR=3.18, p=0.0005). In addition, there was a trend towards increased mortality in those patients whose mother had an established diagnosis of SLE and/or SS at the time of pregnancy (HR=1.78, p=0.081). There was a significantly decreased risk for mortality in Caucasians compared to minorities when analyzing deaths occurring after birth (HR=0.45, p=0.014). A higher ventricular nadir rate (HR=0.92, p<0.0001) and later week of delivery (HR=0.78, p<0.0001) were protective. In a multivariable analysis of predictors in offspring born alive using Cox models, the only significant predictors of mortality were hydrops (HR=7.83, p=0.0007), EFE (HR=17.31, p<0.0001) and a maternal diagnosis of SLE and/or SS at the time of pregnancy (HR=2.85, p=0.02). A higher ventricular nadir rate was protective (HR=0.94, p<0.0001). There was a trend towards a later week of delivery (HR=0.88, p=0.06) associating with a decrease in mortality.
Table 4 displays the case fatality rates for various cardiac-NL manifestations. Overall, isolated advanced CHB was associated with a 7.8% (15/193) case fatality rate whereas the concomitant presence of DCM or EFE more than quadrupled the case fatality rate.
Table 4.
Case Fatality Rate Among Manifestations of Cardiac-NL
| N | Advanced Block | EFE | DCM | Mortality |
|---|---|---|---|---|
| 193 | + | -- | -- | 15 (17.8%) |
| 12 | + | + | -- | 5 (41.7%) |
| 30 | + | -- | + | 11 (36.7%) |
| 9 | + | + | + | 9 (100%) |
| 6 | -- | + | -- | 0 (0%) |
| 6 | -- | -- | + | 4 (66.7%) |
| 0 | -- | + | + | NA |
N= Numbers of cases with available echocardiographic data. (+) represents the presence and (−) the absence of each respective manifestation. Advanced block= 2nd and/or 3rd degree heart block, EFE=endocardial fibroelastosis, DCM= dilated cardiomyopathy.
Higher Case Fatality Rate Among Non-Caucasian Mothers
Table 5 shows the case fatality rate stratified by race/ethnicity. Specifically, 14.3% of children with cardiac–NL born to Caucasian mothers died compared to a case fatality rate of 32.1% observed for African Americans mothers, 25.0% for Hispanic mothers, 26.7% for Asian mothers and 22.2% for Mixed Race mothers. There was a significantly higher case fatality rate in minorities compared to Caucasians in the overall group and for the children that died after birth, but not for fetuses dying in utero. Although the association of race/ethnicity and mortality was not maintained in multivariable analyses, Caucasian fetuses were at lower risk of hydrops (p=0.05), EFE (p=0.05) and carditis (p=0.03), variables highly predictive of mortality.
Table 5.
Case Fatality by Race/Ethnicity
| Caucasian N=245 | Black N=28 | Hispanic N=28 | Asian N=15 | Other N=9 |
|---|---|---|---|---|
| 35 (14.3%) | 9 (32.1%) | 7 (25.0%) | 4 (26.7%) | 2 (22.2%) |
Other=American-Indian and mixed race mothers
Morbidity Associated with Cardiac-NL
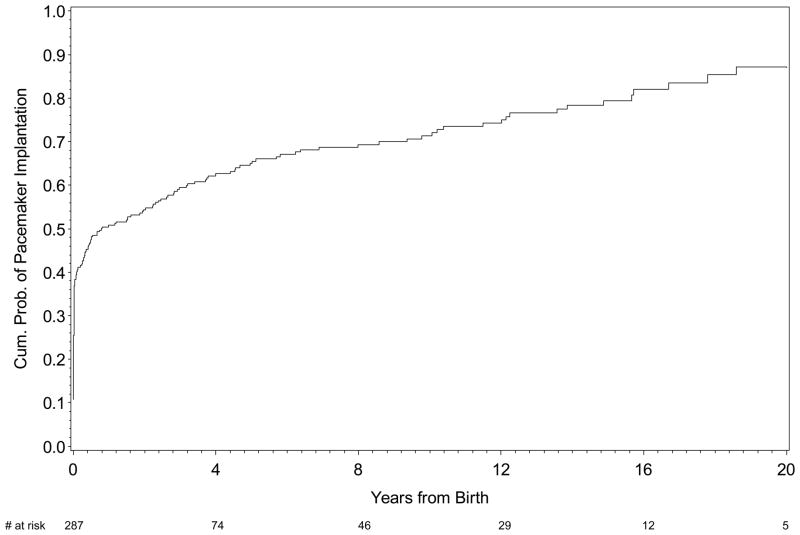
Represented in Figure 2 is the cumulative probability of pacemaker implantation following a live birth. By one year, approximately 50% of the patients were paced; the majority of which occurred in the first month of life. At ten years the cumulative probability of requiring a pacemaker was approximately 70%. Two fetuses were paced in utero but died shortly after birth. Additionally, four children received a cardiac transplantation, one of whom required two.
Figure 2.
The Kaplan-Meier curves reflecting probability of pacemaker implantation. Of note, two cases of cardiac-NL that were paced in utero were omitted from the analysis.
DISCUSSION
In this large US-based registry of cardiac-NL restricted to maternal anti-SSA/Ro exposure, there were 57 (17.5%) deaths with approximately one-third of these offspring dying in utero. There was a significantly higher case fatality rate in minorities compared to Caucasians. In addition, there was a trend towards increased mortality in children of mothers who had an established diagnosis of SLE and/or SS at the time of pregnancy which became significant in the multivariable analysis. Several fetal echocardiographic risk factors were associated with a statistically significant increase in mortality: the presence of hydrops, DCM, EFE, and valvular dysfunction. A higher ventricular nadir rate was protective. In multivariable analysis the only significant echocardiographic predictors of mortality were hydrops and EFE. Separate analyses on fetuses dying in utero and children dying after birth revealed similar echocardiographic predictors of mortality.
In the analyses limited to in utero deaths, predictors of mortality, in addition to those identified by echocardiogram, included earlier gestational age at the time cardiac-NL was first detected. This finding suggests that earlier injury results in more extensive damage to the cardiac structures. If an earlier event targets the majority of the fetal heart, it may result in a more severe lesion such as cardiomyopathy. In a later event in which the exposed and vulnerable targets are restricted to the isolated conduction system tissues, the insult may not be lethal. The association of in utero death with more recent year of pregnancy, while modest, was initially counterintuitive. This may reflect increased awareness of anti-SSA/Ro attributed cardiac disease which might otherwise have been categorized as an unexplained fetal demise. Additional predictors of in utero mortality included the use of fluorinated steroids and/or terbutaline. Each of these medications is generally prescribed in the more severe cases, which is the likely reason for their association with mortality, rather than direct causality. In the analysis limited to children who died after birth, as expected, preterm delivery was associated with an increased mortality. A later week of delivery and a higher ventricular nadir rate were protective, the latter remaining in the multivariable analysis of predictors for mortality in live born children.
With regard to morbidity, pacemakers were required in the majority of children, with most paced in the neonatal period. Pacemaker placement was not associated with mortality in the overall analysis or live birth analysis, suggesting that pacemaker-induced cardiomyopathy may not be a common cause of mortality in this cohort.
Previous data on risk factors associated with mortality, while available, are limited by the heterogeneity of the cohorts, since not all children have documented exposure to maternal anti-SSA/Ro-SSB/La antibodies. Moreover, most reports reflect cases seen at tertiary medical centers and thus data may be skewed toward the sickest patients. Impaired left ventricular function has been a common denominator associated with poor prognosis. In a study based in France, all six deaths in anti-SSA/Ro exposed children with CHB were associated with a dilated cardiomyopathy [23]. In an earlier U.S. based multicenter case series of CHB, the development of cardiomyopathy after birth occurred in sixteen fetuses; four died and six required transplantation [6]. Antibodies were confirmed in ten of the mothers. Recently, data from the Association for European Pediatric Cardiology identified impaired left ventricular function to be associated with mortality in 162 children with CHB, of which 80% were exposed to anti-SSA/SSB antibodies [26]. In our cohort approximately half the patients with DCM died.
In recent years, EFE has emerged not only as an extension of the cardiac pathogenicity associated with anti-SSA/Ro, but as a risk factor predicting mortality. Data from the RRNL confirm and extend these earlier observations in that half the patients with EFE died. Jaeggi initially demonstrated the poor prognostic significance of EFE in five patients [24]. In another study of 13 patients with CHB exposed to anti-SSA/Ro antibodies that developed EFE, 11 (85%) either died or underwent cardiac transplantation [7]. Emphasizing the injurious effect of anti-SSA/Ro on the endocardium per se, two fetuses with EFE absent any conduction abnormalities died and one child with isolated EFE received a transplant [8]. However, the severity of EFE was not confirmed in a recent case series of five children with isolated EFE, in which four children were alive at four years, three of whom had normal heart function [9]. In another study comprised of 20 cases of anti-SSA associated EFE, an 80% survival rate at a median follow-up of 3 years was observed in those treated with IVIG and steroids at the time of diagnosis [31].
A novel finding, not previously addressed by any study of cardiac-NL, was the higher case fatality rate in children born to non-Caucasian mothers (which comprise 25% of the RRNL), an observation which was consistent across each of the racial/ethnic groups. Overall, non-Caucasians had a significantly higher case fatality rate than Caucasians, although the association of race/ethnicity and mortality was not maintained in multivariable analyses. This was likely due to the observation that Caucasian fetuses were at lower risk of hydrops and EFE, variables highly predictive of mortality. One possible explanation for this is that more extensive cardiac injury occurs in minorities. Candidate neonatal factors could include genetics, a possibility difficult to explore given low numbers of affected children in each minority group. To date, genetic studies have been limited to Caucasians [32]. The absence of racial/ethnic information in any of the published cohorts precludes comparison of these data to other studies. Further studies are needed to validate this association and to determine whether access to medical care accounts for this disparity.
Another previously unreported finding in this study is that associated hepatic/hematologic-NL, but not cutaneous-NL, is associated with an increased morbidity in live born children. The liver abnormalities may have been due to hepatic congestion secondary to cardiac failure. However, the finding of a mononuclear infiltrate in several livers evaluated on autopsy supports an organ specific inflammatory process similar to that proposed for the initial phase of cardiac injury. Cytopenias may add to the overall burden of disease by decreasing oxygenation of tissues, increasing the risk of bleeding, and increasing the risk of infection. Moreover, cytopenias may represent increased pathogenicity of the autoantibodies. In addition, mortality was associated with a maternal diagnosis of SLE or SS at the time of pregnancy. This observation was unexpected since it predicted that mothers with known rheumatic disease and the presence of autoantibodies prior to pregnancy might be more likely to have had optimal surveillance. Although medications such as non fluorinated steroids, which would more often have been prescribed to patients with established rheumatic disease, might have been an explanation for the increased mortality rates, this was not found to be the case. Perhaps maternal illness per se conferred a less favorable in utero environment as it is generally accepted that SLE associates with premature birth [33]. Finally, the association of SLE and/or SS with mortality could possibly have been a reflection of maternal ethnicity since non-Caucasians may be more frequently represented in mothers with SLE [34, 35]. However, this was not observed: SLE mothers in this study comprised an equal distribution of Caucasian and non-Caucasian.
There are several limitations to this study, all of which are inherent in rare diseases. The low numbers of minorities make it difficult to discern why they have a higher case fatality rate. The race of the mother was used as a proxy for the child given the data available, as the father’s race was not a mandate of the RRNL and not systematically solicited. The low numbers of in utero deaths (18) limit the statistical power for related analyses. While the data presented suggest that the probability of in utero death occurring before 20 weeks is zero, this observation may be misleading since women enrolled in the Registry are often totally asymptomatic and unaware of the presence of anti-SSA/Ro antibodies until detection of cardiac-NL. As such, obstetrical evaluation beyond routine care may not have occurred prior to the early to mid second trimester. Moreover, death before 20 weeks may be due to many causes, and without proof of a cardiac disorder, unambiguous attribution to cardiac-NL was not possible. The data in this study were largely collected in a retrospective manner and in some pregnancies not all of the data were available, which reduced the available sample size for the multivariable analyses. In addition, patients with available data may not be a random sample of the underlying study population which could potentially lead to biased estimates of relative risk. The exact etiology of the cardiac-NL death was unknown in seven cases, however five of them occurred within six months of birth, suggesting that cardiac-NL was a contributing cause.
The significant influence of carditis on mortality is potentially biased since its diagnosis is dependent on histologic evaluation of tissue. Predictably, biopsies were largely performed in the sickest of cases in which the vast majority had associated DCM and/or EFE on echocardiography. Of the five cases of carditis in which the child lived, one required cardiac transplantation. In addition, carditis was also seen on several autopsies. The finding of a mononuclear infiltrate supports the hypothesis that an inflammatory process involving more than the conduction system contributes to the increase in mortality.
In summary, the overall case fatality rate of NL was 17.5%, pacing was required in approximately 70% by ten years of age and 4 children required cardiac transplantation. Mortality was predicted by echocardiographic abnormalities consistent with antibody associated disease beyond the AV node. The case fatality rate was higher in children born to non-Caucasian mothers, which requires further investigation.
The cardiac manifestations of neonatal lupus (cardiac-NL) include advanced conduction disease and rarely an isolated cardiomyopathy. This study, which included three hundred and twenty-five offspring exposed to maternal anti-SSA/Ro antibodies with cardiac-NL, was used to determine the mortality and morbidity and associated risk factors in a multi-racial/ethnic US-based registry. The case fatality rate was 17.5%. A third of the cases died in utero. The cumulative probability of survival at 10 years for a child born alive was 86% (most dying within a year of birth). Fetal echocardiographic risk factors associated with a statistically significant increase in mortality in a multivariate analysis included hydrops, endocardial fibroelastosis (EFE), an earlier diagnosis of cardiac-NL and a lower ventricular rate. Overall, isolated advanced heart block was associated with a 7.8% case fatality rate whereas the concomitant presence of dilated cardiomyopathy or endocardial fibroelastosis more than quadrupled the case fatality rate. There was a significantly higher case fatality rate in minorities compared to Caucasians, who were at a lower risk of hydrops and EFE. Pacing was required in 70% by ten years and 4 children underwent cardiac transplantation. Data from this cohort reveal nearly one-fifth of fetuses who develop cardiac-NL die from complications which are predicted by echocardiographic abnormalities consistent with antibody associated disease beyond the AV node.
Acknowledgments
The authors would like to acknowledge Amanda Zink for assistance in preparing the manuscript and the families who have enrolled in the RRNL whose information made this study possible.
Funding Sources:
This research was funded by the National Institute of Arthritis and Musculoskeletal and Skin Disease contract N01-AR-4-2220-11-0-1 for the Research Registry for Neonatal Lupus and grant 5R37 AR-42455-19 to Dr. Buyon. Dr. Amit Saxena was also funded by the American Heart Association Founders Affiliate Clinical Research Program Award #11CRP7950008 and the 2011-2012/2013 Pfizer Fellowships in Rheumatology/Immunology from Pfizer’s Medical and Academic Partnerships program.
Footnotes
Disclosures: none
References
- 1.McCue CM, Mantakas ME, Tingelstad JB. Congenital heart block in newborns of mothers with connective tissue disease. Circulation. 1977;56:82–90. doi: 10.1161/01.cir.56.1.82. [DOI] [PubMed] [Google Scholar]
- 2.Chameides L, Truex RC, Vetter V, Rashkind WJ, Galioto FM, Jr, Noonan JA. Association of maternal systemic lupus erythematosus with congenital complete heart block. N Engl J Med. 1977;297:1204–1207. doi: 10.1056/NEJM197712012972203. [DOI] [PubMed] [Google Scholar]
- 3.Scott JS, Maddison PJ, Taylor PV, Esscher E, Scott O, Skinner RP. Connective-tissue disease, antibodies to ribonucleoprotein, and congenital heart block. N Engl J Med. 1983;309:209–212. doi: 10.1056/NEJM198307283090403. [DOI] [PubMed] [Google Scholar]
- 4.Reed BR, Lee LA, Harmon C, Wolfe R, Wiggins J, Peebles C, Weston WL. Autoantibodies to SS-A/Ro in infants with congenital heart block. J Pediatr. 1983;103:889–91. doi: 10.1016/s0022-3476(83)80707-0. [DOI] [PubMed] [Google Scholar]
- 5.Jaeggi ET, Hornberger LK, Smallhorn JF, Fouron JC. Prenatal diagnosis of complete atrioventricular block associated with structural heart disease: combined experience of two tertiary care centers and review of the literature. Ultrasound Obstet Gynecol. 2005;26:16–21. doi: 10.1002/uog.1919. [DOI] [PubMed] [Google Scholar]
- 6.Moak JP, Barron KS, Hougen TJ, Wiles HB, Balaji S, Sreeram N, Cohen MH, Nordenberg A, Van Hare GF, Friedman RA, Perez M, Cecchin F, Schneider DS, Nehgme RA, Buyon JP. Congenital heart block: development of late-onset cardiomyopathy, a previously underappreciated sequela. J Am Coll Cardiol. 2001;37:238–242. doi: 10.1016/s0735-1097(00)01048-2. [DOI] [PubMed] [Google Scholar]
- 7.Nield LE, Silverman ED, Smallhorn JF, Mullen JB, Silverman NH, Finley JP, Law YM, Human DG, Seaward PG, Hamilton RM, Hornberger LK. Endocardial fibroelastosis associated with maternal anti-Ro and anti-La antibodies in the absence of atrioventricular block. Circulation. 2002;40:796–802. doi: 10.1016/s0735-1097(02)02004-1. [DOI] [PubMed] [Google Scholar]
- 8.Nield LE, Silverman ED, Smallhorn JF, Taylor GP, Mullen JB, Benson LN, Hornberger LK. Endocardial fibroelastosis associated with maternal anti-Ro and anti-La antibodies in the absence of atrioventricular block. J Am Coll Cardiol. 2002;40:796–802. doi: 10.1016/s0735-1097(02)02004-1. [DOI] [PubMed] [Google Scholar]
- 9.Guettrot-Imbert G, Cohen L, Fermont L, Villain E, Francès C, Thiebaugeorges O, Foliguet B, Leroux G, Cacoub P, Amoura Z, Piette JC, Costedoat-Chalumeau N. A new presentation of neonatal lupus: 5 cases of isolated mild endocardial fibroelastosis associated with maternal Anti-SSA/Ro and Anti-SSB/La antibodies. J Rheumatol. 2011;38:378–86. doi: 10.3899/jrheum.100317. [DOI] [PubMed] [Google Scholar]
- 10.Brucato A, Frassi M, Franceschini F, Cimaz R, Faden D, Pisoni MP, Muscarà M, Vignati G, Stramba-Badiale M, Catelli L, Lojacono A, Cavazzana I, Ghirardello A, Vescovi F, Gambari PF, Doria A, Meroni PL, Tincani A. Risk of Congenital Complete Heart Block in Newborns of Mothers with Anti-Ro/SSA Antibodies Detected by Counterimmunoelectrophoresis. A prospective Study of 100 Women. Arthritis Rheum. 2001;44:1832–1835. doi: 10.1002/1529-0131(200108)44:8<1832::AID-ART320>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 11.Cimaz R, Spence DL, Hornberger L, Silverman ED. Incidence and spectrum of neonatal lupus erythematosus: a prospective study of infants born to mothers with anti-Ro autoantibodies. J Pediatr. 2003;142:678–83. doi: 10.1067/mpd.2003.233. [DOI] [PubMed] [Google Scholar]
- 12.Costedoat-Chalumeau N, Amoura Z, Lupoglazoff JM, Huong DL, Denjoy I, Vauthier D, Sebbouh D, Fain O, Georgin-Lavialle S, Ghillani P, Musset L, Wechsler B, Duhaut P, Piette JC. Outcome of pregnancies in patients with anti-SSA/Ro antibodies: a study of 165 pregnancies, with special focus on electrocardiographic variations in the children and comparison with a control group. Arthritis Rheum. 2004;50:3187–94. doi: 10.1002/art.20554. [DOI] [PubMed] [Google Scholar]
- 13.Friedman DM, Kim MY, Copel JA, Davis C, Phoon CK, Glickstein JS, Buyon JP PRIDE Investigators. Utility of Cardiac Monitoring in Fetuses at Risk for Congenital Heart Block. The PR interval and Dexamethasone evaluation (PRIDE) Prospective Study. Circulation. 2008;117:485–493. doi: 10.1161/CIRCULATIONAHA.107.707661. [DOI] [PubMed] [Google Scholar]
- 14.Waltuck J, Buyon J. Autoantibody-associated congenital heart block: Outcome in mothers and children. Annals Int Med. 1994;120:544–51. doi: 10.7326/0003-4819-120-7-199404010-00003. [DOI] [PubMed] [Google Scholar]
- 15.Buyon JP, Hiebert R, Copel J, Craft J, Friedman D, Katholi M, Lee LA, Provost TT, Reichlin M, Rider L, Rupel A, Saleeb S, Weston WL, Skovron ML. Autoimmune-associated congenital heart block: Mortality, morbidity, and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol. 1998;31:1658–66. doi: 10.1016/s0735-1097(98)00161-2. [DOI] [PubMed] [Google Scholar]
- 16.Julkunen H, Eronen M. The Rate of Recurrence of Isolated Congenital Heart Block: A Population Based Study. Arthritis Rheum. 2001;44:487–8. doi: 10.1002/1529-0131(200102)44:2<487::AID-ANR70>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 17.Gladman G, Silverman ED, Yuk-Law, Luy L, Boutin C, Laskin C, Smallhorn JF. Fetal echocardiographic screening of pregnancies of mothers with anti-Ro and/or anti-La antibodies. Am J Perinatol. 2002;19:73–80. doi: 10.1055/s-2002-23555. [DOI] [PubMed] [Google Scholar]
- 18.Solomon DG, Rupel A, Buyon JP. Birth order, gender and recurrence rate in autoantibody-associated congenital heart block: implications for pathogenesis and family counseling. Lupus. 2003;12:646–647. doi: 10.1191/0961203303lu425xx. [DOI] [PubMed] [Google Scholar]
- 19.Llanos C, Izmirly PM, Katholi M, Clancy RM, Friedman DM, Kim MY, Buyon JP. Recurrence Rates of Cardiac Manifestations Associated with Neonatal Lupus and Maternal/Fetal Risk Factors. Arthritis Rheum. 2009;60:3091–7. doi: 10.1002/art.24768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izmirly PM, Llanos C, Lee LA, Askanase A, Kim MY, Buyon JP. Cutaneous manifestations of neonatal lupus and risk of subsequent congenital heart block. Arthritis Rheum. 2010;62:1153–7. doi: 10.1002/art.27333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rivera TL, Izmirly PM, Birnbaum BK, Byrne P, Brauth JB, Katholi M, Kim MY, Fischer J, Clancy RM, Buyon JP. Disease progression in mothers of children enrolled in the Research Registry for Neonatal Lupus. Ann Rheum Dis. 2009;68:828–35. doi: 10.1136/ard.2008.088054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaeggi ET, Hornberger LK, Smallhorn JF, Fouron JC. Prenatal diagnosis of complete atrioventricular block associated with structural heart disease: combined experience of two tertiary care centers and review of the literature. Ultrasound Obstet Gynecol. 2005;26:16–21. doi: 10.1002/uog.1919. [DOI] [PubMed] [Google Scholar]
- 23.Villain E, Coastedoat-Chalumeau N, Marijon E, Boudjemline Y, Piette JC, Bonnet D. Presentation and prognosis of complete atrioventricular block in childhood, according to maternal antibody status. J Am Coll Cardiol. 2006;48:1682–7. doi: 10.1016/j.jacc.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 24.Jaeggi ET, Hamilton RM, Silverman ED, Zamora SA, Hornberger LK. Outcome of children with fetal, neonatal or childhood diagnosis of isolated congenital atrioventricular block. A single institution’s experience of 30 years. J Am Coll Cardiol. 2002;39:130–7. doi: 10.1016/s0735-1097(01)01697-7. [DOI] [PubMed] [Google Scholar]
- 25.Lopes LM, Tavares GM, Damiano AP, Lopes MA, Aiello VD, Schultz R, Zugaib M. Perinatal outcome of fetal atrioventricular block: one-hundred-sixteen cases from a single institution. Circulation. 2008;118:1268–75. doi: 10.1161/CIRCULATIONAHA.107.735118. [DOI] [PubMed] [Google Scholar]
- 26.Eliasson H, Gardiner HM, Sharland G, Mellander M, Sonesson SE. Isolated atrioventricular block in the fetus: a retrospective multicentre study of 175 patients. Scandinavian Journal of Immunology. 2010;72:263. [abstract] [Google Scholar]
- 27.Lee LA. Transient autoimmunity related to maternal autoantibodies: neonatal lupus. Autoimmun Rev. 2005;4:207–13. doi: 10.1016/j.autrev.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Clancy RM, Buyon JP, Ikeda K, Nozawa K, Argyle DA, Friedman DM, Chan EK. Maternal antibody responses to the 52-kd SSA/RO p200 peptide and the development of fetal conduction defects. Arthritis Rheum. 2005;52:3079–86. doi: 10.1002/art.21289. [DOI] [PubMed] [Google Scholar]
- 29.Stagopan J, Ben-Porat L, Berwick M, Robson M, Kutler D, Auerbach A. A note on competing risks in survival analysis. British Journal of Cancer. 2004;91:1229–1235. doi: 10.1038/sj.bjc.6602102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee EW, Wei LJ, Amato DA. Cox-type tegression analysis for large numbers of small groups of correlated failure time observations. In: Klein JP, Goel PK, editors. Survival Analysis: State of the Art. Dordrecht: Kluwer Academic Publishers; 1992. pp. 237–247. [Google Scholar]
- 31.Trucco SM, Jaeggi E, Cuneo B, Moon-Grady AJ, Silverman E, Silverman N, Hornberger LK. Use of intravenous gamma globulin and corticosteroids in the treatment of maternal autoantibody-mediated cardiomyopathy. J Am Coll Cardiol. 2011;57:715–23. doi: 10.1016/j.jacc.2010.09.044. [DOI] [PubMed] [Google Scholar]
- 32.Clancy RM, Marion MC, Kaufman KM, Ramos PS, Adler A, Harley JB, Langefeld CD, Buyon JP International Consortium on Systemic Lupus Erythematosus Genetics. Identification of candidate loci at 6p21 and 21q22 in a genome-wide association study of cardiac manifestations of neonatal lupus. Arthritis Rheum. 2010;62:3415–24. doi: 10.1002/art.27658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doria A, Tincani A, Lockshin M. Challenges of lupus pregnancies. Rheumatology (Oxford) 2008;47(Suppl 3):iii9–12. doi: 10.1093/rheumatology/ken151. [DOI] [PubMed] [Google Scholar]
- 34.Petri M. Epidemiology of systemic lupus erythematosus. Best Pract Res Clin Rheumatol. 2002;16:847–58. doi: 10.1053/berh.2002.0259. [DOI] [PubMed] [Google Scholar]
- 35.Lim SS, Drenkard C. Epidemiology of systemic lupus erythematosus: capturing the butterfly. Curr Rheumatol Rep. 2008;10:265–72. doi: 10.1007/s11926-008-0043-4. [DOI] [PubMed] [Google Scholar]