Abstract
The c.1550 g→t mutation in the POLG gene causing the G517V substitution has been reported by many groups to be associated with a variety of mitochondrial diseases, including autosomal dominant and recessive forms of ataxia neuropathy, myopathy and microcephaly, progressive external ophthalmoplegia, diabetes, strokes, hypotonia, and epilepsy. However, the variable disease presentation and age of onset raises suspicion of its pathogenicity. Because of the varied reported associated symptoms and request from physicians to address the consequence of this mutation, we have carried out the biochemical analysis of the purified recombinant human DNA polymerase γ protein harboring the G517V substitution. These analyses revealed that the G517V mutant enzyme retained 80-90% of wild-type DNA polymerase activity, in addition to its functional interaction with the p55 accessory subunit. DNA binding by the mutant was also only slightly lower than the wild-type enzyme. Our data suggest that the G517V mutation by itself in pol γ most likely does not have a role in mitochondrial disorders.
1. Introduction
Mitochondrial DNA is replicated and repaired by DNA polymerase γ (pol γ). In mammalian cells, pol γ is a heterotrimer composed of a large 140 kDa catalytic subunit associated with two 55 kDa accessory proteins as a homodimer (Graziewicz et al., 2006; Kaguni, 2004). The catalytic subunit contains the DNA polymerase active site, a 3′-5′ exonuclease for proofreading, and an intrinsic 5′-dRP lyase activity needed for single nucleotide base excision repair. The p55 homodimer accessory subunit binds asymmetrically to the catalytic subunit and imparts high processivity on the complex by increasing its affinity to DNA (Lee et al., 2009; Lim et al., 1999). The catalytic subunit is encoded by a 23 exon gene on chromosome 15, 15q25 (Ropp and Copeland, 1996). In 2001, the first set of POLG mutations were identified in three families with progressive external opthalmoplegia, (PEO) (Van Goethem et al., 2001). This was followed by several publications reporting signs of PEO and ataxia-neuropathy with mtDNA deletions. In 2004, POLG mutations were identified in two unrelated pedigrees of Alpers-Huttenlocher syndrome (AHS) that caused mtDNA depletion (Naviaux and Nguyen, 2004).
To date more than 160 pathogenic mutations have been reported in the POLG gene as well as several single nucleotide polymorphisms (Stumpf and Copeland, 2011) (see http://tools.niehs.nih.gov/polg/). These mutations are associated with at least six varied disease states that affect humans starting from soon after birth until late in life (Saneto and Naviaux, 2010; Wong et al., 2008). Early infant and childhood presentations of POLG related disorders cause mtDNA depletion and include AHS and myocerebrohepatopathy spectrum disorder with recessive inheritance. Later onset (after 10 yrs of age) POLG mutations usually cause mtDNA deletions and include ataxia-neuropathy, myoclonus epilepsy myopathy sensory ataxia, and autosomal recessive and dominant forms of PEO.
Biochemical analysis of the recombinant human pol γ harboring disease substitutions has been a useful tool for understanding the consequence and mechanism of POLG-related disorders. For example, analysis of four autosomal dominant PEO mutations, G923D, R943H, Y955C, and A957S, demonstrated varied degree of defect, but correlated with the severity of the disease as well as age of onset (Graziewicz et al., 2004). Analysis of pol γ with most common disease mutation, A467T, indicates that the Ala to Thr substitution imparts instability, decline of polymerase activity, and a defect of interacting with the p55 accessory subunit (Chan et al., 2005). More recently, biochemical analysis of four AHS mutations in highly conserved amino acids in the thumb subdomain showed nearly total loss of polymerase activity, concordant with the early presentation and severity of AHS in combination with other POLG mutations (Kasiviswanathan et al., 2009).
One such mutation that has been frequently reported in a variety of conditions and age of presentation is the G517V mutation. The G517V mutation was originally reported in a large study of patients with POLG mutations (Horvath et al., 2006). In this study, the authors identified the G517V mutation as an autosomal dominant mutation in three individuals over three generations in one family with neuropathy or epilepsy. The mutation was also found in 1 control subject out of 672 alleles from German control subjects.
The G517V mutation has since been identified in many patients with varying symptoms. Sarzi et al. reported the G517V heterozygous mutation in a patient with psychomotor retardation that died from Leigh syndrome (Sarzi et al., 2007). Her father also carried this mutation but was asymptomatic. Wong et al. found the G517V mutation as a single heterozygous mutation in 5 unrelated subjects (Wong et al., 2008). These patients displayed variable disease presentation including Leigh-like signs, neuropathy, myopathy, sideroblastic anemia and Pearson syndrome to Kearns-Sayre syndrome. The age of onset in these five patients also varied from 2-16 years of age. Furthermore, the G517V mutation was found as a compound heterozygote in two 1-year old patients, one with D1196N with myopathy and ragged red fibers and the second patient with a R1128H mutation that suffered from microcephaly. However, neither patient demonstrated the typical signs of early POLG-related disorders like AHS.
In 2009, Blok et al. reported four mitochondrial disease patients with the single heterozygous G517V mutation in POLG (Blok et al., 2009). The patients ranged in age from 4-40 years and displayed signs of chronic PEO with ataxia and hypotonia (in the 4 yr old), a single unexplained attack of status epilepticus in a 10 yr old, a 34 yr old with cerebellar ataxia, dystonia and mild retardation, and a 40 year old with mental retardation and chorea while giving birth (Blok et al., 2009).
The G517V POLG mutation was also found as a single heterozygous mutation in a 8.5 month old boy with electron transport deficiency and his 7 year old half sister (Burusnukul and de los Reyes, 2009). The 8.5 month old boy was examined for myoclonic jerks, developmental delay, and epilepsy, and he was found to have impaired fasting glucose tolerance, decreased complex 1 activity (9% of normal), and deceased complex 3 activity (32% of normal). No mitochondrial DNA point mutations or deletions were detected. His 7 year old half sister had symptoms of seizures, migraines and was developmentally delayed.
In another infant case, a 6 month old boy was admitted with myoclonic epilepsy that resembled mitochondrial disease (Bolszak et al., 2009). The G517V mutation was found in this patient in trans with the R722H POLG mutation. The boy was treated with various anti –seizure drugs including valproate. At age 6 and later the brain MRI was normal. The 46 year old mother that carried the G517V mutation was asymptomatic.
Hopkins et al. identified the G517V heterozygous mutation in twin sisters, their sister and mother in a proband that developed type I diabetes, adrenal insufficiency, hypothyroidism and psychiatric problems and concluded an autosomal dominance mode of inheritance (Hopkins et al., 2010). Finally, Schulte et al identified the G517V mutation in a single 44 yr old patient with ataxia plus ophthalmoplegia without neuropathy (Schulte et al., 2009).
Thus, the G517V mutation has been reported as a single heterozygous POLG mutation in patients ranging in age from 8 months to 50 years. As a compound heterozygous mutation with other POLG mutations the patients have been around 1 year of age but the symptoms were very different and one patient apparently recovered. To gain a better understanding of this mutation and determine if the Gly to Val mutation elicits a change in pol γ’s enzymatic activity, we biochemically characterized the recombinant wild type pol γ and G517V variant. We found that the G517V pol γ behaved similarly to the wild type enzyme suggesting that the mutation has no pathological consequence.
2. Materials and Methods
2.1. Construction of G517V mutant pol γ
The G517V mutation in the cDNA encoding the exonuclease deficient (Exo−) pol γ (POLG) was generated using the QuikChange site-directed mutagenesis kit (Stratagene) with the pQVSL11.4 baculoviral transfer vector encoding p140 Exo− without its mitochondrial targeting sequence (Lim and Copeland, 2001) as the template. The exonuclease deficient pol γ without additional mutation is denoted wild-type (WT) in this study. The oligonucleotides used to introduce the G517V mutation (underlined sequence) in POLG are 5′-CCA GCA AGT TGC CCA TCG AGG TGG CTG-3′ and 5′-CAG CCA CCT CGA TGG GCA ACT TGC TGG-3′. The mutation was confirmed by sequencing the pol γ insert in the baculovirus transfer vector.
2.2. Expression and purification
The WT and G517V mutant of the His6 affinity-tagged recombinant catalytic subunit of human pol γ were produced in baculovirus-infected Sf9 cells and the proteins were purified to homogeneity as described previously (Graziewicz et al., 2004; Kasiviswanathan et al., 2010; Longley et al., 1998). The His6 affinity-tagged p55 accessory subunit was expressed in E. coli and purified to homogeneity as described previously (Kasiviswanathan et al., 2010; Longley et al., 2006). After purification, the proteins were frozen in small aliquots in liquid nitrogen and stored at −80°C.
2.3. Enzymatic assays
DNA polymerase activity was determined using the standard pol γ assay with poly(dA)-oligo(dT)12-18 (GE Healthcare) as the primer-template substrate (Kasiviswanathan et al., 2010). For determining steady-state kinetic values, the same assay was performed in the presence and absence of the p55 accessory subunit using poly(dA)-oligo(dT)12-18 as a substrate, with reactions containing 25 mM NaCl as previously described (Lim et al., 1999). The two-subunit form of pol γ was reconstituted as previously described (Lim et al., 1999).
The primer extension analysis of WT and G517V mutant proteins utilized a 5′-32P-labeled 35-mer oligonucleotide (5′-CCA GTG CCA AGC TTG CAT GCC TGC AGG TCG ACT CT-3′), singly-primed M13 ssDNA substrate as described (Longley et al., 1998), without the pre-incubation step. The 10 μl reaction contained 25 mM HEPES-KOH, pH 7.6, 5 mM 2-mercaptoethanol, 5 mM MgCl2, 0.05 mg/ml heat-treated BSA, 0 or 100 mM NaCl, 25 μM dNTPs, 25 fmol of the labeled oligonucleotide, 50 fmol of purified WT or G517V mutant enzyme in the presence or absence of 100 fmol of the p55 accessory subunit. Following incubation at 37°C for 20 min, reactions were terminated and products were analyzed using denaturing polyacrylamide gel electrophoresis, as described (Longley et al., 1998). Gels were dried, exposed to a phosphor screen and visualized with a Typhoon 9400 PhosphorImager (Molecular Dynamics).
2.4. DNA binding assay
The dissociation constant (Kd) for DNA binding for the WT and mutant forms of p140 was determined by electrophoretic mobility shift assay (EMSA), essentially as previously described (Lim et al., 2003). Briefly, a double-stranded primer-template substrate was constructed by hybridizing a 5′-32P-labeled 35-mer (5′-CCA GTG CCA AGC TTG CAT GCC TGC AGG TCG ACT CT-3′) to a 1.2-fold molar excess of an unlabeled, complementary 50-mer (5′-GGT CAC GGT TCG AAC GTA CGG ACG TCC AGC TGA GAT CTC CTA GGG GCC CA-3′). Reaction mixtures (20 μl) were assembled at room temperature and contained 10 mM Tris-HCl (pH 8.0), 0.2 mg/ml acetylated BSA, 2 mM dithiothreitol, 1 pmol of primer-template, and 0, 0.1, 0.3, 0.5, 1, 1.5, 2 or 3 pmol of WT or G517V mutant pol γ protein. After a 5 min incubation, 5 μl of 5X loading buffer (10 mM Tris-HCl (pH 8.0), 0.1% bromphenol blue, 50% glycerol) was added and aliquots of the reaction mixture were subjected to electrophoresis for 1 hr at 180 V at 4°C through an 8% TBE polyacrylamide gel (Invitrogen) in 0.5X TBE (45 mM Tris, 45 mM boric acid, 1 mM EDTA). The gels were dried and exposed on a phosphor storage screen. Radioactivity was imaged on a Typhoon 9400 phosphorimager (GE Healthcare, Piscataway, New Jersey), and bands were quantified with NIH Image software.
3. Results
3.1. The G517V substitution has no major effect on pol γ’s catalytic efficiency
In order to determine the role of G517V substitution on the specific activity of pol γ, polymerase assays were carried out using poly(dA)-oligo(dT)12-18 substrate, as described previously (Graziewicz et al., 2004). The results from this analysis suggested that the G517V mutant retained around 80% activity in comparison to the WT pol γ (111 units/ng for G517V vs. 137 units/ng for WT enzyme, Table 1). In addition, steady-state kinetic measurements performed on the WT and G517V mutant using poly(dA)-oligo(dT)12-18 as a substrate with varying concentrations of dTTP revealed that the overall catalytic efficiency (kcat/Km) of the mutant enzyme was 92% of the WT enzyme activity (kcat/Km = 1.03 μM−1s−1 for WT pol γ vs. kcat/Km = 0.95 μM−1s−1 for G517V pol γ, from Table 1). The addition of p55 accessory subunit had only modest effect on the steady state kinetic parameters of both the WT and mutant enzymes as the mutant retained 95% of the WT enzyme’s catalytic efficiency (compare kcat/Km = 0.44 μM−1s−1 for WT pol γ with kcat/Km = 0.42 μM−1s−1 for G517V pol γ, from Table 1).
Table 1.
Specific activities, steady-state kinetic parameters and DNA binding affinities of WT and G517V pol γ enzymes were determined as described in Materials and Methods. The average values of three independent experiments are shown with errors expressed as standard deviations. ND, not determined.
| Enzyme | Activity (units/ng) |
Steady state kinetics | DNA binding Kd (nM) |
||
|---|---|---|---|---|---|
| Km (μM dTTP) | kcat (s−1) | kcat/Km | |||
| WT | 137 ± 7 | 7.1 ± 0.5 | 7.3 ± 0.3 | 1.03 ± 0.07 | 35 ± 8 |
| WT + p55 | ND | 4.6 ± 0.3 | 2.0 ± 0.1 | 0.44 ± 0.04 | ND |
| G517V | 111 ± 4 | 5.3 ± 0.3 | 5.1 ± 0.2 | 0.95 ± 0.02 | 46 ± 6 |
| G517V + p55 | ND | 4.5 ± 0.7 | 1.9 ± 0.2 | 0.42 ± 0.03 | ND |
3.2. DNA binding affinity of the G517V mutant is similar to the WT enzyme
It has been previously shown that WT pol γ tightly binds to the 3′-end of primer template substrates. Since the G517V mutant enzyme exhibited catalytic activity similar to the WT enzyme, we expected that the mutant enzyme should also bind the 3′end of the primer template with high affinity. To determine the affinity to DNA, electrophoretic mobility shift assays (EMSA) were performed with the WT and G517V mutant form of pol γ. A radiolabeled 35mer/50mer primer-template substrate was incubated with different concentrations of WT or G517V mutant, followed by native polyacrylamide gel electrophoresis to separate protein-DNA complexes from free DNA (Fig 1). The apparent Kd (DNA) values subsequently calculated from the reciprocal plots of the fraction of DNA shifted at various enzyme concentrations revealed that the WT enzyme had strong affinity to DNA (Kd (DNA) = 35 nM, Table 1) as previously reported. The Kd (DNA) for the G517V mutant enzyme was 46 nM (Table 1), which is only 1.3-fold less than the WT pol γ.
Figure 1. DNA binding affinity of WT and G517V pol γ proteins.
Representative gels showing electrophoretic mobility shift assays performed using (A) WT and (B) G517V mutant pol γ enzymes as described in Methods to estimate the Kd (DNA) values reported in table 1. Lanes 1-8 contained 1 pmol substrate; Lanes 1-8 had 0, 0.1, 0.3, 0.5, 1, 1.5, 2 or 3 pmol of WT (A) or G517V mutant (B) pol γ protein respectively. S, substrate; P, protein-DNA complex.
3.3. G517V mutant interacts with the p55 accessory subunit
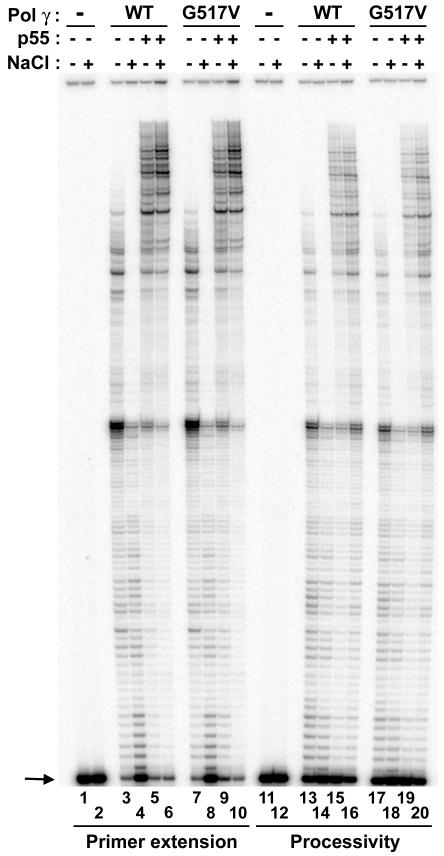
The p55 accessory subunit has been shown to physically interact with the pol γ catalytic subunit and this interaction functionally translates to enhanced processivity and DNA binding for the catalytic subunit. Hence, to check whether p55 stimulates the functional activity of the WT and G517V mutant form of the catalytic subunit, primer extension and processivity assays were carried out. The difference between the primer extension and processivity assay is that the former permits multiple binding events for the polymerase to the 3′-end of the primer terminus substrate, while the latter is a single binding event between the polymerase and DNA substrate. The single binding event can be achieved by adding a DNA trap to the reaction, onto which the polymerase will bind after dissociating from the end-labeled primer template substrate.
Primer extension analysis revealed that the WT enzyme extended approximately 100 nucleotides in the absence of NaCl (Fig 2, lane 3) and the activity was moderately inhibited in the presence of 100 mM NaCl (Fig 2, lane 4). However, the addition of p55 to the reaction stimulated the ability of WT pol γ to extend primers both in the absence and presence of NaCl (Fig 2, compare lanes 3 and 4 to lanes 5 and 6). The G517V mutant pol γ exhibited similar extension patterns both in the absence and presence of NaCl and p55 (Fig 1, compare lanes 3-6 with lanes 7-10). Similar results were obtained with the processivity assays, however the overall amount of extended products were lower compared to the primer extension analysis for both the WT and the G517V mutant enzymes (Fig 2, compare lanes 3-6 with lanes 13-16 and lanes 7-10 with lanes 17-20). These experiments demonstrate that the G517V mutant pol γ functionally interactes with the p55 accessory subunit in a manner indistinguishable from the WT enzyme.
Figure 2. Functional interaction of WT and G517V pol γ with the p55 accessory subunit.
Primer extension (lanes 1-10) and processivity (lanes 11-20) assays were performed using WT and G517V pol γ enzymes in the presence and absence of p55 on singly primed M13 DNA as described in Methods. Reactions contained 25 fmol substrate (all lanes), 50 fmol pol γ exo− (lanes 3-6 and 13-16, WT; lanes 7-10 and 17-20, G517V), 100 fmol p55 accessory subunit (lanes 5, 6, 9, 10, 15, 16, 19 and 20). Activity was measured at 0 mM NaCl (odd numbered lanes) and 100 mM NaCl (even numbered lanes). Lanes 1, 2, 11 and 12 had no enzyme. Arrow indicates position of 35-mer primer.
4. Discussion
Due to the controversial description and varied signs and symptoms reports associated with the POLG G517V mutation, we felt compelled to carry out a thorough kinetic and biochemical analysis of this mutant variant and compare it to the wild type DNA pol γ. We found that the G517V polymerase had similar biochemical properties as the wild type enzyme. Comparisons of the kcat/Km ratios indicate that the G517V variant had over 90% WT enzyme activity. Such subtle differences in vitro are interpreted as within the error of the assay and do not indicate a significant difference. A sensitive measurement of the interaction of the p55 accessory subunit with the catalytic subunit is to test the effect of p55 on pol γ’s processivity. Processivity is the number of nucleotides incorporated per DNA binding event, and we showed in 1999 that the p55 subunit greatly enhances the processivity of the holoenzyme by increasing its affinity to DNA (Lim et al., 1999). We found that the activity profiles of the G517V mutant and wild type enzyme appear identical in the presence of p55 (Fig. 2), indicating no detectable defect in physical and functional association of the mutant enzyme with the accessory subunit.
Glycine 517 of human DNA pol γ is conserved only in mammals but not in Xenopus, Drosophila and other lower eukaryotic organisms such as S. cerevisiae or S. pombe (Fig 3A). Inspection of the crystal structure of the holoenzyme (Lee et al., 2009) indicates that Gly517 is located in the AID (Accessory Interacting Determinant, amino acid residues 511-570) subdomain that interacts with the p55 accessory subunit (Fig. 3B). Sequence alignment of POLG from various organisms revealed that S. cerevisiae, S. pombe and other unicellular eukaryotes that don’t have an accessory subunit (Lucas et al., 2004) also lack the AID subdomain. However, the Gly517 in human pol γ is not in a position to make direct contact with the accessory subunit or DNA and hence it is difficult to predict the consequence of a Val substitution at this position (Fig 3B).
Figure 3. Amino acid alignment and three-dimensional structure of the human DNA polymerase γ holoenzyme with Gly517 highlighted.

A. Clustal multiple alignment of a segment of the DNA pol γ amino acid sequence around the Gly517 area. Blue highlighted sequences are conserved and amino acid 517 is shown in red and marked with an asterisk. B. Three-dimensional structure of the human DNA polymerase holoenzyme. The p55 accessory subunit homodimer is colored in green, the pol γ catalytic subunit in blue where light blue depicts the polymerase active site and dark blue structure contains the 3′→5′ exonuclease. Gly517 is shaded in yellow and is located on the solvent accessible surface of the AID (Accessory Interacting Determinant) subdomain away from the p55 accessory subunit.
Several reports regarding the G517V mutation classified the role of this mutation in mitochondrial disease as unclear (Blok et al., 2009; Wong et al., 2008). Furthermore, G517V has been detected at a frequency of 1.1% in a Spanish control group (Rivera et al., 2010). However, the pathogenic A467T mutation exists at a frequency of 0.6% in some European populations (Van Goethem et al., 2003), which suggest that high frequency alone is not a good measure of the lack of pathogenicity. This study corroborates these suspicions and further demonstrates no biochemical defect from this mutation. Our biochemical analysis indicates that the G517V mutation as a heterozygous mutation is most likely not involved in the reported symptoms or plays a very minor role in the disease progression. However, similar to the E1143G single nucleotide polymorphism and its affect on the W748S mutation, the G517V mutation in conjunction with other pol γ mutations may play a role in modulating a disease phenotype (Chan et al., 2006). We conclude that the G517V mutation by itself in pol γ is either a neutral polymorphism and/or displays only minor defects, inconsistent with its proposed role in disease. This work stresses the need for comprehensive genetic analysis as well as biochemical analysis before a pathological role of a mutation can be assigned.
Acknowledgements
We thank Dr. Tammy Collins and Maggie Humble for critically reading this manuscript. This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (ES 065078 and ES 065080).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blok MJ, Van den Bosch BJ, Jongen E, Hendrickx A, de Die-Smulders CE, Hoogendijk JE, Brusse E, de Visser M, Poll-The BT, Bierau J, de Coo IF, Smeets HJ. The unfolding clinical spectrum of POLG mutations. J Med Genet. 2009;46:776–785. doi: 10.1136/jmg.2009.067686. [DOI] [PubMed] [Google Scholar]
- Bolszak M, Anttonen AK, Komulainen T, Hinttala R, Pakanen S, Sormunen R, Herva R, Lehesjoki AE, Majamaa K, Rantala H, Uusimaa J. Digenic mutations in severe myoclonic epilepsy of infancy. Epilepsy Res. 2009;85:300–304. doi: 10.1016/j.eplepsyres.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Burusnukul P, de los Reyes EC. Phenotypic variations in 3 children with POLG1 mutations. J Child Neurol. 2009;24:482–486. doi: 10.1177/0883073808324539. [DOI] [PubMed] [Google Scholar]
- Chan SSL, Longley MJ, Copeland WC. The common A467T mutation in the human mitochondrial DNA polymerase (POLG) compromises catalytic efficiency and interaction with the accessory subunit. J Biol Chem. 2005;280:31341–31346. doi: 10.1074/jbc.M506762200. [DOI] [PubMed] [Google Scholar]
- Chan SSL, Longley MJ, Copeland WC. Modulation of the W748S mutation in DNA polymerase {gamma} by the E1143G polymorphism in mitochondrial disorders. Human molecular genetics. 2006;15:3473–3483. doi: 10.1093/hmg/ddl424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziewicz MA, Longley MJ, Bienstock RJ, Zeviani M, Copeland WC. Structure-function defects of human mitochondrial DNA polymerase in autosomal dominant progressive external ophthalmoplegia. Nat Struct Mol Biol. 2004;11:770–776. doi: 10.1038/nsmb805. [DOI] [PubMed] [Google Scholar]
- Graziewicz MA, Longley MJ, Copeland WC. DNA polymerase gamma in Mitochondrial DNA Replication and Repair. Chemical Reviews. 2006;106:383–405. doi: 10.1021/cr040463d. [DOI] [PubMed] [Google Scholar]
- Hopkins SE, Somoza A, Gilbert DL. Rare autosomal dominant POLG1 mutation in a family with metabolic strokes, posterior column spinal degeneration, and multi-endocrine disease. J Child Neurol. 2010;25:752–756. doi: 10.1177/0883073809343313. [DOI] [PubMed] [Google Scholar]
- Horvath R, Hudson G, Ferrari G, Futterer N, Ahola S, Lamantea E, Prokisch H, Lochmuller H, McFarland R, Ramesh V, Klopstock T, Freisinger P, Salvi F, Mayr JA, Santer R, Tesarova M, Zeman J, Udd B, Taylor RW, Turnbull D, Suomalainen A, Zeviani M, Chinnery PF. Phenotypic spectrum associated with mutations of the mitochondrial polymerase {gamma} gene. Brain. 2006;129:1674–1684. doi: 10.1093/brain/awl088. [DOI] [PubMed] [Google Scholar]
- Kaguni LS. DNA polymerase gamma, the mitochondrial replicase. Annu Rev Biochem. 2004;73:293–320. doi: 10.1146/annurev.biochem.72.121801.161455. [DOI] [PubMed] [Google Scholar]
- Kasiviswanathan R, Longley MJ, Chan SS, Copeland WC. Disease mutations in the human mitochondrial DNA polymerase thumb subdomain impart severe defects in MtDNA replication. J Biol Chem. 2009;284:19501–19510. doi: 10.1074/jbc.M109.011940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasiviswanathan R, Longley MJ, Young MJ, Copeland WC. Purification and functional characterization of human mitochondrial DNA polymerase gamma harboring disease mutations. Methods. 2010;51:379–384. doi: 10.1016/j.ymeth.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Kennedy WD, Yin YW. Structural insight into processive human mitochondrial DNA synthesis and disease-related polymerase mutations. Cell. 2009;139:312–324. doi: 10.1016/j.cell.2009.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SE, Copeland WC. Differential incorporation and removal of antiviral deoxynucleotides by human DNA polymerase gamma. J Biol Chem. 2001;276:23616–23623. doi: 10.1074/jbc.M101114200. [DOI] [PubMed] [Google Scholar]
- Lim SE, Longley MJ, Copeland WC. The mitochondrial p55 accessory subunit of human DNA polymerase gamma enhances DNA binding, promotes processive DNA synthesis, and confers N-ethylmaleimide resistance. J Biol Chem. 1999;274:38197–38203. doi: 10.1074/jbc.274.53.38197. [DOI] [PubMed] [Google Scholar]
- Lim SE, Ponamarev MV, Longley MJ, Copeland WC. Structural Determinants in Human DNA Polymerase gamma Account for Mitochondrial Toxicity from Nucleoside Analogs. J Mol Biol. 2003;329:45–57. doi: 10.1016/s0022-2836(03)00405-4. [DOI] [PubMed] [Google Scholar]
- Longley MJ, Clark S, Yu Wai Man C, Hudson G, Durham SE, Taylor RW, Nightingale S, Turnbull DM, Copeland WC, Chinnery PF. Mutant POLG2 Disrupts DNA Polymerase gamma Subunits and Causes Progressive External Ophthalmoplegia. Am J Hum Genet. 2006;78:1026–1034. doi: 10.1086/504303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longley MJ, Ropp PA, Lim SE, Copeland WC. Characterization of the native and recombinant catalytic subunit of human DNA polymerase gamma: identification of residues critical for exonuclease activity and dideoxynucleotide sensitivity. Biochemistry. 1998;37:10529–10539. doi: 10.1021/bi980772w. [DOI] [PubMed] [Google Scholar]
- Lucas P, Lasserre JP, Plissonneau J, Castroviejo M. Absence of accessory subunit in the DNA polymerase gamma purified from yeast mitochondria. Mitochondrion. 2004;4:13–20. doi: 10.1016/j.mito.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Naviaux RK, Nguyen KV. POLG Mutations associated with Alpers’ Syndrome and Mitochondrial DNA Depletion. Ann Neurol. 2004;55:706–712. doi: 10.1002/ana.20079. [DOI] [PubMed] [Google Scholar]
- Rivera H, Merinero B, Martinez-Pardo M, Arroyo I, Ruiz-Sala P, Bornstein B, Serra-Suhe C, Gallardo E, Marti R, Moran MJ, Ugalde C, Perez-Jurado LA, Andreu AL, Garesse R, Ugarte M, Arenas J, Martin MA. Marked mitochondrial DNA depletion associated with a novel SUCLG1 gene mutation resulting in lethal neonatal acidosis, multi-organ failure, and interrupted aortic arch. Mitochondrion. 2010;10:362–368. doi: 10.1016/j.mito.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Ropp PA, Copeland WC. Cloning and characterization of the human mitochondrial DNA polymerase, DNA polymerase gamma. Genomics. 1996;36:449–458. doi: 10.1006/geno.1996.0490. [DOI] [PubMed] [Google Scholar]
- Saneto RP, Naviaux RK. Polymerase gamma disease through the ages. Dev Disabil Res Rev. 2010;16:163–174. doi: 10.1002/ddrr.105. [DOI] [PubMed] [Google Scholar]
- Sarzi E, Bourdon A, Chretien D, Zarhrate M, Corcos J, Slama A, Cormier-Daire V, de Lonlay P, Munnich A, Rotig A. Mitochondrial DNA depletion is a prevalent cause of multiple respiratory chain deficiency in childhood. J Pediatr. 2007;150:531–534. doi: 10.1016/j.jpeds.2007.01.044. 534 e531-536. [DOI] [PubMed] [Google Scholar]
- Schulte C, Synofzik M, Gasser T, Schols L. Ataxia with ophthalmoplegia or sensory neuropathy is frequently caused by POLG mutations. Neurology. 2009;73:898–900. doi: 10.1212/WNL.0b013e3181b78488. [DOI] [PubMed] [Google Scholar]
- Stumpf JD, Copeland WC. Mitochondrial DNA replication and disease: insights from DNA polymerase gamma mutations. Cell Mol Life Sci. 2011;68:219–233. doi: 10.1007/s00018-010-0530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Goethem G, Dermaut B, Lofgren A, Martin JJ, Van Broeckhoven C. Mutation of POLG is associated with progressive external ophthalmoplegia characterized by mtDNA deletions. Nature genetics. 2001;28:211–212. doi: 10.1038/90034. [DOI] [PubMed] [Google Scholar]
- Van Goethem G, Martin JJ, Dermaut B, Lofgren A, Wibail A, Ververken D, Tack P, Dehaene I, Van Zandijcke M, Moonen M, Ceuterick C, De Jonghe P, Van Broeckhoven C. Recessive POLG mutations presenting with sensory and ataxic neuropathy in compound heterozygote patients with progressive external ophthalmoplegia. Neuromuscul Disord. 2003;13:133–142. doi: 10.1016/s0960-8966(02)00216-x. [DOI] [PubMed] [Google Scholar]
- Wong LJ, Naviaux RK, Brunetti-Pierri N, Zhang Q, Schmitt ES, Truong C, Milone M, Cohen BH, Wical B, Ganesh J, Basinger AA, Burton BK, Swoboda K, Gilbert DL, Vanderver A, Saneto RP, Maranda B, Arnold G, Abdenur JE, Waters PJ, Copeland WC. Molecular and clinical genetics of mitochondrial diseases due to POLG mutations. Hum Mutat. 2008;29:E150–E172. doi: 10.1002/humu.20824. [DOI] [PMC free article] [PubMed] [Google Scholar]